Algae Extract Increases Seed Production of Soybean Plants and Alters Nitrogen Metabolism
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental Design and Treatments
2.2. Samplings
2.3. Enzyme Activities
2.4. Nitrogen Compounds and Pigments
2.5. Nutrient Leaf Concentration
2.6. Gene Expression by RT-qPCR
2.7. Yield-Related Components
2.8. Statistical Analyzes
3. Results
3.1. Soybean Growth and Productivity
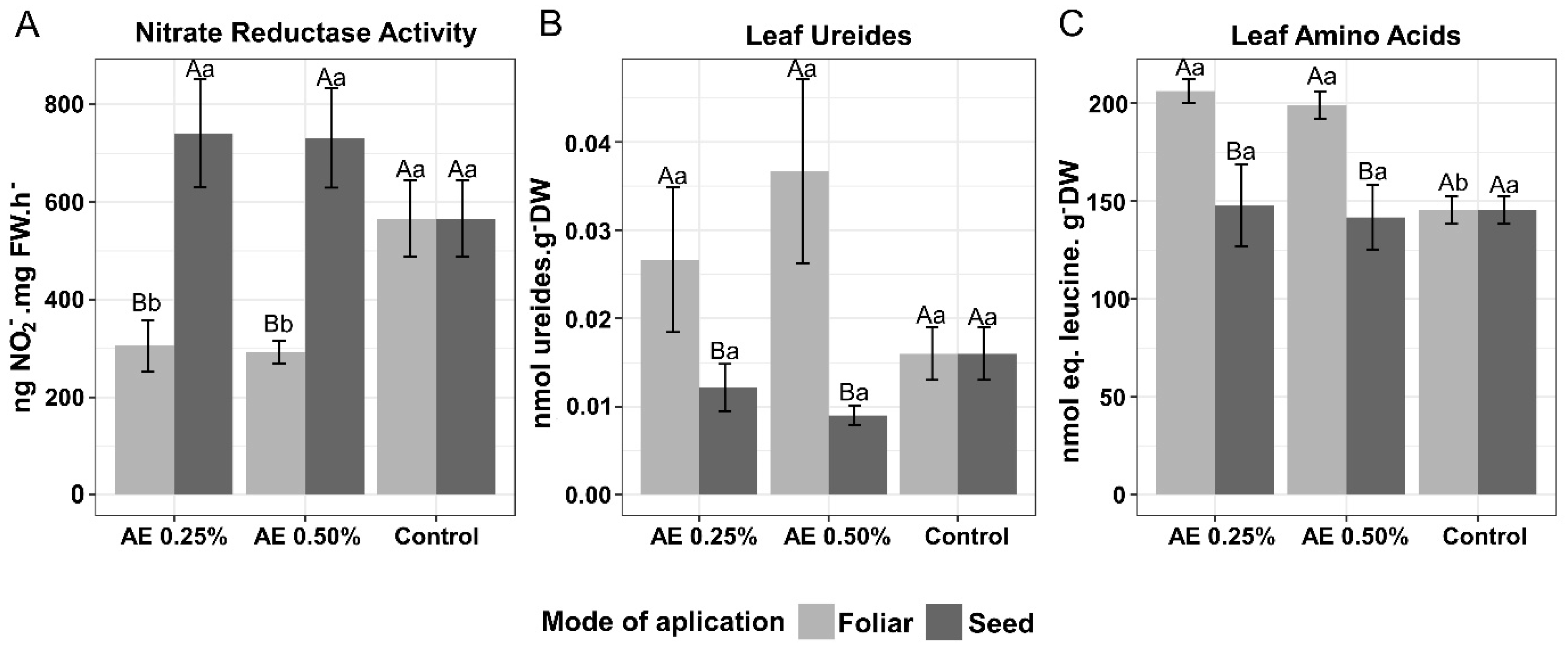
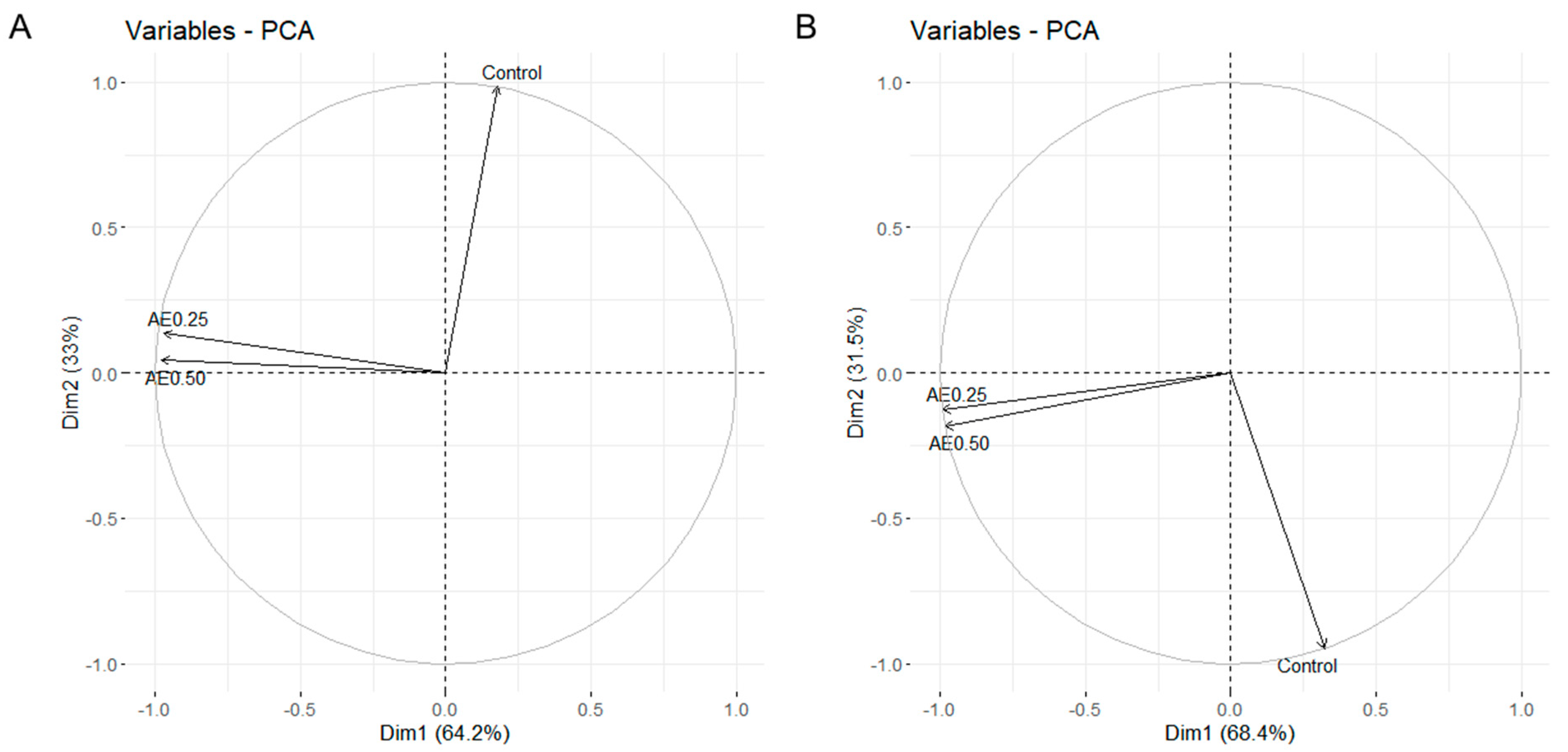
3.2. Enzyme Activities, Nitrogen Compounds and Pigments
3.3. Leaf Nutrient Content
3.4. Gene Expression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CONAB—Companhia Nacional de Abastecimento. Acompanhamento da Safra Brasileira de Grãos; Safra 2021/22, 9º Levantamento; Conab: Brasília, Brazil, 2022; p. 59. [Google Scholar]
- Tamagno, S.; Balboa, G.R.; Assefa, Y.; Kovács, P.; Casteel, S.N.; Salvagiotti, F.; García, F.O.; Stewart, W.M.; Ciampitti, I.A. Nutrient partitioning and stoichiometry in soybean: A synthesis-analysis. Field Crop. Res. 2017, 200, 18–27. [Google Scholar] [CrossRef]
- Cafaro La Menza, N.; Monzon, J.P.; Lindquist, J.L.; Arkebauer, T.J.; Knops, J.M.H.; Unkovich, M.; Specht, J.E.; Grassini, P. Insufficient nitrogen supply from symbiotic fixation reduces seasonal crop growth and nitrogen mobilization to seed in highly productive soybean crops. Plant Cell Environ. 2020, 43, 1958–1972. [Google Scholar] [CrossRef]
- Hungria, M.; Nogueira, M.A. Fixação biológica do nitrogênio. In Bioinsumos na Cultura da Soja; Meyer, M.C., Ed.; Embrapa: Brasília, Brazil, 2022; p. 550. ISBN 978-65-87380-96-4. [Google Scholar]
- Hungria, M.; Mendes, I.C. Nitrogen fixation with soybean: The perfect symbiosis? In Biological Nitrogen Fixation; Wiley Online Library: Vitural, 2015; pp. 1009–1024. [Google Scholar]
- Krapp, A. Plant nitrogen assimilation and its regulation: A complex puzzle with missing pieces. Curr. Opin. Plant Biol. 2015, 25, 115–122. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [PubMed]
- Shargool, D.; Jain, J.C.; McKay, G. Ornithine biosynthesis, and arginine biosynthesis and degradation in plant cells. Phytochemistry 1988, 27, 1571–1574. [Google Scholar] [CrossRef]
- Dixon, N.E.; Riddles, P.W.; Gazzola, C.; Blakeley, R.L.; Zerner, B. Jack bean urease (EC 3.5.1.5). V. On the mechanism of action of urease on urea, formamide, acetamide, N-methylurea, and related compounds. Can. J. Biochem. 1980, 58, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- De Pascale, S.; Rouphael, Y.; Colla, G. Plant biostimulants: Innovative tool for enhancing plant nutrition in organic farming. Eur. J. Hortic. Sci. 2017, 82, 277–285. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- EBIC—European Biostimulant Industry Council. Available online: https://biostimulants.eu/ (accessed on 14 January 2022).
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The use of biostimulants for enhancing nutrient uptake. Adv. Agron. 2015, 130, 141–174. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Win, T.T.; Barone, G.D.; Secundo, F.; Fu, P. Algal biofertilizers and plant growth stimulants for sustainable agriculture. Ind. Biotechnol. 2018, 14, 203–211. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- du Jardin, P.; Xu, L.; Geelen, D. Agricultural functions and action mechanisms of plant biostimulants (PBs): An introduction. In The Chemical Biology of Plant Biostimulants; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; pp. 1–30. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Pohl, A.; Kalisz, A.; Sekara, A. Seaweed extracts’ multifactorial action: Influence on physiological and biochemical status of Solanaceae plants. Acta Agrobot. 2019, 72, 1758. [Google Scholar] [CrossRef]
- Omidbakhshfard, M.A.; Sujeeth, N.; Gupta, S.; Omranian, N.; Guinan, K.J.; Brotman, Y.; Nikoloski, Z.; Fernie, A.R.; Mueller-Roeber, B.; Gechev, T.S. A biostimulant obtained from the seaweed Ascophyllum nodosum protects Arabidopsis thaliana from severe oxidative stress. Int. J. Mol. Sci. 2020, 21, 474. [Google Scholar] [CrossRef]
- Shukla, P.S.; Shotton, K.; Norman, E.; Neily, W.; Critchley, A.T.; Prithiviraj, B. Seaweed extract improve drought tolerance of soybean by regulating stress-response genes. AoB Plants 2018, 10, plx051. [Google Scholar] [CrossRef]
- Goñi, O.; Fort, A.; Quille, P.; McKeown, P.C.; Spillane, C.; O’Connell, S. Comparative transcriptome analysis of two Ascophyllum nodosum extract biostimulants: Same seaweed but different. J. Agric. Food Chem. 2016, 64, 2980–2989. [Google Scholar] [CrossRef] [PubMed]
- De Saeger, J.; Van Praet, S.; Vereecke, D.; Park, J.; Jacques, S.; Han, T.; Depuydt, S. Toward the molecular understanding of the action mechanism of Ascophyllum nodosum extracts on plants. J. Appl. Phycol. 2020, 32, 573–597. [Google Scholar] [CrossRef]
- Thibodeau, P.S.; Jaworski, E.G. Patterns of nitrogen utilization in the soybean. Planta 1975, 127, 133–147. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 29–31. [Google Scholar]
- Souza, S.C.R.; Mazzafera, P.; Sodek, L. Flooding of the root system in soybean: Biochemical and molecular aspects of N metabolism in the nodule during stress and recovery. Amino Acids 2016, 48, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Foy, C.D.; Fleming, A.L. Aluminum tolerances of two wheat genotypes related to nitrate reductase activities. J. Plant Nutr. 1982, 5, 1313–1333. [Google Scholar] [CrossRef]
- Rhodes, D.; Rendon, G.A.; Stewart, G.R. The control of glutamine synthetase level in Lemna minor L. Planta 1975, 125, 201–211. [Google Scholar] [CrossRef]
- Dougall, D.K. Evidence for the presence of glutamate synthase in extracts of carrot cells cultures. Biochem. Biophys. Res. Commun. 1974, 58, 639–646. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Yemm, W.E.; Cocking, E.; Ricketts, E.R. The determination of amino acids with ninhydrin. Analyst 1955, 80, 209–214. [Google Scholar] [CrossRef]
- Vogels, G.D.; Van Der Drift, C. Differential analyses of glyoxylate derivatives. Anal. Biochem. 1970, 33, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- de Souza, S.C.R.; Sodek, L.; Polacco, J.C.; Mazzafera, P. Urease deficiency alters nitrogen metabolism and gene expression in urease-null soybean without affecting growth or productivity under nitrate supply. Acta Physiol. Plant. 2020, 42, 34. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ferreira, E.; Cavalcanti, P.; Nogueira, D. ExpDes: An R Package for ANOVA and Experimental Designs. Appl. Math. 2014, 5, 2952–2958. [Google Scholar] [CrossRef]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant biostimulants: Importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress—A review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- Kocira, S.; Szparaga, A.; Kuboń, M.; Czerwińska, E.; Piskier, T. Morphological and biochemical responses of Glycine max (L.) Merr. to the use of seaweed extract. Agronomy 2019, 9, 93. [Google Scholar] [CrossRef]
- Egli, D.B.; Bruening, W.P. Source-sink relationships, seed sucrose levels and seed growth rates in soybean. Ann. Bot. 2001, 88, 235–242. [Google Scholar] [CrossRef]
- Misra, A.N.; Biswal, U.C. Effect of phytohormones on chlorophyll degradation during aging of chloroplasts in vivo and in vitro. Protoplasma 1980, 105, 1–8. [Google Scholar] [CrossRef]
- Cortleven, A.; Schmülling, T. Regulation of chloroplast development and function by cytokinin. J. Exp. Bot. 2015, 66, 4999–5013. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant properties of seaweed extracts in plants: Implications towards sustainable crop Production. Plants 2021, 10, 531. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, K.; Gopalakrishnan, V.A.K.; Kumar, R.; Ghosh, A. Transcriptional analysis of maize leaf tissue treated with seaweed extract under drought stress. Front. Sustain. Food Syst. 2021, 5, 774978. [Google Scholar] [CrossRef]
- Jannin, L.; Arkoun, M.; Etienne, P.; Laîné, P.; Goux, D.; Garnica, M.; Fuentes, M.; Francisco, S.S.; Baigorri, R.; Cruz, F.; et al. Brassica napus growth is promoted by Ascophyllum nodosum (L.) Le Jol. seaweed extract: Microarray analysis and physiological characterization of N, C, and S metabolisms. J. Plant Growth Regul. 2013, 32, 31–52. [Google Scholar] [CrossRef]
- Rathore, S.S.; Chaudhary, D.R.; Boricha, G.N.; Ghosh, A.; Bhatt, B.P.; Zodape, S.T.; Patolia, J.S. Effect of seaweed extract on the growth, yield and nutrient uptake of soybean (Glycine max) under rainfed conditions. S. Afr. J. Bot. 2009, 75, 351–355. [Google Scholar] [CrossRef]
- Nelson, W.; Staden, J. The effect of seaweed concentrate on growth of nutrient-stressed greenhouse cucumbers. HortScience 1984, 19, 81–82. [Google Scholar] [CrossRef]
- Turan, M.; Köse, C. Seaweed extracts improve copper uptake of grapevine. Acta Agric. Scand. Sect. B Soil Plant Sci. 2004, 54, 213–220. [Google Scholar] [CrossRef]
- Ritchie, S.; Hanway, J.J.; Thompson, H.E.; Benson, G.O. How a Soybean Plant Develops; Iowa State University of Science and Technology Cooperative Extension Service: Ames, IA, USA, 1982; 20p. [Google Scholar]



| Gene | ID Phytozome | Primer F | Primer R |
|---|---|---|---|
| Nitrate reductase (NR) | Glyma.13G084000 | GCGATTTTGAAGGACCCAGA | TTGCGATTTCCACCACGTAC |
| Nitrite reductase (NiR) | Glyma.02G132100 | ACATTGGTTTCATGGGGTGC | ACAACAGGCACCAAGTCCTT |
| Glutamine synthetase (GS) | Glyma.02G127500 | TGGGGAAGCAATGGAGAAGA | AACCAAGCCGAGTGACCAAT |
| Glutamate synthetase (GOGAT) | Glyma.14G162300 | TTGCAGAGAAGTTGGGTGTG | CTTGCCGTCCCTCTGAAATC |
| 10 Nitrate transporter 1 (NTR1) | Glyma.02G011600 | AGGCTTGTATGAGGTTCCTGT | ACACTCCAAAAGCGACAGTT |
| Asparagine synthetase | Glyma.11g38130 | TAGGCTCACTGTTCCTGGAG | ACCCTTGTTGTTCTGGTTTTCA |
| Arginase (ARGAH) | Glyma.17G097800 | TGAGAGAACTCGCTGCAAAG | TGAGAGAACTCGCTGCAAAG |
| Urease (UreUU) | Glyma 2.0 | ATCAAAGGTGGTGAGGTTGC | ATCAAAGGTGGTGAGGTTGC |
| AE 0.25% | AE 0.50% | Control | |
|---|---|---|---|
| Leaf dry weight (g) | 23.14 a | 24.46 a | 21.24 b |
| Stem dry weight (g) | 28.53 b | 32.62 a | 28.10 b |
| Root dry weight (g) | 40.52 a | 35.30 a | 40.99 a |
| Total nodules dry weight (g) | 3.91 a | 3.91 a | 3.68 a |
| Dry weight per nodule (mg) | 4.84 a | 4.82 a | 4.87 a |
| Nodules per plant | 811.38 a | 845.13 a | 758.88 a |
| AE 0.25% | AE 0.50% | Control | |
|---|---|---|---|
| Plant height (cm) | 73.33 ab | 72.42 a | 68.83 b |
| Number of nodes per plant | 69.08 ab | 70.5 a | 64.83 b |
| Pods per plant | 141.67 a | 147.17 a | 120.33 b |
| Pods dry weight per plant (g) | 59.71 a | 61.83 a | 46.10 b |
| Seeds/plant | 343.22 a | 351.97 a | 284.02 b |
| Seeds dry weight per plant (g) | 39.54 a | 39.91 a | 28.7 b |
| Seeds per pod | 2.42 a | 2.40 a | 2.37 a |
| Individual seed dry weight (g) | 0.12 a | 0.11 a | 0.10 b |
| AE 0.25% | AE 0.50% | Control | |
|---|---|---|---|
| GS Activity (ΔAbsorbance.µg protein−1·h−1) | 1.32 a | 0.63 b | 0.85 ab |
| GOGAT Activity (ΔAbsorbance.µg protein−1·h−1) | 6.44 a | 8.69 a | 8.04 a |
| Leaf Nitrate (µg NO3−·g− fresh weight) | 1.36 a | 1.39 a | 1.35 a |
| Xylem Sap Nitrate (µg NO3− µL) | 0.61 a | 0.58 a | 0.64 a |
| Xylem Sap Ureides (nmol ureides·mL−) | 0.016 b | 0.016 b | 0.036 a |
| Xylem Sap Amino Acids (nmol eq. leucine·mL−) | 2.28 b | 2.22 b | 3.89 a |
| Chlorophyll a | 0.39 a | 0.38 a | 0.39 a |
| Chlorophyll b | 0.37 b | 0.47 a | 0.40 ab |
| Chlorophyll total | 0.76 a | 0.85 a | 0.79 a |
| Carotenoids | 0.083 a | 0.042 b | 0.073 ab |
| Treatments | N | P | Ca | K | Mg | S | |||
|---|---|---|---|---|---|---|---|---|---|
| Foliar | Seed | Foliar | Seed | Foliar | Seed | ||||
| AE 0.25% | 40.83 a | 1.79 a | 17.30 a | 18.87 Aa | 16.13 Bab | 3.52 Ab | 3.43 Aa | 2.00 Aa | 1.47 Ba |
| AE 0.50% | 39.43 a | 1.81 a | 17.53 a | 17.70 Aa | 15.00 Bb | 3.89 Aa | 3.30 Ba | 1.53 Ab | 1.43 Aa |
| Control | 41.42 a | 1.81 a | 17.45 a | 17.15 Aa | 17.15 Aa | 3.37 Ab | 3.37 Aa | 1.58 Ab | 1.58 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engel, D.C.H.; Feltrim, D.; Rodrigues, M.; Baptistella, J.L.C.; Mazzafera, P. Algae Extract Increases Seed Production of Soybean Plants and Alters Nitrogen Metabolism. Agriculture 2023, 13, 1296. https://doi.org/10.3390/agriculture13071296
Engel DCH, Feltrim D, Rodrigues M, Baptistella JLC, Mazzafera P. Algae Extract Increases Seed Production of Soybean Plants and Alters Nitrogen Metabolism. Agriculture. 2023; 13(7):1296. https://doi.org/10.3390/agriculture13071296
Chicago/Turabian StyleEngel, Daniele Caroline Hörz, Daniela Feltrim, Mayara Rodrigues, João Leonardo Corte Baptistella, and Paulo Mazzafera. 2023. "Algae Extract Increases Seed Production of Soybean Plants and Alters Nitrogen Metabolism" Agriculture 13, no. 7: 1296. https://doi.org/10.3390/agriculture13071296
APA StyleEngel, D. C. H., Feltrim, D., Rodrigues, M., Baptistella, J. L. C., & Mazzafera, P. (2023). Algae Extract Increases Seed Production of Soybean Plants and Alters Nitrogen Metabolism. Agriculture, 13(7), 1296. https://doi.org/10.3390/agriculture13071296







