Maize On-Farm Stressed Area Identification Using Airborne RGB Images Derived Leaf Area Index and Canopy Height
Abstract
1. Introduction
- Airborne RGB images were used to prepare temporal crop-LAI and crop-height maps, based on previously published techniques;
- A process-based crop model (APSIM) was simulated for both optimal and actual farm conditions to obtain height and LAI values;
- These simulated values, also referred to as synthetic values, were then used to build models for generating a crop healthiness index;
- The models were subsequently evaluated with the maps prepared using airborne RGB images and validated with the field observations.
2. Materials and Methods
2.1. Data
2.2. APSIM Model
2.3. Linear Model for Crop Healthiness
2.4. Random Forest Model for Crop Healthiness
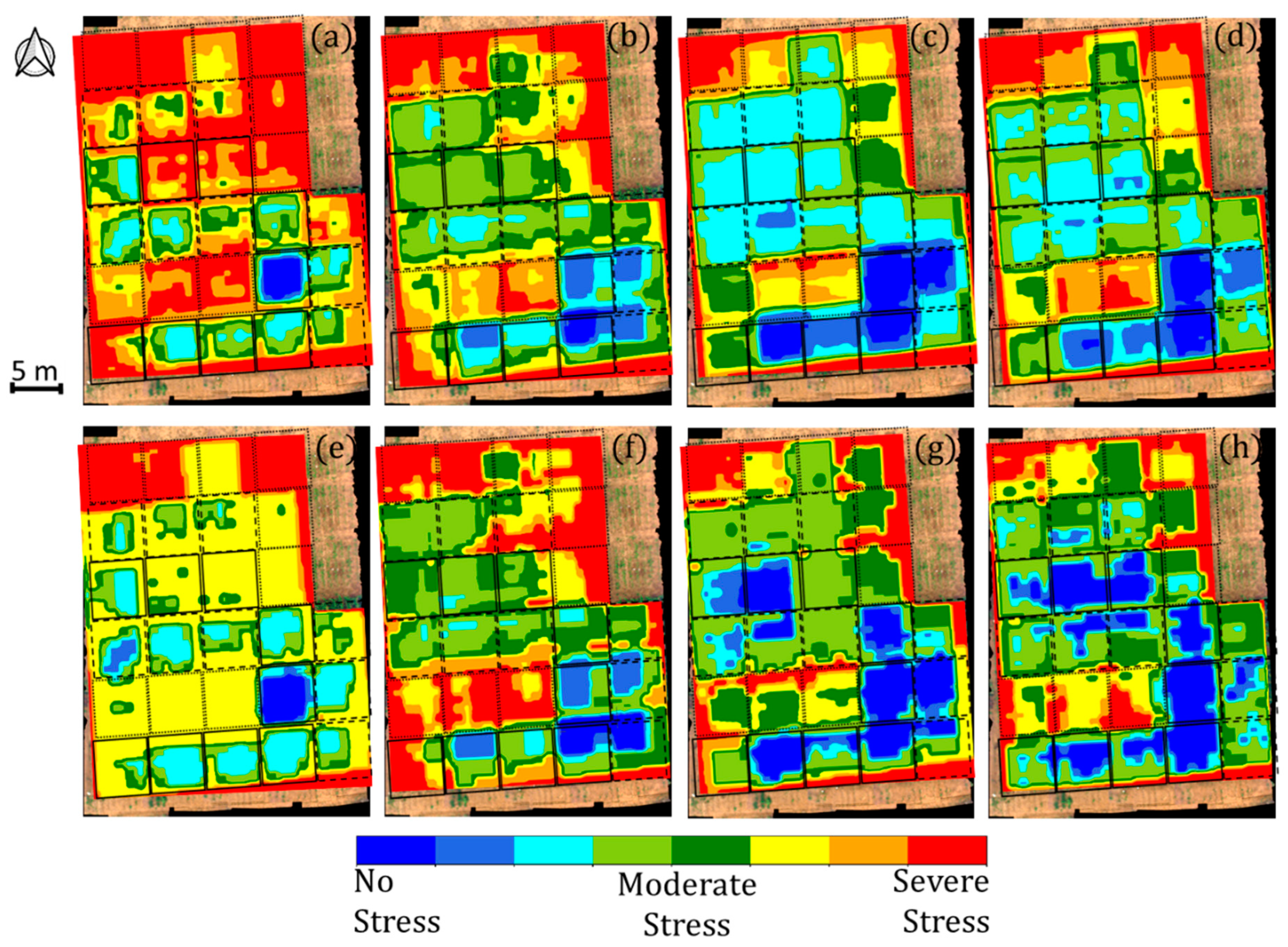
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional review board statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glendining, M.J.; Dailey, A.G.; Williams, A.G.; Van Evert, F.K.; Goulding, K.W.T.; Whitmore, A.P. Is it possible to increase the sustainability of arable and ruminant agriculture by reducing inputs? Agric. Syst. 2009, 99, 117–125. [Google Scholar] [CrossRef]
- Maraveas, C.; Piromalis, D.; Arvanitis, K.G.; Bartzanas, T.; Loukatos, D. Applications of IoT for optimized greenhouse environment and resources management. Comput. Electron. Agric. 2022, 198, 106993. [Google Scholar] [CrossRef]
- Ragavi, V.; Subburaj, J.; Keerthana, P.; Soundaryaveni, C. Smart Agriculture to increase Farmers Profitability using Internet of Things. Indian J. Sci. Technol. 2019, 12, 1–6. [Google Scholar] [CrossRef]
- Daponte, P.; De Vito, L.; Glielmo, L.; Iannelli, L.; Liuzza, D.; Picariello, F.; Silano, G. A Review on the Use of Drones for Precision Agriculture; IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 275, p. 012022. [Google Scholar]
- Adrian, A.M.; Norwood, S.H.; Mask, P.L. Producers’ perceptions and attitudes toward precision agriculture technologies. Comput. Electron. Agric. 2005, 48, 256–271. [Google Scholar] [CrossRef]
- Broge, N.H.; Mortensen, J.V. Deriving green crop area index and canopy chlorophyll density of winter wheat from spectral reflectance data. Remote Sens. Environ. 2002, 81, 45–57. [Google Scholar] [CrossRef]
- Radočaj, D.; Šiljeg, A.; Marinović, R.; Jurišić, M. State of Major Vegetation Indices in Precision Agriculture Studies Indexed in Web of Science: A Review. Agriculture 2023, 13, 707. [Google Scholar] [CrossRef]
- Sun, H. Crop Vegetation Indices. In Encyclopedia of Smart Agriculture Technologies; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–7. [Google Scholar]
- Mwinuka, P.R.; Mourice, S.K.; Mbungu, W.B.; Mbilinyi, B.P.; Tumbo, S.D.; Schmitter, P. UAV-based multispectral vegetation indices for assessing the interactive effects of water and nitrogen in irrigated horticultural crops production under tropical sub-humid conditions: A case of African eggplant. Agric. Water Manag. 2022, 266, 107516. [Google Scholar] [CrossRef]
- Ihuoma, S.O.; Madramootoo, C.A. Sensitivity of spectral vegetation indices for monitoring water stress in tomato plants. Comput. Electron. Agric. 2019, 163, 104860. [Google Scholar] [CrossRef]
- Zhang, L.; Han, W.; Niu, Y.; Chávez, J.L.; Shao, G.; Zhang, H. Evaluating the sensitivity of water stressed maize chlorophyll and structure based on UAV derived vegetation indices. Comput. Electron. Agric. 2021, 185, 106174. [Google Scholar] [CrossRef]
- Townshend, J.R.; Goff, T.E.; Tucker, C.J. Multitemporal dimensionality of images of normalized difference vegetation index at continental scales. IEEE Trans. Geosci. Remote Sens. 1985, 6, 888–895. [Google Scholar] [CrossRef]
- Becker, F.; Choudhury, B.J. Relative sensitivity of normalized difference vegetation Index (NDVI) and microwave polarization difference Index (MPDI) for vegetation and desertification monitoring. Remote Sens. Environ. 1988, 24, 297–311. [Google Scholar] [CrossRef]
- Pettorelli, N. The normalized difference vegetation index; Oxford University Press: Hong Kong, China, 2013. [Google Scholar]
- Suárez, L.; Zarco-Tejada, P.J.; Sepulcre-Cantó, G.; Pérez-Priego, O.; Miller, J.R.; Jiménez-Muñoz, J.C.; Sobrino, J. Assessing canopy PRI for water stress detection with diurnal airborne imagery. Remote Sens. Environ. 2008, 112, 560–575. [Google Scholar] [CrossRef]
- Han, M.; Zhang, H.; DeJonge, K.C.; Comas, L.H.; Trout, T.J. Estimating maize water stress by standard deviation of canopy temperature in thermal imagery. Agric. Water Manag. 2016, 177, 400–409. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Zohaib, A.; Tanveer, M.; Naeem, M.; Ali, I.; Tabassum, T.; Nazir, U. Growth and development responses of crop plants under drought stress: A review. Zemdirbyste 2017, 104, 267–276. [Google Scholar] [CrossRef]
- Baret, F.; Houles, V.; Guerif, M. Quantification of plant stress using remote sensing observations and crop models: The case of nitrogen management. J. Exp. Bot. 2007, 58, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Wittstruck, L.; Jarmer, T.; Trautz, D.; Waske, B. Estimating LAI From Winter Wheat Using UAV Data and CNNs. IEEE Geosci. Remote Sens. Lett. 2022, 19, 3141497. [Google Scholar] [CrossRef]
- Yucky, E.D.D.; Putrada, A.G.; Abdurohman, M. IoT drone camera for a paddy crop health detector with RGB comparison. In Proceedings of the 2021 9th International Conference on Information and Communication Technology (ICoICT), Yogyakarta, Indonesia, 3–5 August 2021; IEEE: Piscataway, NJ, USA; pp. 155–159. [Google Scholar]
- Garza, B.N.; Ancona, V.; Enciso, J.; Perotto-Baldivieso, H.L.; Kunta, M.; Simpson, C. Quantifying Citrus Tree Health Using True Color UAV Images. Remote Sens. 2020, 12, 170. [Google Scholar] [CrossRef]
- Candiago, S.; Remondino, F.; De Giglio, M.; Dubbini, M.; Gattelli, M. Evaluating Multispectral Images and Vegetation Indices for Precision Farming Applications from UAV Images. Remote Sens. 2015, 7, 4026–4047. [Google Scholar] [CrossRef]
- Catania, P.; Roma, E.; Orlando, S.; Vallone, M. Evaluation of Multispectral Data Acquired from UAV Platform in Olive Orchard. Horticulturae 2023, 9, 133. [Google Scholar] [CrossRef]
- Franke, J.; Menz, G. Multi-temporal wheat disease detection by multi-spectral remote sensing. Precis. Agric. 2007, 8, 161–172. [Google Scholar] [CrossRef]
- Shafi, U.; Mumtaz, R.; Iqbal, N.; Zaidi, S.M.H.; Zaidi, S.A.R.; Hussain, I.; Mahmood, Z. A Multi-Modal Approach for Crop Health Mapping Using Low Altitude Remote Sensing, Internet of Things (IoT) and Machine Learning. IEEE Access 2020, 8, 112708–112724. [Google Scholar] [CrossRef]
- Khan, A.; Vibhute, A.D.; Mali, S.; Patil, C. A systematic review on hyperspectral imaging technology with a machine and deep learning methodology for agricultural applications. Ecol. Informatics 2022, 69, 101678. [Google Scholar] [CrossRef]
- Krishna, G.; Sahoo, R.N.; Singh, P.; Patra, H.; Bajpai, V.; Das, B.; Kumar, S.; Dhandapani, R.; Vishwakarma, C.; Pal, M.; et al. Application of thermal imaging and hyperspectral remote sensing for crop water deficit stress monitoring. Geocarto Int. 2021, 36, 481–498. [Google Scholar] [CrossRef]
- Vasavi, P.; Punitha, A.; Rao, T.V.N. Crop leaf disease detection and classification using machine learning and deep learning algorithms by visual symptoms: A review. Int. J. Electr. Comput. Eng. 2022, 12, 2079–2086. [Google Scholar] [CrossRef]
- Raj, R.; Walker, J.P.; Pingale, R.; Nandan, R.; Naik, B.; Jagarlapudi, A. Leaf area index estimation using top-of-canopy airborne RGB images. Int. J. Appl. Earth Obs. Geoinf. 2021, 96, 102282. [Google Scholar] [CrossRef]
- Keating, B.A.; Carberry, P.S.; Hammer, G.L.; Probert, M.E.; Robertson, M.J.; Holzworth, D.; Huth, N.I.; Hargreaves, J.N.; Meinke, H.; Hochman, Z.; et al. An overview of APSIM, a model designed for farming systems simulation. Eur. J. Agron. 2003, 18, 267–288. [Google Scholar] [CrossRef]
- Luo, S.; Wang, C.; Xi, X.; Nie, S.; Fan, X.; Chen, H.; Yang, X.; Peng, D.; Lin, Y.; Zhou, G. Combining hyperspectral imagery and LiDAR pseudo-waveform for predicting crop LAI, canopy height and above-ground biomass. Ecol. Indic. 2019, 102, 801–812. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, S.; Li, X.; Cunha, M.; Jayavelu, S.; Cammarano, D.; Fu, Y. Machine Learning-Based Approaches for Predicting SPAD Values of Maize Using Multi-Spectral Images. Remote Sens. 2022, 14, 1337. [Google Scholar] [CrossRef]
- Belgiu, M.; Drăguţ, L. Random forest in remote sensing: A review of applications and future directions. ISPRS J. Photogramm. Remote Sens. 2016, 114, 24–31. [Google Scholar] [CrossRef]
- Bolton, D.K.; Friedl, M.A. Forecasting crop yield using remotely sensed vegetation indices and crop phenology metrics. Agric. For. Meteorol. 2013, 173, 74–84. [Google Scholar] [CrossRef]
- Sanches, G.M.; Duft, D.G.; Kölln, O.T.; Luciano, A.C.D.S.; De Castro, S.G.Q.; Okuno, F.M.; Franco, H.C.J. The potential for RGB images obtained using unmanned aerial vehicle to assess and predict yield in sugarcane fields. Int. J. Remote Sens. 2018, 39, 5402–5414. [Google Scholar] [CrossRef]
- Nielsen, D.C.; Halvorson, A.D. Nitrogen Fertility Influence on Water Stress and Yield of Winter Wheat. Agron. J. 1991, 83, 1065–1070. [Google Scholar] [CrossRef]
- Wilhelm, W.W.; Ruwe, K.; Schlemmer, M.R. Comparison of Three Leaf Area Index Meters in a Corn Canopy. Crop Sci. 2000, 40, 1179–1183. [Google Scholar] [CrossRef]
- Gano, B.; Dembele, J.S.B.; Ndour, A.; Luquet, D.; Beurier, G.; Diouf, D.; Audebert, A. Using UAV Borne, Multi-Spectral Imaging for the Field Phenotyping of Shoot Biomass, Leaf Area Index and Height of West African Sorghum Varieties under Two Contrasted Water Conditions. Agronomy 2021, 11, 850. [Google Scholar] [CrossRef]
- Zhang, L.; Niu, Y.; Zhang, H.; Han, W.; Li, G.; Tang, J.; Peng, X. Maize Canopy Temperature Extracted From UAV Thermal and RGB Imagery and Its Application in Water Stress Monitoring. Front. Plant Sci. 2019, 10, 1270. [Google Scholar] [CrossRef]
- Peng, X.; Han, W.; Ao, J.; Wang, Y. Assimilation of LAI Derived from UAV Multispectral Data into the SAFY Model to Estimate Maize Yield. Remote Sens. 2021, 13, 1094. [Google Scholar] [CrossRef]
- Holzman, M.E.; Rivas, R.; Piccolo, M.C. Estimating soil moisture and the relationship with crop yield using surface temperature and vegetation index. Int. J. Appl. Earth Obs. Geoinformation 2014, 28, 181–192. [Google Scholar] [CrossRef]
- Al-Gaadi, K.A.; Hassaballa, A.A.; Tola, E.; Kayad, A.G.; Madugundu, R.; Alblewi, B.; Assiri, F. Prediction of Potato Crop Yield Using Precision Agriculture Techniques. PLoS ONE 2016, 11, e0162219. [Google Scholar] [CrossRef]
- Ji, Z.; Pan, Y.; Zhu, X.; Wang, J.; Li, Q. Prediction of Crop Yield Using Phenological Information Extracted from Remote Sensing Vegetation Index. Sensors 2021, 21, 1406. [Google Scholar] [CrossRef]

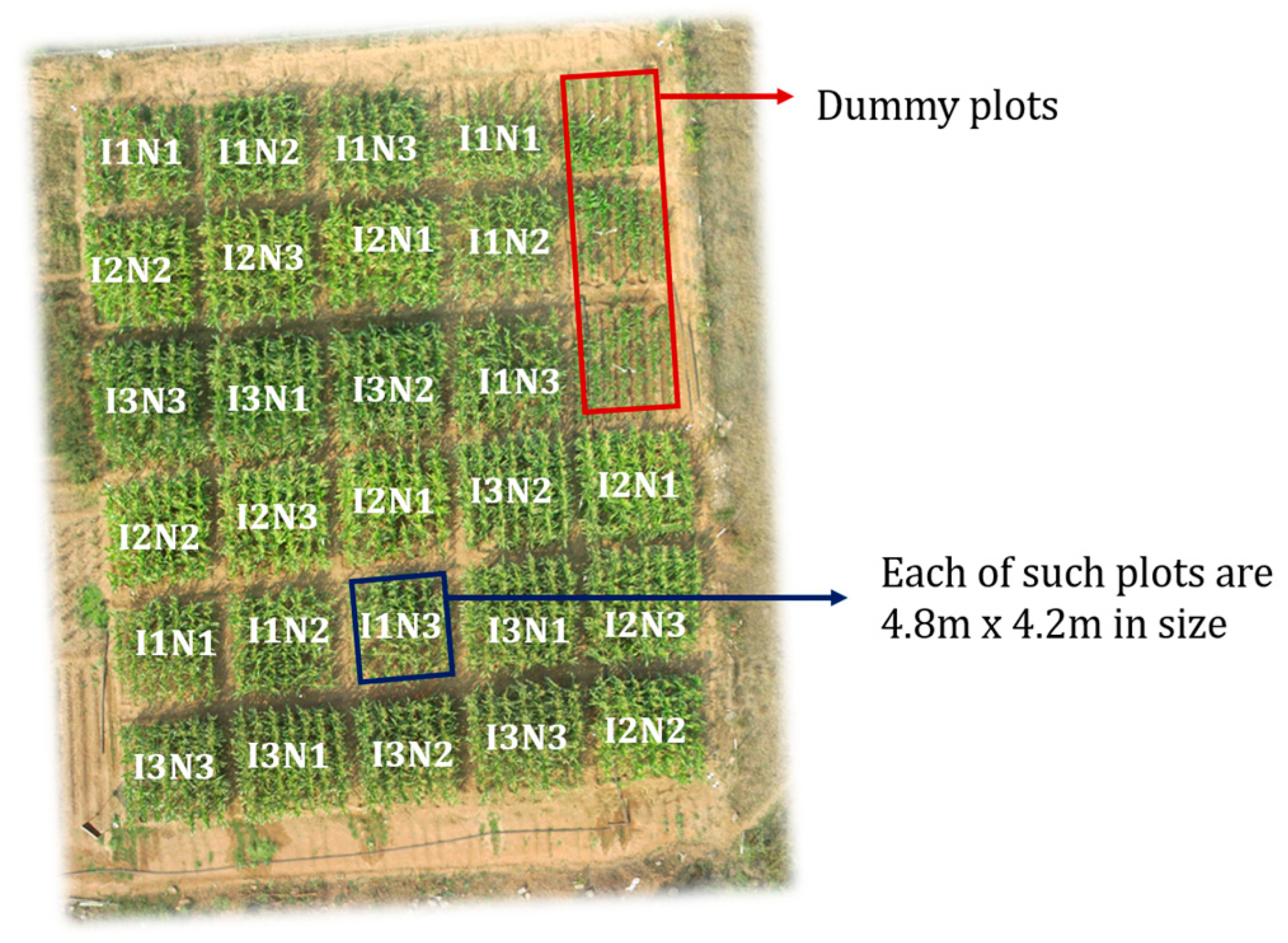
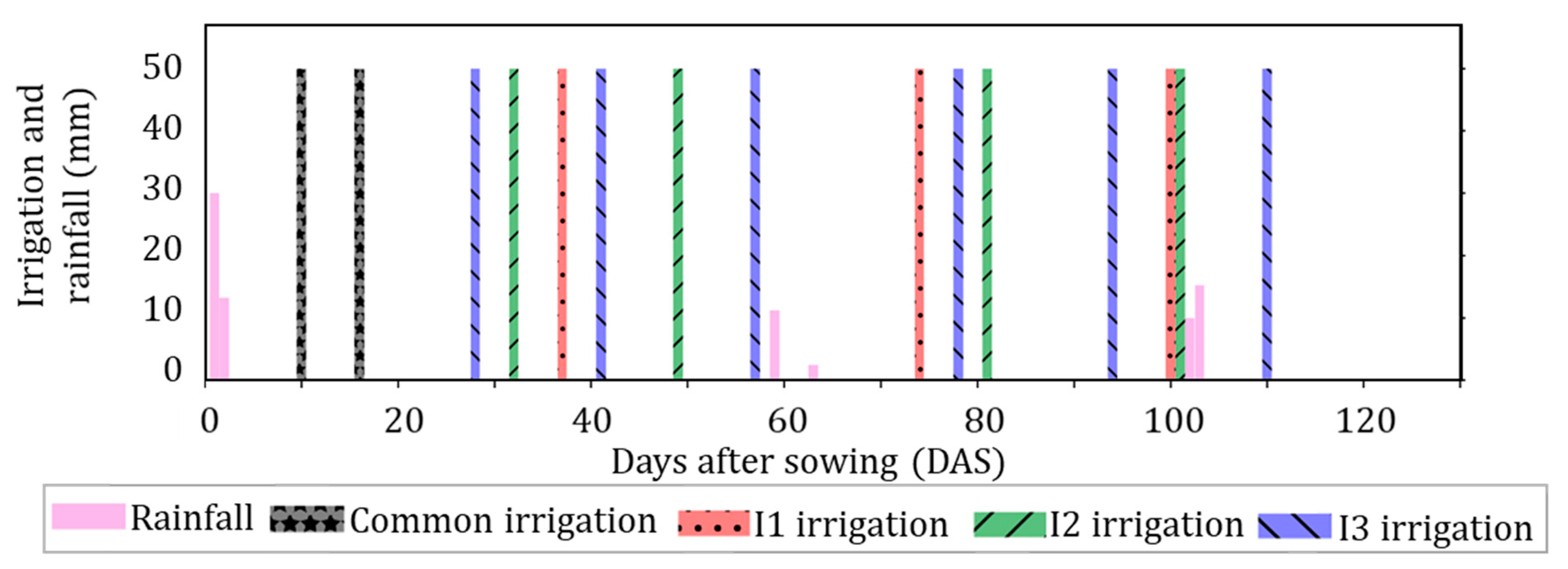
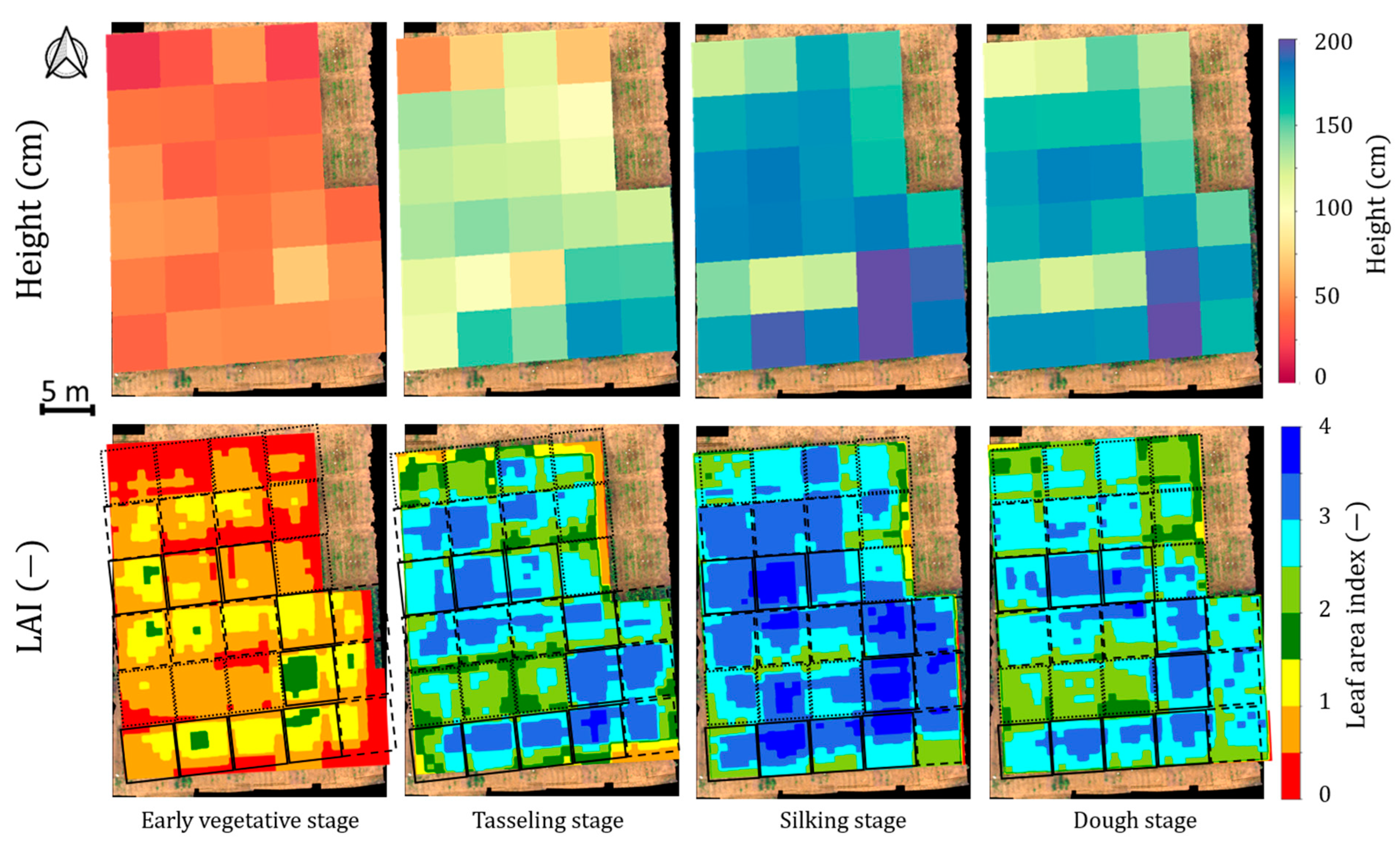
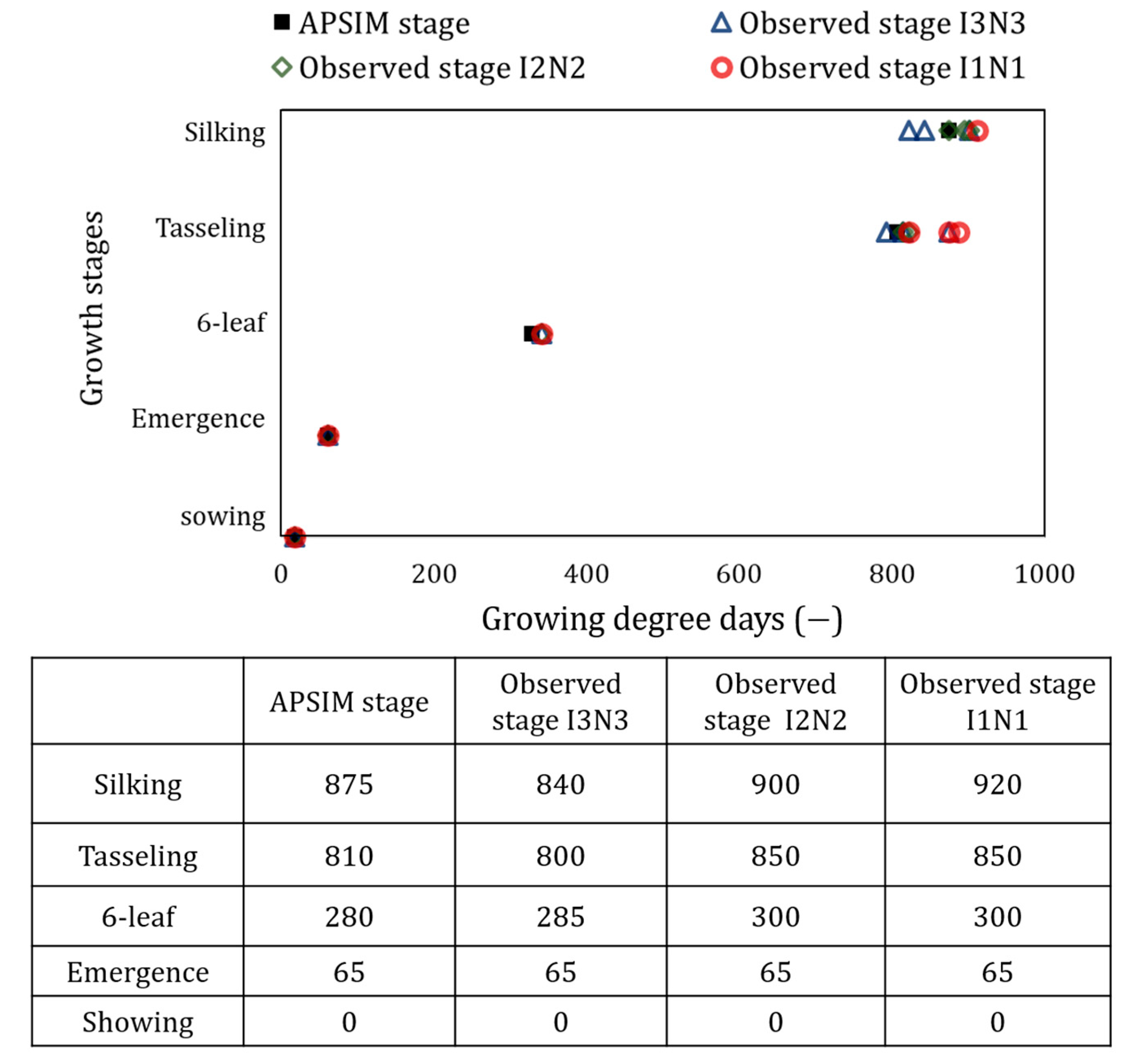
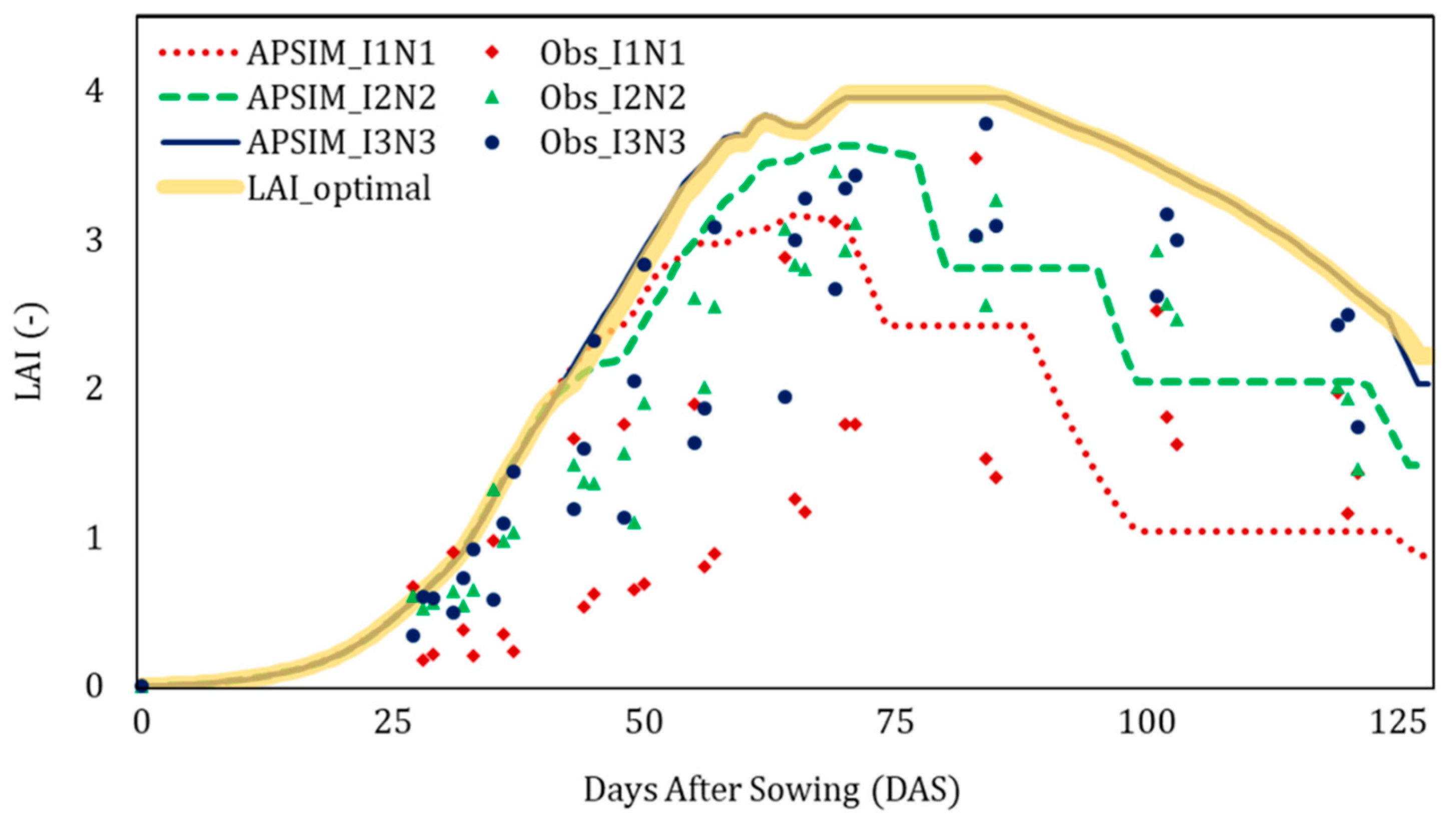
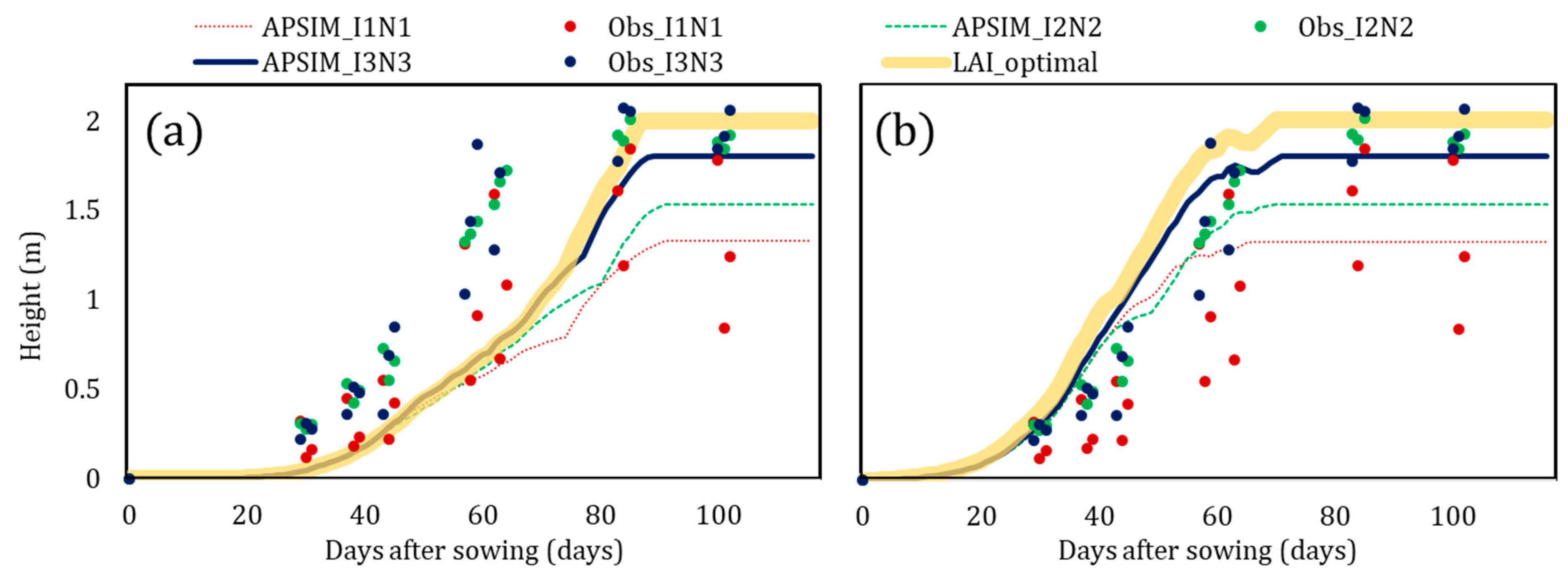
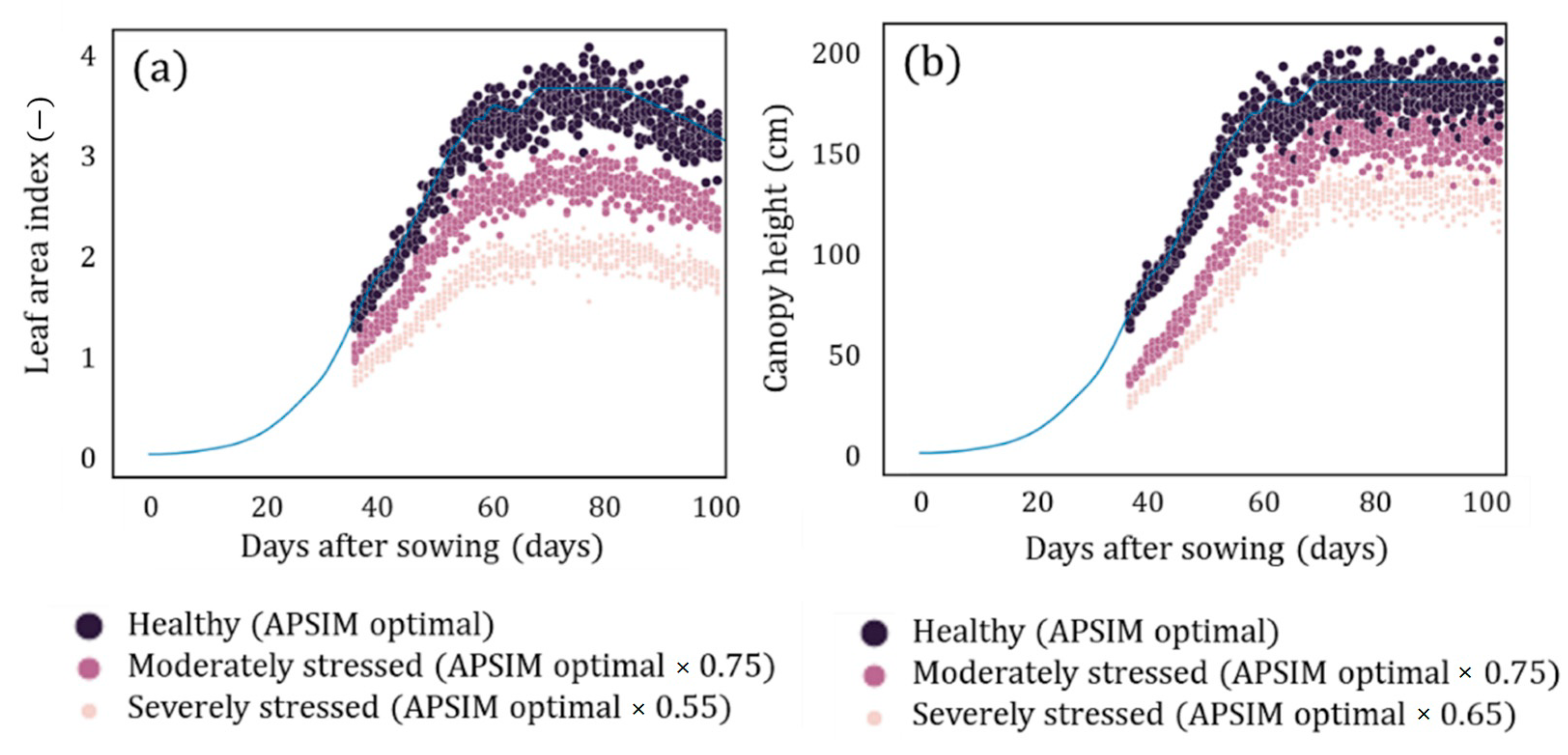

| Data | Details | Source |
|---|---|---|
| Temporal canopy height map | A farm map with plot-wise (4.2 m × 4.8 m) height values | The map has been developed using Raj et al., 2021, [29] protocol |
| Temporal canopy LAI map | A farm map with high spatial resolution (1 m × 1 m) LAI values | The map has been developed using Raj et al., 2021, [29] protocol |
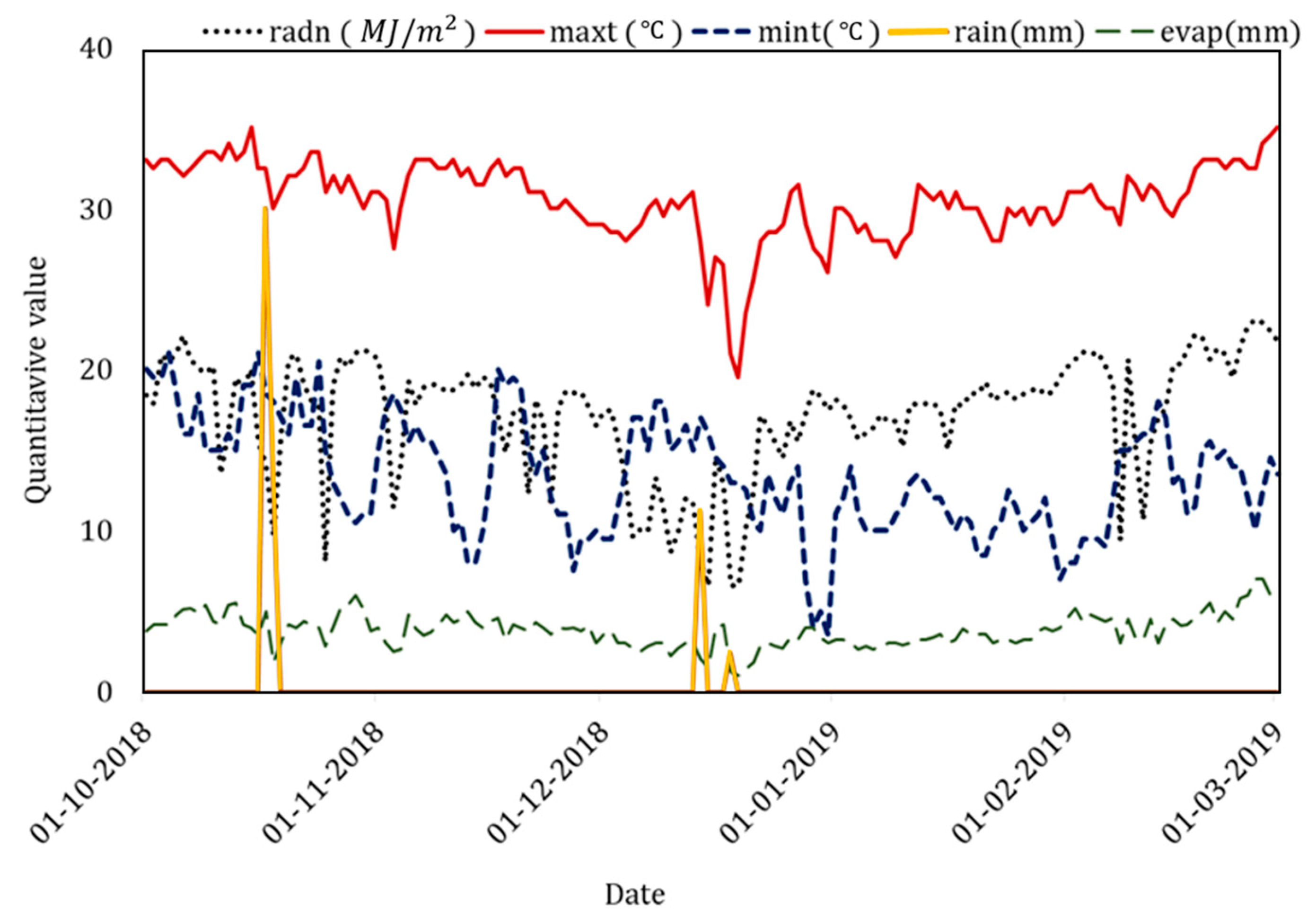
| Weather data | Daily solar irradiance, rainfall, evaporation, minimum and maximum temperature | Automatic weather station beside farm and managed by the India Meteorological Department (IMD) |
| Soil properties | Soil type, soil composition, soil depth, field capacity | On-farm physical investigation |
| Crop yield | Plot-wise crop yield weighted at crop maturity | On-farm weighing of the maize cobs |
| Soil Depth (cm) | Soil Type | Clay (%) | Sand (%) | Silt (%) | OC (%) | EC (%) | Gravel (%) | BD (g/cc) | WP (% Vol) | FC (% Vol) | Sat (% Vol) | AW (cm/cm) | SHC (mm/h) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 | Sandy loam | 18 | 79 | 3.2 | 0.6 | 0.5 | 33 | 1.5 | 11.1 | 17.6 | 42.5 | 0.05 | 24.8 |
| 30 | 16 | 73 | 11.4 | 0.1 | 0.2 | 37 | 1.6 | 9.5 | 16.3 | 38.8 | 0.05 | 17.5 | |
| 40 | 18 | 69 | 13.4 | 0.1 | 0.4 | 60 | 1.6 | 10.7 | 18.3 | 38.7 | 0.04 | 8.6 | |
| 50 | 18 | 67 | 15.2 | 0.1 | 0.3 | 37 | 1.6 | 10.8 | 18.4 | 36.9 | 0.06 | 10.1 | |
| 60 | 18 | 69 | 13.2 | 0.1 | 0.2 | 36 | 1.5 | 10.7 | 18.7 | 40.6 | 0.06 | 16.8 | |
| 70 | 17 | 69 | 13.4 | 0.2 | 0.3 | 33 | 1.5 | 10.2 | 18.6 | 42.6 | 0.07 | 23.6 | |
| 80 | 18 | 65 | 16.9 | 0.2 | 0.4 | 35 | 1.4 | 10.9 | 20.4 | 45.0 | 0.07 | 24.9 | |
| 90 | 20 | 69 | 11.4 | 0.1 | 0.4 | 27 | 1.6 | 12.0 | 19.7 | 49.8 | 0.06 | 14.4 | |
| 100 | 18 | 69 | 13.2 | 0.1 | 0.4 | 48 | 1.5 | 10.8 | 19.1 | 42.9 | 0.05 | 17.9 |
| Parameter | Healthy Condition | Severe Stress Condition |
|---|---|---|
| LAI | ) | |
| Canopy height |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raj, R.; Walker, J.P.; Jagarlapudi, A. Maize On-Farm Stressed Area Identification Using Airborne RGB Images Derived Leaf Area Index and Canopy Height. Agriculture 2023, 13, 1292. https://doi.org/10.3390/agriculture13071292
Raj R, Walker JP, Jagarlapudi A. Maize On-Farm Stressed Area Identification Using Airborne RGB Images Derived Leaf Area Index and Canopy Height. Agriculture. 2023; 13(7):1292. https://doi.org/10.3390/agriculture13071292
Chicago/Turabian StyleRaj, Rahul, Jeffrey P. Walker, and Adinarayana Jagarlapudi. 2023. "Maize On-Farm Stressed Area Identification Using Airborne RGB Images Derived Leaf Area Index and Canopy Height" Agriculture 13, no. 7: 1292. https://doi.org/10.3390/agriculture13071292
APA StyleRaj, R., Walker, J. P., & Jagarlapudi, A. (2023). Maize On-Farm Stressed Area Identification Using Airborne RGB Images Derived Leaf Area Index and Canopy Height. Agriculture, 13(7), 1292. https://doi.org/10.3390/agriculture13071292








