Appraisal of Functions and Role of Selenium in Heavy Metal Stress Adaptation in Plants
Abstract
1. Introduction
2. Methodology
3. Selenium Uptake and Translocation
4. Selenium and Heavy Metals
5. Selenium Interaction with Osmolytes under Heavy Metal Stress
6. Selenium Interplay with Phytohormones under Heavy Metal Stress
7. Molecular and Proteomic Interaction of Selenium under Heavy Metals
8. Selenium Interplay with Secondary Metabolites (Phenolics, N-Containing Metabolites and Terpenes)
9. Selenium Interaction with Mineral Nutrients under Heavy Metal Stress
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hasan, M.M.; Uddin, M.N.; Ara-Sharmeen, F.I.; Alharby, H.; Alzahrani, Y.; Hakeem, K.R. Assisting phytoremediation of heavy metals using chemical amendments. Plants 2019, 8, 295. [Google Scholar] [CrossRef]
- Rather, B.A.; Mir, I.R.; Masood, A.; Anjum, N.A.; Khan, N.A. Nitric oxide pre-treatment advances seed germination and alleviates copper-induced photosynthetic inhibition in Indian mustard. Plants 2020, 9, 776. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Rai, P.; Guerriero, G.; Sharma, S.; Corpas, F.J.; Singh, V.P. Silicon induces adventitious root formation in rice under arsenate stress with involvement of nitric oxide and indole-3-acetic acid. J. Exp. Bot. 2021, 72, 4457–4471. [Google Scholar] [CrossRef] [PubMed]
- Alsafran, M.; Saleem, M.H.; Al Jabri, H.; Rizwan, M.; Usman, K. Principles and applicability of integrated remediation strategies for heavy metal Removal/Recovery from contaminated environments. J. Plant Growth Regul. 2022. [Google Scholar] [CrossRef]
- Sharma, J.K.; Kumar, N.; Singh, N.P.; Santal, A.R. Phytoremediation technologies and their mechanism for removal of heavy metal from contaminated soil: An approach for a sustainable environment. Front. Plant Sci. 2023, 14, 1076876. [Google Scholar] [CrossRef]
- Khan, M.Y.; Prakash, V.; Yadav, V.; Chauhan, D.K.; Prasad, S.M.; Ramawat, N.; Sharma, S. Regulation of cadmium toxicity in roots of tomato by indole acetic acid with special emphasis on reactive oxygen species production and their scavenging. Plant Physiol. Biochem. 2019, 142, 193–201. [Google Scholar] [CrossRef]
- Noor, I.; Sohail, H.; Sun, J.; Nawaz, M.A.; Li, G.; Hasanuzzaman, M.; Liu, J. Heavy metal and metalloid toxicity in horticultural plants: Tolerance mechanism and remediation strategies. Chemosphere 2022, 303, 135196. [Google Scholar] [CrossRef]
- Asgher, M.; Khan, M.I.R.; Anjum, N.A.; Khan, N.A. Minimising toxicity of cadmium in plants—Role of plant growth regulators. Protoplasma 2015, 252, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 1, 1–18. [Google Scholar] [CrossRef]
- Saini, S.; Kaur, N.; Pati, P.K. Phytohormones: Key players in the modulation of heavy metal stress tolerance in plants. Ecotoxicol. Environ. Saf. 2021, 223, 112578. [Google Scholar] [CrossRef] [PubMed]
- Asgher, M.; Ahmed, S.; Sehar, Z.; Gautam, H.; Gandhi, S.G.; Khan, N.A. Hydrogen peroxide modulates activity and expression of antioxidant enzymes and protects photosynthetic activity from arsenic damage in rice (Oryza sativa L.). J. Hazard. Mater. 2021, 401, 123365. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, M.K.; Majumdar, A.; Barla, A.; Bose, S.; Srivastava, S. An assessment ofarsenic hazard in groundwater–soil–rice system in two villages of Nadia district, West Bengal, India. Environ. Geochem. Health 2019, 41, 2381–2395. [Google Scholar] [CrossRef]
- Tyagi, N.; Raghuvanshi, R.; Upadhyay, M.K.; Srivastava, A.K.; Suprasanna, P.; Srivastava, S. Elemental (As, Zn, Fe and Cu) analysis and health risk assessment of rice grains and rice based food products collected from markets from different cities of Gangetic basin, India. J. Food Compos. Anal. 2020, 93, 103612. [Google Scholar] [CrossRef]
- Asgher, M.; Sehar, Z.; Rehaman, A.; Rashid, S.; Ahmed, S.; Per, T.S.; Alyemeni, M.N.; Khan, N.A. Exogenously-applied L-glutamic acid protects photosynthetic functions and enhances arsenic tolerance through increased nitrogen assimilation and antioxidant capacity in rice (Oryza sativa L.). Environ. Pollut. 2022, 301, 119008. [Google Scholar] [CrossRef]
- Asgher, M.; Khan, M.I.R.; Anjum, N.A.; Verma, S.; Vyas, D.; Per, T.S.; Khan, N.A. Ethylene and polyamines in counteracting heavy metal phytotoxicity: A crosstalk perspective. J. Plant Growth Regul. 2019, 37, 1050–1065. [Google Scholar] [CrossRef]
- Hasan, M.M.; Alharbi, B.M.; Alhaithloul, H.A.S.; Abdulmajeed, A.M.; Alghanem, S.M.; Al-Mushhin, A.A.M.; Jahan, M.S.; Corpas, F.J.; Fang, X.-W.; Soliman, M.H. Spermine-mediated tolerance to selenium toxicity in wheat (Triticum aestivum L.) depends on endogenous nitric oxide synthesis. Antioxidants 2021, 10, 1835. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Raza, A.; Hawrylak-Nowak, B.; Matraszek-Gawron, R.; Mahmud, J.A.; Nahar, K.; Fujita, M. Selenium in plants: Boon or Bane? Environ. Exp. Bot. 2020, 178, 104170. [Google Scholar] [CrossRef]
- Tamaoki, M.; Freeman, J.L.; Pilon-Smits, E.A.H. Cooperative ethylene and jasmonic acid signaling regulates selenite resistance in Arabidopsis. Plant Physiol. 2008, 146, 1219–1230. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; García-Caparrós, P.; Parvin, K.; Zulfiqar, F.; Ahmed, N.; Fujita, M. Selenium supplementation and crop plant tolerance to metal/metalloid toxicity. Front. Plant Sci. 2022, 12, 792770. [Google Scholar] [CrossRef]
- Tavakoli, S.; Enteshari, S.; Yousefifard, M. The effect of selenium on physiologic and morphologic properties of Melissa officinalis L. Iran. J. Plant Physiol. 2020, 10, 3125–3134. [Google Scholar]
- Pazurkiewicz-Kocot, K.; Galas, W.; Kita, A. The effect of selenium on the accumulation of some metals in Zea mays L. plants treated with indole-3-acetic acid. Cell. Mol. Biol. Lett. 2003, 8, 97–104. [Google Scholar]
- Li, D.; Zhou, C.; Ma, J.; Wu, Y.; Kang, L.; An, Q.; Pan, C. Nanoselenium transformation and inhibition of cadmium accumulation by regulating the lignin biosynthetic pathway and plant hormone signal transduction in pepper plants. J. Nanobiotech. 2021, 19, 316. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Liu, T.; Li, Y.; Li, F. Selenium reduces cadmium uptake into rice suspension cells by regulating the expression of lignin synthesis and cadmium-related genes. Sci. Total Environ. 2018, 644, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.W.; Ha, J.; Liang, S.X.; Kang, W.J. Protective role of selenium on garlic growth under cadmium stress. Commun. Soil Sci. Plan. 2010, 41, 1195–1204. [Google Scholar] [CrossRef]
- Alyemeni, M.N.; Ahanger, M.A.; Wijaya, L.; Alam, P.; Bhardwaj, R.; Ahmad, P. Selenium mitigates cadmium-induced oxidative stress in tomato (Solanum lycopersicum L.) plants by modulating chlorophyll fluorescence, osmolyte accumulation, and antioxidant system. Protoplasma 2018, 255, 459–469. [Google Scholar] [CrossRef]
- Babashpour-Asl, M.; Farajzadeh-Memari-Tabrizi, E.; Yousefpour-Dokhanieh, A. Foliar-applied selenium nanoparticles alleviate cadmium stress through changes in physio-biochemical status and essential oil profile of coriander (Coriandrum sativum L.) leaves. Environ. Sci. Pollut. Res. 2022, 29, 80021–80031. [Google Scholar] [CrossRef]
- Zeeshan, M.; Hu, Y.X.; Iqbal, A.; Salam, A.; Liu, Y.X.; Muhammad, I.; Ahmad, S.; Khan, A.H.; Hale, B.; Wu, H.Y.; et al. Amelioration of AsV toxicity by concurrent application of ZnO-NPs and Se-NPs is associated with differential regulation of photosynthetic indexes, antioxidant pool and osmolytes content in soybean seedling. Ecotoxicol. Environ. Saf. 2021, 225, 112738. [Google Scholar] [CrossRef] [PubMed]
- Atarodi, B.; Fotovat, A.; Khorassani, R.; Keshavarz, P. Study of wheat (Triticum aestivum L.) response to selenium application under cadmium stress. Environ. Stress. Crop Sci. 2019, 12, 291–305. [Google Scholar]
- Saeedi, M.; Soltani, F.; Babalar, M.; Izadpanah, F.; Wiesner-Reinhold, M.; Baldermann, S. Selenium fortification alters the growth, antioxidant characteristics and secondary metabolite profiles of cauliflower (Brassica oleracea var. botrytis) cultivars in hydroponic culture. Plants 2021, 10, 1537. [Google Scholar] [CrossRef]
- Chu, J.; Yao, X.; Zhang, Z. Responses of wheat seedlings to exogenous selenium supply under cold stress. J. Biol. Trace Elem. Res. 2010, 136, 355–363. [Google Scholar] [CrossRef]
- Handa, N.; Kohli, S.K.; Sharma, A.; Thukral, A.K.; Bhardwaj, R.; Abd Allah, E.F.; Alqarawi, A.A.; Ahmad, P. Selenium modulates dynamics of antioxidative defence expression, photosynthetic attributes and secondary metabolites to mitigate chromium toxicity in Brassica juncea L. Environ. Exp. Bot. 2019, 161, 180–192. [Google Scholar] [CrossRef]
- Tian, M.; Xu, X.; Liu, F.; Fan, X.; Pan, S. Untargeted metabolomics reveals predominant alterations in primary metabolites of broccoli sprouts in response to pre-harvest selenium treatment. Food Res. Int. 2018, 111, 205–211. [Google Scholar] [CrossRef]
- Barickman, T.C.; Kopsell, D.A.; Sams, C.E. Selenium influences glucosinolate and isothiocyanates and increases sulfur uptake in Arabidopsis thaliana and rapid-cycling Brassica oleracea. J. Agric. Food Chem. 2013, 61, 202–209. [Google Scholar] [CrossRef]
- Sams, C.E.; Panthee, D.R.; Charron, C.S.; Kopsell, D.A.; Yuan, J.S. Selenium regulates gene expression for glucosinolate and carotenoid biosynthesis in Arabidopsis. J. Am. Soc. Hortic. Sci. 2011, 136, 23–34. [Google Scholar] [CrossRef]
- Amirabad, A.S.; Behtash, F.; Vafaee, Y. Selenium mitigates cadmium toxicity by preventing oxidative stress and enhancing photosynthesis and micronutrient availability on radish (Raphanus sativus L.) cv. Cherry Belle. Environ. Sci. Pollut. Res. 2020, 27, 12476–12490. [Google Scholar] [CrossRef] [PubMed]
- Aqib, M.; Nawaz, F.; Majeed, S.; Ghaffar, A.; Ahmad, K.S.; Shehzad, M.A.; Usmani, M.M. Physiological insights into sulfate and selenium interaction to improve drought tolerance in mung bean. Physiol. Mol. Bio. Plants 2021, 27, 1073–1087. [Google Scholar] [CrossRef]
- Zhu, Y.; Dong, Y.; Zhu, N.; Jina, H. Foliar application of biosynthetic nano-selenium alleviates the toxicity of Cd, Pb, and Hg in Brassica chinensis by inhibiting heavy metal adsorption and improving antioxidant system in plant. Ecotoxicol. Environ. Saf. 2022, 240, 113681. [Google Scholar] [CrossRef]
- Gu, X.; Wen, X.; Yi, N.; Liu, Y.; Wu, J.; Li, H.; Liu, G. Effect of foliar application of silicon, selenium and zinc on heavy metal accumulation in wheat grains in field studies. Environ. Pollut. Bioavail. 2022, 34, 246–252. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Selenium in higher plants: Physiological role, antioxidant metabolism and abiotic stress tolerance. J. Plant Sci. 2010, 5, 354–375. [Google Scholar] [CrossRef]
- Atici, O.; Agar, G.; Battal, P. Changes in phytohormone contents in chickpea seeds germinating under lead or zinc stress. Algologia 2005, 49, 215–222. [Google Scholar] [CrossRef]
- Maksymiec, W.; Krupa, Z. The effects of short-term exposition to Cd, excess Cu ions and jasmonate on oxidative stress appearing in Arabidopsis thaliana. Environ. Exp. Bot. 2006, 57, 187–194. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Sesin, V.; Kisiala, A.; Emery, R.N. Phytohormonal roles in plant responses to heavy metal stress: Implications for using macrophytes in phytoremediation of aquatic ecosystems. Environ. Toxicol. Chem. 2021, 40, 7–22. [Google Scholar] [CrossRef]
- Malheiros, R.S.P.; Costa, L.C.; Ávila, R.T.; Pimenta, T.M.; Teixeira, L.S.; Brito, F.A.L.; Ribeiro, D.M. Selenium downregulates auxin and ethylene biosynthesis in rice seedlings to modify primary metabolism and root architecture. Planta 2019, 250, 333–345. [Google Scholar] [CrossRef]
- Malheiros, R.S.P.; Gonçalves, F.C.M.; Brito, F.A.L.; Zsögön, A.; Ribeiro, D.M. Selenomethionine induces oxidative stress and modifies growth in rice (Oryza sativa L.) seedlings through effects on hormone biosynthesis and primary metabolism. Ecotoxicol. Environ. Saf. 2020, 189, 109942. [Google Scholar] [CrossRef]
- El-Badri, A.M.; Batool, M.; Wang, C.; Hashem, A.M.; Tabl, K.M.; Nishawy, E.; Wang, B. Selenium and zinc oxide nanoparticles modulate the molecular and morpho-physiological processes during seed germination of Brassica napus under salt stress. Ecotoxicol. Environ. Saf. 2021, 225, 112695. [Google Scholar] [CrossRef]
- Luo, Y.; Wei, Y.; Sun, S.; Wang, J.; Wang, W.; Han, D.; Fu, Y. Selenium modulates the level of auxin to alleviate the toxicity of cadmium in tobacco. Int. J. Mol. Sci. 2019, 20, 3772. [Google Scholar] [CrossRef]
- Hassan, M.; Israr, M.; Mansoor, S.; Hussain, S.A.; Basheer, F.; Azizullah, A.; Ur Rehman, S. Acclimation of cadmium-induced genotoxicity and oxidative stress in mung bean seedlings by priming effect of phytohormones and proline. PLoS ONE 2021, 16, e0257924. [Google Scholar] [CrossRef]
- Ahmad, P.; Raja, V.; Ashraf, M.; Wijaya, L.; Bajguz, A.; Alyemeni, M.N. Jasmonic acid (JA) and gibberellic acid (GA3) mitigated Cd-toxicity in chickpea plants through restricted Cd uptake and oxidative stress management. Sci. Rep. 2021, 11, 19768. [Google Scholar] [CrossRef]
- Alves, L.R.; Dos Reis, A.R.; Prado, E.R.; Lavres, J.; Pompeu, G.B.; Azevedo, R.A.; Gratao, P.L. New insights into cadmium stressful-conditions: Role of ethylene on selenium-mediated antioxidant enzymes. Ecotoxicol. Environ. Saf. 2019, 186, 109747. [Google Scholar] [CrossRef]
- Rahman, S.U.; Li, Y.; Hussain, S.; Hussain, B.; Riaz, L.; Ashraf, M.N.; Cheng, H. Role of phytohormones in heavy metal tolerance in plants: A review. Ecol. Indic. 2023, 146, 109844. [Google Scholar] [CrossRef]
- Frankenberger, J.W.T.; Karlson, U. Campaigning for bioremediation. Chemtech 1994, 24, 45–51. [Google Scholar]
- White, P.J. Selenium metabolism in plants. Biochim. Biophys. Acta-Gen. Subj. 2018, 1862, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Gupta, S. An overview of selenium uptake, metabolism, and toxicity in plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef]
- Parker, D.R.; Feist, L.J.; Varvel, T.W. Selenium phytoremediation potential of Stanleya pinnata. Plant Soil 2003, 249, 157–165. [Google Scholar] [CrossRef]
- Zayed, A.; Lytle, C.M.; Terry, N. Accumulation and volatilization of different chemical species of selenium by plants. Planta 1998, 206, 284–292. [Google Scholar] [CrossRef]
- White, P.J.; Bowen, H.C.; Parmaguru, P.; Fritz, M.; Spracklen, W.P.; Spiby, R.E.; Meacham, M.C.; Mead, A.; Harriman, M.; Trueman, L.J.; et al. Interactions between selenium and sulfur nutrition in Arabidopsis thaliana. J. Exp. Bot. 2004, 55, 1927–1937. [Google Scholar] [CrossRef]
- Sors, T.G.; Ellis, D.R.; Salt, D.E. Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth. Res. 2005, 86, 373–389. [Google Scholar] [CrossRef]
- Arvy, M.P. Selenate and selenite uptake and translocation in bean plants (Phaseolus vulgaris). J. Exp. Bot. 1993, 44, 1083–1087. [Google Scholar] [CrossRef]
- Freeman, J.L.; Quinn, C.F.; Marcus, M.A.; Fakra, S.; Pilon-Smits, E.A. Selenium-tolerant diamondback moth disarms hyperaccumulator plant defense. Curr. Biol. 2006, 16, 2181–2192. [Google Scholar] [CrossRef]
- Dumont, E.; Vanhaecke, F.; Cornelis, R. Selenium speciation from food source to metabolites: A critical review. Anal. Bioanal. Chem. 2006, 385, 1304–1323. [Google Scholar] [CrossRef]
- Brown, T.A.; Shrift, A. Selenium: Toxicity and tolerance in higher plants. Biol. Rev. 1982, 57, 59–84. [Google Scholar] [CrossRef]
- Zhang, L.; Chu, C. Selenium uptake, transport, metabolism, reutilization, and biofortification in rice. Rice 2022, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, Y.; Li, K.; Wan, Y.; Wang, Q.; Zhuang, Z.; Guo, Y.; Li, H. Uptake, translocation and biotransformation of selenium nanoparticles in rice seedlings (Oryza sativa L.). J. Nanobiotechnol. 2020, 18, 103. [Google Scholar] [CrossRef]
- Trippe, R.C., III; Richard, C.; Pilon-Smits, E.A.H. Selenium transport and metabolism in plants: Phytoremediation and biofortification implications. J. Hazard. Mater. 2021, 404, 124178. [Google Scholar] [CrossRef]
- Lobanov, A.V.; Hatfield, D.L.; Gladyshev, V.N. Eukaryotic selenoproteins and selenoproteomes. Biochim. Biophys. Acta-Gen. Subj. 2009, 1790, 1424–1428. [Google Scholar] [CrossRef]
- Nancharaiah, Y.V.; Lens, P.N.L. Selenium biomineralization for biotechnological applications. Trends Biotechnol. 2015, 33, 323–330. [Google Scholar] [CrossRef]
- Costa, L.C.; Luz, L.M.; Nascimento, V.L.; Araujo, F.F.; Santos, M.N.S.; França, C.F.M.; Silva, T.P.; Fugate, K.K.; Finger, F.L. Selenium-ethylene interplay in postharvest life of cut flowers. Front. Plant Sci. 2020, 11, 584698. [Google Scholar] [CrossRef]
- Conte, F.; Copat, C.; Longo, S.; Oliveri Conti, G.; Grasso, A.; Arena, G.; Ferrante, M. First data on trace elements in Haliotistuberculata (Linnaeus, 1758) from southern Italy: Safety issues. Food Chem. Toxicol. 2015, 81, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Copat, C.; Grasso, A.; Fiore, M.; Cristaldi, A.; Zuccarello, P.; Signorelli, S.S.; Ferrante, M. Trace elements in seafood from the Mediterranean sea: An exposure risk assessment. Food Chem. Toxicol. 2018, 115, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhao, R.; Zhao, W.; Fu, R.; Guo, J.; Bi, N.; Zhang, J. Effects of arbuscular mycorrhizal fungi on maize (Zea mays L.) and sorghum (Sorghum bicolor L. Moench) grown in rare earth elements of mine tailings. Appl. Soil Ecol. 2013, 72, 85–92. [Google Scholar] [CrossRef]
- Angulo-Bejarano, P.I.; Puente-Rivera, J.; Cruz-Ortega, R. Metal and metalloid toxicity in plants: An overview on molecular aspects. Plants 2021, 10, 635. [Google Scholar] [CrossRef]
- He, P.; Lv, X.; Wang, G. Effects of Se and Zn supplementation on the antagonism against Pb and Cd in vegetables. Environ. Int. 2004, 30, 167–172. [Google Scholar] [CrossRef]
- Nawaz, F.; Ashraf, M.Y.; Ahmad, R.; Waraich, E.A.; Shabbir, R.N. Selenium (Se) regulates seedling growth in wheat under drought stress. Adv. Chem. 2014, 2014, 143567. [Google Scholar] [CrossRef]
- Gui, J.Y.; Rao, S.; Huang, X.; Liu, X.; Cheng, S.; Xu, F. Interaction between selenium and essential micronutrient elements in plants: A systematic review. Sci. Total Environ. 2022, 853, 158673. [Google Scholar] [CrossRef]
- Ismael, M.A.; Elyamine, A.M.; Moussa, M.G.; Cai, M.; Zhao, X.; Hu, C. Cadmium in plants: Uptake, toxicity, and its interactions with selenium fertilizers. Metallomics 2019, 11, 255–277. [Google Scholar] [CrossRef]
- Filek, M.; Koscielniak, J.; Labanowska, M.; Bednarska, E.; Bidzinska, E. Selenium-induced protection of photosynthesis activity in rape (Brassica napus) seedlings subjected to cadmium stress:Fluorescence and EPR measurements. Photosynth. Res. 2010, 105, 27–37. [Google Scholar] [CrossRef]
- Feng, R.; Wei, C.; Tu, S. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Tobiasz, A.; Walas, S.; Filek, M.; Mrowiec, H.; Samsel, K.; Sieprawska, A.; Hartikainen, H. Effect of selenium on distribution of macro- and micro-elements to different tissues during wheat. Biol. Plant. 2014, 58, 370–374. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Exogenous selenium pretreatment protects rapeseed seedlings from cadmium-induced oxidative stress by upregulating antioxidant defense and methylglyoxal detoxification systems. Biol. Trace Elem. Res. 2012, 49, 248–261. [Google Scholar] [CrossRef]
- Lanza, M.G.D.B.; Reis, A.R.d. Roles of selenium in mineral plant nutrition: ROS scavenging responses against abiotic stresses. Plant Physiol. Biochem. 2021, 164, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Sharma, S.; Kaur, S.; Nayyar, H. Selenium in agriculture: A nutrient or contaminant for crops? Arch. Agron. Soil Sci. 2014, 60, 1593–1624. [Google Scholar] [CrossRef]
- Cakir, O.; Candar-Cakir, B.; Zhang, B.H. Small RNA and degradome sequencing reveals important microRNA function in Astragalus chrysochlorus response to selenium stimuli. Plant Biotechnol. J. 2016, 14, 543–556. [Google Scholar] [CrossRef]
- Aroiee, H.; Shekari, L.; Mirshekari, A. Effects of selenium on damage of heavy metals in germination, growth and antioxidant activities of cucumber (Cucumis sativus L.) seedling. Iran. J. Seed Sci. Res. 2019, 6, 269–286. [Google Scholar] [CrossRef]
- Cartes, P.; Jara, A.A.; Pinilla, L.; Rosas, A.; Mora, M.L. Selenium improves the antioxidant ability against aluminium-induced oxidative stress in ryegrass roots. Ann. Appl. Biol. 2010, 156, 297–307. [Google Scholar] [CrossRef]
- Malik, J.A.; Kumar, S.; Thakur, P.; Sharma, S.; Kaur, N.; Kaur, R.; Pathania, D.; Bhandhari, K.; Kaushal, N.; Singh, K.; et al. Promotion of growth in mungbean (Phaseolus aureus Roxb.) by selenium is associated with stimulation of carbohydrate metabolism. Biol. Trace Elem. Res. 2011, 143, 530–539. [Google Scholar] [CrossRef]
- Mroczek-Zdyrska, M.; Strubińska, J.; Hanaka, A. Selenium improves physiological parameters and alleviates oxidative stress in shoots of lead-exposed Vicia faba L. minor plants grown under phosphorus-deficient conditions. J. Plant Growth Regul. 2017, 36, 186–199. [Google Scholar] [CrossRef]
- Wu, Z.; Yin, X.; Bañuelos, G.S.; Lin, Z.Q.; Liu, Y.; Li, M.; Yuan, L. Indications of selenium protection against cadmium and lead toxicity in oilseed rape (Brassica napus L.). Front. Plant Sci. 2016, 7, 1875. [Google Scholar] [CrossRef]
- Ahmad, P.; Abd Allah, E.F.; Hashem, A.; Sarwat, M.; Gucel, S. Exogenous application of selenium mitigates cadmium toxicity in Brassica juncea L. (Czern and Cross) by up-regulating antioxidative system and secondary metabolites. J. Plant Growth Regul. 2016, 35, 936–950. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Dong, Y.Y.; Feng, L.Y.; Deng, Z.L.; Xu, Q.; Tao, Q.; Wang, C.Q.; Chen, Y.E.; Yuan, M.; Yuan, S. Selenium enh;nces cadmium accumulation capability in two mustard family species—Brassica napus and B. Juncea. Plants 2020, 9, 904. [Google Scholar] [CrossRef]
- Azizi, I.; Esmaielpour, B.; Fatemi, H. Effect of foliar application of selenium on morphological and physiological indices of savory ( Satureja hortensis ) under cadmium stress. Food Sci. Nutr. 2020, 8, 6539–6549. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, P.; Nie, Z.; Shi, H.; Li, C.; Wang, Y.; Qin, S.; Qin, X.; Liu, H. Selenium supply alters the subcellular distribution and chemical forms of cadmium and the expression of transporter genes involved in cadmium uptake and translocation in winter wheat (Triticum aestivum). BMC Plant Boil. 2020, 20, 550. [Google Scholar]
- Chauhan, R.; Awasthi, S.; Indoliya, Y.; Chauhan, A.S.; Mishra, S.; Agrawal, L.; Tripathi, R.D. Transcriptome and proteome analyses reveal selenium mediated amelioration of arsenic toxicity in rice (Oryza sativa L.). J. Hazard. Mater. 2020, 390, 122122. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yan, J.; Qin, Y.; Xu, J.; Shohag, M.J.I.; Wei, Y.; Gu, M. Effect of different forms of selenium on the physiological response and the cadmium uptake by rice under cadmium stress. Int. J. Environ. Res. Public Health 2020, 17, 6991. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, R.P.; Singh, P.K.; Awasthi, S.; Chakrabarty, D.; Trivedi, P.K.; Tripathi, R.D. Selenium ameliorates arsenic induced oxidative stress through modulation of antioxidant enzymes and thiols in rice (Oryza sativa L.). Ecotoxicology 2014, 23, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, X.; Li, H.; Bi, J.; Yu, J.; Liu, X.; Rong, Z. Selenium modulates cadmium-induced ultrastructural and metabolic changes in cucumber seedlings. RSC Adv. 2020, 10, 17892–17905. [Google Scholar] [CrossRef]
- Martins, J.P.R.; Souza, A.F.C.; Rodrigues, L.C.A.; Braga, P.C.S.; Gontijo, A.B.P.L.; Falqueto, A.R. Zinc and selenium as modulating factors of the anatomy and physiology of Billbergia zebrina (Bromeliaceae) during in vitro culture. Photosynthetica 2020, 58, 1068–1077. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, S.; Jiang, Z.; Wang, Y.; Zhang, Z. Selenium biofortification modulates plant growth, microelement and heavy metal concentrations, selenium uptake, and accumulation in black-grained wheat. Front. Plant Sci. 2021, 12, 748523. [Google Scholar] [CrossRef]
- Shekari, L.; Aroiee, H.; Mirshekari, A.; Nemati, H. Protective role of selenium on cucumber (Cucumis sativus L.) exposed to cadmium and lead stress during reproductive stage role of selenium on heavy metals stress. J. Plant Nutr. 2019, 42, 529–542. [Google Scholar] [CrossRef]
- Naseem, M.; Anwar-ul-Haq, M.; Wang, X.; Farooq, N.; Awais, M.; Sattar, H.; Ahmed Malik, H.; Mustafa, A.; Ahmad, J.; El-Esawi, M.A. Influence of selenium on growth, physiology, and antioxidant responses in maize varies in a dose-dependent manner. J. Food Qual. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Bakhtiari, M.; Raeisi Sadati, F.; Raeisi Sadati, S.Y. Foliar application of silicon, selenium, and zinc nanoparticles can modulate lead and cadmium toxicity in sage (Salvia officinalis L.) plants by optimizing growth and biochemical status. Environ. Sci. Pollut. Res. 2023, 30, 54223–54233. [Google Scholar] [CrossRef]
- Ros, G.H.; Van Rotterdam, A.M.D.; Bussink, D.W.; Bindraban, P.S. Selenium fertilization strategies for bio-fortification of food: An agro-ecosystem approach. Plant Soil 2016, 404, 99–112. [Google Scholar] [CrossRef]
- Barman, F.; Kundu, R. Foliar application of selenium affecting pollen viability, grain chalkiness, and transporter genes in cadmium accumulating rice cultivar: A pot study. Chemosphere 2023, 313, 137538. [Google Scholar] [CrossRef]
- Rostami, M.; Abbaspour, H. Effect of selenium on growth and physiological traits of basil plant (Ocimum basilicum L.) under arsenic stress conditions. Rev. Agric. Neotrop. 2019, 6, 30–37. [Google Scholar] [CrossRef]
- Mozafariyan, M.; Shekari, L.; Hawrylak-Nowak, B.; Kamelmanesh, M.M. Protective role of selenium on pepper exposed to cadmium stress during reproductive stage. Biol. Trace Elem. Res. 2014, 160, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Wang, L.; Yang, J.; Zhao, P.; Zhu, Y.; Li, Y. Underlying mechanisms responsible for restriction of uptake and translocation of heavy metals (metalloids) by selenium via root application in plants. J. Hazard. Mater. 2020, 402, 23570. [Google Scholar] [CrossRef]
- Kieliszek, M. Selenium–fascinating microelement, properties and sources in food. J. Mol. 2019, 24, 1298. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Abdelrahman, M.; Hosseini, M.S.; Hoveizeh, N.F.; Tran, L.S.P. Alleviation of the effect of salinity on growth and yield of strawberry by foliar spray of selenium-nanoparticles. Environ. Pollut. 2019, 253, 246–258. [Google Scholar] [CrossRef]
- Alam, M.Z.; McGee, R.; Hoque, M.A.; Ahammed, G.J.; Carpenter-Boggs, L. Effect of arbuscular mycorrhizal fungi, selenium and biochar on photosynthetic pigments and antioxidant enzyme activity under arsenic stress in mung bean (Vigna radiata). Front. Physiol. 2019, 10, 193. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Agarwal, R.M. Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L) as influenced by potassium supplementation. Plant Physiol. Biochem. 2017, 115, 449–460. [Google Scholar] [CrossRef]
- Sajedi, N. Evaluation of selenium and salicylic acid effect on physiological and qualitative characteristics of dry-land wheat cultivars. Iran Agric. Res. 2017, 36, 91–100. [Google Scholar]
- Khalofah, A.; Migdadi, H.; El-Harty, E. Antioxidant enzymatic activities and growth response of quinoa (Chenopodium quinoa Willd) to exogenous selenium application. Plants 2021, 10, 719. [Google Scholar] [CrossRef]
- Sytar, O.; Kumari, P.; Yadav, S.; Brestic, M.; Rastogi, A. Phytohormone priming: Regulator for heavy metal stress in plants. J. Plant Growth Regul. 2019, 38, 739–752. [Google Scholar] [CrossRef]
- Ali, M.A.; Asghar, H.N.; Khan, M.Y.; Saleem, M.; Naveed, M.; Niazi, N.K. Alleviation of nickel-induced stress in mungbean through application of gibberellic acid. Int. J. Agric. Biol. 2015, 17, 990–994. [Google Scholar] [CrossRef]
- Jia, H.; Song, Z.; Wu, F.; Ma, M.; Li, Y.; Han, D.; Yang, Y.; Zhang, S.; Cui, H. Low selenium increases the auxin concentration and enhances tolerance to low phosphorous stress in tobacco. Environ. Exp. Bot. 2018, 153, 127–134. [Google Scholar] [CrossRef]
- Alhaithloul, H.A.S.; Abu-Elsaoud, A.M.; Soliman, M.H. Abiotic stress tolerance in crop plants: Role of phytohormones. In Abiotic Stress in Plants; Fahad, S., Saud, S., Chen, Y., Wu, C., Wang, D., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Tamaoki, M.; Freeman, J.L.; Marquès, L.; Pilon-Smits, E.A.H. New insights into the roles of ethylene and jasmonic acid in the acquisition of selenium resistance in plants. Plant Signal. Behav. 2008, 3, 865–867. [Google Scholar] [CrossRef]
- Kolbert, Z.; Ortega, L.; Erdei, L. Involvement of nitrate reductase (NR) in osmotic stress-induced NO generation of Arabidopsis thaliana L. roots. J. Plant Physiol. 2010, 167, 77–80. [Google Scholar] [CrossRef]
- Abedi, S.; Iranbakhsh, A.; Oraghi-Ardebili, Z.; Ebadi, M. Nitric oxide and selenium nanoparticles confer changes in growth, metabolism, antioxidant machinery, gene expression, and flowering in chicory (Cichorium intybus L.): Potential benefits and risk assessment. Environ. Sci. Pollut. Res. Int. 2021, 28, 3136–3148. [Google Scholar] [CrossRef]
- Lu, Q.; Chen, S.; Li, Y.; Zheng, F.; He, B.; Gu, M. Exogenous abscisic acid (ABA) promotes cadmium (Cd) accumulation in Sedum alfredii Hance by regulating the expression of Cd stress response genes. Environ. Sci. Pollut. Res. 2020, 27, 8719–8731. [Google Scholar] [CrossRef]
- Pandey, C.; Gupta, M. Selenium and auxin mitigates arsenic stress in rice (Oryza sativa L.) by combining the role of stress indicators, modulators and genotoxicity assay. J. Hazard. Mater. 2015, 287, 384–391. [Google Scholar] [CrossRef]
- Qiu, C.W.; Zhang, C.; Wang, N.H.; Mao, W.; Wu, F. Strigolactone GR24 improves cadmium tolerance by regulating cadmium uptake, nitric oxide signaling and antioxidant metabolism in barley (Hordeum vulgare L.). Environ. Pollut. 2021, 273, 116486. [Google Scholar] [CrossRef]
- Chen, L.; Hu, W.; Long, C.; Wang, D. Exogenous plant growth regulator alleviate the adverse effects of U and Cd stress in sunflower (Helianthus annuus L.) and improve the efficacy of U and Cd remediation. Chemosphere 2020, 262, 127809. [Google Scholar] [CrossRef] [PubMed]
- Sehar, Z.; Iqbal, N.; Fatma, M.; Rather, B.A.; Albaqami, M.; Khan, N.A. Ethylene suppresses abscisic acid, modulates antioxidant system to counteract arsenic-inhibited photosynthetic performance in the presence of selenium in mustard. Front. Plant Sci. 2022, 13, 852704. [Google Scholar] [CrossRef]
- Shi, W.G.; Liu, W.; Yu, W.; Zhang, Y.; Ding, S.; Li, H.; Mrak, T.; Kraigher, H.; Luo, Z.B. Abscisic acid enhances lead translocation from the roots to the leaves and alleviates its toxicity in Populus × canescens. J. Hazard. Mater. 2019, 362, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Poonam, S.; Kaur, H.; Geetika, S. Effect of jasmonic acid on photosynthetic pigments and stress markers in Cajanus cajan (L.) Mill sp. seedlings under copper stress. Am. J. Plant Sci. 2013, 4, 817–823. [Google Scholar] [CrossRef]
- Singh, S.; Prasad, S.M. Effects of 28-homobrassinoloid on key physiological attributes of Solanum lycopersicum seedlings under cadmium stress: Photosynthesis and nitrogen metabolism. Plant Growth Regul. 2017, 82, 161–173. [Google Scholar] [CrossRef]
- Šípošová, K.; Labancová, E.; Kučerová, D.; Kollárová, K.; Vivodová, Z. Effects of exogenous application of indole-3-butyric acid on maize plants cultivated in the presence or absence of cadmium. Plants 2021, 10, 2503. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Asgher, M.; Per, T.S.; Masood, A.; Fatma, M.; Khan, M.I. Ethylene potentiates sulfur-mediated reversal of cadmium inhibited photosynthetic responses in mustard. Front. Plant Sci. 2016, 7, 1628. [Google Scholar] [CrossRef]
- Tai, Z.; Yin, X.; Fang, Z.; Shi, G.; Lou, L.; Cai, Q. Exogenous GR24 alleviates cadmium toxicity by reducing cadmium uptake in switchgrass (Panicum virgatum) seedlings. Int. J. Environ. Res. Public Health 2017, 14, 852. [Google Scholar] [CrossRef]
- Ahmad, P.; Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alam, P. Exogenous application of nitric oxide modulates osmolyte metabolism, antioxidants, enzymes of ascorbate-glutathione cycle and promotes growth under cadmium stress in tomato. Protoplasma 2018, 255, 79–93. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, A.; Bhardwaj, R. Plant steroidal hormone epibrassinolide regulate–Heavy metal stress tolerance in Oryza sativa L. by modulating antioxidant defense expression. Environ. Exp. Bot. 2016, 122, 1–9. [Google Scholar] [CrossRef]
- Bücker-Neto, L.; Paiva, A.L.S.; Machado, R.D.; Arenhart, R.A.; Margis-Pinheiro, M. Interactions between plant hormones and heavy metals responses. Genet. Mol. Biol. 2017, 40, 373–386. [Google Scholar] [CrossRef]
- Rajewska, I.; Talarek, M.; Bajguz, A. Brassinosteroids and response of plants to heavy metals action. Front. Plant Sci. 2016, 7, 629. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Pan, K. Effect of exogenous abscisic acid on the level of antioxidants in Atractylodes macrocephala Koidz under lead stress. Environ. Sci. Pollut. Res. 2013, 20, 1441–1449. [Google Scholar] [CrossRef]
- Sharma, A.; Sidhu, G.P.S.; Araniti, F.; Bali, A.S.; Shahzad, B.; Tripathi, D.K.; Landi, M. The role of salicylic acid in plants exposed to heavy metals. Molecules 2020, 25, 540. [Google Scholar] [CrossRef]
- Ahsan, N.; Renaut, J.; Komatsu, S. Recent developments in the application of proteomics to the analysis of plant responses to heavy metals. Proteomics 2009, 9, 2602–2621. [Google Scholar] [CrossRef]
- Hossain, Z.; Komatsu, S. Contribution of proteomic studies towards understanding plant heavy metal stress response. Front. Plant Sci. 2013, 3, 310. [Google Scholar] [CrossRef]
- Zeng, F.; Wu, X.; Qiu, B.; Wu, F.; Jiang, L.; Zhang, G. Physiological and proteomic alterations in rice (Oryza sativa L.) seedlings under hexavalent chromium stress. Planta 2014, 240, 291–308. [Google Scholar] [CrossRef]
- Thapa, G.; Sadhukhan, A.; Panda, S.K. Molecular mechanistic model of plant heavy metal tolerance. Biometals 2012, 25, 489–505. [Google Scholar] [CrossRef]
- Behbahani, S.R.; Iranbakhsh, A.; Ebadi, M.; Majd, A.; Ardebili, Z.O. Red elemental selenium nanoparticles mediated substantial variations in growth, tissue differentiation, metabolism, gene transcription, epigenetic cytosine DNA methylation, and callogenesis in bittermelon (Momordica charantia); an in vitro experiment. PLoS ONE 2020, 15, e0235556. [Google Scholar] [CrossRef]
- Handa, N.; Kohli, S.K.; Thukral, A.K.; Arora, S.; Bhardwaj, R. Role of Se(VI) in counteracting oxidative damage in Brassica juncea L. under Cr(VI) stress. Acta Physiol. Plant. 2017, 39, 51. [Google Scholar] [CrossRef]
- Schiavon, M.; Berto, C.; Malagoli, M.; Trentin, A.; Sambo, P.; Dall’Acqua, S.; Pilon-Smits, E.A.H. Selenium biofortification in radish enhances nutritional quality via accumulation of methyl-selenocysteine and promotion of transcripts and metabolites related to glucosinolates, phenolics, and amino acids. Front. Plant Sci. 2016, 7, 1371. [Google Scholar] [CrossRef]
- Dutilleul, C.; Jourdain, A.; Bourguignon, J.; Hugouvieux, V. The Arabidopsis putative selenium-binding protein family: Expression study and characterization of SBP1 as a potential new player in cadmium detoxification processes. Plant Physiol. 2008, 147, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Ma, Q. Transcriptome and proteome profiling revealed molecular mechanism of selenium responses in bread wheat (Triticum aestivum L.). BMC Plant Biol. 2021, 21, 584. [Google Scholar] [CrossRef]
- Kumar, I.; Sharma, R.K. Production of secondary metabolites in plants under abiotic stress: An overview. Significances Bioeng. Biosci. 2018, 2, 196–200. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Akhi, M.Z.; Haque, M.M.; Biswas, M.S. Role of secondary metabolites to attenuate stress damages in plants. In Antioxidants-Benefits, Sources, Mechanisms of Action; IntechOpen: London, UK, 2021. [Google Scholar]
- Ahanger, M.A.; Bhat, J.A.; Siddiqui, M.H.; Rinklebe, J.; Ahmad, P. Silicon and secondary metabolites integration in plants: A significant association in stress tolerance. J. Exp. Bot. 2020, 71, 6758–6774. [Google Scholar] [CrossRef]
- Delfine, S.; Csiky, O.; Seufert, G.; Loreto, F. Fumigation with exogenous monoterpenes of a non-isoprenoid-emitting oak (Quercus suber): Monoterpene acquisition, translocation, and effect on the photosynthetic properties at high temperatures. New Phytol. 2000, 146, 27–36. [Google Scholar] [CrossRef]
- Hassan, A. Effects of mineral nutrients on physiological and biochemical processes related to secondary metabolites production in medicinal herbs. Med. Arom. Plant Sci. Biotechnol. 2012, 6, 105–110. [Google Scholar]
- Nakabayashi, R.; Mori, T.; Saito, K. Alternation of flavonoid accumulation under drought stress in Arabidopsis thaliana. Plant Signal. Behav. 2014, 9, e29518. [Google Scholar] [CrossRef]
- Kováčik, J.; Grúz, J.; Bačkor, M.; Strnad, M.; Repčák, M. Salicylic acid-induced changes to growth and phenolic metabolism in Matricaria chamomilla plants. Plant Cell Rep. 2009, 28, 135–143. [Google Scholar] [CrossRef]
- Manquián-Cerda, K.M.; Escudey, G.; Zúñiga, N.; Arancibia-Miranda, M.; Molina, E.C. Effect of cadmium on phenolic compounds, antioxidant enzyme activity and oxidative stress in blueberry (Vaccinium corymbosum L.) Plantlets grown in vitro. Ecotoxicol. Environ. Saf. 2016, 133, 316–326. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Q.; Lu, H.; Li, J.; Yang, D.; Liu, J.; Yan, C. Phenolic metabolism and related heavy metal tolerance mechanism in Kandelia obovata under Cd and Zn stress. Ecotoxicol. Environ. Saf. 2019, 169, 134–143. [Google Scholar] [CrossRef]
- Tan, P.; Zeng, C.; Wan, C.; Liu, Z.; Dong, X.; Peng, J.; Lin, H.; Li, M.; Liu, Z.; Yan, M. Metabolic profiles of Brassica juncea roots in response to cadmium stress. Metabolites 2021, 11, 383. [Google Scholar] [CrossRef]
- Anjitha, K.S.; Sameena, P.P.; Puthur, J.T. Functional aspects of plant secondary metabolites in metal stress tolerance and their importance in pharmacology. Plant Stress 2021, 2, 100038. [Google Scholar] [CrossRef]
- Kiumarzi, F.; Morshedloo, M.R.; Zahedi, S.M.; Mumivand, H.; Behtash, F.; Hano, C.; Chen, J.T.; Lorenzo, J.M. Selenium nanoparticles (Se-NPs) alleviates salinity damages and improves phytochemical characteristics of pineapple mint (Mentha suaveolens Ehrh.). Plants 2022, 11, 1384. [Google Scholar] [CrossRef]
- Gul, H.; Kinza, S.; Shinwari, Z.K.; Hamayun, M. Effect of selenium on the biochemistry of Zea mays under salt stress. Pak. J. Bot. 2017, 49, 25–32. [Google Scholar]
- Chauhan, R.; Awasthi, S.; Tripathi, P.; Mishra, S.; Dwivedi, S.; Niranjan, A.; Tripathi, R.D. Selenite modulates the level of phenolics and nutrient element to alleviate the toxicity of arsenite in rice (Oryza sativa L.). Ecotoxicol. Environ. Saf. 2017, 138, 47–55. [Google Scholar] [CrossRef]
- Astaneh, R.K.; Bolandnazar, S.; Nahandi, F.Z.; Oustan, S. Effect of selenium application on phenylalanine ammonia-lyase (PAL) activity, phenol leakage and total phenolic content in garlic (Allium sativum L.) under NaCl stress. Inf. Process. Agric. 2018, 5, 339–344. [Google Scholar] [CrossRef]
- Skrypnik, L.; Styran, T.; Savina, T.; Golubkina, N. Effect of selenium application and growth stage at harvest on hydrophilic and lipophilic antioxidants in lamb’s lettuce (Valerianella locusta L. Laterr.). Plants 2021, 10, 2733. [Google Scholar] [CrossRef]
- Chomchan, R.; Siripongvutikorn, S.; Puttarak, P.; Rattanapon, R. Influence of selenium bio-fortification on nutritional compositions, bioactive compounds content and anti-oxidative properties of young ricegrass (Oryza sativa L.). Funct. Foods Health Dis. 2017, 7, 195–209. [Google Scholar] [CrossRef]
- Farman, M.; Nawaz, F.; Majeed, S.; Ahmad, K.S.; Rafeeq, R.; Shehzad, M.A.; Shabbir, R.N.; Usmani, M.M. Interplay between selenium and mineral elements to improve plant growth and development. In Handbook of Bioremediation; Academic Press: Cambridge, MA, USA, 2021; pp. 221–236. [Google Scholar]
- Hawrylak-Nowak, B. Effect of selenium on selected macronutrients in maize plants. J. Elem. 2008, 13, 513–519. [Google Scholar]
- Drahoňovský, J.; Száková, J.; Mestek, O.; Tremlová, J.; Kaňa, A.; Najmanová, J.; Tlustoš, P. Selenium uptake, transformation and inter-element interactions by selected wildlife plant species after foliar selenate application. Environ. Exp. Bot. 2016, 125, 12–19. [Google Scholar] [CrossRef]
- Khattab, H. Metabolic and oxidative responses associated with exposure of Eruca sativa (Rocket) plants to different levels of selenium. Int. J. Agric. Biol. 2004, 6, 1101–1106. [Google Scholar]
- Elkelish, A.A.; Soliman, M.H.; Alhaithloul, H.A.; El-Esawi, M.A. Selenium protects wheat seedlings against salt stress-mediated oxidative damage by up-regulating antioxidants and osmolytes metabolism. Plant Physiol. Biochem. 2019, 137, 144–153. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, C.; Wang, X.; Qing, X.; Wang, P.; Zhang, Y.; Zhang, X.; Zhao, X. Selenium alleviated chromium stress in chinese cabbage (Brassica campestris L. ssp. Pekinensis) by regulating root morphology and metal element uptake. Ecotoxicol. Environ. Saf. 2019, 173, 314–321. [Google Scholar] [CrossRef]
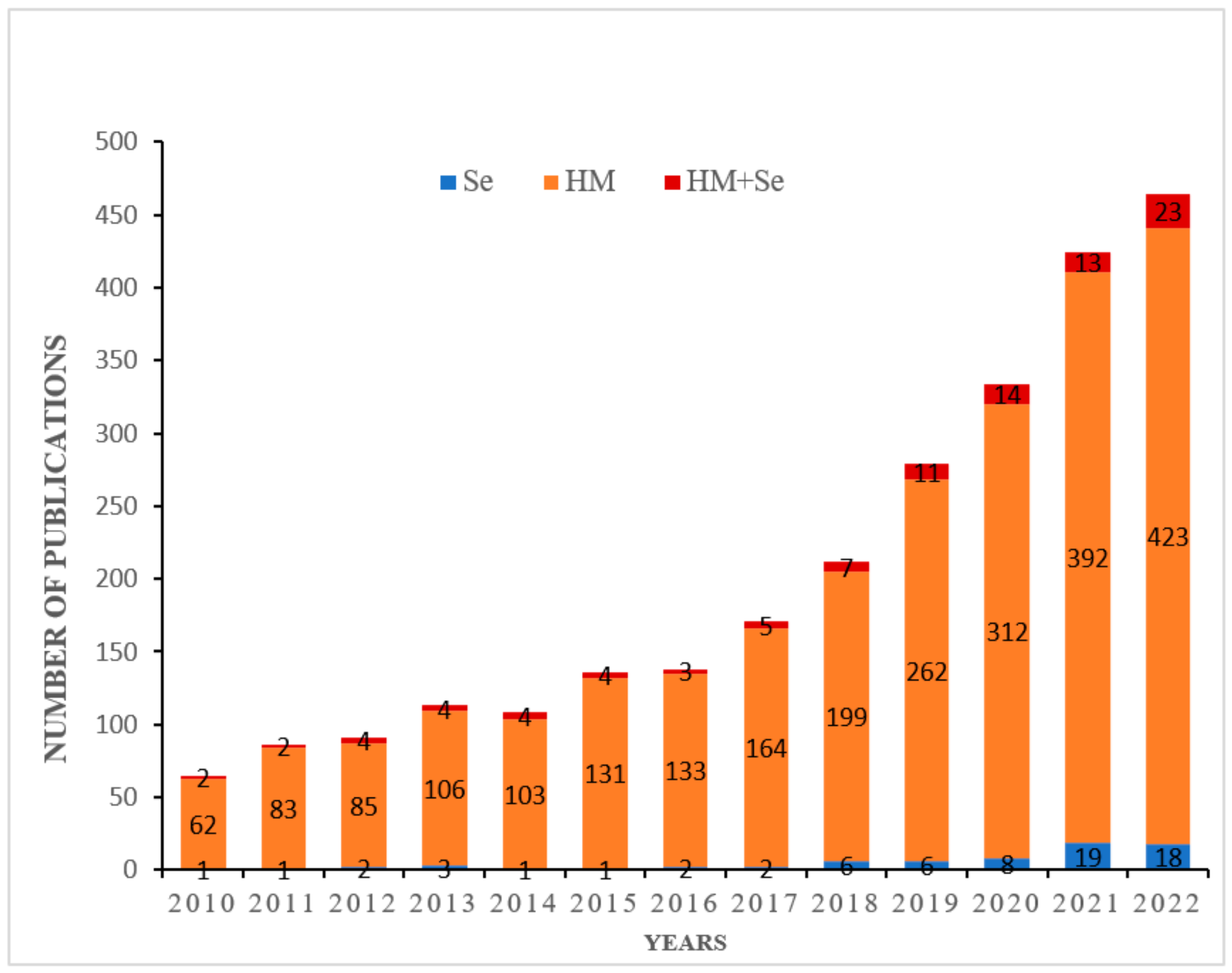
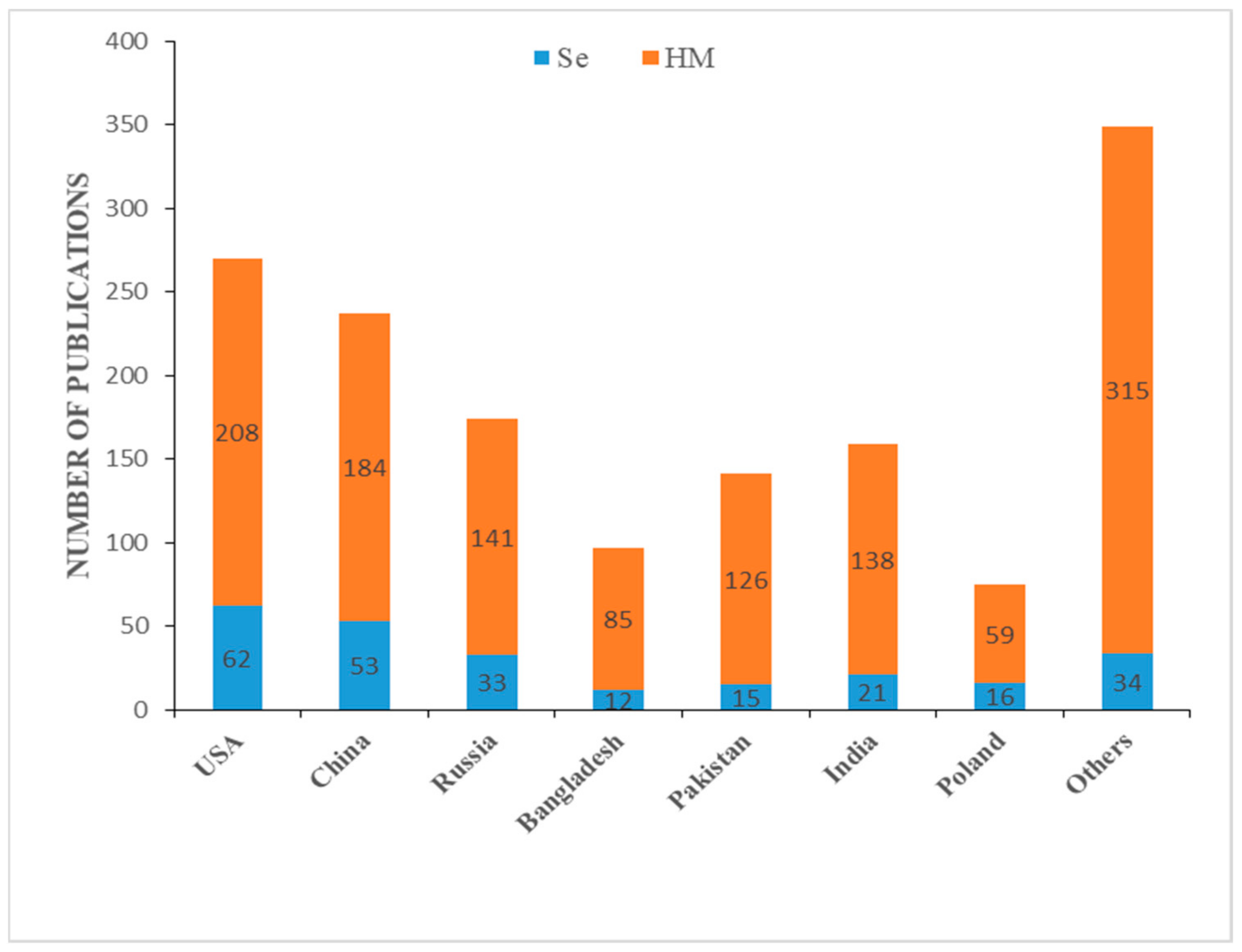
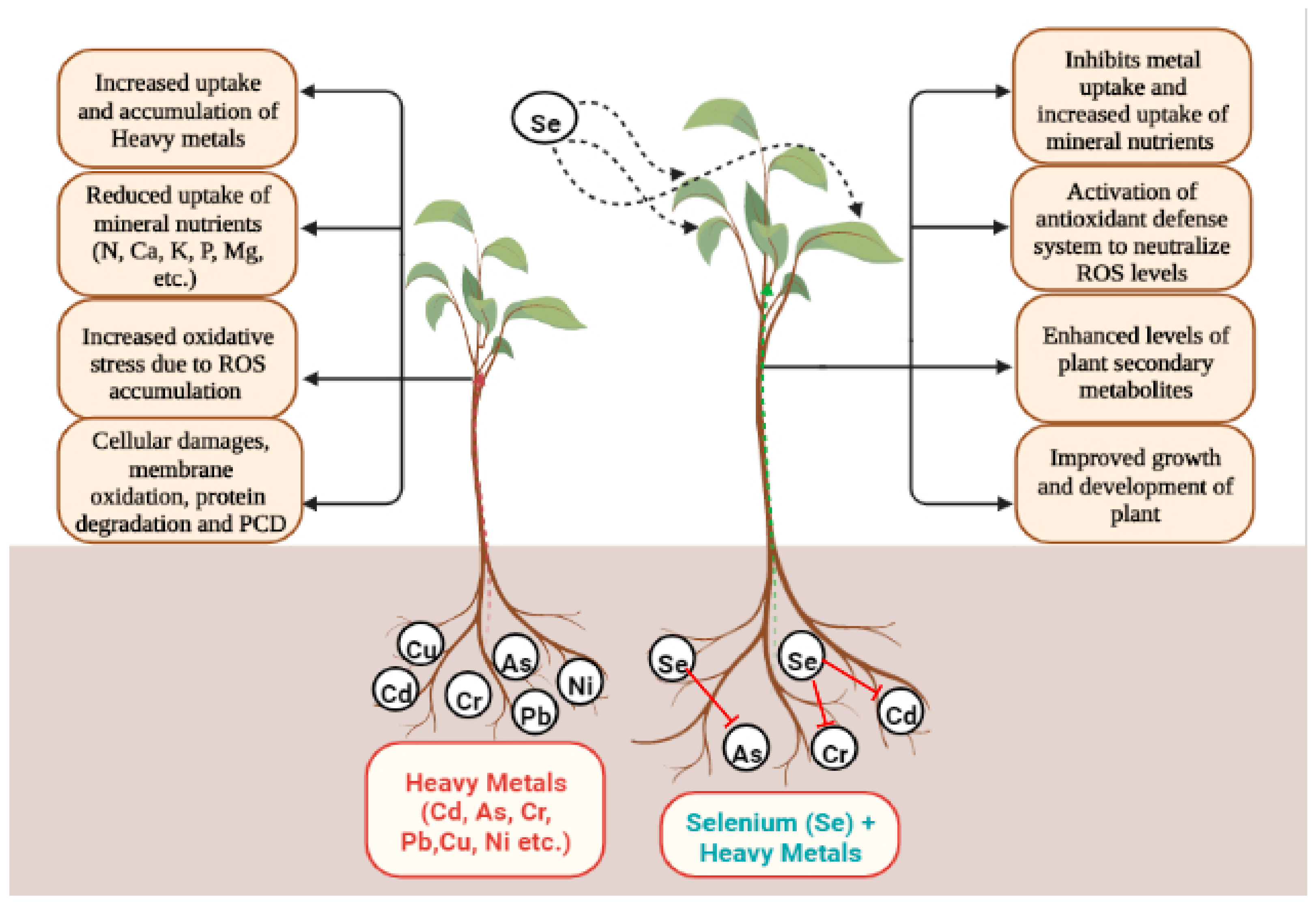

| Species | Heavy Metal Conc. | Se Conc. | Effect of Se on Antioxidants Metabolism | Reference(s) |
|---|---|---|---|---|
| Brassica napus | 5 µM Cd 500 µM Pb | 15 µM | Enhanced SOD and GSH-Px levels to minimize Cd/Pb-induced oxidative stress | [87] |
| Brassica juncea | 300 µM Cr | 4 µM | Strengthen the inheretent defense system by inducing the expression of SOD, CAT, APX, GPOX, GR, GST, DHAR M-DHAR, AsA and GSH against Cr stress. | [31] |
| Brassica juncea | 100 or 200 µM Cd | 50 µM | Increment in CAT, APX and GR activity was observed | [88] |
| Brassica spp. | 50 µM Cd | 3 µM | Induced ROS detoxification, maintained the levels of SOD, CAT and POD | [89] |
| Glycine max | As 25 µM | 25 μM | Reduced As toxicity by improving photosynthesis, antioxidants, and regulation of some defense genes | [27] |
| Vicia faba | 50 µM Pb | 6 µM | Up-regulated CAT, GPOX and GSH-Px levels | [86] |
| Phaseolus aureus | 10 µM As | 5 µM | Reduced As-induced oxidative stress by increasing CAT, APX GR, AsA and GSH levels | [85] |
| Satureja hortensis | 150 µM Cd | 40 µM | Elevated the levels of CAT and POD and reduced Cd toxicity. | [90] |
| Cucumus sativus | 25 µM Cd 200 µM Ni 100 µM Pb | 8 µM | Stimulated antioxidant system by enhancing the activity of CAT, APX and GPOX | [83] |
| Triticum aestivum | Cd 50 µM | 5 and 10 μM | Down-regulation of genes of Cd uptake and transport | [91] |
| Oryza sativa | As 100 µM | 25 μM | Up-regulated various As-tolerant genes and also induced the antioxidant expression | [92] |
| Oryza sativa | 20 µM Cd | 1 µM | Up-regulated CAT and GSH-Px activity and reduced lipid oxidation | [93] |
| Oryza sativa | 25 µM As | 25 µM | Positively enhanced the activities of CAT, APX, GSH-Px, GR, GST and GSH | [94] |
| Lolium perenne | 0.2 µM Al | 5 µM | Reduced lipid peroxidation by Increasing SOD and APX activity | [84] |
| Species | HM Conc. | Phytohormones Conc. | Response | Reference(s) |
|---|---|---|---|---|
| Brassica juncea | 24 µM As | 200 μL/L Ethephon | Improvement of photosynthetic attributes by reduced As and ABA accumulation, increment in antioxidant activity | [123] |
| Sedum alfredii | 100 µM Cd | 0.2 mg/L ABA | Exogenous ABA enhanced endogenous ABA production, which is involved in Cd tolerance by regulating the expression of Cd tolerance genes | [119] |
| Populus × Canescens | 3 µM Pb | 10 μM ABA | Ameliorated the toxic effects of Pb by minimizing oxidative stress and induced expression of genes responsible for Pb resistance | [124] |
| Oryza sativa | 150 μM As | 3 μM IAA | Improved growth by accumulating more amino acids, proteins etc. | [120] |
| Cajanus cajan | 5 µM Cu2+ | 1 nM JA | Improved photosynthesis, antioxidative system and reduced oxidative stress | [125] |
| Solanum lycopersicum | 3 µM Cd | 10 nM HBL | Improved overall growth and productivity of plants, positively regulated N-metabolism. | [126] |
| Zea mays | 50 µM Cd | 10−9 M IBA | Reduced Cd toxicity by inducing ROS detoxification and improved nutrient status of plants | [127] |
| Vigna radiata | 60 µM Ni | 10−4 M GA3 | Improved growth and biomass of plants by reducing the uptake of Ni from soil | [113] |
| Helianthus annuus | 4 µM U/Cd | 500 mg/L IAA | ROS detoxification by inducing antioxidants, increased uptake of U/Cd from soil | [122] |
| Brassica juncea | 50 μM Cd | 200 μL/L Ethephon | Improved growth, induced antioxidants, and amino acids accumulation | [128] |
| Brassica juncea | 1.2 µM Cr | 200 μL/L Ethephon | Mitigated Cr stress by improving photosynthesis, reduced oxidative stress, and also enhanced proline accumulation | [15] |
| Hordeum vulgare | 10 μM Cd | 1 μM GR24 (strigol analogue) | Reduced Cd toxicity by improving photosynthesis, uptake of essential nutrients, and inducing stress markers | [121] |
| Panicum virgatum | 10 µM Cd | 1 μM GR24 (strigol analogue) | Increment in photosynthetic parameters, stimulation of antioxidant system and improvement in the mineral status of plants | [129] |
| Solanum lycopersicum | 150 μM Cd | 100 μM NO | Positive effect on photosynthesis and improved growth, antioxidant system, osmoprotectants and secondary metabolites accumulation, hence involved in Cd tolerance. | [130] |
| Oryza sativa | 5 µM Cr | 0.1 nM 24-EBL | Induced Cd detoxification by enhancing photosynthesis and regulating the genes expression | [131] |
| Plant Species | Se Dose | Secondary Metabolites | Response | Reference(s) |
|---|---|---|---|---|
| Mentha suaveolens | 10 µM | Production of essential oils such as piperitenone oxide, limonene, jasmone etc. | Enhanced growth and SMs production | [158] |
| Brassica juncea | 4 µM | Enhanced levels of phenols, flavonoids, and anthocyanins | Improvement in the photosynthesis thereby growth of plant by inducing the accumulation of antioxidants, SMs, etc., against Cr stress | [31] |
| Brassica oleracea | 25 µM | Affected the production or accumulation of glucoraphanin, an important glucosinolate | Reduced content of glucosinlates precursors, Suppressed the expression of genes involved in glucosinlates biosynthesis | [32] |
| Melissa officinalis | 5 µM | Increased the accretion of essential oils such as z-citral, citral and geranyl acetate | Positive effect on the growth at low concentration | [20] |
| Brassica oleracea | 10 µM | Increment in the levels of phenolic compounds and glucosinolates | Improved growth and yield characteristics of plants by inducing antioxidant and SMs levels | [30] |
| Zea mays | 10 µM | Enhancement of phenols and flavonoids content | Induced accumulation of proteins, sugars and SMs | [159] |
| Oryza sativa | 25 µM | Induced the accumulation of essential phenolics such as gallic, protocatechuic and ferulic (acids) | Increased the uptake of nutrients from the soil and regulated SMs production, hence reducing As toxicity | [160] |
| Brassica juncea | 50 µM | Enhanced phenolic content | Reduced Cd stress by inducing the antioxidant system and SMs status of plants | [88] |
| Allium sativum | 4 µM | Increased total phenolic content | Increased tolerance ability of garlic to salt stress by preventing membrane oxidation, phenols accumulation and regulation of phenylalanine ammonia-lyase activity | [161] |
| Vallerianella locusta | 5 µM | Enhanced the endogenous levels of flavonoids and phenolics | Improved growth, antioxidant activity and accumulation of SMs | [162] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asgher, M.; Rehaman, A.; Islam, S.N.u.; Arshad, M.; Khan, N.A. Appraisal of Functions and Role of Selenium in Heavy Metal Stress Adaptation in Plants. Agriculture 2023, 13, 1083. https://doi.org/10.3390/agriculture13051083
Asgher M, Rehaman A, Islam SNu, Arshad M, Khan NA. Appraisal of Functions and Role of Selenium in Heavy Metal Stress Adaptation in Plants. Agriculture. 2023; 13(5):1083. https://doi.org/10.3390/agriculture13051083
Chicago/Turabian StyleAsgher, Mohd, Abdul Rehaman, Syed Nazar ul Islam, Mohd Arshad, and Nafees A. Khan. 2023. "Appraisal of Functions and Role of Selenium in Heavy Metal Stress Adaptation in Plants" Agriculture 13, no. 5: 1083. https://doi.org/10.3390/agriculture13051083
APA StyleAsgher, M., Rehaman, A., Islam, S. N. u., Arshad, M., & Khan, N. A. (2023). Appraisal of Functions and Role of Selenium in Heavy Metal Stress Adaptation in Plants. Agriculture, 13(5), 1083. https://doi.org/10.3390/agriculture13051083









