Reducing Anion Nutrient Leaching Losses from a Short-Cycle Container-Grown Crop (Tagetes patula) Using Activated Aluminum
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Plant Growth Measurements
2.3. Leachate Collection and Analyses
2.4. Statistical Analysis
3. Results
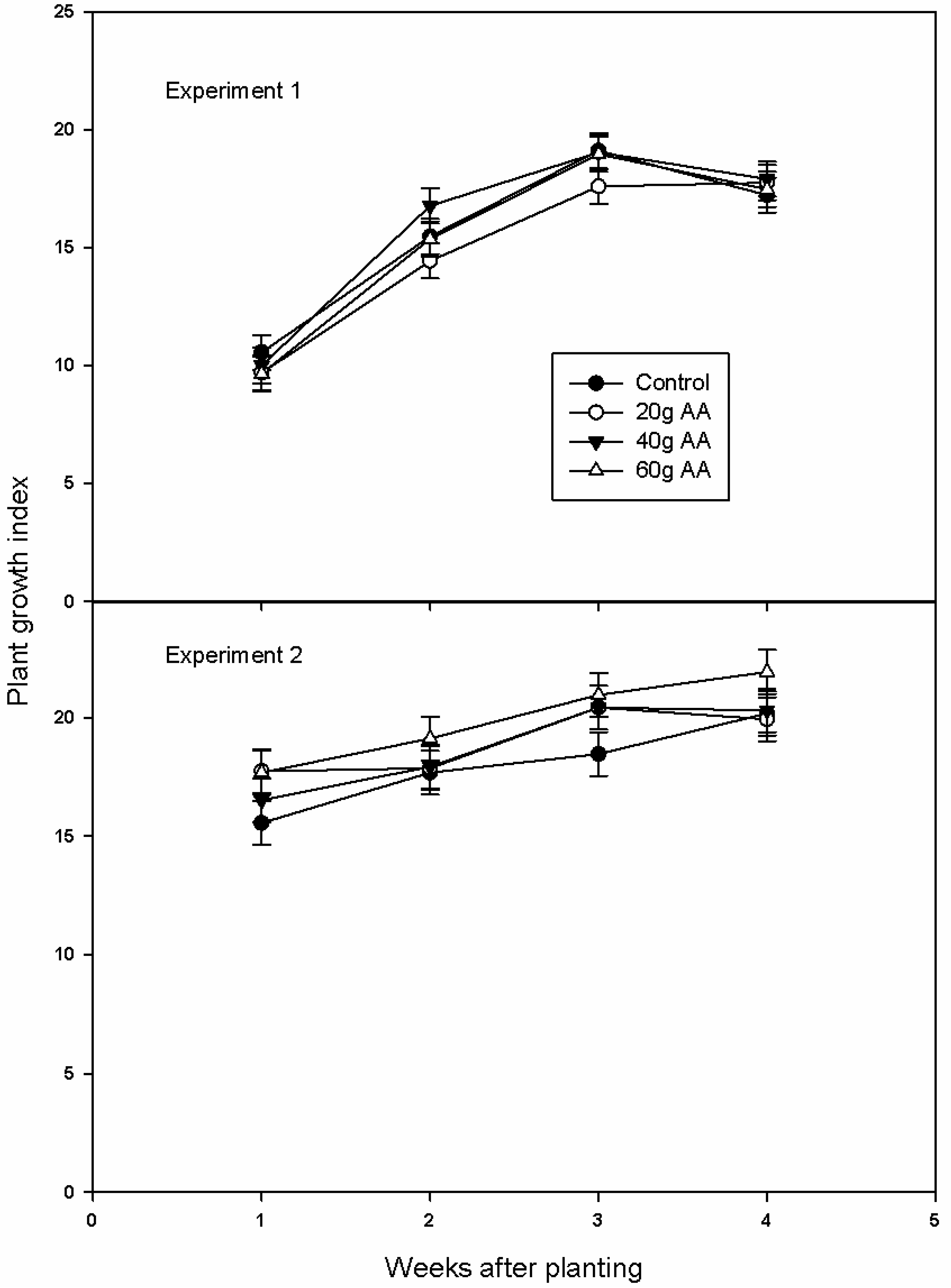
3.1. Marigold Growth and Quality
3.2. Aluminum Leached
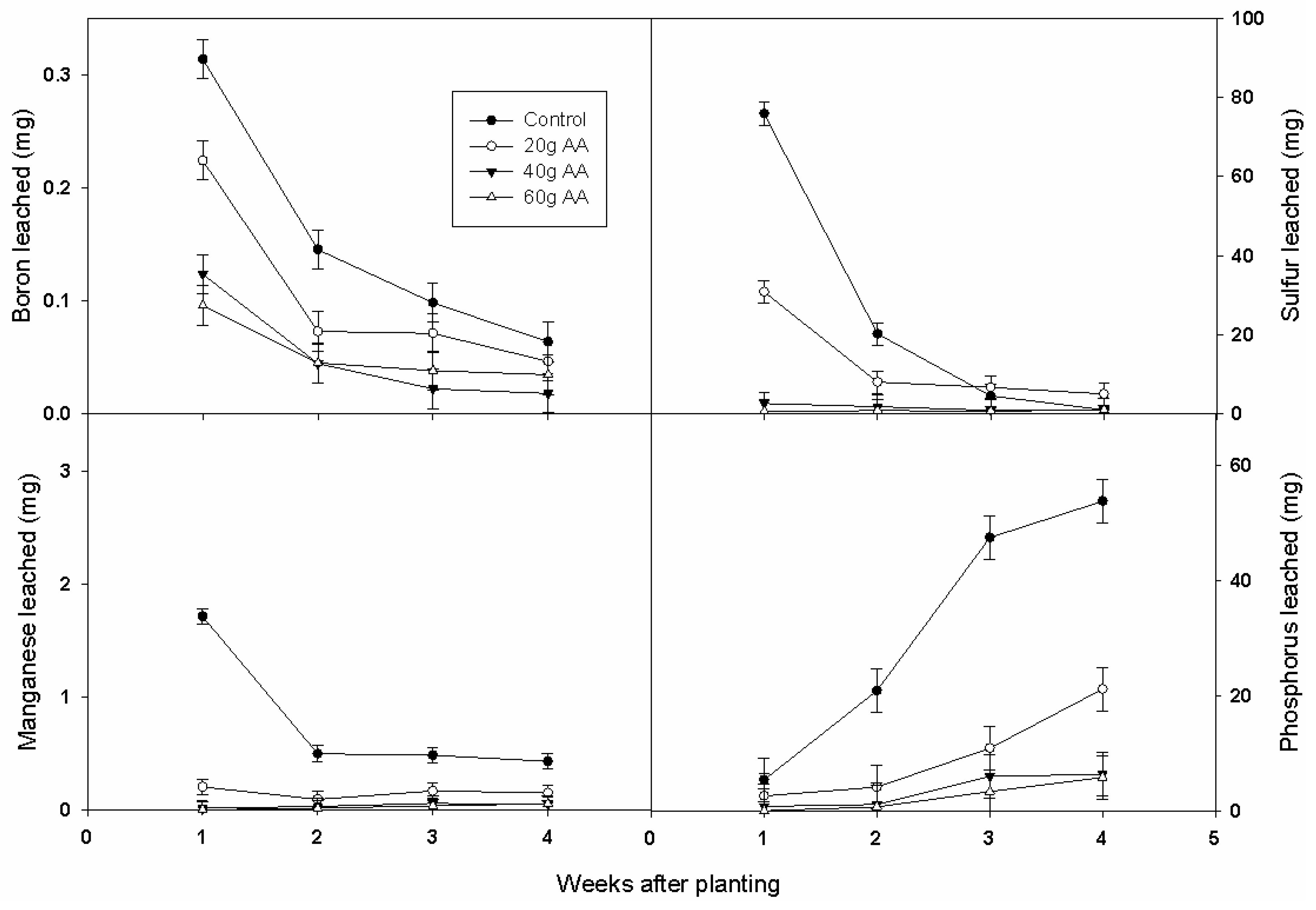
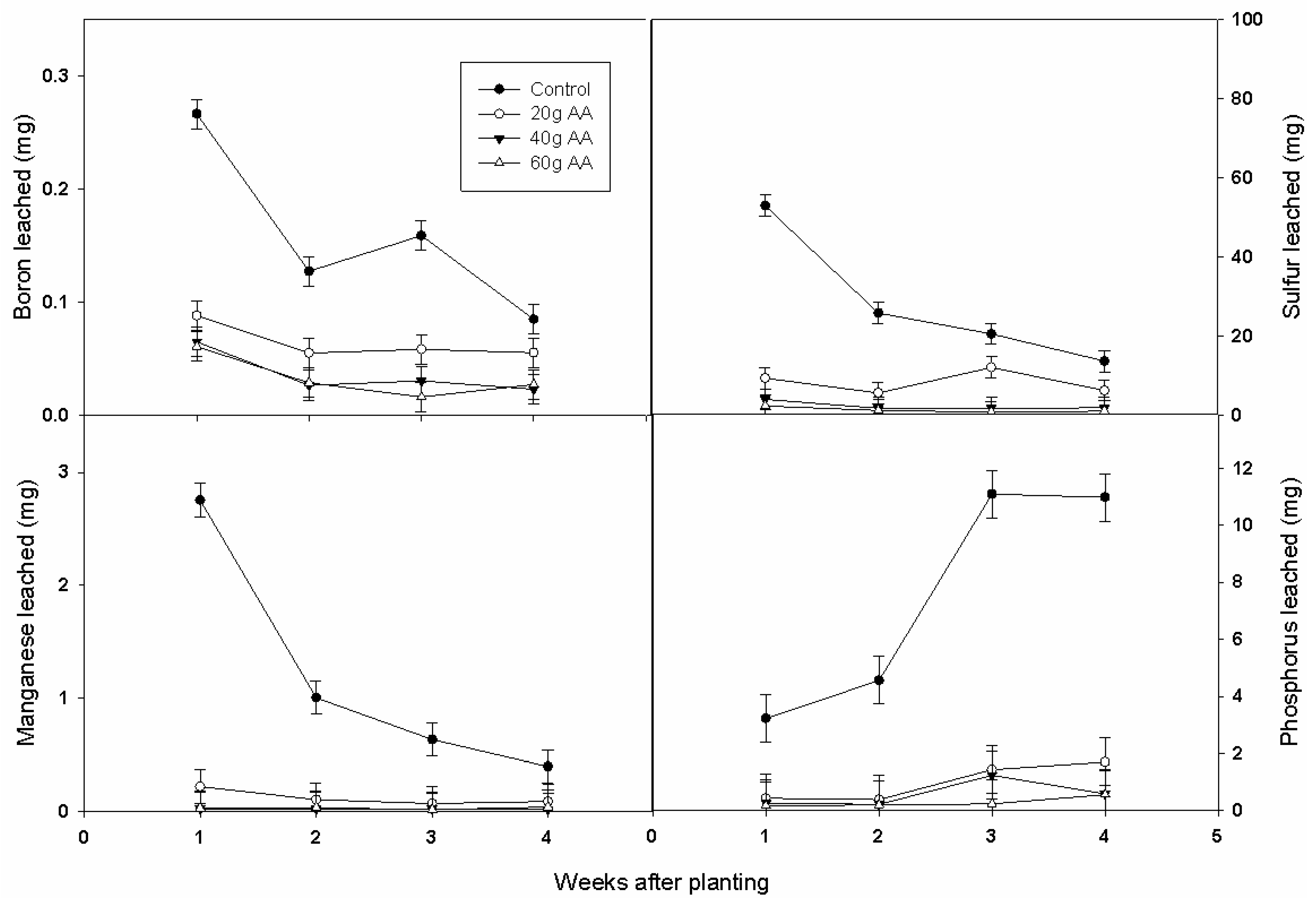
3.3. Nutrient and Leaching Losses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krofft, C.E.; Pickens, J.M.; Newby, A.F.; Sibley, J.L.; Fain, G.B. The effect of leaching fraction-based irrigation on fertilizer longevity and leachate nutrient content in a greenhouse environment. Horticulturae 2020, 6, 43. [Google Scholar] [CrossRef]
- Jahromi, N.B.; Walker, F.; Fulcher, A.; Altland, J.; Wright, W.C. Growth response, mineral nutrition, and water utilization of container-grown woody ornamentals grown in biochar-amended pine bark. HortScience 2018, 53, 347–353. [Google Scholar] [CrossRef]
- Owen, J.S., Jr.; Warren, S.L.; Bilderback, T.E.; Albano, J.P. Phosphorus rate, leaching fraction, and substrate influence on influent quantity, effluent nutrient content, and response of a containerized woody ornamental crop. HortTechnology 2008, 43, 906–912. [Google Scholar] [CrossRef]
- Zhu, H.; Frants, J.M.; Derksen, R.C.; Krause, C.R. Investigation of drainage and plant growth from nursery container substrate. Appl. Eng. Agric. 2007, 23, 289–297. [Google Scholar] [CrossRef]
- Zhu, H.; Zondag, R.H.; Merrick, J.; Demaline, T.; Krause, C.R. Nutrient leaching from container-grown ornamental tree production. J. Environ. Hortic. 2015, 33, 76–83. [Google Scholar] [CrossRef]
- Abdi, D.E.; Owen, J.S., Jr.; Brindley, J.C.; Birnbaum, A.; Cregg, B.M.; Fernandez, R.T. Irrigation return flow and nutrient movement mitigation by irrigation method for container plant production. Irrig. Sci. 2021, 5, 567–585. [Google Scholar] [CrossRef]
- Bilderback, T. Water management is key in reducing nutrient runoff from container nurseries. HortTechnology 2002, 4, 541–544. [Google Scholar] [CrossRef]
- Tyler, H.H.; Warren, S.L.; Bilderback, T.E. Reduced leaching fractions improve irrigation use efficiency and nutrient efficacy. J. Environ. Hortic. 1996, 14, 199–204. [Google Scholar] [CrossRef]
- Majsztrik, J.C.; Fernandez, R.T.; Fisher, P.R.; Hitchcock, D.R.; Lea-Cox, J.; Owen, J.S., Jr.; Oki, L.R.; White, S.A. Water use and treatment in container-grown specialty crop production: A review. Water Air Soil Pollut. 2017, 228, 151. [Google Scholar] [CrossRef]
- Yazdi, M.N.; Sample, D.J.; Scott, D.; Owen, J.S.; Ketabchy, M.; Alamdari, N. Water quality characterization of storm and irrigation runoff from a container nursery. J. Environ. Hortic. 2019, 667, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Agro, E.; Zheng, Y. Controlled-release fertilizer application rates for container nursery crop production in Southwestern Ontario, Canada. HortScience 2014, 11, 1414–1423. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Y.; Caldwell, R.D. Best management practices for minimizing nitrate leaching from container-grown nurseries. Sci. World 2001, 1, 96–102. [Google Scholar] [CrossRef]
- Garber, M.P.; Ruter, J.M.; Midcap, J.T.; Bondari, L. Survey of container nursery irrigation practices in Georgia. HortTechnology 2002, 12, 727–731. [Google Scholar] [CrossRef]
- Yeager, T.; Wright, R.; Fare, D.; Gilliam, C.; Johnson, J.; Bilderback, T.; Zondag, R. Six state survey of container nursery nitrate nitrogen runoff. J. Environ. Hortic. 1993, 11, 206–208. [Google Scholar] [CrossRef]
- Mangiafico, S.S.; Newman, J.; Merhaut, D.J.; Gan, J.; Faber, B.; Wu, L. Nutrients and pesticides in stormwater runoff and soil water in production nurseries and citrus and avocado groves in California. HortTechnology 2009, 19, 360–367. [Google Scholar] [CrossRef]
- Rose, M.A.; Rose, M.; Wang, H. Fertilizer concentration and moisture tension affect growth and foliar N, P, and K contents of two woody ornamentals. HortScience 1999, 34, 246–250. [Google Scholar] [CrossRef]
- Majsztrik, J.C.; Ristvey, A.G.; Ross, D.S.; Lea-Cox, J.D. Comparative water and nutrient application rates among ornamental operations in Maryland. HortScience 2018, 53, 1364–1371. [Google Scholar] [CrossRef]
- Warsaw, A.L.; Fernandez, R.T.; Cregg, B.M.; Andresen, J.A. Container-grown ornamental plant growth and water runoff nutrient content and volume under four irrigation treatments. HortScience 2009, 44, 1573–1580. [Google Scholar] [CrossRef]
- Mangiafico, S.S.; Gan, J.; Wu, L.; Lu, J.; Newman, J.P.; Faber, B.; Merhaut, D.J.; Evans, R. Detention and recycling basins for managing nutrient and pesticide runoff from nurseries. HortScience 2008, 43, 393–398. [Google Scholar] [CrossRef]
- Lu, J.; Wu, L.; Newman, J.; Faber, B.; Gan, J. Degradation of pesticides in nursery recycling pond waters. J. Agric. Food Chem. 2006, 54, 2658–2663. [Google Scholar] [CrossRef]
- Ristvey, A.G.; Belayneh, B.E.; Lea-Cox, J.D. A comparison of irrigation-water containment methods and management strategies between two ornamental production systems to minimize water security threats. Water 2019, 11, 2558. [Google Scholar] [CrossRef]
- Spangler, J.T.; Sample, D.J.; Fox, L.J.; Owen, J.S., Jr.; White, S.A. Floating treatment wetland aided nutrient removal from agricultural runoff using two wetland species. Ecol. Eng. 2019, 127, 468–479. [Google Scholar] [CrossRef]
- Sanders, K.R.; Beasley, J.S. Fertilizer source affects nutrient losses from hybrid bermudagrass during surface runoff. HortTechnology 2019, 6, 952–957. [Google Scholar] [CrossRef]
- Broschat, T.K. Substrate nutrient retention and growth of container-grown plants in clinoptilolic zeolite-amended substrates. HortTechnology 2001, 11, 75–78. [Google Scholar] [CrossRef]
- Owen, J.S., Jr.; Warren, S.L.; Bilderback, T.E.; Albano, J.P. Industrial mineral aggregate amendment affects physical and chemical properties of pine bark substrates. HortScience 2007, 42, 1287–1294. [Google Scholar] [CrossRef]
- Altland, J.E.; Locke, J.C.; Krause, C.R. Influence of pine bark particle size and pH on cation exchange capacity. HortTechnology 2017, 24, 554–559. [Google Scholar] [CrossRef]
- Fields, J.S.; Owen, J.S., Jr.; Altland, J.E.; van Iersel, M.W.; Jackson, B.E. Soilless substrate hydrology can be engineered to influence plant water status for an ornamental containerized crop grown within optimal water potentials. J. Am. Soc. Hortic. Sci. 2018, 143, 268–281. [Google Scholar] [CrossRef]
- Shreckhise, J.H.; Owen, J.S., Jr.; Eick, M.J.; Niemera, A.X.; Altland, J.E.; White, S.A. Dolomite and micronutrient fertilizer affect phosphorus fate in pine bark substrate used for containerized nursery crop production. Soil Sci. Soc. Am. J. 2019, 83, 1410–1420. [Google Scholar] [CrossRef]
- Godoy, A.; Cole, J.C. Phosphorus source affects phosphorus leaching and growth of containerized spiraea. HortScience 2000, 35, 1249–1252. [Google Scholar] [CrossRef]
- Marconi, D.J.; Nelson, P.V. Leaching of applied phosphorus in container media. Sci. Hortic. 1984, 22, 275–285. [Google Scholar] [CrossRef]
- Garelick, H.; Dybowska, A.; Valsami-Jones, E.; Priest, N.D. Remediation technologies for arsenic contaminated drinking waters. J. Soils Sediments 2005, 5, 182–190. [Google Scholar] [CrossRef]
- Nicomel, N.R.; Leus, K.; Folens, K.; Van Der Voort, P.; Du Lang, G. Technologies for arsenic removal from water: Current status and future perspectives. Int. J. Environ. Res. Public Health 2015, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Sorg, T.J.; Chen, A.S.C.; Wang, L.; Kolisz, R. Regenerating an arsenic removal iron-bed adsorptive media system, part 1: The regeneration process. Am. Water Work. Assoc. 2017, 109, 13–24. [Google Scholar] [CrossRef]
- Su, T.; Guan, X.; Gu, G.; Wang, J. Adsorption characteristics of As(V), Se (IV), and V(V) onto activated alumina: Effects of pH, surface loading, and ionic strength. J. Colloid Interface Sci. 2008, 326, 347–353. [Google Scholar] [CrossRef]
- Scott, I.S.P.C.; Penn, C.J.; Huang, C.H. Development of a regeneration technique for aluminum-rich and iron-rich phosphorus sorption materials. Water 2020, 12, 1784. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Feng, C.; Li, J.; Li, G. Adsorption capacity for phosphorus comparison among activated alumina, silica sand and anthracite coal. J. Water Resour. Prot. 2009, 4, 260–264. [Google Scholar] [CrossRef]
- Wu, G.; Liu, G.; Li, X.; Peng, Z.; Zhou, Q.; Qi, T. Enhanced phosphate removal with fine activated alumina synthesized from a sodium aluminate solution: Performance and mechanism. RSC Adv. 2022, 12, 4562–4571. [Google Scholar] [CrossRef]
- Yu, C.; Liu, L.; Wang, X.; Fu, J.; Wu, Y.; Feng, C.; Wu, Y.; Shen, J. Fluoride removal performance of highly porous activated alumina. Journal of sol-gel science and technology. J. Sol-Gel Sci. Technol. 2023, 106, 471–479. [Google Scholar] [CrossRef]
- International Publication Number WO02/083598A1. U.S. Patent No. 5,693,119. Available online: https://patents.google.com/patent/WO2002083598A1/en (accessed on 27 February 2023).
- Patent Number 6287357. Available online: https://patents.justia.com/patent/6287357 (accessed on 27 February 2023).
- He, H.; Li, Y.; He, L.F. Aluminum toxicity and tolerance in Solanaceae plants. S. Afr. J. Bot. 2019, 123, 23–29. [Google Scholar] [CrossRef]
- Panda, S.K.; Baluska, F.; Matsumoto, H. Aluminum stress signaling in plants. Plant Signal. Behav. 2009, 4, 592–597. [Google Scholar] [CrossRef]
- Rahman, M.A.; Lee, S.H.; Hee, C.J.; Kabir, A.H.; Jones, C.S.; Lee, K.W. Importance of mineral nutrition for mitigating aluminum toxicity in plants on acidic soils: Current status and opportunities. Int. J. Mol. Sci. 2018, 19, 3073. [Google Scholar] [CrossRef] [PubMed]
- Shetty, R.; Vidya, C.S.N.; Prakash, N.B.; Lux, A.; Vaculik, M. Aluminum toxicity in plants and its possible mitigation in acid soils by biochar: A review. Sci. Total Environ. 2021, 765, 142744. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.J. Crop production on acidic soils: Overcoming aluminium toxicity and phosphorus deficiency. Ann. Bot. 2010, 106, 183–184. [Google Scholar] [CrossRef]
- Gillespie, C.J.; Antonangelo, J.A.; Zhang, H. The response of soil ph and exchangeable al to alum and lime amendments. Agriculture 2021, 11, 547. [Google Scholar] [CrossRef]
- Silva, S. Aluminum toxicity targets in plants. J. Bot. 2012, 2012, 219462. [Google Scholar] [CrossRef]
- Altland, J.E. Lime rate affects substrate ph and container-grown birch trees. Comm. Soil Sci. Plant Anal. 2019, 50, 93–101. [Google Scholar] [CrossRef]
- Huang, J.; Fisher, P.R.; Argo, W.R. Container substrate-pH response to differing limestone type and particle size. HortScience 2007, 42, 1268–1273. [Google Scholar] [CrossRef]
- Jackson, B.E.; Wright, R.D.; Gruda, N. Container medium pH in a pine tree substrate amended with peatmoss and dolomitic limestone affects plant growth. HortScience 2009, 44, 1983–1987. [Google Scholar] [CrossRef]
- Owen, W.G.; Jackson, B.E.; Fonteno, W.C.; Whipker, B.E. Liming requirements of greenhouse peat based substrates amended with pine wood chips as a perlite alternative. HortTechnology 2020, 30, 219–230. [Google Scholar] [CrossRef]
- Taylor, M.D.; Kreis, R.; Reito, L. Establishing growing substrate pH with compost and limestone and the impact on pH buffering capacity. HortScience 2016, 51, 1153–1158. [Google Scholar] [CrossRef]
- Wefers, K.; Misra, C. Oxides and Hydroxides of Aluminum; Alcoa Laboratories Technical Paper No. 19; Alcoa Research Laboratories: Pittsburgh, PA, USA, 1987. [Google Scholar]
- Irmak, S.; Haman, D.Z.; Irmak, A.; Jones, J.W.; Campbell, K.L.; Crisman, T.L. Measurement and analyses of growth and stress parameters of Viburnum odoratissimum (Ker-gawl) grown in a multi-pot box system. HortScience 2004, 39, 1445–1455. [Google Scholar] [CrossRef]
- García-Gaytán, V.; Hernández-Mendoza, F.; Coria-Téllez, A.V.; García-Morales, S.; Sánchez-Rodríguez, E.; Rojas-Abarca, L.; Daneshvar, H. Fertigation: Nutrition, stimulation and bioprotection of the root in high performance. Plants 2018, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, S.K. Water soluble fertilizers in horticultural crops—An appraisal. Indian J. Agric. Sci. 2016, 86, 1245–1256. [Google Scholar]
- Correll, D.L. The role of phosphorus in the eutrophication of receiving waters: A review. J. Environ. Qual. 1998, 27, 261–266. [Google Scholar] [CrossRef]
- Poudyal, S.; Owen, J.S., Jr.; Sharkey, T.D.; Fernandez, R.T.; Cregg, B. Phosphorus requirement for biomass accumulation is higher compared to photosynthetic biochemistry for three ornamental shrubs. Sci. Hortic. 2021, 275, 109719. [Google Scholar] [CrossRef]
- Massey, M.S.; Zohar, I.; Ippolito, J.A.; Litaor, M.I. Phosphorus sorption to aluminum-based water treatment residuals reacted with dairy wastewater: 2. X-Ray Absorption Spectroscopy. J. Environ. Qual. 2018, 47, 546–553. [Google Scholar] [CrossRef]
- Tanada, S.; Kabayama, M.; Kawasaki, N.; Sakiyama, T.; Nakamura, T.; Araki, M.; Tamura, T. Removal of phosphate by aluminum oxide hydroxide. J. Colloid Interface Sci. 2003, 257, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Phillips, B.L.; Boily, J.F.; Hu, Y.; Hu, Z.; Yang, P.; Feng, X.; Xu, W.; Zhu, M. Phosphate sorption speciation and precipitation mechanisms on amorphous aluminum hydroxide. Soil Syst. 2019, 3, 20. [Google Scholar] [CrossRef]
- Bojórquez-Quintal, E.; Escalante-Magaña, C.; Echevarría-Machado, I.; Martínez-Estévez, M. Aluminum, a friend or foe of higher plants in acid soils. Front. Plant Sci. 2017, 8, 1767. [Google Scholar] [CrossRef]
- Brunner, I.; Sperisen, C. Aluminum exclusion and aluminum tolerance in woody plants. Front. Plant Sci. 2013, 4, 172. [Google Scholar] [CrossRef]
- Amaizah, N.R.; Cakmak, D.; Saljnikov, S.; Roglic, G.; Kokovic, N.; Manoilovic, D. Effect of waste Al-phosphate on soil and plant. Plant Soil Environ. 2013, 59, 130–135. [Google Scholar] [CrossRef]


| Average Cumulative Mass Collected in Leachate (mg) | ||||
|---|---|---|---|---|
| 0 g AA | 20 g AA | 40 g AA | 60 g AA | |
| Aluminum | 1.90 (a) | 1.52 (a) | 1.31 (ab) | 0.89 (b) |
| Phosphorus | 78.9 (a) | 21.6 (b) | 8.53 (b) | 5.78 (b) |
| Boron | 0.63 (a) | 0.34 (b) | 0.18 (c) | 0.17 (c) |
| Manganese | 3.96 (a) | 0.54 (b) | 0.11 (b) | 0.11 (b) |
| Sulfur | 107 (a) | 42.0 (b) | 8.02 (c) | 4.32 (c) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdi, D.E.; Blanchard, J.; Fields, J.S.; Santos, L.; Beasley, L.; Beasley, J. Reducing Anion Nutrient Leaching Losses from a Short-Cycle Container-Grown Crop (Tagetes patula) Using Activated Aluminum. Agriculture 2023, 13, 1028. https://doi.org/10.3390/agriculture13051028
Abdi DE, Blanchard J, Fields JS, Santos L, Beasley L, Beasley J. Reducing Anion Nutrient Leaching Losses from a Short-Cycle Container-Grown Crop (Tagetes patula) Using Activated Aluminum. Agriculture. 2023; 13(5):1028. https://doi.org/10.3390/agriculture13051028
Chicago/Turabian StyleAbdi, Damon E., Jennifer Blanchard, Jeb S. Fields, Leticia Santos, Lily Beasley, and Jeffrey Beasley. 2023. "Reducing Anion Nutrient Leaching Losses from a Short-Cycle Container-Grown Crop (Tagetes patula) Using Activated Aluminum" Agriculture 13, no. 5: 1028. https://doi.org/10.3390/agriculture13051028
APA StyleAbdi, D. E., Blanchard, J., Fields, J. S., Santos, L., Beasley, L., & Beasley, J. (2023). Reducing Anion Nutrient Leaching Losses from a Short-Cycle Container-Grown Crop (Tagetes patula) Using Activated Aluminum. Agriculture, 13(5), 1028. https://doi.org/10.3390/agriculture13051028








