Genetic Differentiation of Red Clover (Trifolium pratense L.) Cultivars and Their Wild Relatives †
Abstract
1. Introduction
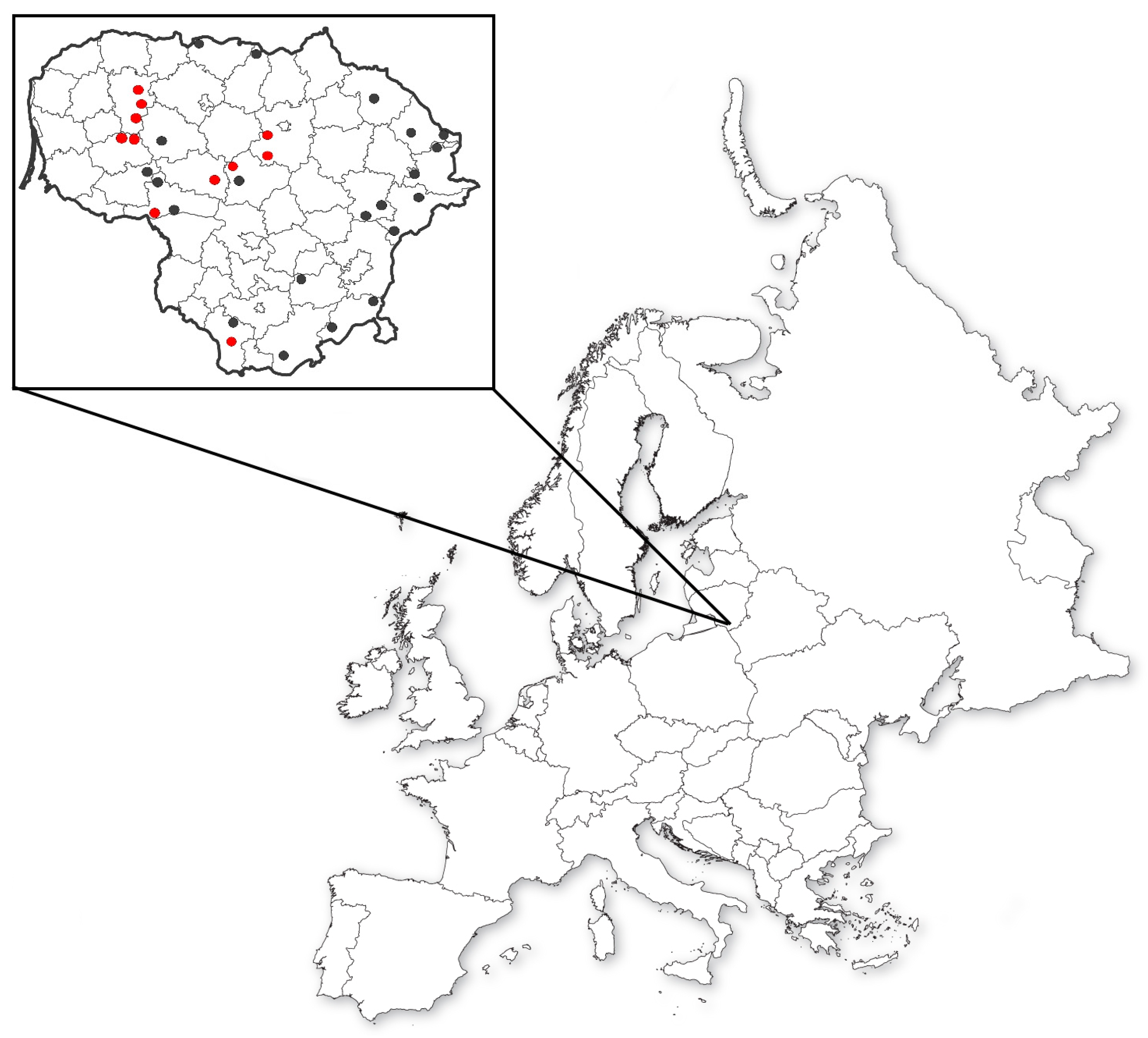
2. Materials and Methods
Statistical Analysis
3. Results and Discussion
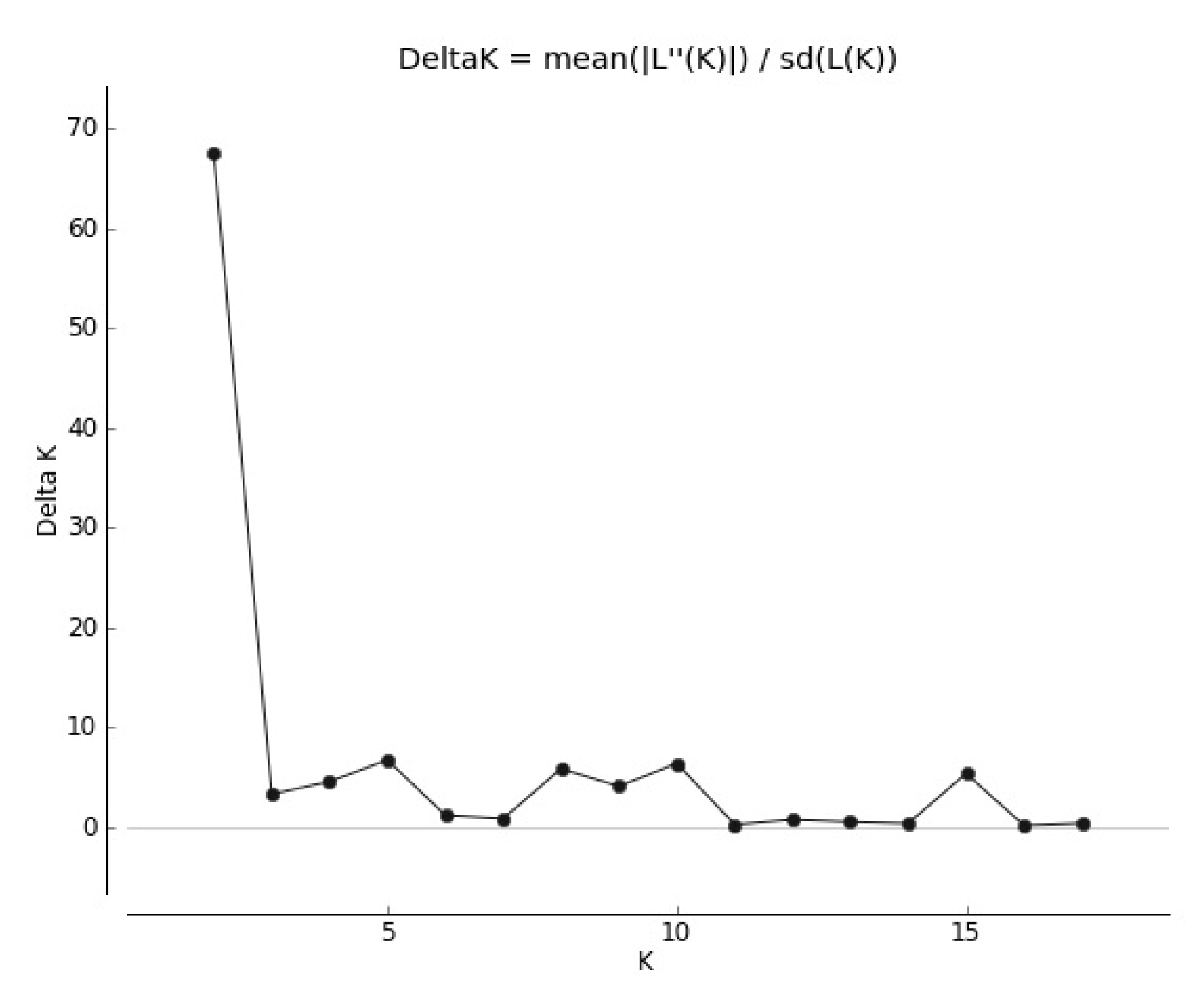
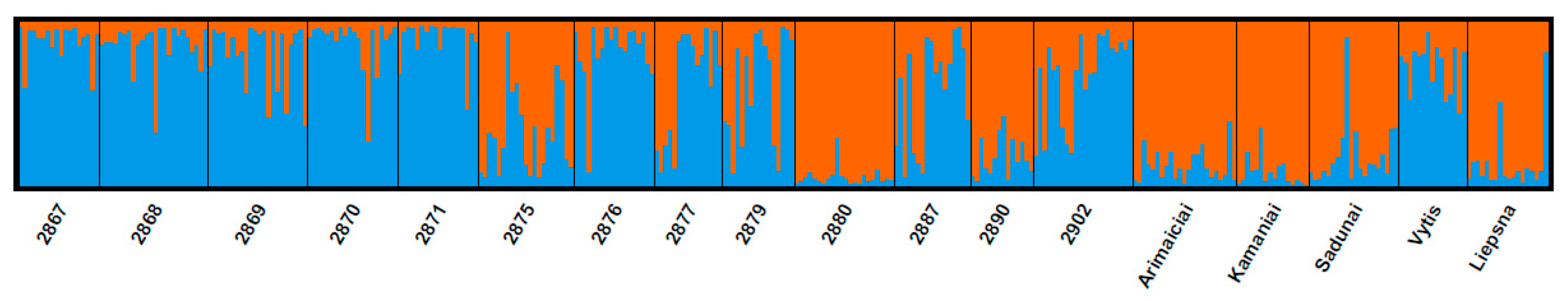
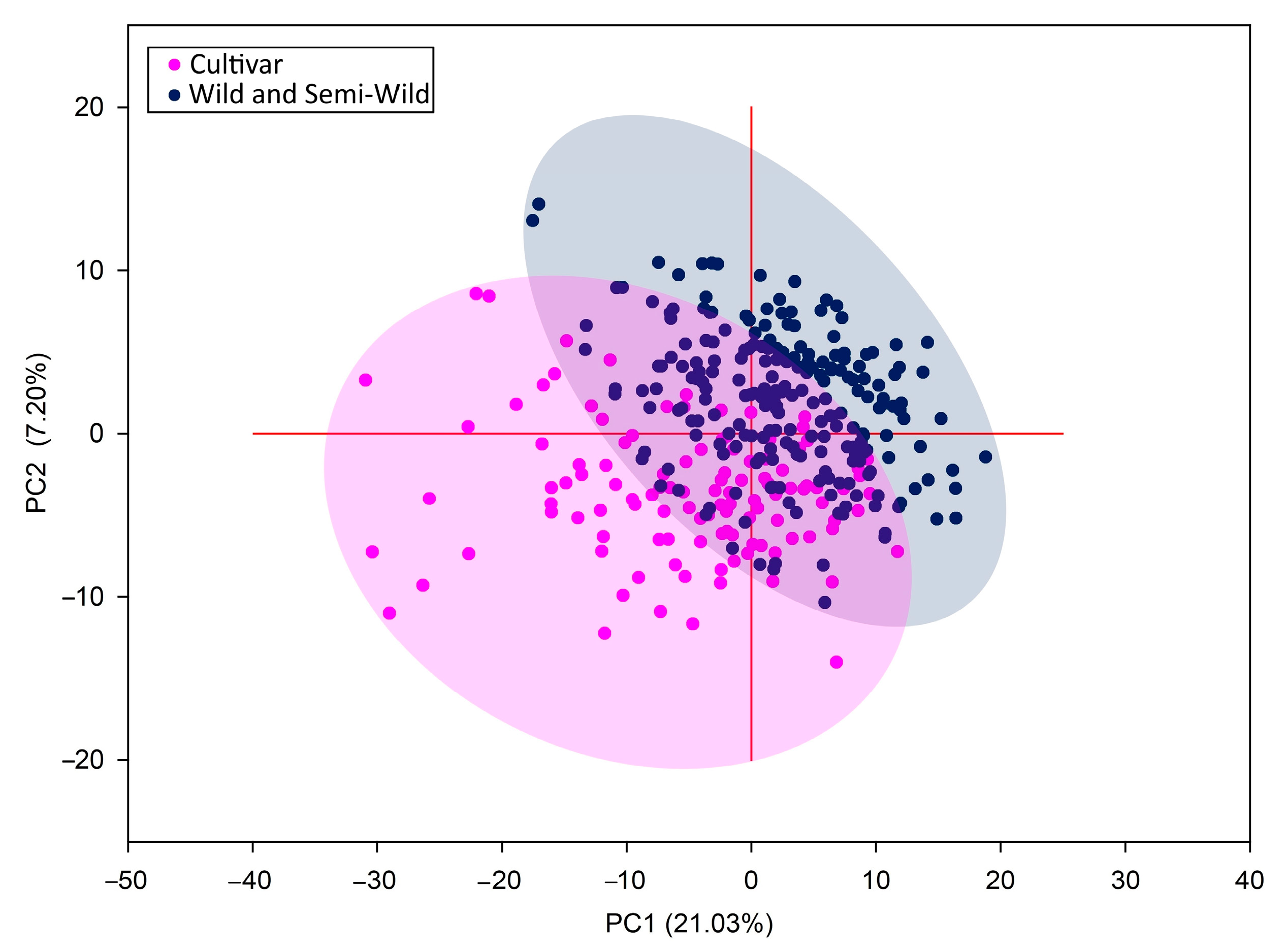
3.1. Genetic Structure of Red Clover Populations
3.2. Genetic Diversity within Populations
3.3. Genetic Diversity among Populations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Pop. Code | Pop. Size., No | Origin | Latitude (N) | Longitude (E) |
|---|---|---|---|---|
| ‘Arimaičiai’ | 23 | Variety (LT) | – | – |
| ‘Kamaniai’ | 16 | Variety (LT) | – | – |
| ‘Liepsna’ | 18 | Variety (LT) | – | – |
| ‘Sadūnai’ | 20 | Variety (LT) | – | – |
| ‘Vytis’ | 15 | Variety (LT) | – | – |
| pop2867 | 18 | Raseiniai distr., Maslauskiškiai. | 55.36811° | 23.32267° |
| pop2868 | 24 | Šilalė distr., Karūziškės II, Medvėgalis mound. | 55.62802° | 22.39114° |
| pop2869 | 22 | Šilalė distr., Paršežeris. | 55.64130° | 22.30454° |
| pop2870 | 20 | Telšiai distr., Mažieji Burbiškiai, Moteraitis mound. | 55.76428° | 22.49596° |
| pop2871 | 18 | Jurbarkas distr., Greičiai. | 55.05878° | 22.64937° |
| pop2875 | 21 | Telšiai distr., Šatrija mound. | 55.87134° | 22.55596° |
| pop2876 | 18 | Telšiai distr., Badmakiai. | 55.96160° | 22.52041° |
| pop2877 | 15 | Kėdainiai distr., Čystapolis. | 55.47403° | 23.66135° |
| pop2879 | 16 | ,Panevėžya distr., Butrimoniai. | 55.49606° | 24.12171° |
| pop2880 | 22 | Panevėžiys distr., Vadatkėliai. | 55.64402° | 24.12658° |
| pop2887 | 17 | Population from plant gene bank. | – | – |
| pop2890 | 14 | Population from plant gene bank. | – | – |
| pop2902 | 22 | Lazdijai distr., Veisiejai region park. | 54.12698° | 23.66182° |

References
- Dluhošová, J.; Ištvánek, J.; Nedělník, J.; Řepková, J. Red clover (Trifolium pratense) and Zigzag clover (T. medium)—A picture of genomic similarities and differences. Front. Plant Sci. 2018, 9, 724. [Google Scholar] [CrossRef] [PubMed]
- Casler, M.D.; Undersander, D.J. Identification of Temperate Pasture Grasses and Legumes, in Horse Pasture Management; Elsevier: Amsterdam, The Netherlands, 2019; pp. 11–35. [Google Scholar]
- Lizarazo, C.I.; Tuulos, A.; Jokela, V.; Mäkelä, P.S.A. Sustainable mixed cropping systems for the boreal-nemoral region. Front. Sustain. Food Syst. 2020, 4, 103. [Google Scholar] [CrossRef]
- Gillet, J.M. Taxonomy and morphology. In Clover Science and Technology; Taylor, N.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1985; Volume 25, pp. 7–69. [Google Scholar]
- Taylor, N.L.; Quesenberry, K.H. Red Clover Science; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; 226p, ISBN 978-0-7923-3887-1. [Google Scholar]
- Brewbaker, J.L.; Carnahan, H.L. Leaf marking alleles in white clover: Uniform nomenclature. J. Hered. 1956, 47, 103–104. [Google Scholar] [CrossRef]
- Bortnem, R.; Boe, A. Frequency of the No Mark Leaflet Allele in Red Clover. Crop Sci. 2002, 42, 634–636. [Google Scholar] [CrossRef]
- Tashiro, R.M.; Han, Y.; Monteros, M.J.; Bouton, J.H.; Parrott, W.A. Leaf trait coloration in white clover and molecular mapping of the red midrib and leaflet number traits. Crop Sci. 2010, 50, 1260–1268. [Google Scholar] [CrossRef]
- Ergon, A.; Bakken, A.K. Breeding for intercropping: The case of red clover persistence in grasslands. Euphytica 2022, 218, 98. [Google Scholar] [CrossRef]
- McKenna, P.; Cannon, N.; Conway, J.; Dooley, J. The use of red clover (Trifolium pratense) in soil fertility-building: A review. F. Crop. Res. 2018, 221, 38–49. [Google Scholar] [CrossRef]
- Schipanski, M.E.; Drinkwater, L.E. Nitrogen fixation of red clover interseeded with winter cereals across a management-induced fertility gradient. Nutr. Cycl. Agroecosystems 2011, 90, 105–119. [Google Scholar] [CrossRef]
- De Vega, J.; Ayling, S.; Hegarty, M.; Kudrna, D.; Goicoechea, J.L.; Ergon, Å; Rognli, O.A.; Jones, C.; Swain, M.; Geurts, R.; et al. Red clover (Trifolium pratense L.) draft genome provides a platform for trait improvement. Sci. Rep. 2015, 5, 17394. [Google Scholar] [CrossRef] [PubMed]
- Egan, L.M.; Hofmann, R.W.; Ghamkhar, K.; Hoyos-Villegas, V. Prospects for trifolium improvement through germplasm characterisation and pre-breeding in New Zealand and Beyond. Front. Plant Sci. 2021, 12, 653191. [Google Scholar] [CrossRef]
- Dabkevičienė, G.; Statkevičiūtė, G.; Mikaliūnienė, J.; Norkevičienė, E.; Kemešytė, V. Production of Trifolium pratense L. and T. hybridum L. tetraploid populations and assessment of their agrobiological characteristics. Zemdirb.-Agric. 2016, 103, 377–384. [Google Scholar] [CrossRef]
- Rauf, S. Induced polyploidy: A tool for forage species improvement. Agriculture 2021, 11, 210. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Łukomska, A.; Gutowska, I.; Kochman, J.; Janił, J.; Janda, K. Edible flowers extracts as a source of bio-active compounds with antioxidant properties—In vitro studies. Appl. Sci. 2021, 11, 2120. [Google Scholar] [CrossRef]
- Jing, S.; Kryger, P.; Markussen, B.; Boelt, B. Pollination and plant reproductive success of two ploidy levels in red clover (Trifolium pratense L.). Front. Plant Sci. 2021, 12, 720069. [Google Scholar] [CrossRef]
- Solberg, S.O.; Yndgaard, F.; Palmè, A. Morphological and phenological consequences of ex situ conservation of natural populations of red clover (Trifolium pratense L.). Plant Genet. Resour. 2017, 15, 97–108. [Google Scholar] [CrossRef]
- Cardinale, B.J. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef]
- Jones, C. Population structure and genetic diversity in red clover (Trifolium pratense L.) germplasm. Sci. Rep. 2020, 10, 8364. [Google Scholar] [CrossRef]
- Egan, L.M.; Hofmann, R.W.; Ghamkhar, K.; Hoyos-Villegas, V. Identification of founding accessions and patterns of relatedness and inbreeding derived from historical pedigree data in a red clover germplasm collection in New Zealand. Crop Sci. 2019, 59, 2100–2108. [Google Scholar] [CrossRef]
- Walisch, T.J.; Colling, G.; Poncelet, M.; Matthies, D. Effects of inbreeding and interpopulation crosses on performance and plasticity of two generations of offspring of a declining grassland plant. Am. J. Bot. 2012, 99, 1300–1313. [Google Scholar] [CrossRef]
- Petrauskas, G.; Stukonis, V.; Norkevičienė, E. Defining a phenotypic variability and productivity in wild type red clover germplasm. J. Agric. Sci. 2020, 12, 52–61. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L.; Doyle, J.A.; Doyle, J.K. A rapid total DNA preparation procedure for fresh plant tissue. Focus 1990, 12, 3–15. [Google Scholar]
- Paplauskienė, V.; Dabkevičienė, G. Genetic variability determination using ISSR-PCR markers in red clover varieties. Biologija 2008, 54, 56–59. [Google Scholar] [CrossRef]
- Paplauskienė, V.; Dabkevičienė, G.; Pašakinskienė, I. Molecular characterization of interspecific clover hybrids using ISSR markers. Zemdirb.-Agric. 2007, 94, 111–119. [Google Scholar]
- The Data Analysis for This Paper Was Generated Using SAS Software; Version [9.4] for Windows; SAS Institute Inc.: Cary, NC, USA, 2017.
- Kopelman, N.M.; Mayzel, J.; Jakobsson, M.; Rosenberg, N.A.; Mayrose, I. Clumpak: A program for identifying clustering modes and packaging population structure inferences across K. Mol. Ecol. Resour. 2015, 15, 1179–1191. [Google Scholar] [CrossRef]
- Nagylaki, T. Fixation indices in subdivided populations. Genetics 1998, 148, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.H.; Rafii, M.Y.; Ramlee, S.I. DNA fingerprinting, fixation-index (Fst), and admixture mapping of selected Bambara groundnut (Vigna subterranea [L.] Verdc.) accessions using ISSR markers system. Sci. Rep. 2021, 11, 14527. [Google Scholar] [CrossRef] [PubMed]
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 1980, 32, 314–331. [Google Scholar]
- Serrote, C.M.L. Determining the polymorphism information content of a molecular marker. Gene 2020, 726, 144175. [Google Scholar] [CrossRef]
- Gholami, S.; Vafaee, Y.; Nazari, F.; Ghorbani, A. Molecular characterization of endangered Iranian terrestrial orchids using ISSR markers and association with floral and tuber-related phenotypic traits. Physiol. Mol. Biol. Plants 2021, 27, 53–68. [Google Scholar] [CrossRef]
- Ulloa, O.; Ortega, F.; Campos, H. Analysis of genetic diversity in red clover (Trifolium pratense L.) breeding populations as revealed by RAPD genetic markers. Genome 2003, 46, 529–535. [Google Scholar] [CrossRef]
- Osterman, J.; Hammenhag, C.; Ortiz, R.; Geleta, M. Insights into the genetic diversity of Nordic red clover (Trifolium pratense) revealed by SeqSNP-based genic markers. Front. Plant Sci. 2021, 12, 748750. [Google Scholar] [CrossRef]
- Vleugels, T.; Amdahl, H.; Roldán-Ruiz, I.; Cnops, G. Factors underlying seed yield in red clover: Review of current knowledge and perspectives. Agronomy 2019, 9, 829. [Google Scholar] [CrossRef]
- Fernández-Otero, C.I.; Ramos-Cabrer, A.M.; López-Díaz, J.E.; Pereira-Lorenzo, S. Evaluating the diversity of ecotypes of red clover (Trifolium pratense L.) from Northwestern Spain by phenotypic traits and microsatellites. Agronomy 2021, 11, 2275. [Google Scholar] [CrossRef]
- Curnow, R.N.; Wright, S. Evolution and the genetics of populations. Variability within and among Natural Populations; University of Chicago Press: Chicago, IL, USA, 1978; Volume 4. [Google Scholar] [CrossRef]
- Wright, S. Evolution in mendelian populations. Genetics 1931, 16, 97–159. [Google Scholar] [CrossRef]
- Gupta, M.; Sharma, V.; Singh, S.K.; Chahota, R.K.; Sharma, T.R. Analysis of genetic diversity and structure in a gene bank collection of red clover (Trifolium pratense L.) using SSR markers. Plant Genet. Resour. 2017, 15, 376–379. [Google Scholar] [CrossRef]
- Shi, J.; Joshi, J.; Tielbörger, K.; Verhoeven, K.J.F.; Macel, M. Costs and benefits of admixture between foreign genotypes and local populations in the field. Ecol. Evol. 2018, 8, 3675–3684. [Google Scholar] [CrossRef]
- Hou, Y.; Lou, A. Population genetic diversity and structure of a naturally isolated plant species, Rhodiola dumulosa (Crassulaceae). PLoS ONE 2011, 6, e24497. [Google Scholar] [CrossRef] [PubMed]
- Nei, M. Genetic distance between populations. Am. Nat. 1972, 106, 283–292. [Google Scholar] [CrossRef]
- Petrauskas, G.; Norkevičienė, E.; Stukonis, V.; Kemešytė, V. Phenotypic traits for wild red clover seed yield under drought conditions. Czech. J. Genet. Plant Breed. 2020, 56, 140–149. [Google Scholar] [CrossRef]
- Kölliker, R.; Herrmann, D.; Boller, B.; Widmer, F. Swiss mattenklee landraces, a distinct and diverse genetic resource of red clover (Trifolium pratense L.). Theor. Appl. Genet. 2003, 107, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.P.; Helgadóttir, A.; Frankow-Lindberg, B.E.; Skøt, L.; Jones, C.; Skøt, K.P. Temporal changes in population genetic diversity and structure in red and white clover grown in three contrasting environments in northern Europe. Ann. Bot. 2012, 110, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Osterman, J.; Hammenhag, C.; Ortiz, R.; Geleta, M. Discovering candidate SNPs for resilience breeding of red clover. Front. Plant Sci. 2021, 13, 997860. [Google Scholar] [CrossRef] [PubMed]
- Zanotto, S. Trait characterization of genetic resources reveals useful variation for the improvement of cultivated Nordic red clover. J. Agron. Crop Sci. 2021, 207, 492–503. [Google Scholar] [CrossRef]

| Primer | Sequence 5′→ 3′ | Annealing °C |
|---|---|---|
| ISSR1 | AGAGAGAGAGAGAGAGAGAGC | 52 |
| ISSR2 | AGAGAGAGAGAGAGAGAGAGT | 50 |
| UBC827 | ACACACACACACACACG | 53 |
| UBC857 | ACACACACACACACACCG | 55 |
| 155H | CACACACACACACAGA | 49 |
| Primer | Fragment Size (bp) | No. of Loci | No. of Polymorphic Loci | Percentage Polymorphic Loci (PPL) % | Polymorphic Information Content (PIC) |
|---|---|---|---|---|---|
| ISSR1 | 500–2500 | 15 | 11 | 73.3 | 0.4882 |
| ISSR2 | 500–2500 | 18 | 14 | 77.8 | 0.4922 |
| UBC827 | 600–2500 | 17 | 13 | 76.5 | 0.4911 |
| UBC857 | 600–2400 | 13 | 10 | 76.9 | 0.4915 |
| 155H | 450–2300 | 16 | 12 | 75.0 | 0.4898 |
| Total/avg. | – – | 79/15.8 | 60/12 | –/75.9 | –/0.4906 |
| df | SS | MS | Est. Var. | Variation % | PhiPT ** | Nm | 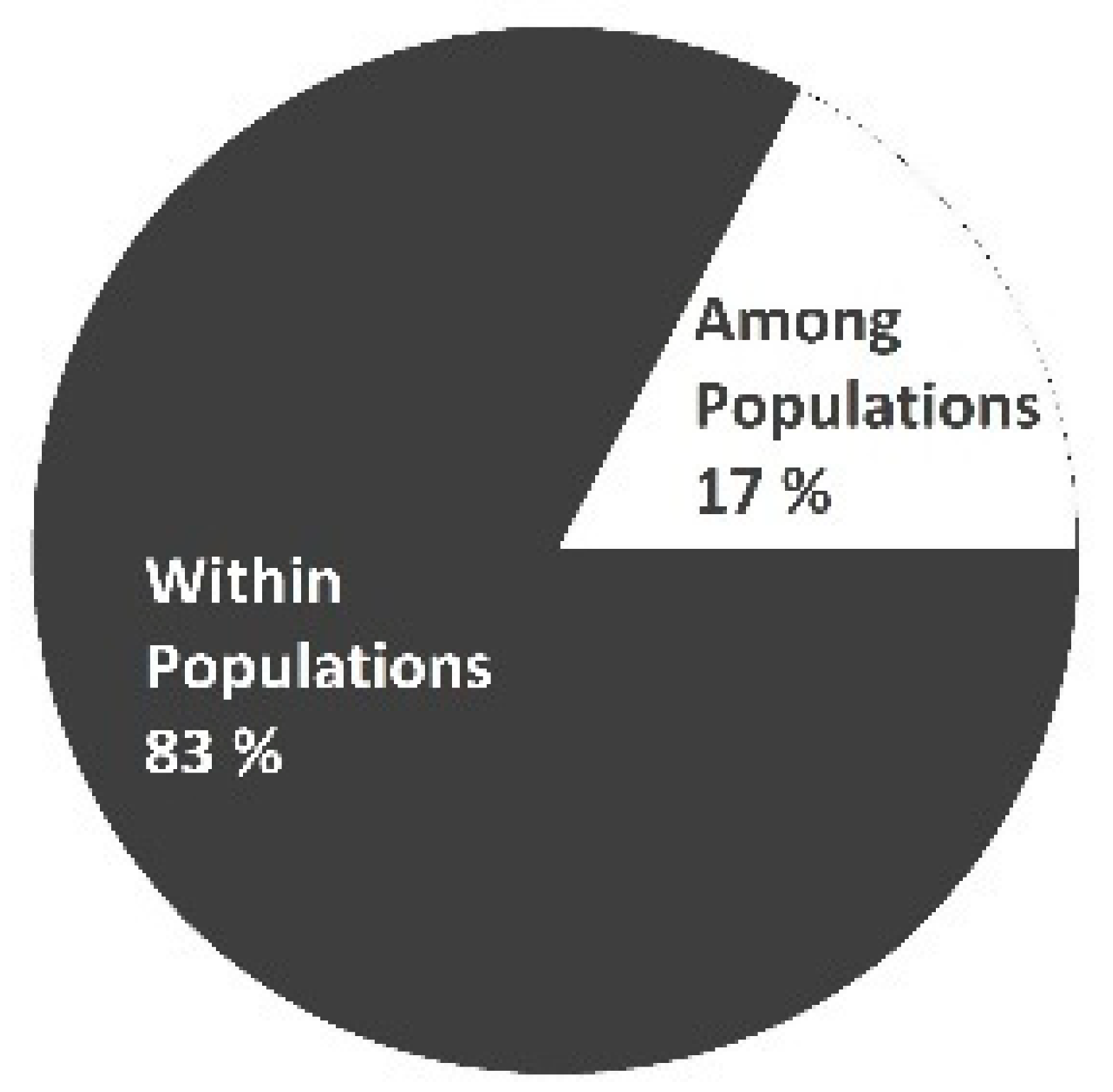 | |
| Among Pops | 17 | 948.549 | 55.797 | 2.364 | 17% | 0.173 | 2.2474 | |
| Within Pops | 321 | 3637.776 | 11.333 | 11.333 | 83% | |||
| Total | 338 | 4586.324 | 13.697 | 100% |
| Population/Variety | N | Na | Ne | PL | PPL % | h | I |
|---|---|---|---|---|---|---|---|
| 2867 | 18 | 1.608 | 1.426 | 59 | 74.68 | 0.2511 | 0.3781 |
| 2868 | 24 | 1.506 | 1.365 | 57 | 72.15 | 0.2233 | 0.3431 |
| 2869 | 22 | 1.595 | 1.422 | 59 | 74.68 | 0.2484 | 0.3749 |
| 2870 | 20 | 1.506 | 1.440 | 55 | 69.62 | 0.2566 | 0.3813 |
| 2871 | 18 | 1.582 | 1.450 | 60 | 75.95 | 0.2635 | 0.3940 |
| 2875 | 21 | 1.570 | 1.474 | 60 | 75.95 | 0.2728 | 0.4047 |
| 2876 | 18 | 1.608 | 1.418 | 59 | 74.68 | 0.2499 | 0.3778 |
| 2877 | 15 | 1.532 | 1.469 | 59 | 74.68 | 0.2736 | 0.4067 |
| 2879 | 16 | 1.557 | 1.424 | 59 | 74.68 | 0.2511 | 0.3794 |
| 2880 | 22 | 1.544 | 1.450 | 59 | 74.68 | 0.2627 | 0.3926 |
| 2887 | 17 | 1.709 | 1.518 | 65 | 82.28 | 0.3008 | 0.4476 |
| 2890 | 14 | 1.506 | 1.384 | 55 | 69.62 | 0.2294 | 0.3471 |
| 2902 | 22 | 1.671 | 1.491 | 64 | 81.01 | 0.2854 | 0.4258 |
| ‘Arimaičiai’ | 23 | 1.595 | 1.458 | 60 | 75.95 | 0.2666 | 0.3980 |
| ‘Kamaniai’ | 16 | 1.658 | 1.471 | 63 | 79.75 | 0.2767 | 0.4145 |
| ‘Sadūnai’ | 20 | 1.494 | 1.446 | 57 | 72.15 | 0.2588 | 0.3853 |
| ‘Vytis’ | 15 | 1.430 | 1.408 | 51 | 64.56 | 0.2360 | 0.3509 |
| ‘Liepsna’ | 18 | 1.722 | 1.512 | 66 | 83.54 | 0.2988 | 0.4465 |
| 2867 | 2868 | 2869 | 2870 | 2871 | 2875 | 2876 | 2877 | 2879 | 2880 | 2887 | 2890 | 2902 | ARI | KAM | SAD | VYT | LIE | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2867 | 0.071 | 0.102 | 0.065 | 0.069 | 0.130 | 0.106 | 0.080 | 0.086 | 0.128 | 0.078 | 0.113 | 0.083 | 0.124 | 0.106 | 0.084 | 0.086 | 0.087 | 2867 | |
| 2868 | 0.070 | 0.076 | 0.062 | 0.092 | 0.109 | 0.064 | 0.057 | 0.103 | 0.058 | 0.090 | 0.082 | 0.087 | 0.085 | 0.075 | 0.102 | 0.090 | 2868 | ||
| 2869 | 0.092 | 0.095 | 0.079 | 0.086 | 0.072 | 0.096 | 0.105 | 0.091 | 0.104 | 0.063 | 0.096 | 0.104 | 0.088 | 0.077 | 0.089 | 2869 | |||
| 2870 | 0.066 | 0.141 | 0.115 | 0.080 | 0.079 | 0.119 | 0.087 | 0.133 | 0.061 | 0.095 | 0.095 | 0.091 | 0.066 | 0.083 | 2870 | ||||
| 2871 | 0.088 | 0.125 | 0.069 | 0.050 | 0.086 | 0.068 | 0.136 | 0.073 | 0.125 | 0.091 | 0.087 | 0.080 | 0.088 | 2871 | |||||
| 2875 | 0.109 | 0.081 | 0.068 | 0.076 | 0.075 | 0.108 | 0.080 | 0.116 | 0.083 | 0.070 | 0.100 | 0.093 | 2875 | ||||||
| 2876 | 0.111 | 0.112 | 0.129 | 0.108 | 0.130 | 0.107 | 0.124 | 0.120 | 0.112 | 0.114 | 0.093 | 2876 | |||||||
| 2877 | 0.059 | 0.095 | 0.050 | 0.058 | 0.068 | 0.083 | 0.066 | 0.074 | 0.070 | 0.073 | 2877 | ||||||||
| 2879 | 0.072 | 0.041 | 0.091 | 0.065 | 0.092 | 0.088 | 0.068 | 0.063 | 0.065 | 2879 | |||||||||
| 2880 | 0.093 | 0.101 | 0.060 | 0.103 | 0.071 | 0.078 | 0.098 | 0.083 | 2880 | ||||||||||
| 2887 | 0.084 | 0.073 | 0.078 | 0.085 | 0.061 | 0.065 | 0.059 | 2887 | |||||||||||
| 2890 | 0.098 | 0.101 | 0.111 | 0.098 | 0.101 | 0.103 | 2890 | ||||||||||||
| 2902 | 0.081 | 0.074 | 0.056 | 0.056 | 0.068 | 2902 | |||||||||||||
| ARI | 0.102 | 0.104 | 0.088 | 0.073 | ARI | ||||||||||||||
| KAM | 0.058 | 0.090 | 0.073 | KAM | |||||||||||||||
| SAD | 0.075 | 0.056 | SAD | ||||||||||||||||
| VYT | 0.072 | VYT | |||||||||||||||||
| LIE | LIE |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrauskas, G.; Norkevičienė, E.; Baistruk-Hlodan, L. Genetic Differentiation of Red Clover (Trifolium pratense L.) Cultivars and Their Wild Relatives. Agriculture 2023, 13, 1008. https://doi.org/10.3390/agriculture13051008
Petrauskas G, Norkevičienė E, Baistruk-Hlodan L. Genetic Differentiation of Red Clover (Trifolium pratense L.) Cultivars and Their Wild Relatives. Agriculture. 2023; 13(5):1008. https://doi.org/10.3390/agriculture13051008
Chicago/Turabian StylePetrauskas, Giedrius, Eglė Norkevičienė, and Lesia Baistruk-Hlodan. 2023. "Genetic Differentiation of Red Clover (Trifolium pratense L.) Cultivars and Their Wild Relatives" Agriculture 13, no. 5: 1008. https://doi.org/10.3390/agriculture13051008
APA StylePetrauskas, G., Norkevičienė, E., & Baistruk-Hlodan, L. (2023). Genetic Differentiation of Red Clover (Trifolium pratense L.) Cultivars and Their Wild Relatives. Agriculture, 13(5), 1008. https://doi.org/10.3390/agriculture13051008







