Fate of Copper in Saline–Alkali Soil with Long-Term Application of Biogas Residue
Abstract
1. Introduction
2. Materials and Methods
2.1. Saline–Alkali Soil and Biogas Residue
2.2. Adsorption Isotherms of Cu
2.3. Analytical Methods
3. Results and Discussion
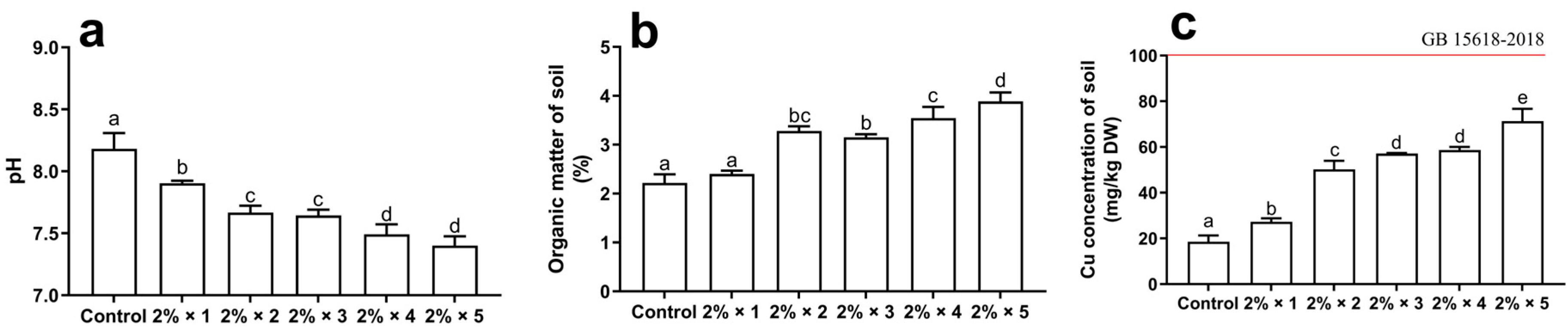
3.1. Basic Properties of Soil
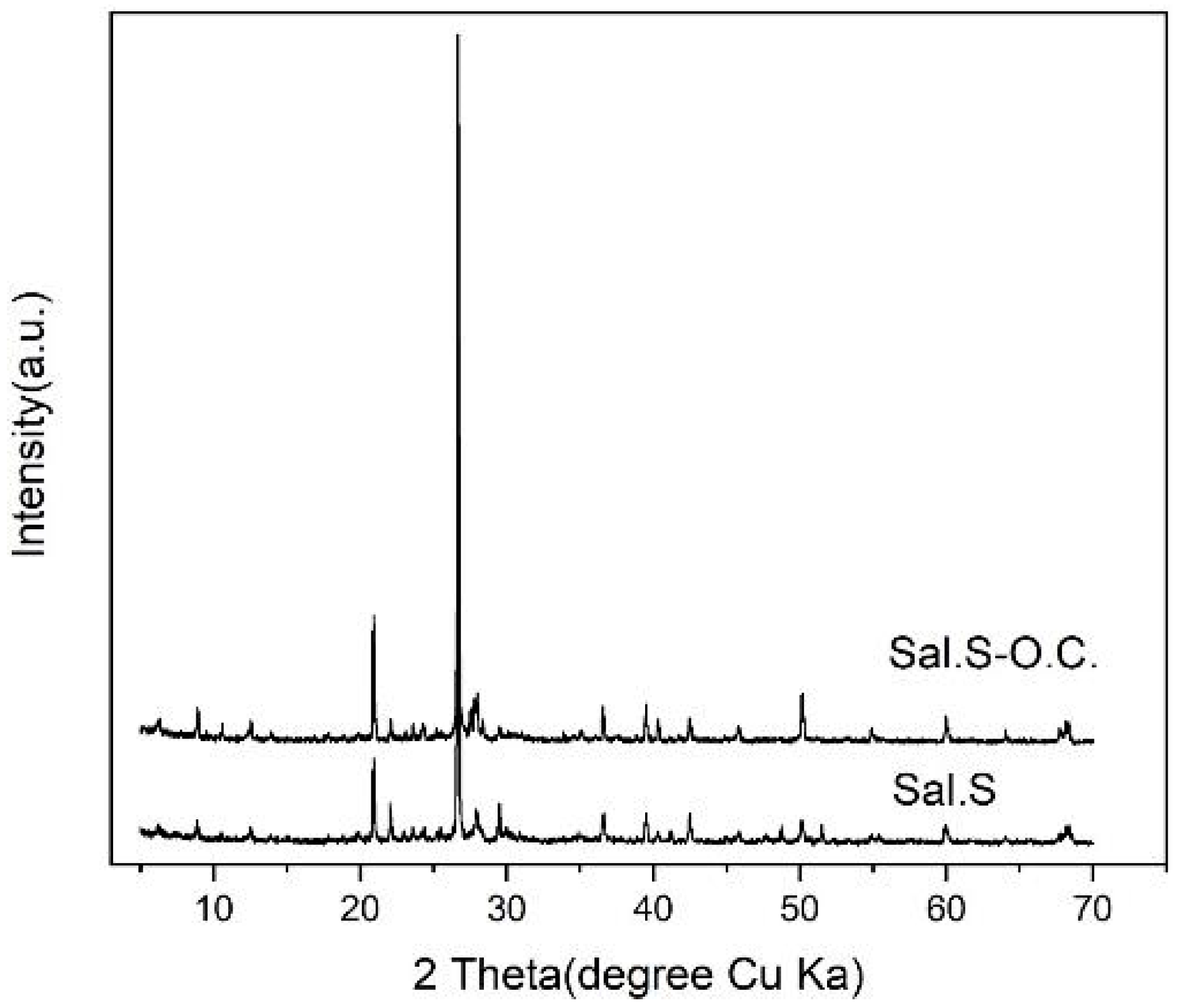
3.2. XRD Analysis for Saline–Alkali Soil
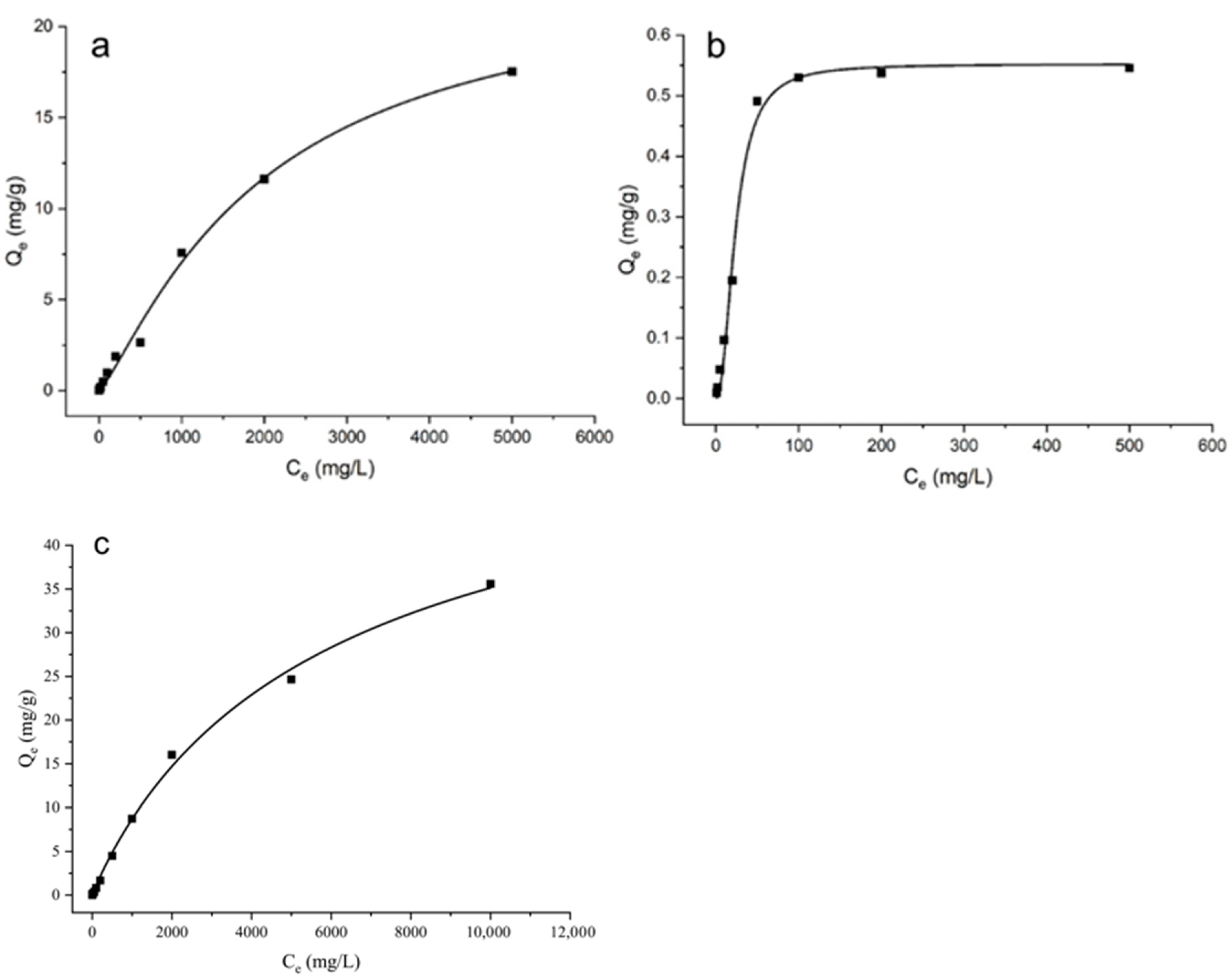
3.3. Adsorption Isotherms for Cu by Saline–Alkali Soils and Biogas Residue
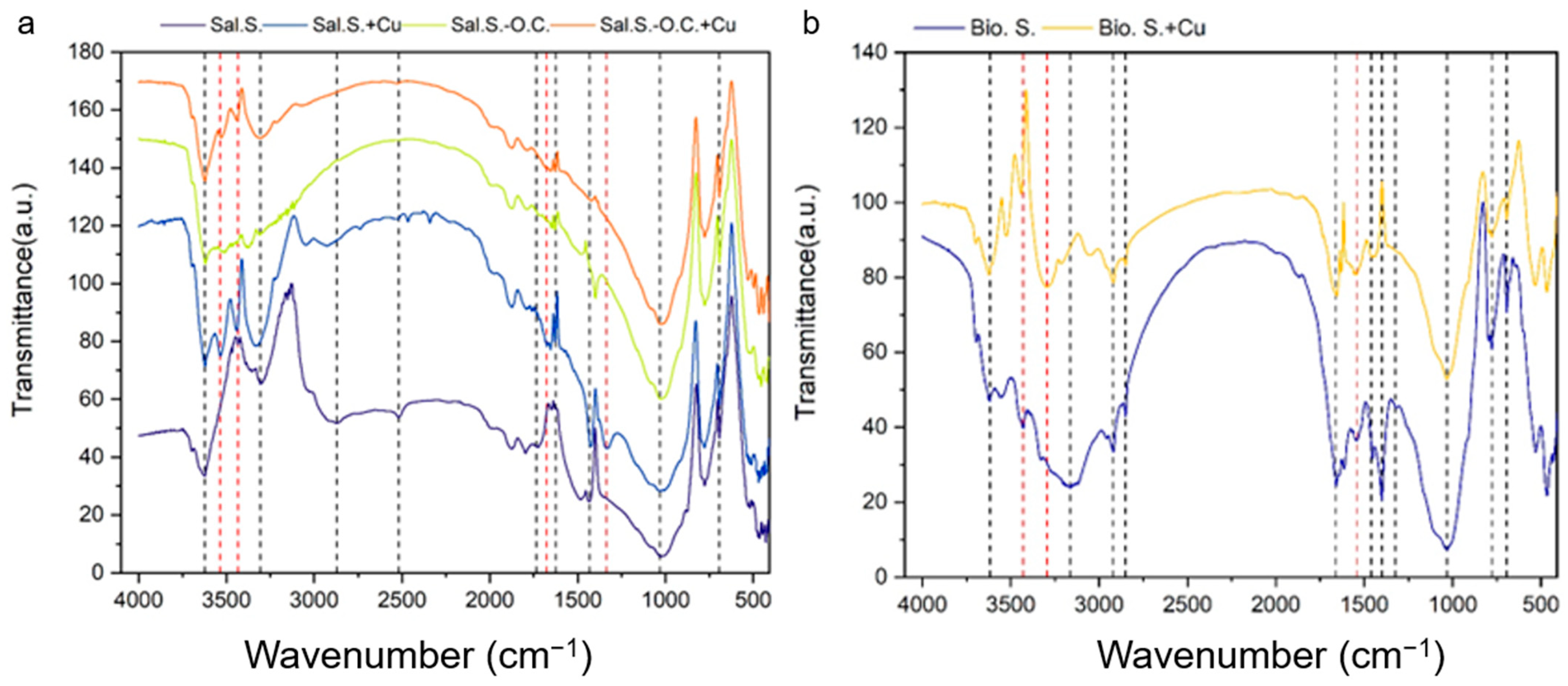
3.4. FTIR Analysis for Saline–Alkali Soil
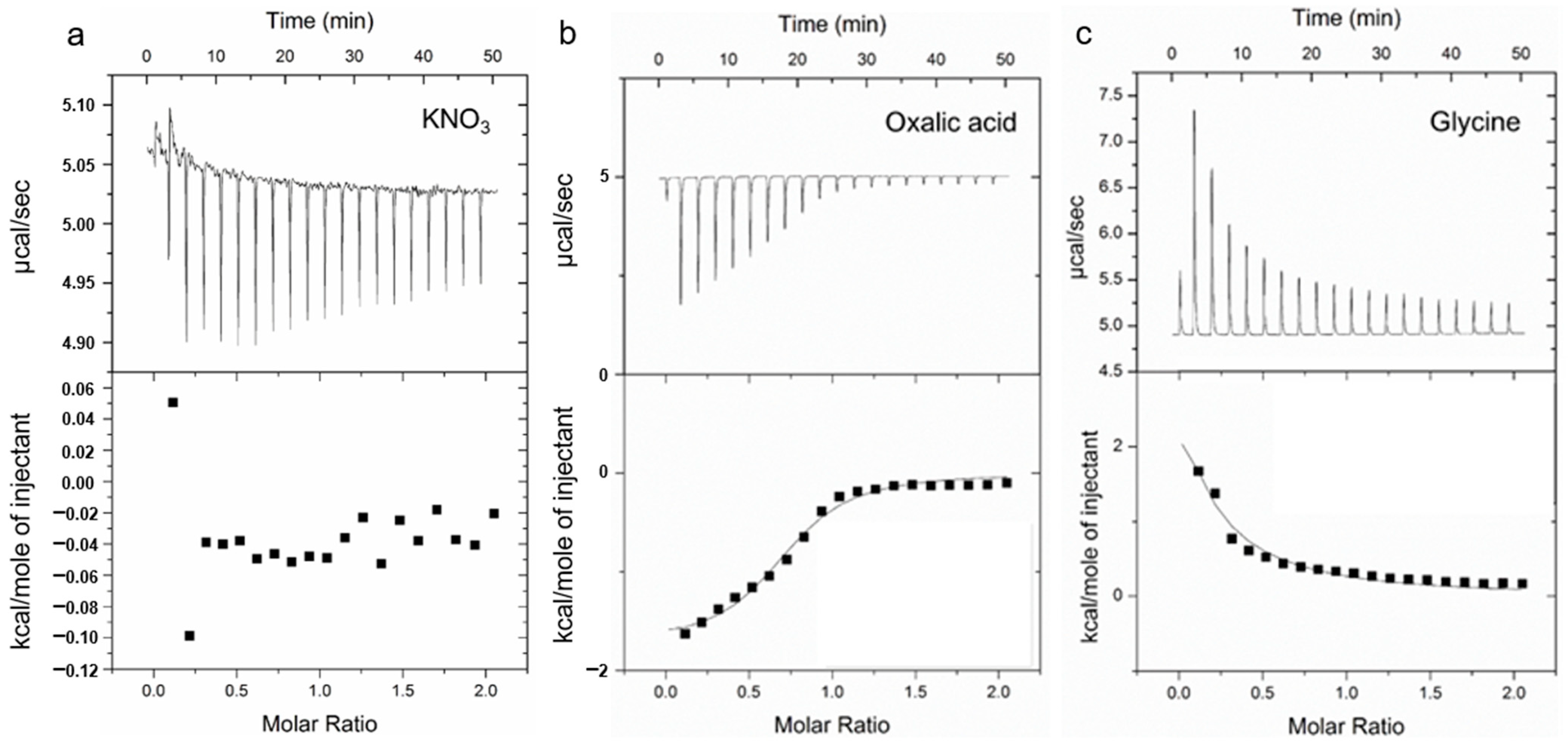
3.5. Thermodynamic Analysis
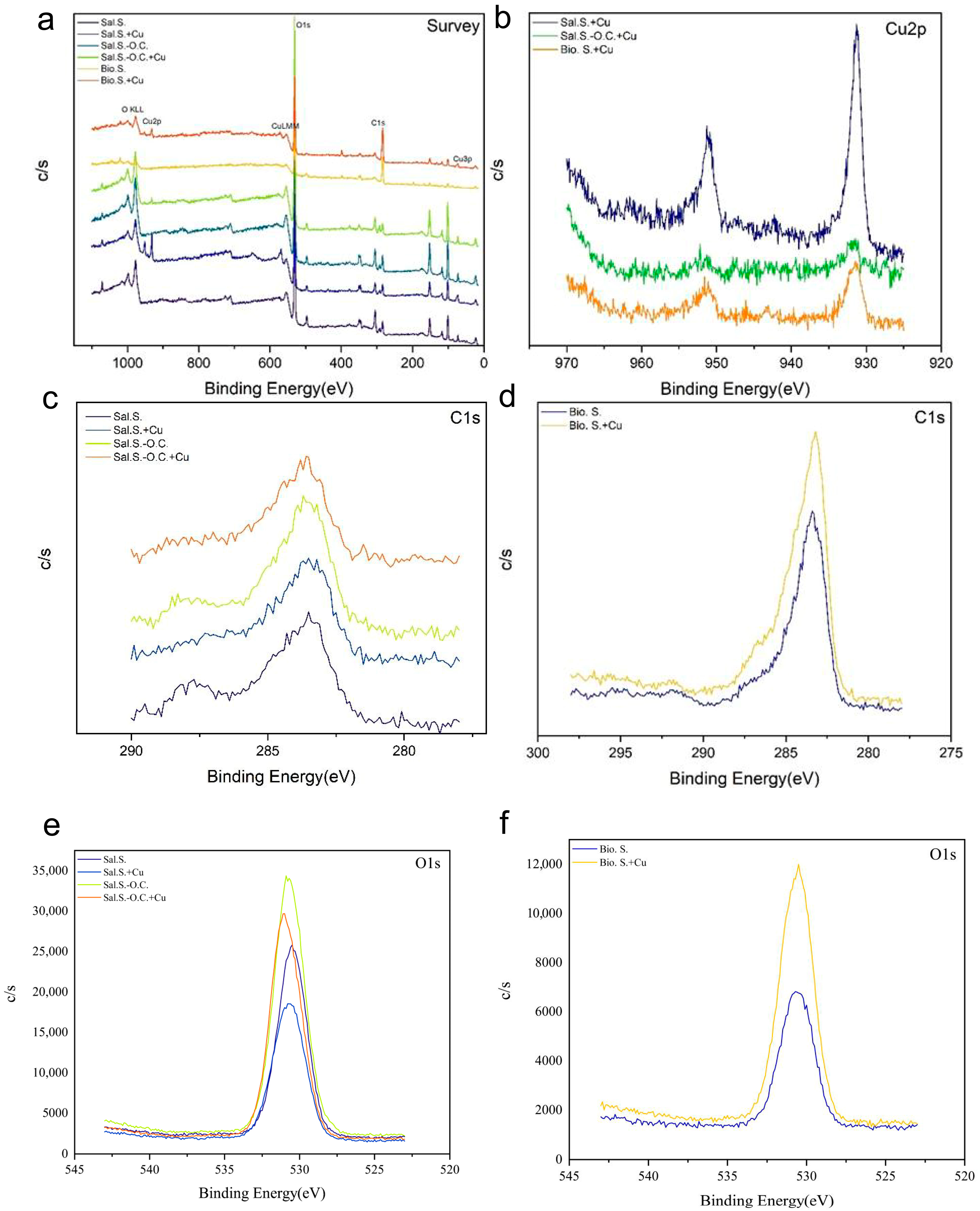
3.6. XPS Analysis of Biogas Residue and Saline–Alkali Soil
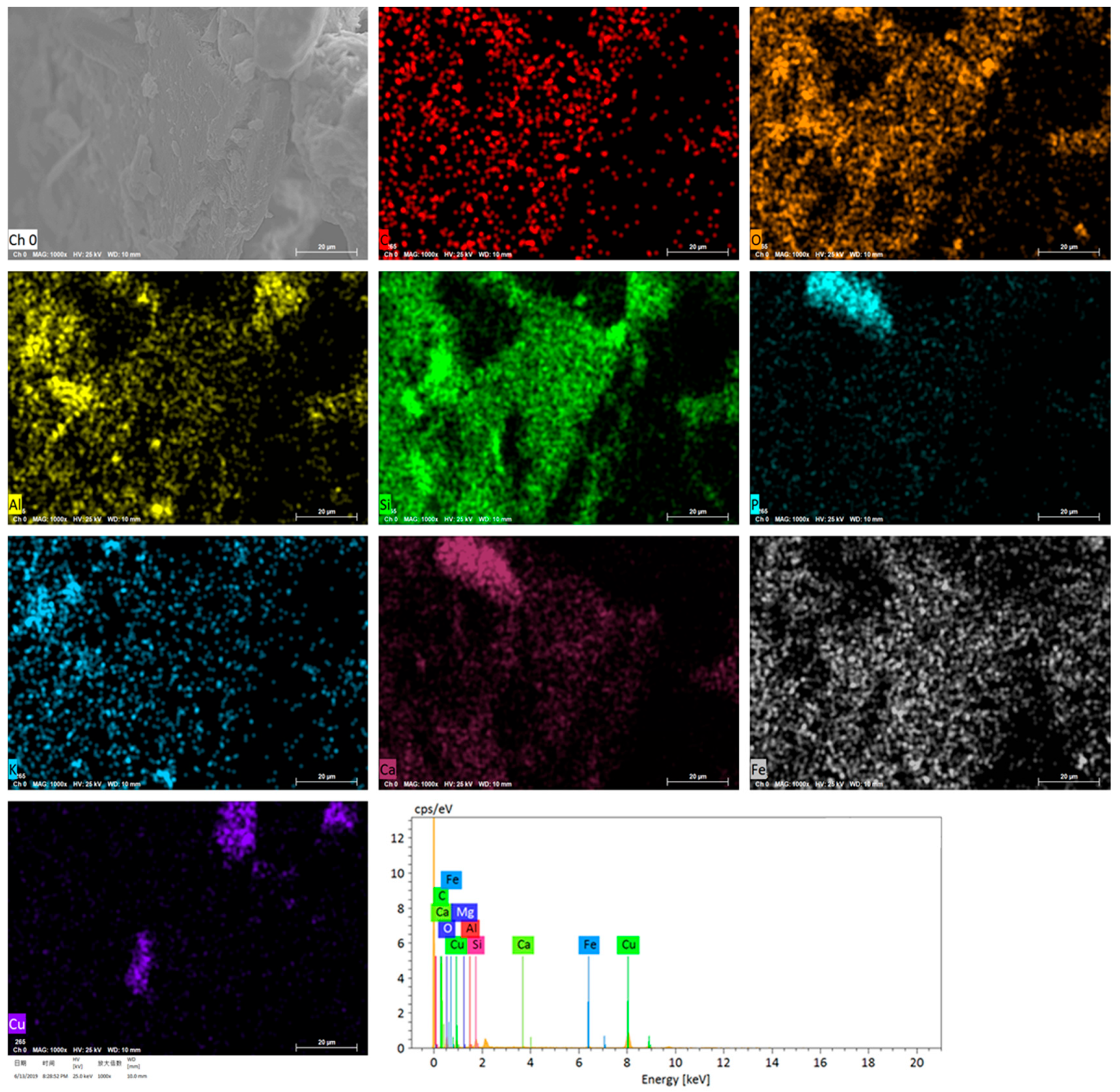
3.7. SEM–EDS Analysis of Distribution of Cu in Alkali Soil and Biogas Residue
3.8. Analysis of Decomposition Process of Biogas Residue in Saline–Alkali Soil Based on FTIR
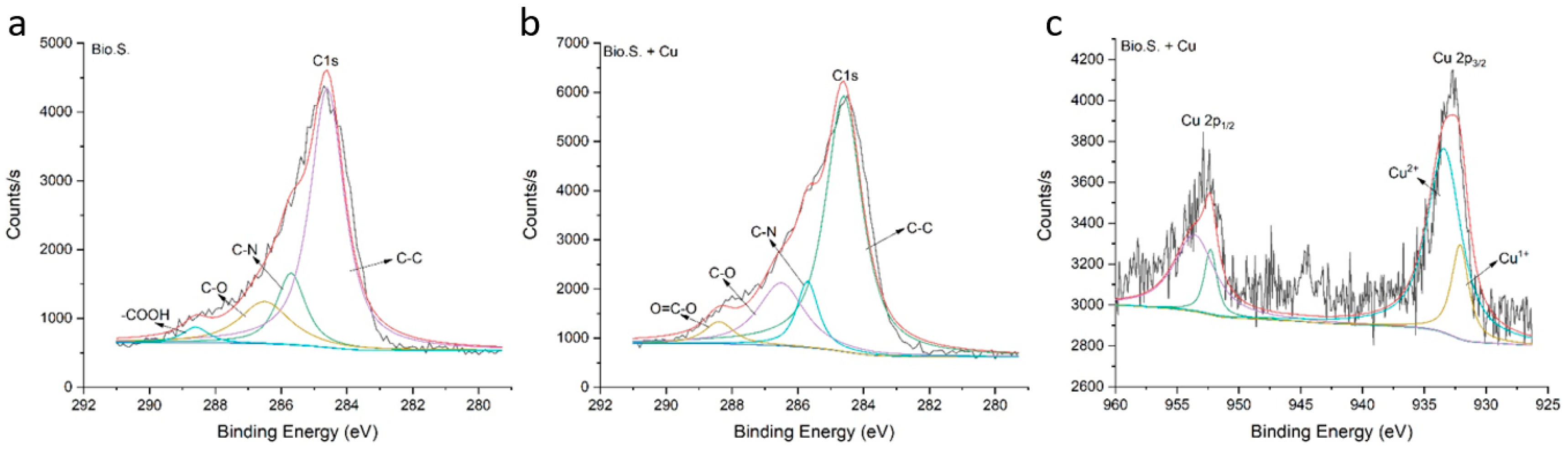
3.9. Behaviour of Cu in Saline–Alkali Soil after Application of Biogas Residue Based on XPS Analysis
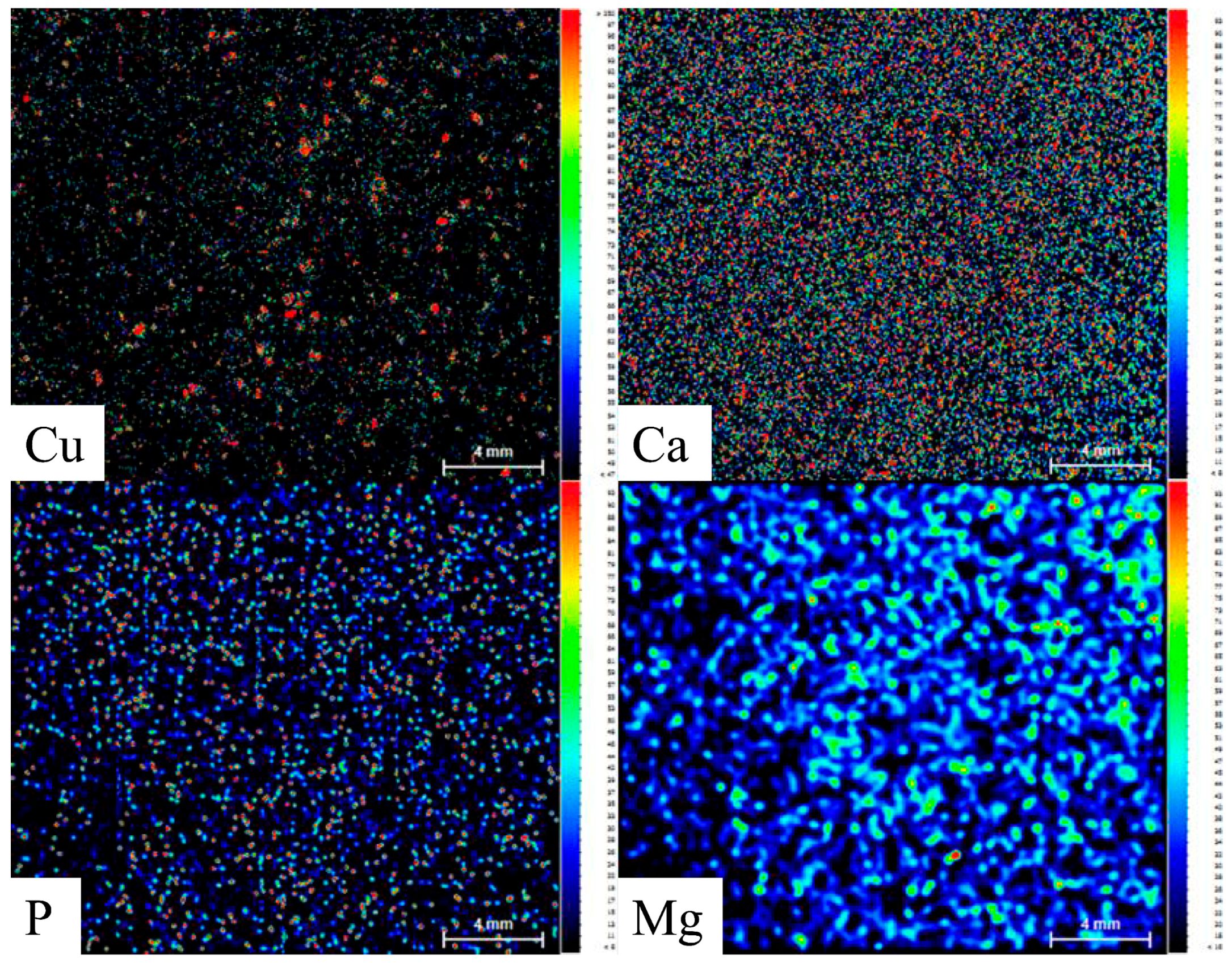
3.10. Distribution of Cu in Saline–Alkali Soil after Continuous Application of Biogas Residue Based on Micro-XRF Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lombi, E.; Susini, J. Synchrotron-based techniques for plant and soil science: Opportunities, challenges and future perspectives. Plant Soil 2009, 320, 1–35. [Google Scholar] [CrossRef]
- Papuga, K.; Kaszubkiewicz, J.; Kawalko, D.; Kreimeyer, M. Effect of Organic Matter Removal by Hydrogen Peroxide on the Determination of Soil Particle Size Distribution Using the Dynamometer Method. Agriculture 2022, 12, 226. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Barbarick, K.A.; Brobst, R.B. Copper and Zinc speciation in a biosolids-amended, semiarid grassland soil. J. Environ. Qual. 2014, 43, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Tella, M.; Bravin, M.N.; Thuries, L.; Cazevieille, P.; Chevassus-Rosset, C.; Collin, B.; Chaurand, P.; Legros, S.; Doelsch, E. Increased zinc and copper availability in organic waste amended soil potentially involving distinct release mechanisms. Environ. Pollut. 2016, 212, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Mondaca, P.; Neaman, A.; Sauve, S.; Salgado, E.; Bravo, M. Solubility, partitioning, and activity of copper-contaminated soils in a semiarid region. J. Plant Nutr. Soil Sci. 2015, 178, 452–459. [Google Scholar] [CrossRef]
- Mamindy-Pajany, Y.; Sayen, S.; Mosselmans, J.F.W.; Guillon, E. Copper, Nickel and Zinc speciation in a biosolid-amended soil: pH adsorption edge, mu-XRF and mu-XANES investigations. Environ. Sci. Technol. 2014, 48, 7237–7244. [Google Scholar] [CrossRef]
- Sarker, T.C.; Incerti, G.; Spaccini, R.; Piccolo, A.; Mazzoleni, S.; Bonanomi, G. Linking organic matter chemistry with soil aggregate stability: Insight from C-13 NMR spectroscopy. Soil Biol. Biochem. 2018, 117, 175–184. [Google Scholar] [CrossRef]
- Liu, L.; Guo, X.; Zhang, C.; Luo, C.; Xiao, C.; Li, R. Adsorption behaviours and mechanisms of heavy metal ions’ impact on municipal waste composts with different degree of maturity. Environ. Technol. 2018, 40, 2962–2976. [Google Scholar] [CrossRef]
- Lockwood, C.L.; Stewart, D.I.; Mortimer, R.J.G.; Mayes, W.M.; Jarvis, A.P.; Gruiz, K.; Burke, I.T. Leaching of copper and nickel in soil-water systems contaminated by bauxite residue (red mud) from Ajka, Hungary: The importance of soil organic matter. Environ. Sci. Pollut. Res. 2015, 22, 10800–10810. [Google Scholar] [CrossRef]
- Fulda, B.; Voegelin, A.; Maurer, F.; Christl, I.; Kretzschmar, R. Copper redox transformation and complexation by reduced and oxidized soil humic acid. 1. X-ray absorption spectroscopy study. Environ. Sci. Technol. 2013, 47, 10903–10911. [Google Scholar] [CrossRef]
- Maurer, F.; Christl, I.; Fulda, B.; Voegelin, A.; Kretzschmar, R. Copper redox transformation and complexation by reduced and oxidized soil humic acid. 2. potentiometric titrations and dialysis cell experiments. Environ. Sci. Technol. 2013, 47, 10912–10921. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, L.; Jiang, M.; Wang, J.; Xia, F.; Shi, L.; Xia, Y.; Chen, C.; Shen, Z.; Chen, Y. Cyclic and safety utilisation of Cu polluted biogas residue in saline-alkali soil. Sci. Total Environ. 2020, 704, 135410. [Google Scholar] [CrossRef]
- Graouer-Bacart, M.; Sayen, S.; Guillon, E. Macroscopic and molecular approaches of enrofloxacin retention in soils in presence of Cu(II). J. Colloid Interface Sci. 2013, 408, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Zachara, J.M.; Smith, S.C.; Resch, C.T.; Cowan, C.E. Cadmium sorption to soil separates containing layer silicates and iron and aluminum-oxides. Soil Sci. Soc. Am. J. 1992, 56, 1074–1084. [Google Scholar] [CrossRef]
- Kahle, M.; Kleber, M.; Jahn, R. Predicting carbon content in illitic clay fractions from surface area, cation exchange capacity and dithionite-extractable iron. Eur. J. Soil Sci. 2002, 53, 639–644. [Google Scholar] [CrossRef]
- Rahman, M.M.; Liu, Y.; Kwag, J.; Ra, C. Recovery of struvite from animal wastewater and its nutrient leaching loss in soil. J. Hazard. Mater. 2011, 186, 2026–2030. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, X.; Wang, W.; Yan, X.; Xia, P.; Chen, J.; Zhao, J. Modeling and optimization of struvite recovery from wastewater and reusing for heavy metals immobilization in contaminated soil. J. Chem. Technol. Biotechnol. 2016, 91, 3045–3052. [Google Scholar] [CrossRef]
- De Godoi, F.C.; Rodriguez-Castellon, E.; Guibal, E.; Beppu, M.M. An XPS study of chromate and vanadate sorption mechanism by chitosan membrane containing copper nanoparticles. Chem. Eng. J. 2013, 234, 423–429. [Google Scholar] [CrossRef]
- Matjie, R.H.; French, D.; Ward, C.R.; Pistorius, P.C.; Li, Z. Behaviour of coal mineral matter in sintering and slagging of ash during the gasification process. Fuel Process. Technol. 2011, 92, 1426–1433. [Google Scholar] [CrossRef]
- Ouhadi, V.R.; Yong, R.N.; Rafiee, F.; Goodarzi, A.R. Impact of carbonate and heavy metals on micro-structural variations of clayey soils. Appl. Clay Sci. 2011, 52, 228–234. [Google Scholar] [CrossRef]
- Vippola, M.; Ahmaniemi, S.; Keränen, J.; Vuoristo, P.; Lepistö, T.; Mäntylä, T.; Olsson, E. Aluminum phosphate sealed alumina coating: Characterization of microstructure. Mater. Sci. Eng. A 2002, 323, 1–8. [Google Scholar] [CrossRef]
- Graeser, S.; Postl, W.; Bojar, H.B.; Armbruster, T.; Raber, T.; Ettinger, K.; Walter, F. Struvite-(K), KMgPO46H2O, the potassium equivalent of struvite a new mineral. Eur. J. Miner. 2008, 20, 629–633. [Google Scholar] [CrossRef]
- Lou, K.; Rajapaksha, A.U.; Ok, Y.S.; Chang, S.X. Sorption of copper(II) from synthetic oil sands process-affected water (OSPW) by pine sawdust biochars: Effects of pyrolysis temperature and steam activation. J. Soil Sediment 2016, 16, 2081–2089. [Google Scholar] [CrossRef]
- Yao, C. Extended and improved Langmuir equation for correlating adsorption equilibrium data. Sep. Purif. Technol. 2000, 19, 237–242. [Google Scholar] [CrossRef]
- Lord, R.; Sakrabani, R. Ten-year legacy of organic carbon in non-agricultural (brownfield) soils restored using green waste compost exceeds 4 per mille per annum: Benefits and trade-offs of a circular economy approach. Sci. Total Environ. 2019, 686, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- De Souza, C.D.C.B.; Amaral Sobrinho, N.M.B.D.; Lima, E.S.A.; Lima, J.D.O.; Carmo, M.G.F.D.; García, A.C. Relation between changes in organic matter structure of poultry litter and heavy metals solubility during composting. J. Environ. Manag. 2019, 247, 291–298. [Google Scholar] [CrossRef]
- Guo, X.; Li, C.; Zhu, Q.; Huang, T.; Cai, Y.; Li, N.; Liu, J.; Tan, X. Characterization of dissolved organic matter from biogas residue composting using spectroscopic techniques. Waste Manag. 2018, 78, 301–309. [Google Scholar] [CrossRef]
- Huang, J.; Huang, Z.; Zhou, J.; Li, C.; Yang, Z.; Ruan, M.; Li, H.; Zhang, X.; Wu, Z.; Qin, X.; et al. Enhancement of heavy metals removal by microbial flocculant produced by Paenibacillus polymyxa combined with an insufficient hydroxide precipitation. Chem. Eng. J. 2019, 374, 880–894. [Google Scholar] [CrossRef]
- Fan, Y.; Zheng, C.; Huo, A.; Wang, Q.; Shen, Z.; Xue, Z.; He, C. Investigating the binding properties between antimony(V) and dissolved organic matter (DOM) under different pH conditions during the soil sorption process using fluorescence and FTIR spectroscopy. Ecotoxicol. Environ. Saf. 2019, 181, 34–42. [Google Scholar] [CrossRef]
- Iftime, M.M.; Ailiesei, G.L.; Ungureanu, E.; Marin, L. Designing chitosan based eco-friendly multifunctional soil conditioner systems with urea controlled release and water retention. Carbohydr. Polym. 2019, 223, 115040. [Google Scholar] [CrossRef]
- Uchimiya, M.; Bannon, D.I.; Wartelle, L.H. Retention of heavy metals by carboxyl functional groups of biochars in small arms range soil. J. Agric. Food Chem. 2012, 60, 1798–1809. [Google Scholar] [CrossRef]
- Schilling, M.; Cooper, W.T. Identification of copper binding sites in soil organic matter through chemical modifications and13C CP-MAS NMR spectroscopy. Environ. Sci. Technol. 2004, 38, 5059–5063. [Google Scholar] [CrossRef]
- Yang, K.; Miao, G.; Wu, W.; Lin, D.; Pan, B.; Wu, F.; Xing, B. Sorption of Cu2+ on humic acids sequentially extracted from a sediment. Chemosphere 2015, 138, 657–663. [Google Scholar] [CrossRef]
- Karlsson, T.; Elgh-Dalgren, K.; Skyllberg, U.; Sveriges, L. Modeling copper(II) complexation in a peat soil based on spectroscopic structural information. Soil Sci. Soc. Am. J. 2008, 72, 1286. [Google Scholar] [CrossRef]
- Shi, J.; Wu, Q.; Zheng, C.; Yang, J. The interaction between particulate organic matter and copper, zinc in paddy soil. Environ. Pollut. 2018, 243, 1394–1402. [Google Scholar] [CrossRef]
- Strawn, D.G.; Baker, L.L. Speciation of Cu in a contaminated agricultural soil measured by XAFS, μ-XAFS, and μ-XRF. Environ. Sci. Technol. 2008, 42, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Town, R.M.; Powell, H. Ion-selective electrode potentiometric studies on the complexation of copper(ii) by soil-derived humic and fulvic-acids. Anal. Chim. Acta. 1993, 279, 221–233. [Google Scholar] [CrossRef]
- Yang, R.; Tao, J.; Huang, Q.; Tie, B.; Lei, M.; Yang, Y.; Du, H. Co-adsorption of Cd(II) and Sb(III) by ferrihydrite: A combined XPS and ITC study. J. Soil Sediment 2019, 19, 1319–1327. [Google Scholar] [CrossRef]
- Alam, M.S.; Gorman-Lewis, D.; Chen, N.; Flynn, S.L.; Ok, Y.S.; Konhauser, K.O.; Alessi, D.S. Thermodynamic Analysis of Nickel(ll) and Zinc(ll) Adsorption to Biochar. Environ. Sci. Technol. 2018, 52, 6246–6255. [Google Scholar] [CrossRef]
- Chen, J.W.; Jiao, Y.; Wang, X.D. Thermodynamic studies on gas-based reduction of vanadium titano-magnetite pellets. Int. J. Miner. Metall. Mater. 2019, 26, 822–830. [Google Scholar] [CrossRef]
- Makowska, J.; Wyrzykowski, D.; Pilarski, B.; Neubauer, D.; Kamysz, E.; Tesmar, A.; Chmurzyński, L. Copper(II) coordination properties of GxG peptides: Key role of side chains of central residues on coordination of formed systems; combined potentiometric and ITC studies. J. Chem. Thermodyn. 2019, 128, 336–343. [Google Scholar] [CrossRef]
- North, M.L.; Wilcox, D.E. The shift from entropic Cu2+ binding to enthalpic Cu+ binding determines the reduction thermodynamics of blue copper proteins. J. Am. Chem. Soc. 2019, 141, 14329–14339. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Huang, Q.; Zhou, M.; Tie, B.; Lei, M.; Wei, X.; Liu, X.; Yang, Y. Sorption of Cu(II) by Al hydroxide organo–mineral coprecipitates: Microcalorimetry and NanoSIMS observations. Chem. Geol. 2018, 499, 165–171. [Google Scholar] [CrossRef]
- Fan, J.; Zeng, Y.; Sun, J. The transformation and migration of selenium in soil under different Eh conditions. J. Soil Sediment 2018, 18, 2935–2947. [Google Scholar] [CrossRef]
- Gerin, P.A.; Genet, M.J.; Herbillon, A.J.; Delvaux, B. Surface analysis of soilmaterial by X-ray photoelectron spectroscopy. Eur. J. Soil Sci. 2003, 54, 589–603. [Google Scholar] [CrossRef]
- Yao, T.; Chen, R.; Feng, Y.; Lin, X. Application of bioinformatics to spectral analysis: Soil organic carbon structure distinguished by X-ray photoelectron spectroscopy. Anal. Bioanal. Chem. 2019, 411, 2481–2485. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, Z.; Feng, L.; Luo, X.; Wu, P.; Cui, L.; Mao, X. Modified cellulose by polyethyleneimine and ethylenediamine with induced Cu(II) and Pb(II) adsorption potentialities. Carbohydr. Polym. 2018, 202, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ko, T. Feasibility application of Cu-contaminated soil on the removal of H2S from hot coal gas. Mater. Trans. 2015, 56, 445–449. [Google Scholar] [CrossRef]
- Li, C.; Yan, A.; Xie, X.; Zhang, J. Adsorption of Cu(II) on soil humin: Batch and spectroscopy studies. Environ. Earth. Sci. 2019, 78, 487. [Google Scholar] [CrossRef]
- Fulda, B.; Voegelin, A.; Ehlert, K.; Kretzschmar, R. Redox transformation, solid phase speciation and solution dynamics of copper during soil reduction and reoxidation as affected by sulfate availability. Geochim. Cosmochim. Acta 2013, 123, 385–402. [Google Scholar] [CrossRef]
- Qi, Y.; Zhu, J.; Fu, Q.; Hu, H.; Rong, X.; Huang, Q. Characterization and Cu sorption properties of humic acid from the decomposition of rice straw. Environ. Sci. Pollut. R. 2017, 24, 23744–23752. [Google Scholar] [CrossRef]
- Boiocchi, M.; Bonizzoni, M.; Ciarrocchi, C.; Fabbrizzi, L.; Invernici, M.; Licchelli, M. Anion recognition in water, including sulfate, by a bicyclam bimetallic receptor: A process governed by the enthalpy/entropy compensatory relationship. Chem. Eur. J. 2018, 24, 5659–5666. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Koopal, L.K.; Fang, L.; Xiong, J.; Tan, W. Proton and copper binding to humic acids analyzed by XAFS Spectroscopy and isothermal titration calorimetry. Environ. Sci. Technol. 2018, 52, 4099–4107. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.F.; Xue, J.Y. A study of adsorption and absorption mechanisms of copper in palygorskite. Clay Miner. 2008, 43, 195–203. [Google Scholar] [CrossRef]
- Jefferson, W.A.; Hu, C.; Liu, H.; Qu, J. Reaction of aqueous Cu–Citrate with MnO2 birnessite: Characterization of Mn dissolution, oxidation products and surface interactions. Chemosphere 2015, 119, 1–7. [Google Scholar] [CrossRef]
- Ma, L.; Xu, R.; Jiang, J. Adsorption and desorption of Cu(II) and Pb(II) in paddy soils cultivated for various years in the subtropical China. J. Environ. Sci. 2010, 22, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhu, X.; Qian, W.; Tang, H.; Jiang, J.; Yu, Y.; Xu, R. Effect of tea polyphenols on copper adsorption and manganese release in two variable-charge soils. J. Geochem. Explor. 2018, 190, 374–380. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Wang, S.; Dong, P.; Zhang, X.; Zhang, L.; Chen, C.; Xu, X.; Xia, Y.; Shen, Z.; Shi, L.; et al. Fate of Copper in Saline–Alkali Soil with Long-Term Application of Biogas Residue. Agriculture 2023, 13, 915. https://doi.org/10.3390/agriculture13040915
Liu B, Wang S, Dong P, Zhang X, Zhang L, Chen C, Xu X, Xia Y, Shen Z, Shi L, et al. Fate of Copper in Saline–Alkali Soil with Long-Term Application of Biogas Residue. Agriculture. 2023; 13(4):915. https://doi.org/10.3390/agriculture13040915
Chicago/Turabian StyleLiu, Binhao, Shengxiao Wang, Pengcheng Dong, Xinzhe Zhang, Long Zhang, Chen Chen, Xihui Xu, Yan Xia, Zhenguo Shen, Liang Shi, and et al. 2023. "Fate of Copper in Saline–Alkali Soil with Long-Term Application of Biogas Residue" Agriculture 13, no. 4: 915. https://doi.org/10.3390/agriculture13040915
APA StyleLiu, B., Wang, S., Dong, P., Zhang, X., Zhang, L., Chen, C., Xu, X., Xia, Y., Shen, Z., Shi, L., & Chen, Y. (2023). Fate of Copper in Saline–Alkali Soil with Long-Term Application of Biogas Residue. Agriculture, 13(4), 915. https://doi.org/10.3390/agriculture13040915









