Comparative Primary Metabolite Profiling of Setaria viridis Reveals Potential Markers to Water Limitation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Water Treatments
2.2. Sampling, Yield and Stomata Conductance
2.3. Metabolite Extraction and Gas Chromatography Coupled with Mass Spectrometry Analysis (GC-MS)
2.4. sPLS-DA Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Reduced Water Availability Decreases Biomass and Stomatal Conductance in Setaria
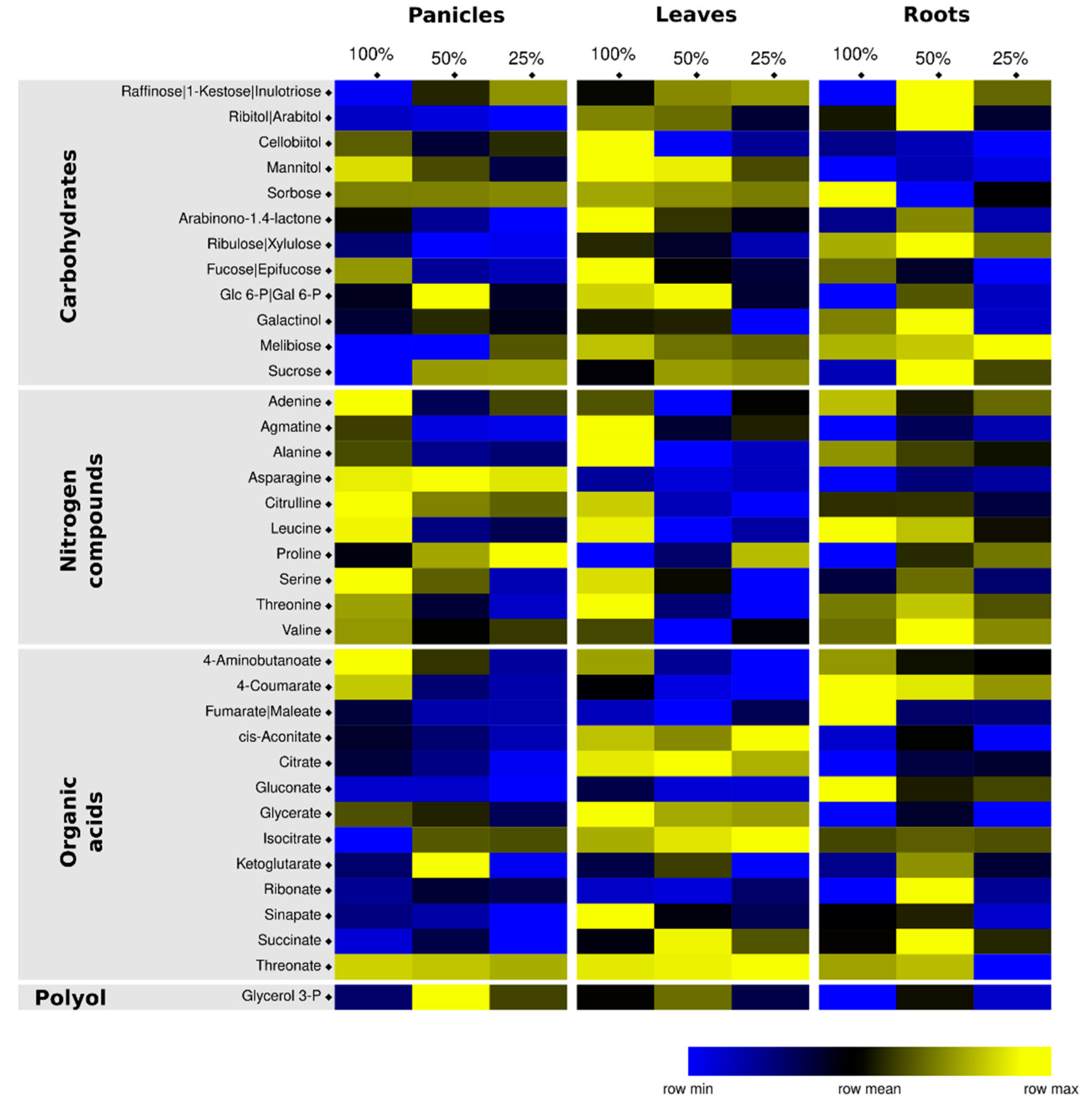
3.2. Metabolomics Analysis Highlights Organ-Specific Changes in Response to Drought
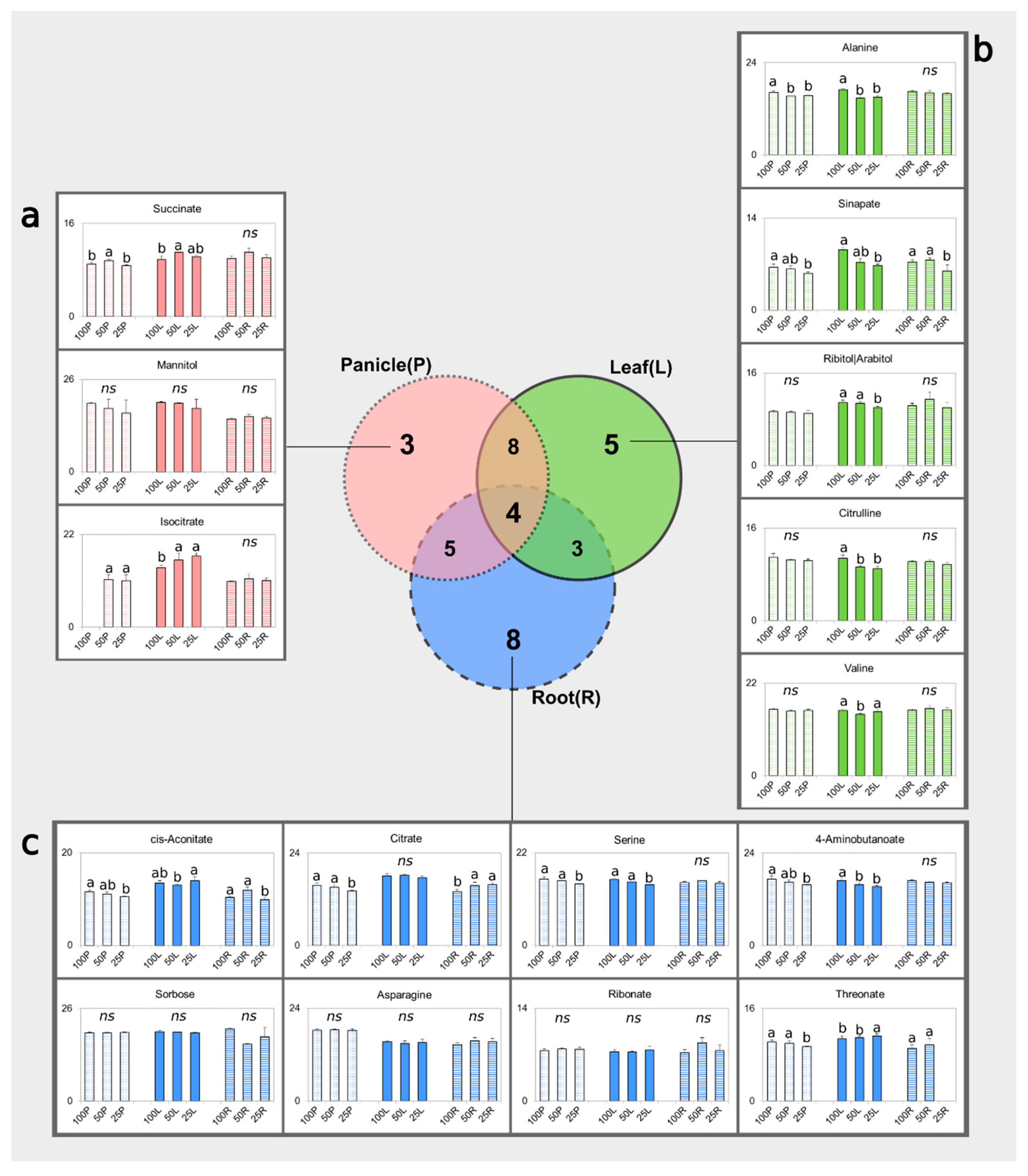
3.3. Identification of Potential Water-Deprivation Bioindicators
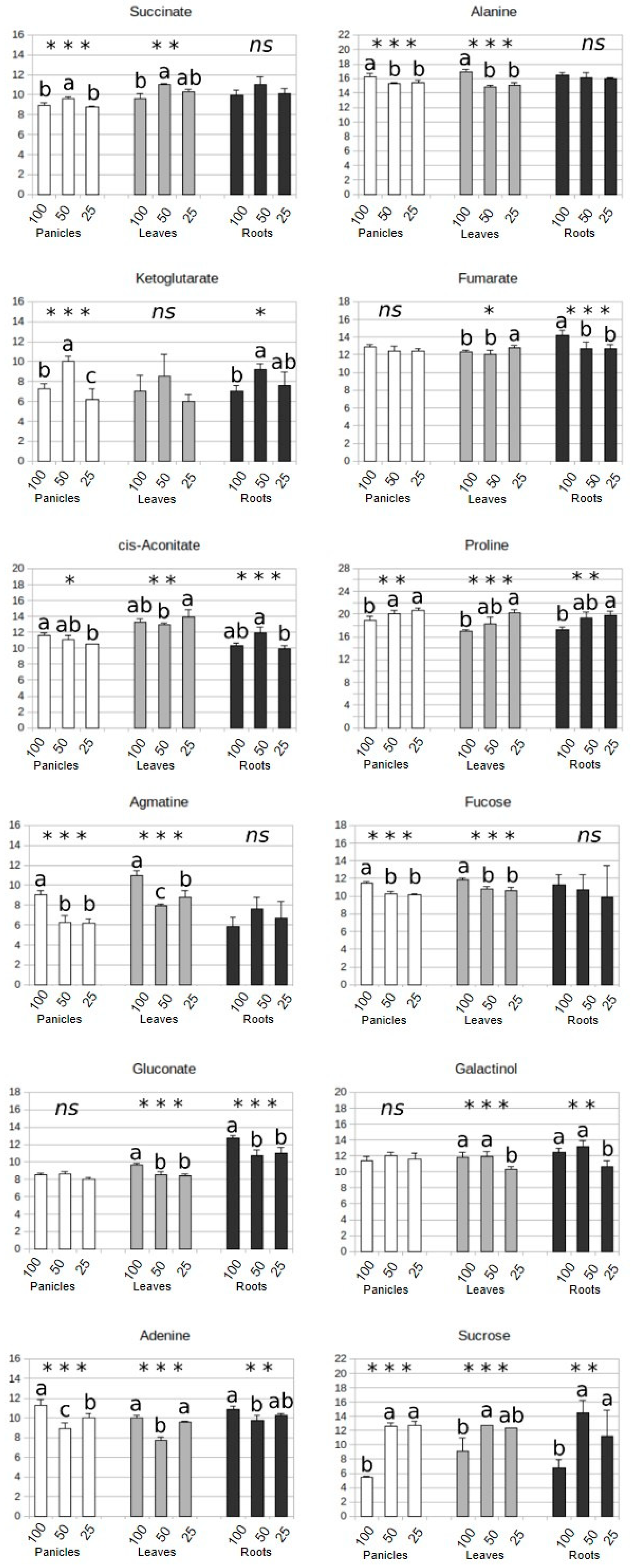
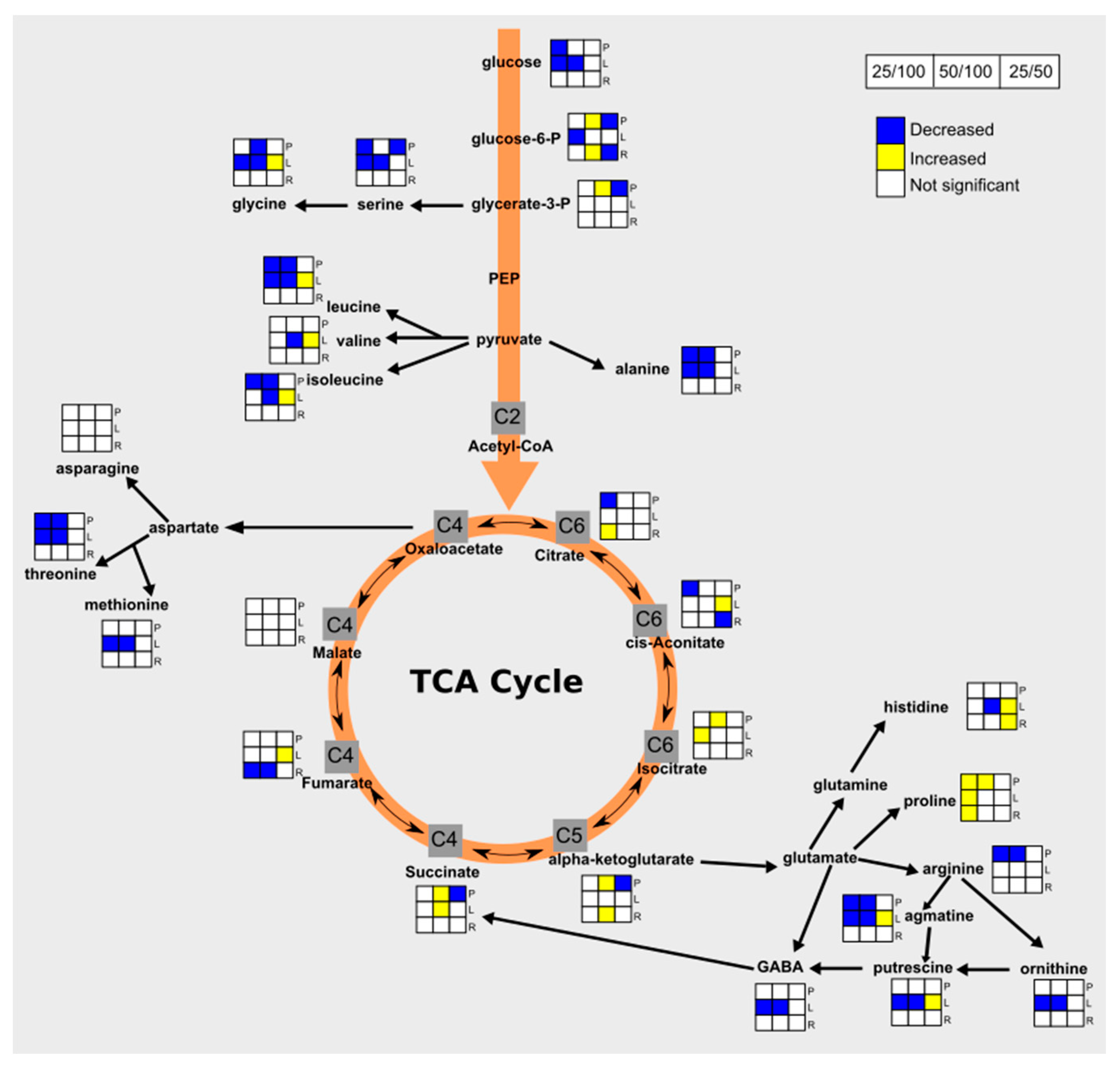
3.4. Drought Impacts Free Amino Acid Levels and TCA Pathway
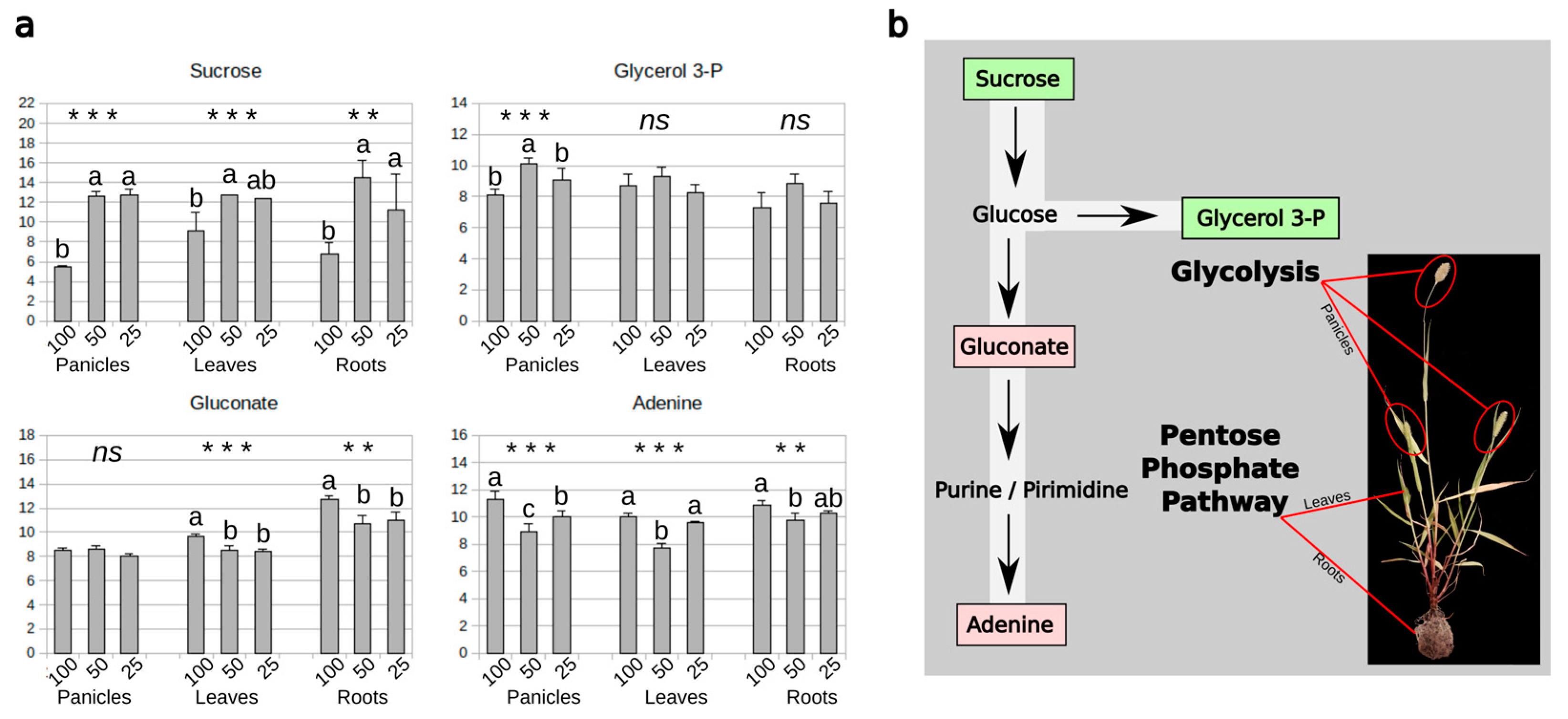
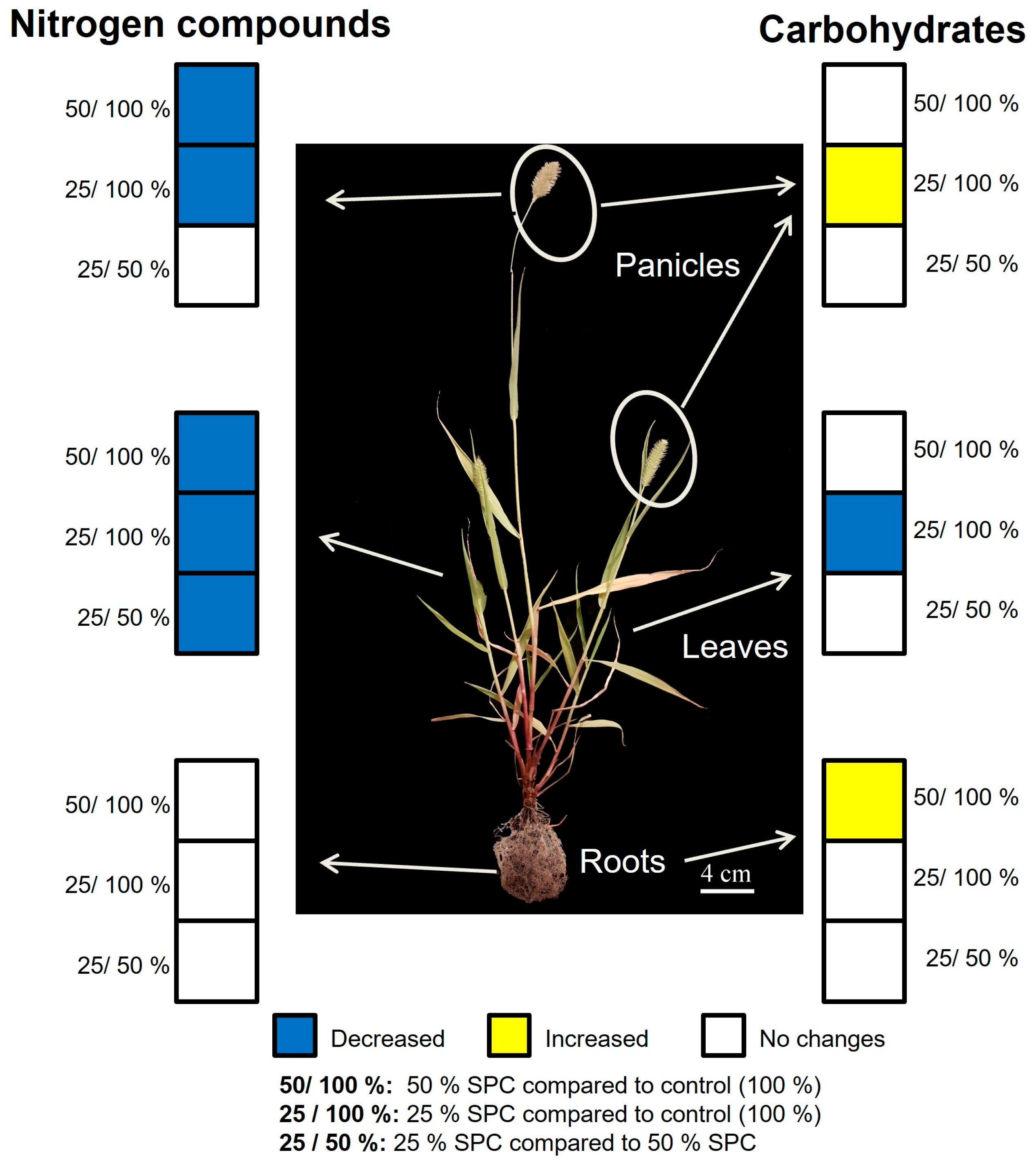
3.5. Carbohydrate and Nitrogen Compound Responses to Drought
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obata, T.; Fernie, A.R. The Use of Metabolomics to Dissect Plant Responses to Abiotic Stresses. Cell. Mol. Life Sci. 2012, 69, 3225–3243. [Google Scholar] [CrossRef]
- Gargallo-Garriga, A.; Sardans, J.; Pérez-Trujillo, M.; Rivas-Ubach, A.; Oravec, M.; Vecerova, K.; Urban, O.; Jentsch, A.; Kreyling, J.; Beierkuhnlein, C.; et al. Opposite Metabolic Responses of Shoots and Roots to Drought. Sci. Rep. 2014, 4, 6829. [Google Scholar] [CrossRef]
- Dresselhaus, T.; Hückelhoven, R. Biotic and Abiotic Stress Responses in Crop Plants. Agronomy 2018, 8, 267. [Google Scholar] [CrossRef]
- Suzuki, N.; Mittler, R. Reactive Oxygen Species and Temperature Stresses: A Delicate Balance between Signaling and Destruction. Physiol. Plant. 2006, 126, 45–51. [Google Scholar] [CrossRef]
- Fàbregas, N.; Fernie, A.R. The Metabolic Response to Drought. J. Exp. Bot. 2019, 70, 1077–1085. [Google Scholar] [CrossRef]
- Llanes, A.; Andrade, A.; Alemano, S.; Luna, V. Metabolomic Approach to Understand Plant Adaptations to Water and Salt Stress. In Plant Metabolites and Regulation Under Environmental Stress; Elsevier: Amsterdam, The Netherlands, 2018; pp. 133–144. ISBN 978-0-12-812689-9. [Google Scholar]
- Shulaev, V.; Cortes, D.; Miller, G.; Mittler, R. Metabolomics for Plant Stress Response. Physiol. Plant. 2008, 132, 199–208. [Google Scholar] [CrossRef]
- Wu, D.; Cai, S.; Chen, M.; Ye, L.; Chen, Z.; Zhang, H.; Dai, F.; Wu, F.; Zhang, G. Tissue Metabolic Responses to Salt Stress in Wild and Cultivated Barley. PLoS ONE 2013, 8, e55431. [Google Scholar] [CrossRef]
- Kang, Z.; Babar, M.A.; Khan, N.; Guo, J.; Khan, J.; Islam, S.; Shrestha, S.; Shahi, D. Comparative Metabolomic Profiling in the Roots and Leaves in Contrasting Genotypes Reveals Complex Mechanisms Involved in Post-Anthesis Drought Tolerance in Wheat. PLoS ONE 2019, 14, e0213502. [Google Scholar] [CrossRef]
- Sardans, J.; Gargallo-Garriga, A.; Urban, O.; Klem, K.; Walker, T.W.N.; Holub, P.; Janssens, I.A.; Peñuelas, J. Ecometabolomics for a Better Understanding of Plant Responses and Acclimation to Abiotic Factors Linked to Global Change. Metabolites 2020, 10, 239. [Google Scholar] [CrossRef]
- Steinfath, M.; Strehmel, N.; Peters, R.; Schauer, N.; Groth, D.; Hummel, J.; Steup, M.; Selbig, J.; Kopka, J.; Geigenberger, P.; et al. Discovering Plant Metabolic Biomarkers for Phenotype Prediction Using an Untargeted Approach: Discovering Plant Metabolic Biomarkers. Plant Biotechnol. J. 2010, 8, 900–911. [Google Scholar] [CrossRef]
- Doust, A.; Diao, X. (Eds.) Plant Genetics and Genomics: Crops and Models. In Genetics and Genomics of Setaria; Springer International Publishing: Cham, Switzerland, 2017; Volume 19, ISBN 978-3-319-45103-9. [Google Scholar]
- Petti, C.; Shearer, A.; Tateno, M.; Ruwaya, M.; Nokes, S.; Brutnell, T.; DeBolt, S. Comparative Feedstock Analysis in Setaria Viridis L. as a Model for C4 Bioenergy Grasses and Panicoid Crop Species. Front. Plant Sci. 2013, 4, 181. [Google Scholar] [CrossRef]
- Saha, P.; Blumwald, E. Spike-Dip Transformation of Setaria Viridis. Plant J. 2016, 86, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Giavalisco, P.; Li, Y.; Matthes, A.; Eckhardt, A.; Hubberten, H.-M.; Hesse, H.; Segu, S.; Hummel, J.; Köhl, K.; Willmitzer, L. Elemental Formula Annotation of Polar and Lipophilic Metabolites Using 13C, 15N and 34S Isotope Labelling, in Combination with High-Resolution Mass Spectrometry. Plant J. 2011, 68, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Roessner, U.; Luedemann, A.; Brust, D.; Fiehn, O.; Linke, T.; Willmitzer, L.; Fernie, A.R. Metabolic Profiling Allows Comprehensive Phenotyping of Genetically or Environmentally Modified Plant Systems. Plant Cell 2001, 13, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Weckwerth, W.; Wenzel, K.; Fiehn, O. Process for the Integrated Extraction, Identification and Quantification of Metabolites, Proteins and RNA to Reveal Their Co-Regulation in Biochemical Networks. Proteomics 2004, 4, 78–83. [Google Scholar] [CrossRef]
- Cuadros-Inostroza, Á.; Caldana, C.; Redestig, H.; Kusano, M.; Lisec, J.; Peña-Cortés, H.; Willmitzer, L.; Hannah, M.A. TargetSearch—A Bioconductor Package for the Efficient Preprocessing of GC-MS Metabolite Profiling Data. BMC Bioinform. 2009, 10, 428. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. Using MetaboAnalyst 3.0 for Comprehensive Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2016, 55, 14.10.1–14.10.91. [Google Scholar] [CrossRef]
- Flexas, J.; Barbour, M.M.; Brendel, O.; Cabrera, H.M.; Carriquí, M.; Díaz-Espejo, A.; Douthe, C.; Dreyer, E.; Ferrio, J.P.; Gago, J.; et al. Mesophyll Diffusion Conductance to CO2: An Unappreciated Central Player in Photosynthesis. Plant Sci. 2012, 193–194, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Time, A.; Garrido, M.; Acevedo, E. Water Relations and Growth Response to Drought Stress of Prosopis Tamarugo Phil. A Review. J. Soil Sci. Plant Nutr. 2018, 18, 329–343. [Google Scholar] [CrossRef]
- Saha, P.; Sade, N.; Arzani, A.; Rubio Wilhelmi, M.d.M.; Coe, K.M.; Li, B.; Blumwald, E. Effects of Abiotic Stress on Physiological Plasticity and Water Use of Setaria Viridis (L.). Plant Sci. 2016, 251, 128–138. [Google Scholar] [CrossRef]
- Worley, B.; Powers, R. Multivariate Analysis in Metabolomics. Curr. Metabolomics 2012, 1, 92–107. [Google Scholar] [CrossRef]
- Lee, L.C.; Liong, C.-Y.; Jemain, A.A. Partial Least Squares-Discriminant Analysis (PLS-DA) for Classification of High-Dimensional (HD) Data: A Review of Contemporary Practice Strategies and Knowledge Gaps. Analyst 2018, 143, 3526–3539. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xin, Z.; Yang, T.; Ma, X.; Zhang, Y.; Wang, Z.; Ren, Y.; Lin, T. Metabolomics Response for Drought Stress Tolerance in Chinese Wheat Genotypes (Triticum Aestivum). Plants 2020, 9, 520. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Zhang, Y.; Liu, A.; Li, D.; Wang, X.; Dossa, K.; Zhou, R.; Yu, J.; Zhang, Y.; Wang, L.; et al. Transcriptomic and Metabolomic Profiling of Drought-Tolerant and Susceptible Sesame Genotypes in Response to Drought Stress. BMC Plant Biol. 2019, 19, 267. [Google Scholar] [CrossRef]
- Liquet, B.; Cao, K.-A.L.; Hocini, H.; Thiébaut, R. A Novel Approach for Biomarker Selection and the Integration of Repeated Measures Experiments from Two Assays. BMC Bioinform. 2012, 13, 325. [Google Scholar] [CrossRef]
- Ruiz-Perez, D.; Guan, H.; Madhivanan, P.; Mathee, K.; Narasimhan, G. So You Think You Can PLS-DA? BMC Bioinform. 2020, 21, 2. [Google Scholar] [CrossRef]
- Lea, P.J.; Sodek, L.; Parry, M.A.J.; Shewry, P.R.; Halford, N.G. Asparagine in Plants. Ann. Appl. Biol. 2007, 150, 1–26. [Google Scholar] [CrossRef]
- Tegeder, M.; Masclaux-Daubresse, C. Source and Sink Mechanisms of Nitrogen Transport and Use. New Phytol. 2018, 217, 35–53. [Google Scholar] [CrossRef]
- Merchant, A.; Richter, A.A. Polyols as Biomarkers and Bioindicators for 21st Century Plant Breeding. Funct. Plant Biol. 2011, 38, 934. [Google Scholar] [CrossRef]
- Seki, M.; Umezawa, T.; Urano, K.; Shinozaki, K. Regulatory Metabolic Networks in Drought Stress Responses. Curr. Opin. Plant Biol. 2007, 10, 296–302. [Google Scholar] [CrossRef]
- Li, Z.; Yu, J.; Peng, Y.; Huang, B. Metabolic Pathways Regulated by Abscisic Acid, Salicylic Acid and γ-Aminobutyric Acid in Association with Improved Drought Tolerance in Creeping Bentgrass (Agrostis Stolonifera). Physiol. Plant. 2017, 159, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Ryan, P.R.; Delhaize, E. Aluminium Tolerance in Plants and the Complexing Role of Organic Acids. Trends Plant Sci. 2001, 6, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Dodd, I.C. Root-To-Shoot Signalling: Assessing The Roles of ‘Up’ In the Up and Down World of Long-Distance Signalling In Planta. Plant Soil 2005, 274, 251–270. [Google Scholar] [CrossRef]
- Lee, H.-J.; Ha, J.-H.; Park, C.-M. Underground Roots Monitor Aboveground Environment by Sensing Stem-Piped Light. Commun. Integr. Biol. 2016, 9, e1261769. [Google Scholar] [CrossRef]
- Bowne, J.B.; Erwin, T.A.; Juttner, J.; Schnurbusch, T.; Langridge, P.; Bacic, A.; Roessner, U. Drought Responses of Leaf Tissues from Wheat Cultivars of Differing Drought Tolerance at the Metabolite Level. Mol. Plant 2012, 5, 418–429. [Google Scholar] [CrossRef]
- Ullah, N.; Yüce, M.; Neslihan Öztürk Gökçe, Z.; Budak, H. Comparative Metabolite Profiling of Drought Stress in Roots and Leaves of Seven Triticeae Species. BMC Genom. 2017, 18, 969. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.P.; Palmer, W.M.; Brown, C.; Abel, C.; Lunn, J.E.; Furbank, R.T.; Grof, C.P.L. A Developing Setaria Viridis Internode: An Experimental System for the Study of Biomass Generation in a C4 Model Species. Biotechnol. Biofuels 2016, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Koch, K. Sucrose Metabolism: Regulatory Mechanisms and Pivotal Roles in Sugar Sensing and Plant Development. Curr. Opin. Plant Biol. 2004, 7, 235–246. [Google Scholar] [CrossRef]
- Guo, L.-X.; Shi, C.-Y.; Liu, X.; Ning, D.-Y.; Jing, L.-F.; Yang, H.; Liu, Y.-Z. Citrate Accumulation-Related Gene Expression and/or Enzyme Activity Analysis Combined With Metabolomics Provide a Novel Insight for an Orange Mutant. Sci. Rep. 2016, 6, 29343. [Google Scholar] [CrossRef]
- Marček, T.; Hamow, K.Á.; Végh, B.; Janda, T.; Darko, E. Metabolic Response to Drought in Six Winter Wheat Genotypes. PLoS ONE 2019, 14, e0212411. [Google Scholar] [CrossRef]
- Nataraja, K.N.; Parvathi, M.S. Tolerance to Drought Stress in Plants: Unravelling the Signaling Networks. In Drought Stress Tolerance in Plants, Vol 2; Hossain, M.A., Wani, S.H., Bhattacharjee, S., Burritt, D.J., Tran, L.-S.P., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 71–90. ISBN 978-3-319-32421-0. [Google Scholar]
- Lemoine, R.; Camera, S.L.; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.-L.; Laloi, M.; Coutos-Thévenot, P.; Maurousset, L.; et al. Source-to-Sink Transport of Sugar and Regulation by Environmental Factors. Front. Plant Sci. 2013, 4, 272. [Google Scholar] [CrossRef]
- Ghate, T.; Barvkar, V.; Deshpande, S.; Bhargava, S. Role of ABA Signaling in Regulation of Stem Sugar Metabolism and Transport under Post- Flowering Drought Stress in Sweet Sorghum. Plant Mol. Biol. Report. 2019, 37, 303–313. [Google Scholar] [CrossRef]
- Slewinski, T.L. Non-Structural Carbohydrate Partitioning in Grass Stems: A Target to Increase Yield Stability, Stress Tolerance, and Biofuel Production. J. Exp. Bot. 2012, 63, 4647–4670. [Google Scholar] [CrossRef] [PubMed]
- Cho, L.-H.; Pasriga, R.; Yoon, J.; Jeon, J.-S.; An, G. Roles of Sugars in Controlling Flowering Time. J. Plant Biol. 2018, 61, 121–130. [Google Scholar] [CrossRef]
- Das, A.; Rushton, P.; Rohila, J. Metabolomic Profiling of Soybeans (Glycine Max L.) Reveals the Importance of Sugar and Nitrogen Metabolism under Drought and Heat Stress. Plants 2017, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Parida, A.K.; Panda, A.; Rangani, J. Metabolomics-Guided Elucidation of Abiotic Stress Tolerance Mechanisms in Plants. In Plant Metabolites and Regulation Under Environmental Stress; Elsevier: Amsterdam, The Netherlands, 2018; pp. 89–131. ISBN 978-0-12-812689-9. [Google Scholar]
- Varshney, R.K.; Pandey, M.K.; Chitikineni, A. (Eds.) Advances in Biochemical Engineering/Biotechnology. In Plant Genetics and Molecular Biology; Springer International Publishing: Cham, Switzerland, 2018; Volume 164, ISBN 978-3-319-91312-4. [Google Scholar]
- Singh, O.V.; Kumar, R. Biotechnological Production of Gluconic Acid: Future Implications. Appl. Microbiol. Biotechnol. 2007, 75, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Shetty, K. Role of Proline-Linked Pentose Phosphate Pathway in Biosynthesis of Plant Phenolics for Functional Food and Environmental Applications: A Review. Process Biochem. 2004, 39, 789–804. [Google Scholar] [CrossRef]
- Silva, S.F.; Miranda, M.T.; Cunha, C.P.; Domingues, A.P., Jr.; Aricetti, J.A.; Caldana, C.; Machado, E.C.; Ribeiro, R.V. Metabolic Profiling of Drought Tolerance: Revealing How Citrus Rootstocks Modulate Plant Metabolism under Varying Water Availability. Environ. Exp. Bot. 2023, 206, 105169. [Google Scholar] [CrossRef]
- Diab, H.; Limami, A. Reconfiguration of N Metabolism upon Hypoxia Stress and Recovery: Roles of Alanine Aminotransferase (AlaAT) and Glutamate Dehydrogenase (GDH). Plants 2016, 5, 25. [Google Scholar] [CrossRef]
- Wang, X.; Cai, X.; Xu, C.; Wang, Q.; Dai, S. Drought-Responsive Mechanisms in Plant Leaves Revealed by Proteomics. Int. J. Mol. Sci. 2016, 17, 1706. [Google Scholar] [CrossRef]
- Miyashita, Y.; Dolferus, R.; Ismond, K.P.; Good, A.G. Alanine Aminotransferase Catalyses the Breakdown of Alanine after Hypoxia in Arabidopsis Thaliana: AlaAT in Arabidopsis. Plant J. 2007, 49, 1108–1121. [Google Scholar] [CrossRef] [PubMed]
- Taji, T.; Ohsumi, C.; Iuchi, S.; Seki, M.; Kasuga, M.; Kobayashi, M.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Important Roles of Drought- and Cold-Inducible Genes for Galactinol Synthase in Stress Tolerance in Arabidopsis Thaliana. Plant J. 2002, 29, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, M.G.; Ishizaki, T.; Valencia, M.; Ogawa, S.; Dedicova, B.; Ogata, T.; Yoshiwara, K.; Maruyama, K.; Kusano, M.; Saito, K.; et al. Overexpression of an Arabidopsis Thaliana Galactinol Synthase Gene Improves Drought Tolerance in Transgenic Rice and Increased Grain Yield in the Field. Plant Biotechnol. J. 2017, 15, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Asthir, B. Proline: A Key Player in Plant Abiotic Stress Tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Chmielewska, K.; Rodziewicz, P.; Swarcewicz, B.; Sawikowska, A.; Krajewski, P.; Marczak, Ł.; Ciesiołka, D.; Kuczyńska, A.; Mikołajczak, K.; Ogrodowicz, P.; et al. Analysis of Drought-Induced Proteomic and Metabolomic Changes in Barley (Hordeum Vulgare L.) Leaves and Roots Unravels Some Aspects of Biochemical Mechanisms Involved in Drought Tolerance. Front. Plant Sci. 2016, 7, 1108. [Google Scholar] [CrossRef]
- Delauney, A.J.; Verma, D.P.S. Proline Biosynthesis and Osmoregulation in Plants. Plant J. 1993, 4, 215–223. [Google Scholar] [CrossRef]
- Urano, K.; Maruyama, K.; Ogata, Y.; Morishita, Y.; Takeda, M.; Sakurai, N.; Suzuki, H.; Saito, K.; Shibata, D.; Kobayashi, M.; et al. Characterization of the ABA-Regulated Global Responses to Dehydration in Arabidopsis by Metabolomics. Plant J. 2009, 57, 1065–1078. [Google Scholar] [CrossRef]
- Hossain, M.A.; Wani, S.H.; Bhattacharjee, S.; Burritt, D.J.; Tran, L.-S.P. (Eds.) Drought Stress Tolerance in Plants, Vol 1; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-28897-0. [Google Scholar]
- Pires, M.V.; Pereira Júnior, A.A.; Medeiros, D.B.; Daloso, D.M.; Pham, P.A.; Barros, K.A.; Engqvist, M.K.M.; Florian, A.; Krahnert, I.; Maurino, V.G.; et al. The Influence of Alternative Pathways of Respiration That Utilize Branched-Chain Amino Acids Following Water Shortage in Arabidopsis: Influence of the ETF/ETFQO Pathway under Water Stress. Plant Cell Environ. 2016, 39, 1304–1319. [Google Scholar] [CrossRef]
- Huang, T.; Jander, G. Abscisic Acid-Regulated Protein Degradation Causes Osmotic Stress-Induced Accumulation of Branched-Chain Amino Acids in Arabidopsis Thaliana. Planta 2017, 246, 737–747. [Google Scholar] [CrossRef]
- Virlouvet, L.; Jacquemot, M.-P.; Gerentes, D.; Corti, H.; Bouton, S.; Gilard, F.; Valot, B.; Trouverie, J.; Tcherkez, G.; Falque, M.; et al. The ZmASR1 Protein Influences Branched-Chain Amino Acid Biosynthesis and Maintains Kernel Yield in Maize under Water-Limited Conditions. Plant Physiol. 2011, 157, 917–936. [Google Scholar] [CrossRef]
- Malatrasi, M.; Corradi, M.; Svensson, J.T.; Close, T.J.; Gulli, M.; Marmiroli, N. A Branched-Chain Amino Acid Aminotransferase Gene Isolated from Hordeum Vulgare Is Differentially Regulated by Drought Stress. Theor. Appl. Genet. 2006, 113, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Araújo, W.L.; Ishizaki, K.; Nunes-Nesi, A.; Larson, T.R.; Tohge, T.; Krahnert, I.; Witt, S.; Obata, T.; Schauer, N.; Graham, I.A.; et al. Identification of the 2-Hydroxyglutarate and Isovaleryl-CoA Dehydrogenases as Alternative Electron Donors Linking Lysine Catabolism to the Electron Transport Chain of Arabidopsis Mitochondria. Plant Cell 2010, 22, 1549–1563. [Google Scholar] [CrossRef] [PubMed]
- Sweetlove, L.J.; Beard, K.F.M.; Nunes-Nesi, A.; Fernie, A.R.; Ratcliffe, R.G. Not Just a Circle: Flux Modes in the Plant TCA Cycle. Trends Plant Sci. 2010, 15, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Fait, A.; Fromm, H.; Walter, D.; Galili, G.; Fernie, A.R. Highway or Byway: The Metabolic Role of the GABA Shunt in Plants. Trends Plant Sci. 2008, 13, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Bown, A.W.; Shelp, B.J. The Metabolism and Functions of [Gamma]-Aminobutyric Acid. Plant Physiol. 1997, 115, 1–5. [Google Scholar] [CrossRef]
- Kinnersley, A.M.; Turano, F.J. Gamma Aminobutyric Acid (GABA) and Plant Responses to Stress. Crit. Rev. Plant Sci. 2000, 19, 479–509. [Google Scholar] [CrossRef]
- Coruzzi, G.M.; Zhou, L. Carbon and Nitrogen Sensing and Signaling in Plants: Emerging ‘Matrix Effects’. Curr. Opin. Plant Biol. 2001, 4, 247–253. [Google Scholar] [CrossRef]
- Pinheiro, C.; António, C.; Ortuño, M.F.; Dobrev, P.I.; Hartung, W.; Thomas-Oates, J.; Ricardo, C.P.; Vanková, R.; Chaves, M.M.; Wilson, J.C. Initial Water Deficit Effects on Lupinus Albus Photosynthetic Performance, Carbon Metabolism, and Hormonal Balance: Metabolic Reorganization Prior to Early Stress Responses. J. Exp. Bot. 2011, 62, 4965–4974. [Google Scholar] [CrossRef]
- De Miguel, M.; Guevara, M.Á.; Sánchez-Gómez, D.; de María, N.; Díaz, L.M.; Mancha, J.A.; Fernández de Simón, B.; Cadahía, E.; Desai, N.; Aranda, I.; et al. Organ-Specific Metabolic Responses to Drought in Pinus Pinaster Ait. Plant Physiol. Biochem. 2016, 102, 17–26. [Google Scholar] [CrossRef]
- Loka, D.; Harper, J.; Humphreys, M.; Gasior, D.; Wootton-Beard, P.; Gwynn-Jones, D.; Scullion, J.; Doonan, J.; Kingston-Smith, A.; Dodd, R.; et al. Impacts of Abiotic Stresses on the Physiology and Metabolism of Cool-Season Grasses: A Review. Food Energy Secur. 2019, 8, e00152. [Google Scholar] [CrossRef]
- Shi, H.; Wang, B.; Yang, P.; Li, Y.; Miao, F. Differences in Sugar Accumulation and Mobilization between Sequential and Non-Sequential Senescence Wheat Cultivars under Natural and Drought Conditions. PLoS ONE 2016, 11, e0166155. [Google Scholar] [CrossRef] [PubMed]
- Volenec, J.J.; Nelson, C.J. Carbon Metabolism in Forage Plants. In Forages; John Wiley & Sons, Ltd.: London, UK, 2020; pp. 65–84. ISBN 978-1-119-43666-9. [Google Scholar]
- Perlikowski, D.; Augustyniak, A.; Masajada, K.; Skirycz, A.; Soja, A.M.; Michaelis, Ä.; Wolter, G.; Kosmala, A. Structural and Metabolic Alterations in Root Systems under Limited Water Conditions in Forage Grasses of Lolium-Festuca Complex. Plant Sci. 2019, 283, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Galili, G. The Aspartate-Family Pathway of Plants: Linking Production of Essential Amino Acids with Energy and Stress Regulation. Plant Signal. Behav. 2011, 6, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kang, R.; Liu, Y.; Wu, K.-X.; Yan, X.; Song, Y.; Pan, L.-B.; Tang, Z.-H. Differential Metabolite Accumulation in Different Tissues of Gleditsia Sinensis under Water Stress and Rehydration Conditions. Forests 2020, 11, 542. [Google Scholar] [CrossRef]

| Parameters | Water Treatments | ||
|---|---|---|---|
| 100% (Control) | 50% SPC | 25% SPC | |
| Plant dry weight (g) | 0.033 ± 0.006 a | 0.016 ± 0.007 b | 0.018 ± 0.016 b |
| Stomatal conductance (mmol m−2 s−1) | 1.36 ± 0.31 a | 0.052 ± 0.016 b | 0.067 ± 0.014 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guedes, F.A.d.F.; Nascimento, L.B.d.S.; Costa, M.P.; Macrae, A.; Alves-Ferreira, M.; Caldana, C.; Reinert, F. Comparative Primary Metabolite Profiling of Setaria viridis Reveals Potential Markers to Water Limitation. Agriculture 2023, 13, 660. https://doi.org/10.3390/agriculture13030660
Guedes FAdF, Nascimento LBdS, Costa MP, Macrae A, Alves-Ferreira M, Caldana C, Reinert F. Comparative Primary Metabolite Profiling of Setaria viridis Reveals Potential Markers to Water Limitation. Agriculture. 2023; 13(3):660. https://doi.org/10.3390/agriculture13030660
Chicago/Turabian StyleGuedes, Fernanda Alves de Freitas, Luana Beatriz dos Santos Nascimento, Mara Priscila Costa, Andrew Macrae, Marcio Alves-Ferreira, Camila Caldana, and Fernanda Reinert. 2023. "Comparative Primary Metabolite Profiling of Setaria viridis Reveals Potential Markers to Water Limitation" Agriculture 13, no. 3: 660. https://doi.org/10.3390/agriculture13030660
APA StyleGuedes, F. A. d. F., Nascimento, L. B. d. S., Costa, M. P., Macrae, A., Alves-Ferreira, M., Caldana, C., & Reinert, F. (2023). Comparative Primary Metabolite Profiling of Setaria viridis Reveals Potential Markers to Water Limitation. Agriculture, 13(3), 660. https://doi.org/10.3390/agriculture13030660









