Design of Device for Optical Luminescent Diagnostic of the Seeds Infected by Fusarium
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Obtainment and Analysis of the Spectra
3.2. Calculation of Regression Models
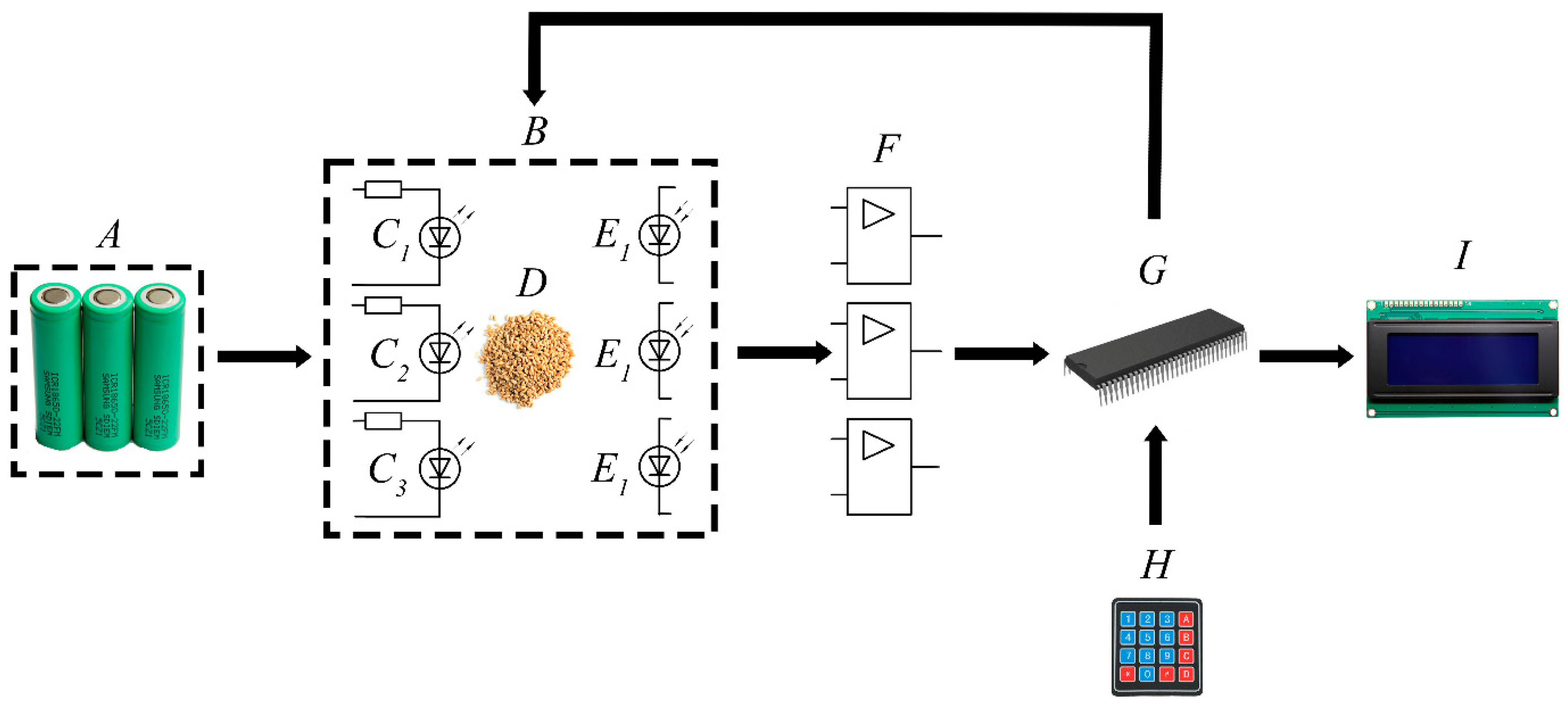
3.3. Device of Photoluminescent Diagnostics of Seeds
- The selected seeds (D) are placed in a dark, light-tight chamber (B).
- Photoluminescence is excited for 20 microseconds by two (for wheat or barley) or one radiation source (for oats).
- Luminescence is detected 0.75–1.0 microseconds after the radiation source is turned off by two photodetectors (for wheat or barley) or one (for oats).
- The received analog electrical signal from the receivers (photodiodes E) is amplified by an amplifier (F), converted into a digital signal, and fed to the microcontroller (G).
- On the microcontroller, the degree of infection is calculated taking into account the photo signal and a priori information (calibration Equations (2)–(4)).
- The received and processed signal is fed to the indicator (I). Then a decision is made on subsequent operations with seeds.
3.4. Justification of the Choice of the Element Base of the Device
- (1)
- Easy operation and minimal labor intensity.
- (2)
- Minimum permissible measurement error.
- (3)
- The weight of the device is not more than 3.5 kg to ensure maximum operator mobility.
- (4)
- Safety during operation.
- (5)
- Battery life of 5 h.
- (6)
- A light-tight chamber when measuring the seed material should ensure that there is no radiation from the external light background, as well as minimal reflections of the flow from the walls when the LEDs are working.
- (7)
- Convenience of cleaning the light-tight chamber from agricultural crops.
- (8)
- The result of measuring the diagnostic parameter, namely the degree of infection, should be displayed on the indicator for the operator.
- (9)
- Low cost of an express diagnostic device for maximum accessibility to users.
- -
- laboratory and field tests of the developed device.
- -
- extension of the application of the developed method to other agricultural plants and other diseases.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alemu, K. Detection of Diseases, Identification and Diversity of Viruses: A. J. Biol. Agric. Healthc. 2015, 5, 132–141. [Google Scholar]
- Mohd Ali, M.; Bachik, N.A.; Muhadi, N.A.; Yusof, T.; Gomes, C. Non-destructive techniques of detecting plant diseases: A review. Physiol. Mol. Plant Pathol. 2019, 108, 101426. [Google Scholar]
- Mahlein, A.-K. Plant Disease Detection by Imaging Sensors–Parallels and Specific Demands for Precision Agriculture and Plant Phenotyping. Plant Dis. 2016, 100, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Mahlein, A.-K.; Alisaac, E.A.; Masri, A.; Behmann, J.; Dehne, H.-W.; Oerke, E.-C. Comparison and Combination of Thermal, Fluorescence and Hyperspectral Imaging for Monitoring Fusarium Head Blight of Wheat on Spikelet Scale. Sensors 2019, 19, 2281. [Google Scholar] [CrossRef]
- Mahlein, A.-K.; Kuska, M.T.; Thomas, S.; Wahabzada, M.; Behmann, J.; Rascher, U.; Kersting, K. Quantitative and qualitative phenotyping of disease resistance of crops by hyperspectral sensors: Seamless interlocking of phytopathology, sensors, and machine learning is needed! Curr. Opin. Plant Biol. 2019, 50, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Makmuang, S.; Nootchanat, S.; Ekgasit, S.; Wongravee, K. Non-destructive method for discrimination of weedy rice using near infrared spectroscopy and modified self-organizing maps (SOMs). Comput. Electron. Agric. 2021, 191, 106522. [Google Scholar] [CrossRef]
- Tsakanikas, P.; Fengou, L.-C.; Manthou, E.; Lianou, A.; Panagou, E.Z.; Nychas, G.E. A unified spectra analysis workflow for the assessment of microbial contamination of ready-to-eat green salads: Comparative study and application of non-invasive sensors. Comput. Electron. Agric. 2018, 155, 212–219. [Google Scholar] [CrossRef]
- Johannes, A.; Picon, A.; Alvarez-Gila, A.; Echazarra, J.; Rodriguez-Vaamonde, S.; Díez Navajas, A.; Ortiz-Barredo, A. Automatic plant disease diagnosis using mobile capture devices, applied on a wheat use case. Comput. Electron. Agric. 2017, 138, 200–209. [Google Scholar] [CrossRef]
- Haagsma, M.; Page, G.F.M.; Johnson, J.S.; Still, C.; Waring, K.M.; Sniezko, R.A.; Selker, J.S. Using Hyperspectral Imagery to Detect an Invasive Fungal Pathogen and Symptom Severity in Pinus strobiformis Seedlings of Different Genotypes. Remote Sens. 2020, 12, 4041. [Google Scholar] [CrossRef]
- Haagsma, M.; Page, G.F.M.; Johnson, J.S.; Still, C.; Waring, K.M.; Sniezko, R.A.; Selker, J.S. Model selection and timing of acquisition date impacts classification accuracy: A case study using hyperspectral imaging to detect white pine blister rust over time. Comput. Electron. Agric. 2021, 191, 106555. [Google Scholar] [CrossRef]
- Zhang, D.-Y.; Chen, G.; Yin, X.; Hu, R.-J.; Gu, C.-Y.; Pan, Z.-G.; Zhou, X.-G.; Chen, Y. Integrating spectral and image data to detect Fusarium head blight of wheat. Comput. Electron. Agric. 2020, 175, 105588. [Google Scholar] [CrossRef]
- Heim, R.H.J.; Wright, I.J.; Chang, H.C.; Carnegie, A.J.; Pegg, G.S.; Lancaste, E.K.; Falster, D.S.; Oldeland, J. Detecting myrtle rust (Austropuccinia psidii) on lemon myrtle trees using spectral signatures and machine learning. Plant Pathol. 2018, 67, 1114–1121. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, X.; You, Z.; Zhang, L. Leaf image based cucumber disease recognition using sparse representation classification. Comput. Electron. Agric. 2017, 134, 135–141. [Google Scholar] [CrossRef]
- Marin, D.B.; Ferraz, G.A.S.; Santana, L.S.; Barbosa, B.D.S.; Barata, R.A.P.; Osco, L.P.; Ramos, A.P.M.; Guimarães, P.H.S. Detecting coffee leaf rust with UAV-based vegetation indices and decision tree machine learning models. Comput. Electron. Agric. 2021, 190, 106476. [Google Scholar] [CrossRef]
- Jiang, Z.; Dong, Z.; Jiang, W.; Yang, Y. Recognition of rice leaf diseases and wheat leaf diseases based on multi-task deep transfer learning. Comput. Electron. Agric. 2021, 186, 106184. [Google Scholar] [CrossRef]
- Bhandari, M.; Ibrahim, A.M.H.; Xue, Q.; Jung, J.; Chang, A.; Rudd, J.C.; Maeda, M.; Rajan, N.; Neely, H.; Landivar, J. Assessing winter wheat foliage disease severity using aerial imagery acquired from small Unmanned Aerial Vehicle (UAV). Comput. Electron. Agric. 2020, 176, 105665. [Google Scholar] [CrossRef]
- Bauriegel, E.; Herppich, W.B. Hyperspectral and Chlorophyll Fluorescence Imaging for Early Detection of Plant Diseases, with Special Reference to Fusarium spec. Infections on Wheat. Agriculture 2014, 4, 32–57. [Google Scholar] [CrossRef]
- Tischler, Y.K.; Thiessen, E.; Hartung, E. Early optical detection of infection with brown rust in winter wheat by chlorophyll fluorescence excitation spectra. Comput. Electron. Agric. 2018, 146, 77–85. [Google Scholar] [CrossRef]
- Fahey, T.; Pham, H.; Gardi, A.; Sabatini, R.; Stefanelli, D.; Goodwin, I.; Lamb, D.W. Active and Passive Electro-Optical Sensors for Health Assessment in Food Crops. Sensors 2021, 21, 171. [Google Scholar] [CrossRef]
- Cherney, J.H.; Digman, M.F.; Cherney, D.J. Handheld NIRS for forage evaluation. Comput. Electron. Agric. 2021, 190, 106469. [Google Scholar] [CrossRef]
- Acosta, J.; Castillo, M.S.; Hodge, G.R. Comparison of benchtop and handheld near-infrared spectroscopy devices to determine forage nutritive value. Crop Sci. 2020, 60, 3410–3422. [Google Scholar] [CrossRef]
- Berzaghi, P.; Cherney, J.H.; Casler, M.D. Prediction performance of portable near infrared reflectance instruments using preprocessed dried, ground forage samples. Comput. Electron. Agric. 2021, 182, 106013. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Wang, J.; Song, Z.; Rehman, T.U.; Bureetes, T.; Ma, D.; Chen, Z.; Neeno, S.; Jin, J. Leaf Scanner: A portable and low-cost multispectral corn leaf scanning device for precise phenotyping. Comput. Electron. Agric. 2019, 167, 105069. [Google Scholar] [CrossRef]
- Song, D.; Qiao, L.; Gao, D.; Li, S.; Li, M.; Sun, H.; Ma, J. Development of crop chlorophyll detector based on a type of interference filter optical sensor. Comput. Electron. Agric. 2021, 187, 106260. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, C.; Taha, M.F.; Qiu, Z.; He, Y. Determination of leaf water content with a portable NIRS system based on deep learning and information fusion analysis. Trans. ASABE 2021, 64, 127–135. [Google Scholar] [CrossRef]
- Giménez-Gallego, J.; González-Teruel, J.D.; Soto-Valles, F.; Jiménez-Buendía, M.; Navarro-Hellín, H.; Torres-Sánchez, R. Intelligent thermal image-based sensor for affordable measurement of crop canopy temperature. Comput. Electron. Agric. 2021, 188, 106319. [Google Scholar] [CrossRef]
- Yau, W.K.; Ng, O.-E.; Lee, S.W. Portable device for contactless, non-destructive and in situ outdoor individual leaf area measurement. Comput. Electron. Agric. 2021, 187, 106278. [Google Scholar] [CrossRef]
- Gong, A.; Wu, X.; Qiu, Z.; He, Y. A handheld device for leaf area measurement. Comput. Electron. Agric. 2013, 98, 74–80. [Google Scholar] [CrossRef]
- Buelvas, R.M.; Adamchuk, V.I.; Leksono, E.; Tikasz, P.; Lefsrud, M.; Holoszkiewicz, J. Biomass estimation from canopy measurements for leafy vegetables based on ultrasonic and laser sensors. Comput. Electron. Agric. 2019, 164, 104896. [Google Scholar] [CrossRef]
- Perez, R.M.; Cheein, F.A.; Rosell-Polo, J.R. Flexible system of multiple RGB-D sensors for measuring and classifying fruits in agri-food Industry. Comput. Electron. Agric. 2017, 139, 231–242. [Google Scholar] [CrossRef]
- Tugnolo, A.; Giovenzana, V.; Beghi, R.; Grassi, S.; Alamprese, C.; Casson, A.; Casiraghi, E.; Guidetti, R. A diagnostic visible/near infrared tool for a fully automated olive ripeness evaluation in a view of a simplified optical system. Comput. Electron. Agric. 2021, 180, 105887. [Google Scholar] [CrossRef]
- Yang, B.; Guo, W.; Huang, X.; Du, R.; Liu, Z. A portable, low-cost and sensor-based detector on sweetness and firmness grades of kiwifruit. Comput. Electron. Agric. 2020, 179, 105831. [Google Scholar] [CrossRef]
- Long, D.S.; McCallum, J.D. Adapting a relatively low-cost reflectance spectrometer for on-combine sensing of grain protein concentration. Comput. Electron. Agric. 2020, 174, 105467. [Google Scholar] [CrossRef]
- Pankin, D.; Povolotckaia, A.; Kalinichev, A.; Povolotskiy, A.; Borisov, E.; Moskovskiy, M.; Gulyaev, A.; Lavrov, A.; Izmailov, A. Complex Spectroscopic Study for Fusarium Genus Fungi Infection Diagnostics of “Zalp” Cultivar Oat. Agronomy 2021, 11, 2402. [Google Scholar] [CrossRef]
- Bashilov, A.M.; Efremenkov, I.Y.; Belyakov, M.V.; Lavrov, A.V.; Gulyaev, A.A.; Gerasimenko, S.A.; Borzenko, S.I.; Boyko, A.A. Determination of Main Spectral and Luminescent Characteristics of Winter Wheat Seeds Infected with Pathogenic Microflora. Photonics 2021, 8, 494. [Google Scholar] [CrossRef]
- Belyakov, M.V.; Moskovskiy, M.N.; Litvinov, M.A.; Lavrov, A.V.; Khamuev, V.G.; Efremenkov, I.Y.; Gerasimenko, S.A. Method of Optical Diagnostics of Grain Seeds Infected with Fusarium. Appl. Sci. 2022, 12, 4824. [Google Scholar] [CrossRef]
- Pedrós, R.; Moya, I.; Goulas, Y.; Jacquemoud, S. Chlorophyll Fluorescence Emission Spectrum inside a Leaf. Photochem. Photobiol. Sci. 2008, 7, 498. [Google Scholar] [CrossRef]
- Simons, R.M.; Blatchley III, E.R.; Linden, K.G. Far UV-C in the 200-225 nm Range, and Its Potential for Disinfection Applications. Available online: https://www.iuva.org/resources/covid-19/Far%20UV-C%20in%20the%20200%20_%20225%20nm%20range,%20and%20its%20potential%20for%20disinfection%20applications.pdf. (accessed on 30 August 2022).
- Karadağ, K.; Emin Tenekeci, M.; Taşaltın, R.; Bilgili, A. Detection of pepper fusarium disease using machine learning algorithms based on spectral reflectance. Sustain. Comput. Inform. Syst. 2020, 28, 100299. [Google Scholar] [CrossRef]
- Sanjay, M.; Kalpana, B. Early Mass Diagnosis of Fusarium Wilt in Banana Cultivations using an E-Nose Integrated Autonomous Rover System. Int. J. Appl. Sci. Biotechnol. 2017, 5, 5–261. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, D.; Du, S.; Huang, L.; Zhao, H.; Liang, D.; Gu, C.; Yang, X. A Rapidly Diagnosis and Application System of Fusarium Head Blight Based on Smartphone. In Proceedings of the 8th International Conference on Agro-Geoinformatics (Agro-Geoinformatics), Istanbul, Turkey, 16–19 July 2019; pp. 1–5. [Google Scholar]
- Zhang, D.; Wang, Z.; Jin, N.; Gu, C.; Chen, Y.; Huang, Y. Evaluation of Efficacy of Fungicides for Control of Wheat Fusarium Head Blight Based on Digital Imaging. IEEE Access 2020, 8, 109876–109890. [Google Scholar] [CrossRef]
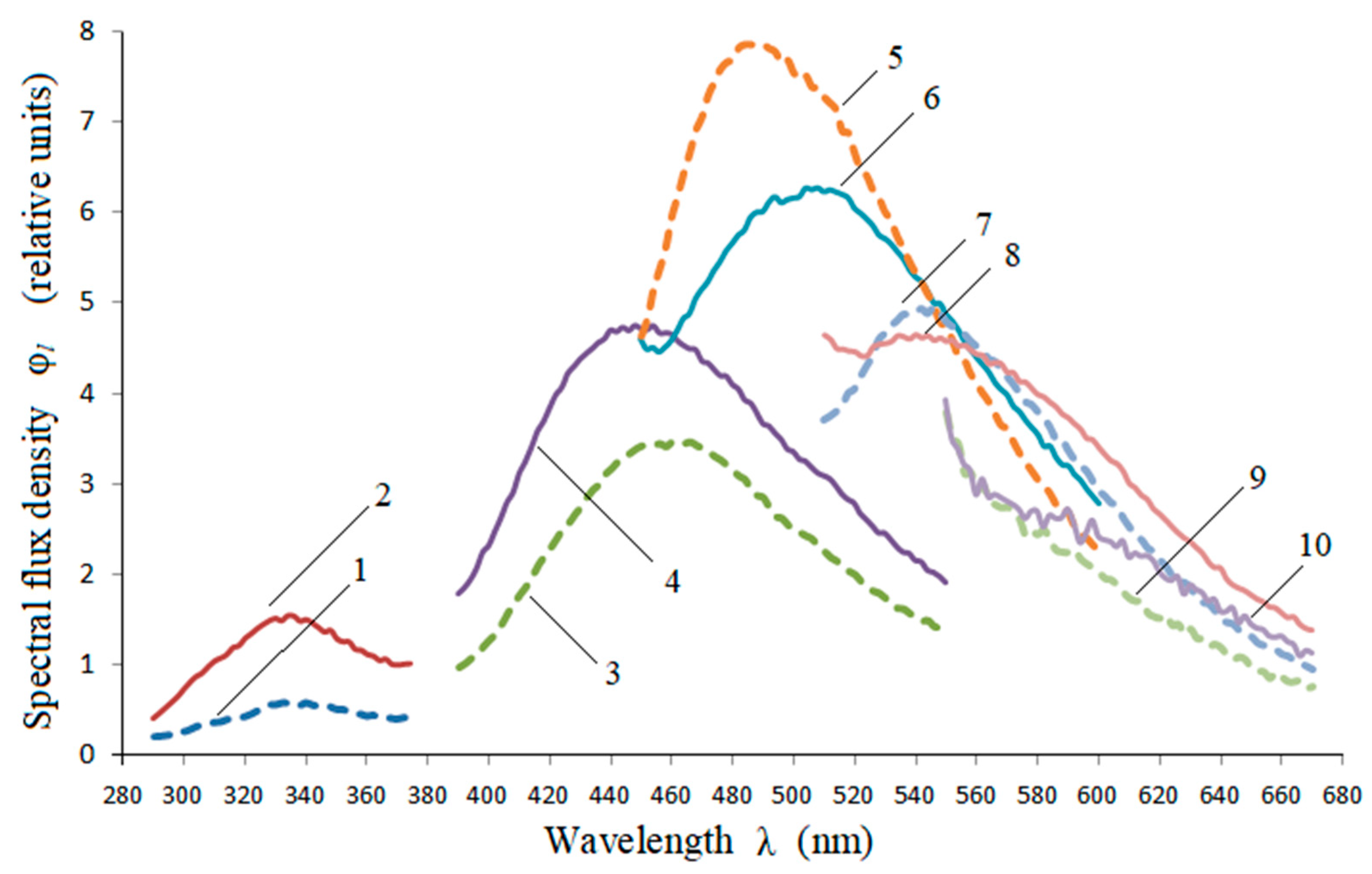
| Parameter | λe = 232 nm | λe = 362 nm | λe = 424 nm | λe = 485 nm |
|---|---|---|---|---|
| Wheat | ||||
| R2 | 0.94 | 0.84 | 0.83 | 0.91 |
| SΦ | 1.50 | 0.40 | 0.13 | 0.09 |
| Barley | ||||
| R2 | 0.94 | 0.95 | 0.63 | 0.29 |
| SΦ | 2.06 | 1.44 | 0.29 | 0.11 |
| Oats | ||||
| R2 | 0.50 | 0.62 | 0.88 | 0.97 |
| SΦ | 1.33 | 0.19 | 0.31 | 0.17 |
| LED Name [Data Source] | Radiation Wavelength λ, nm | Forward Current I, mA | Forward Voltage U, V | Angle of Radiation φ, ˚ | Flow of Radiation Φe, W |
|---|---|---|---|---|---|
| VLMU3510-365-130 | 362 | 700 | 4.0 | 160 | 0.95 |
| NICHIA NCSU276A | 365 | 700 | 4.0 | 160 | 0.9 |
| NICHIA NVSU233B | 365 | 1400 | 3.85 | 160 | 1.4 |
| NICHIA NCSU033C | 365 | 700 | 4.0 | 160 | 0.75 |
| CREELED424 | 424 | 700 | 4.0 | 150 | 0.8 |
| LHUV-0420-0550 | 420 | 1000 | 3.5 | 150 | 1.0 |
| LZ4-00UA00-00U6 | 400 | 1000 | 3.9 | 120 | 5.45 |
| XPEBBL-L1-0000-00201 | 485 | 1000 | 3.5 | 140 | 3.5 |
| MLEBLU-A1-0000-000T01 | 480 | 350 | 3.5 | 150 | 0.8 |
| L1CUBLU100000000 | 485 | 350 | 3.5 | 150 | 0.034 |
| Sources of Radiation | Kls |
|---|---|
| VLMU3510-365-130 | 0.98 |
| NICHIA NCSU276A | 0.97 |
| NICHIA NVSU233B | 0.98 |
| NICHIA NCSU033C | 0.98 |
| CREELED424 | 0.95 |
| LHUV-0420-0550 | 0.94 |
| LZ4-00UA00-00U6 | 0.73 |
| XPEBBL-L1-0000-00201 | 0.90 |
| MLEBLU-A1-0000-000T01 | 0.93 |
| L1CUBLU100000000 | 0.92 |
| Photodiode Name [Data Source] | Spectral Range λ, nm | Sensitivity S, A/W | Dark Current Id, fA |
|---|---|---|---|
| VEMD5510CF | 440–620 | 0.2 | 2 × 105 |
| FGAP71 | 150–550 | 0.12 | 4 × 104 |
| Hamamatsu S8265 | 340–720 | 0.3 | 2 × 104 |
| Hamamatsu S1133 | 320–730 | 0.4 | 1 × 104 |
| Hamamatsu S7686 | 480–660 | 0.38 | 2 × 104 |
| BWR21R | 420–675 | 0.009 | 2 × 107 |
| VBPW34S | 430–1100 | 0.004 | 3 × 107 |
| VEMD5510CF | 440–620 | 0.2 | 2 × 105 |
| SFH 2711 | 470–670 | 0.001 | 2 × 105 |
| SLD-70 BG2A | 400–700 | 0.001 | 1 × 108 |
| Radiation Receiver | kld |
|---|---|
| VEMD5510CF | 0.68 |
| FGAP71 | 0.66 |
| Hamamatsu S8265 | 0.75 |
| Hamamatsu S1133 | 0.79 |
| Hamamatsu S7686 | 0.49 |
| BWR21R | 0.87 |
| VBPW34S | 0.26 |
| VEMD5510CF | 0.76 |
| SFH 2711 | 0.79 |
| SLD-70 BG2A | 0.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moskovskiy, M.N.; Belyakov, M.V.; Dorokhov, A.S.; Boyko, A.A.; Belousov, S.V.; Noy, O.V.; Gulyaev, A.A.; Akulov, S.I.; Povolotskaya, A.; Efremenkov, I.Y. Design of Device for Optical Luminescent Diagnostic of the Seeds Infected by Fusarium. Agriculture 2023, 13, 619. https://doi.org/10.3390/agriculture13030619
Moskovskiy MN, Belyakov MV, Dorokhov AS, Boyko AA, Belousov SV, Noy OV, Gulyaev AA, Akulov SI, Povolotskaya A, Efremenkov IY. Design of Device for Optical Luminescent Diagnostic of the Seeds Infected by Fusarium. Agriculture. 2023; 13(3):619. https://doi.org/10.3390/agriculture13030619
Chicago/Turabian StyleMoskovskiy, Maksim N., Mikhail V. Belyakov, Alexey S. Dorokhov, Andrey A. Boyko, Sergey V. Belousov, Oleg V. Noy, Anatoly A. Gulyaev, Sergey I. Akulov, Anastasia Povolotskaya, and Igor Yu. Efremenkov. 2023. "Design of Device for Optical Luminescent Diagnostic of the Seeds Infected by Fusarium" Agriculture 13, no. 3: 619. https://doi.org/10.3390/agriculture13030619
APA StyleMoskovskiy, M. N., Belyakov, M. V., Dorokhov, A. S., Boyko, A. A., Belousov, S. V., Noy, O. V., Gulyaev, A. A., Akulov, S. I., Povolotskaya, A., & Efremenkov, I. Y. (2023). Design of Device for Optical Luminescent Diagnostic of the Seeds Infected by Fusarium. Agriculture, 13(3), 619. https://doi.org/10.3390/agriculture13030619








