Abstract
Eastern redbud, Cercis canadensis L., is a popular ornamental tree in the U.S. and has flower colors of purple-type, red-type, and white-type. Most redbuds cultivars have purple-type flowers. Red-types and white-types are much less common. These unusual flower colors have become an important trait for incorporation into novel redbud cultivars. Eastern redbud seedlings require 3–5 years before blooming, making flower color selection a slow and expensive process. The ability to select seedlings for flower color type would significantly increase the efficiency of the breeding process. Redbud flower color is dominated by anthocyanin content; leaf petioles often show visible pink to purple color, indicating the potential presence of anthocyanin. In this study, anthocyanin profiles of 14 cultivars and 25 progenies of ‘Appalachian Red’ (red-type) × ‘Oklahoma’ (purple-type) were determined using HPLC. The petiole anthocyanin profiles were strongly indicative of plant flower-color types. Both peonidin-3-glucoside and malvidin-3-glucoside were dominant in petioles from all purple-type plants. In contrast, malvidin-3-glucoside was absent from petioles of red-type plants, and neither peonidin-3-glucoside nor malvidin-3-glucoside was detected among white-type cultivars. These results indicate that the presence or absence of peonidin-3-glucoside and malvidin-3-glucoside in petioles can be a physiological marker for identifying redbud flower color types.
1. Introduction
Redbud (Cercis spp. L.) is a popular small ornamental tree of the legume family with a variety of garden uses [1,2,3]. Redbud was the fifth most valuable deciduous flowering tree in the United States in 2019, occupying ≈8% of sale value for all deciduous flowering trees [4]. The economic value of redbud increased in the U.S. from USD 21.8 to USD 28.4 million from 2009 to 2019 [4,5,6]. The eastern redbud has a wide native range in eastern North America, extending from Toronto, Canada, through the southeastern United States and as far south as northeastern Mexico [7]. Among all Cercis species, the eastern redbud (C. canadensis L.) fills a unique niche in the U.S. market as a North American native plant with diverse ornamental traits. Showy spring blooms, heart-shaped foliage, adaptability to diverse environmental conditions, and a variety of cultivars exhibiting additional ornamental characteristics have made the eastern redbud popular in the North American nursery industry [3].
Because of its wide habitat range and long history of human selection, eastern redbud cultivars show a wide diversity [7,8,9] in traits such as flower color [10], tree architecture [2,11], leaf color [2,11], and environment tolerances [12]. Flower traits are one of the most important ornamental traits for Cercis cultivars. Based on the color type, most redbud cultivars are purple-type flowers, while red-types and white-types are much less common [10]. The most common flower color among eastern redbud cultivars is pink–purple (or light purple-type), as can be found in ‘Ace of Hearts’ and ‘Traveller’ (Figure 1D) [10]. Some cultivars, such as ‘Merlot’ (Figure 1G), have a darker purple-type flower color [10]. The red-type flower color is a comparatively rare trait that has been reported in two eastern redbud cultivars: ‘Crosswicks Red’, which is a pink red-type (described as red-pink in [10]), and ‘Appalachian Red’ (Figure 1C), which is a dark red-type (described as red-purple in [10]). Some ecotypes or cultivars, such as C. canadensis var. alba (Figure 1A), ‘Texas White’, and ‘NC 2017-108’ (White Pom Poms) have white flower color. The unusual flower colors have become an important trait for incorporation into novel redbud cultivars. As eastern redbud seedlings require 3–5 years before blooming, flower color selection is a slow and expensive process [2]. The ability to select redbuds at the seedling stage for flower color would significantly increase the efficiency of the breeding process.

Figure 1.
Flowers of C. canadensis cultivars or genotypes: (A) ‘var alba’ (white-type), (B) ‘Crosswick’s Red’ (red-type), (C) ‘Appalachian Red’ (red-type), (D) ‘Traveller’ (purple-type), (E) ‘Ruby Falls’ (purple-type), (F) ‘Forest Pansy’ (purple-type), and (G) ‘Oklahoma’ (purple-type).
Eastern redbud flower color is linked with anthocyanin pigment composition of the floral parts, including calyx, petiole, banner, wings, and keel [10]. In previous research, nine anthocyanin pigments in redbud flowers were identified in 12 cultivars/genotypes using high-performance liquid chromatography (HPLC) [10]. Among the tested cultivars, the white-type flowers showed absent or deficient content of the nine anthocyanins in their flowers [10] and had been reported as a recessive gene [11]. The red-flowered redbud cultivars were dominant in malvidin 3,5-diglucoside and the purple-flowered cultivars were dominant in both cyanidin 3-glucoside and malvidin-3-glucoside. Both malvidin-3-glucoside and peonidin-3-glucoside were detected in all purple-type and red-type flowers but amounts as a percent of total anthocyanins were significantly lower in flower tissue of red-type cultivars (0 to 1.1%) than in purple-type cultivars (1 to 19.7%) [10].
Redbud leaf petioles often show visible pink to purple color, even in seedlings (Figure 2), indicating the potential presence of anthocyanin pigments. Anthocyanin profiles of petioles could be a physiological marker for flower color-type breeding in early selection if different flower color-type plants show unique anthocyanin profiles as is found in floral tissue. A selection based on petiole tissue instead of flower color could significantly increase the efficiency of the breeding process because petiole tissue is available most of the year from the seedling to the adult phases. To investigate the potential of using the anthocyanin content of the petiole as a physiological marker for flower color, anthocyanin profiles of petiole tissue for different flower color-types are needed.
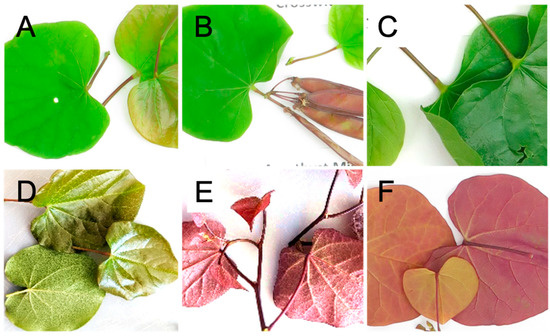
Figure 2.
Leaves and petioles of C. canadensis cultivars: (A) ‘Appalachian Red’, (B) ‘Crosswick’s Red’, (C) ‘Traveller’, (D) ‘Oklahoma’, (E) ‘Ruby Falls’, (F) Flame Thrower.
Understanding the relationship between petiole anthocyanin profile and the flower color is critical for developing the physiological marker to predict flower color for Cercis Breeding. In this study, peonidin-3-glucoside and malvidin-3-glucoside content in petiole tissues of 14 cultivars and 22 progenies of ‘Appalachian Red’ (red-type) × ‘Oklahoma’ (purple-type) with segregated flower color-types were determined by HPLC. The relationship of anthocyanin content to flower color-type type and leaf color of tested plants was also investigated.
2. Materials and Methods
2.1. Plant Material
The study included a total of 14 C. canadensis cultivars and varieties (Table 1), including 10 purple-types (‘Pink Pom Poms’, ‘Oklahoma’, ‘Traveller’, ‘Gold Crown’, ‘Hearts of Gold’, Golden Falls®, ‘Merlot’, ‘Forest Pansy’, ’Ruby Falls’, and Flamethrower®), two red-types (‘Appalachian Red’ and ‘Crosswicks Red’), and two white-types (C. canadensis var. alba and ‘Texas White’) [13]. A total of 25 hybrid progenies of ‘Appalachian Red’ × ‘Oklahoma’, including two F1 progenies (purple-types), and 23 F2 progenies (12 purple-types and 11 red-types), were also included in the study. Leaf samples of plant material were collected in Raleigh, NC, from the JC Raulston Arboretum and NC State’s Main Campus and in Jackson Spring, NC, from the Sandhills Research Station as listed in Table 1. Stems with 4 or more attached leaves were cut from plants, placed in plastic bags, and transported cold to Kannapolis, NC. Leaves from the most recently fully expanded stage to the 4th leaf on the stem were used for anthocyanin analysis (Figure 2). Hybridizations of potted parent plants were conducted in the North Carolina State University (NCSU) Horticulture Field Lab (HFL) greenhouse in Raleigh, NC, USA, in 2013. Seeds were scarified by submersion in concentrated (18.4 M) sulfuric acid for 30 min, with gentle stirring every 2 min for the first 10 min of the treatment, followed by stirring at 5 min intervals thereafter [14]. The acid-scarified seeds were then rinsed and cold-stratified at 4 °C for approximately two months before being sown. F1 plants were grown in the greenhouse in 2014 until being transplanted into a field isolation block in the winter of 2014 at the Sandhills Research Station, Jackson Springs, NC, USA.

Table 1.
Eastern redbud (C. canadensis L.) plant material, flower types, and sources.
To create the F2 generation, multiple selected F1 trees were dug from the field, planted in a 95-L pot, and brought to the greenhouse for crossing. In March 2017, branches containing flower buds were cut from multiple F1 trees in the field and brought to the greenhouse for flowering. Mixed pollen from the cut branches was bulked and used to pollinate the multiple selected F1 trees. F2 seed was harvested in the fall of 2017, acid-scarified, cold-stratified, and then sown in January 2018. Resultant seedlings were grown in the greenhouse in individual pots to a height of approximately 15 cm and transplanted to the field in May 2018 at the Sandhills Research Station, Jackson Springs, NC (USA). Trees were evaluated for flower type in March 2022, and petiole tissues were collected in June 2022. All petiole tissues of hybrids were collected on the same day, and petiole tissues of cultivars were collected the next day.
2.2. HPLC Analysis
For each collected cultivar or hybrid (Table 1), petiole samples were collected and tested in three independent HPLC analyses. Petioles were cut into 5 mm sections and anthocyanins extracted with acidified methanol (formic acid:methanol:deionized water, 3:60:37 v/v/v) using 0.2 g tissue per 1.5 mL solvent. Samples were vortexed for 30 sec, sonicated at room temperature for 20 min (Branson ultrasonicator, Fisher Scientific), and then centrifuged at 10,600× g for 20 min at 4 °C. Supernatant aliquots of 1 mL were filtered through 0.2 µm PTFE membranes (Fisher Scientific, Pittsburg, PA, USA) into 2 mL amber vials (Agilent), flushed with nitrogen gas, and capped. Filtered samples (30 µL) were injected into a Hitachi LaChrom HPLC (Hitachi Ltd., Tokoyo, Japan), equipped with a UV-VS diode array detector (DAD), controlled temperature auto sampler (4 °C), and column compartment (30 °C). D-2000 software (Hitachi Ltd., Tokoyo, Japan) was used as the system run controller and for data processing. Anthocyanin separation was performed using a using a reversed phase C18 column (Synergi 4 µ Hydro-RP 80 Å, 6 × 250 um, Phenomenex, Torrance, CA, USA). The mobile phase consisted of 5% formic acid in water (A) and 100% methanol (B) with a flow rate of 1 mL/min using a step gradient of 0 min, 10% B; 5 min, 15% B; 15 min, 20% B; 20 min, 25% B; 25 min, 30% B; 45 min, 60% B; 47 min, 10% B; 60 min, 10% B. Compound concentrations were estimated using standard curves generated by injecting 5 µL of 0.0625–0.5 mg/mL preparations of cyanidin 3-gluocoside, cyanidin 3,5-diglucoside, malvidin-3-glucoside, peonidin-3-glucoside, peonidin 3,5-diglucoside, and malvidin 3,5-diglucoside as external standards (Chromadex, Irvine, CA, USA; Sigma, St. Louis, MO, USA). Compound identification was performed based on retention time compared to authentic standards and previously published reports [15]. Each sample was run in duplicate, and content was reported as mg of cyanidin 3-glucoside. Sums of anthocyanins were calculated to obtain total anthocyanin content, and each anthocyanin calculated as a percent of the total anthocyanin content. Estimated concentrations of malvidin-3-glucoside, peonidin-3-glucoside, and the sum of the two anthocyanins were translated into mg/100 g fwt, and the result was listed in Table 2. The malvidin-3-glucoside, peonidin-3-glucoside, and sum of the two anthocyanins of petiole tissue from white-type, purple-type, and red-type flower plants were compared by Wilcoxon Rank Sum t-Test, and the results were listed in Table 3.

Table 2.
Average Content of Peonidin-3-Glucoside and Malvidin-3-Glucoside in Petiole Tissue of Eastern Redbud (Cercis canadensis L.) determined by HPLC.

Table 3.
Concentrations of peonidin-3-glucoside and malvidin-3-glucoside in petiole tissues of eastern redbud (Cercis canadensis L.) with purple-type, red-type, and white-type flowers.
3. Results
3.1. Anthocyanin Content and Profile in Petiole Tissues
The dominant anthocyanins found in petioles of redbud leaves were peonidin-3-glucoside and malvidin-3-glucoside. Presence and concentrations of the two anthocyanins varied widely among petiole tissues of the selected plant material (Table 2). Peonidin-3-glucoside values ranged from non-detectable (<0.001 mg/100 g fwt) in white-flowered genotypes to 11.74 mg/100 g fwt in purple-flowered genotypes, and the average concentration among samples was 2.01 mg/100 g fwt. Malvidin-3-glucoside values ranged from non-detectable in white or red-flowered genotypes to 13.33 mg/100 g fwt in purple-flowered genotypes, with an average concentration among samples of 1.25 mg/100 g fwt. The average total anthocyanin content among all tested samples was 3.26 mg/100 g fwt. In white-type plants, anthocyanins were below detection limits. Flame Thrower® had the highest total anthocyanin content 22.64 mg/100 g fwt. Average content with standard error of both peonidin-3-glucoside and malvidin-3-glucoside contents as well as the sum total of these two anthocyanins are listed for each cultivar or hybrid in Table 2.
3.2. Relationship of Anthocyanin Content in Petiole Tissue to Flower Color-Type
The presence and concentration of the two anthocyanins in petiole tissue were strongly correlated to the flower color-type (Table 3). Peonidin-3-glucoside concentration in petiole tissues of purple-type, red-type, and white-type flower plants was 2.48, 2.17, and 0 mg/100 g fwt respectively. The differences in petiole peonidin-3-glucoside concentration of white-type compared to red-purple or purple-red were statistically significant (p < 0.01), but were not significantly different between the purple-types and red-types using the Wilcoxon Rank Sum t-Test. Levels of malvidin-3-glucoside in petiole tissue showed distinct differences between flower color-types. Malvidin-3-glucoside content in petiole tissues of purple-type, red-type, and white-type flower plants were 2.36, 0, and 0 mg/100 g fwt respectively. The difference between the purple-type and other two color-types was statistically significant (p < 0.01) by Wilcoxon Rank Sum t-Test. The results are shown in Table 3.
3.3. Relationship of Anthocyanin Content in Petiole Tissue to Foliage Color
The total concentration of the two anthocyanins in petiole tissues was strongly correlated to purple foliage color (Table 4). In this study, four out of the 39 cultivars and hybrids used had purple coloration in at least some of their leaves, including ‘Forest Pansy’, ‘Merlot’, ‘Ruby Falls’, and Flame Thrower®, and all four cultivars had purple-type flower color. The petiole tissue of these four cultivars had peonidin-3-glucoside, malvidin-3-glucoside, and total anthocyanin concentrations that were significantly higher (p < 0.01) than the green-leaved cultivars and hybrids, as determined by independent Wilcoxon Rank Sum t-Test (Table 4). The concentration of peonidin-3-glucoside in petiole tissues of the plants with purple foliage versus non-purple foliage was 8.58 and 1.28 mg/100 g fwt. The concentration of malvidin-3-glucoside in petiole tissues of the plants with purple foliage versus non-purple foliage was 8.28 and 0.45 mg/100 g fwt. The total concentration of peonidin-3-glucoside and malvidin-3-glucoside in petiole tissues of the purple leaf cultivars and non-purple leaf plants are 16.46 and 1.73 mg/100 g fwt. Significant differences in malvidin-3-glucoside and peonidin-3-glucoside content in petiole tissue between red and no-purple leaf (including green and yellow leave cultivar) plants were observed (p < 0.01).

Table 4.
Concentration of peonidin-3-glucoside, malvidin-3-glucoside, and total anthocyanin in petiole tissue of Eastern redbud (Cercis canadensis L.) for plants with different foliage colors.
Unlike purple foliage color, anthocyanin content of petiole tissue was not statistically correlated to the yellow foliage color in this study (Table 5). Only three of the 39 cultivars and hybrids in this study had yellow coloration in at least some of their leaves, including ‘Hearts of Gold’, Golden Falls®, and Flame Thrower®. In addition, Flame Thrower® is a cultivar that combines yellow and purple foliage traits, resulting in orange coloration in some of its leaves. The concentrations of malvidin-3-glucoside in petiole tissues of the yellow leaf cultivars versus the non-yellow-leaf were 5.76 and 1.10 mg/100 g fwt, respectively. However, if Flame Thrower® is excluded, the malvidin-3-glucoside in petiole tissue of the yellow foliage cultivars averages 0.71 mg/100 g fwt, indicating that Flame Thrower® is an outlier that greatly impacted the analysis. Similar phenomena were observed in the peonidin-3-glucoside and total anthocyanin contents (Table 5). With the small sample size and high impact of a single data point (Flame Thrower®), the correlation of anthocyanin content in petiole tissue of plants with yellow versus non-yellow foliage remains unclear.

Table 5.
Concentration of malvidin-3-glucoside, peonidin-3-glucoside, and total anthocyanin in petiole tissue of Eastern redbud (Cercis canadensis L.) with yellow and non-yellow foliage.
4. Discussion
4.1. Anthocyanin Profile of Petiole Tissue as a Physiological Marker for Flower Color-Types
Results showed that the anthocyanin profile of petiole tissue could be used as a physiological marker for flower color-types in eastern redbud selection. In the present study, the presence and absence of peonidin-3-glucoside and malvidin-3-glucoside in the petiole tissue correlated with flower color-type without exception. Peonidin-3-glucoside is present in the petiole tissue of all purple-type and red-type flower plants and is absent from white-type flower plants. In contrast, malvidin-3-glucoside is present in the petiole tissues of all purple-type flower plants and is absent from red-type and white-type flower plants, again with almost no exceptions (Table 2 and Table 3). The only minor outlier is that one of the three HPLC replicates of ‘Texas White’ samples showed 0.01 mg/100 g of malvidin-3-glucoside and peonidin-3-glucoside. This result could have been caused by mechanical error or minor contamination in sample preparation. However, this error does not impact flower color-type prediction as the measured content here is drastically lower than every tested sample from purple-type flower plants. The average petiole malvidin-3-glucoside content of purple-type flower plants was 2.36 mg/100 g fwt, and the lowest content was 0.12 mg/100 g fwt (discovered in Pink Pom Pom). Thus, we recommend that the content of malvidin-3-glucoside and peonidin-3-glucoside in petiole tissue can be used as a physiological marker for flower color-type.
Physiological markers can be developed and used in plant breeding processes when an easily measurable physiological index is discovered to have a solid correlation to a trait for which phenotyping is complicated and/or expensive. For example, the seed melatonin content of pepper, Capsicum annuum, can be used as a physiological marker for selecting higher chilling stress tolerance genotypes [16]. Several physiological traits, including the whole-plant stable isotope ratios of nitrogen (δ15N) and shoot glutamine synthetase activity, can be used to select salt-tolerant durum wheat, Triticum durum, genotypes [17]. For ornamental trees, phenotyping flower traits is typically costly in terms of time and resources due to long juvenile phases. Molecular markers are recommended for earlier selection to conquer this problem if whole genome sequence data and breeding resources are available, such as with reblooming markers for lilacs [18] and hydrangeas [19]. However, accessibility and resources for developing molecular markers for many perennial ornamental plants are still limited. Thus, any available physiological marker could be very useful.
The present study shows a method for developing a physiological marker for flower color for a perennial ornamental tree species by analysis of the anthocyanin profile of non-flower tissue, which could be used in early selection. Anthocyanin content from various plant parts has been recommended as an indicator of flower color in common primrose, Primula vulgaris [20]. In corn, abundant anthocyanins in non-grain portions of the plant were found in the dark purple kernel genotype plants, and the visible differences in the color intensity of leaf sheath, anther, husk, and tassel color are linked to their genotypes [21]. In eastern redbud, it has already been shown that cotyledon color of the juvenile phase seedling can be used in the primary selection for gold-colored foliage in the adult phase [11]. However, differences in anthocyanin content in petiole tissue are not directly visible by human eye. This study used HPLC analysis to develop anthocyanin profiles of petiole tissue and found that anthocyanin content in these tissues was highly correlated with flower color-type. To our knowledge, this is the first report to use an anthocyanin profile of a non-reproduction organ to predict flower color-types for a Cercis species.
4.2. Relationship of Anthocyanin Profile of Petiole Tissue to Foliage Color
Results showed anthocyanin content of petiole tissue was statistically different between redbud plants with and without purple foliage. Unsurprisingly, cultivars with purple foliage had significantly higher anthocyanin content in their petiole tissue than in cultivars without any purple foliage. The inherited trait for purple leaves in C. canadensis is a recessive single locus [21] that results in the accumulation of anthocyanins in the leaf tissues, including the petiole. Thus, the accumulation of different anthocyanins could result in different types of purple foliage. However, because all tested cultivars with purple foliage also had purple-type flowers, it is still unknown if peonidin-3-glucoside and malvidin-3-glucoside composition in leaf tissue will contribute to visible differences. Early research in the color of red-type flower redbud petal tissue showed that a higher a/b ratio in the color measurement of Hunter coordinate L*a*b*, and malvidin-3-glucoside pigment itself also showed a significantly lower a/b ratio in the color measurement [10]. In the color measurement of the Hunter coordinate L*a*b* system, the a/b ratio could reflect the spectrum from purple and red to orange. Investigation on whether the absence of malvidin-3-glucoside results in visible color differences and ornamental for cultivars with purple foliage is still needed.
4.3. Relationship of Anthocyanin Profile of Petiole Tissue to Flower Color
Understanding the anthocyanidin contents of different redbud tissue might provide an extra economic value for this ornamental plant [22]. The anthocyanidins most common in plants include cyanidin, pelargonidin, and delphinidin. The addition of a methyl group to cyanidin and delphinidin yields the pigments peonidin, petunidin, and malvidin [23]. Redbud flowers are edible [22]. A strong body of research indicates the importance of anthocyanins for multiple human health properties [24]. Additionally, anthocyanins are valuable as natural colorants and aid in efficiency in semiconductor cells [25].
A pleiotropic relationship between vegetative and floral organs in dicots has been proposed [26], where genes controlling color in one organ are conserved in other organs. This effect appears evident in white-flowered redbuds, where leaf petioles also have little to no detectable anthocyanin. What is unclear is the relationship between petiole color and flower color of red or purple-flowered redbuds. While the pigment malvidin-3,5-diglucoside dominates in red-colored redbud flowers, peonidin-3-glucoside was dominant in petiole tissue. Although in small amounts in red flowers of redbud, malvidin-3-glucoside was distinctly absent in the petioles. Peonidin-3-glucoside and malvidin-3-glucoside peaks were persistent in all petiole tissue from purple-flowered redbud genotypes.
Malvidin-3-glucoside contributes about 6 to 20% of the anthocyanins found in purple redbud flowers [10], and this pathway may be dominant in both vegetative and floral tissue of this redbud type. In purified extracts, peonidin-3-glucoside imparts a red to red-blue color, depending on concentration, malvidin-3-glucoside imparts a blue-red color, and cyanidin 3-glucoside a bright red color [27]. Purple-type flowers of redbud have twice as much cyanidin 3-glucoside than malvidin-3-glucoside and this mix of red and blue-red may impart the visual purple color. Malvidin 3,5-diglucoside imparts a lighter blue-red color than malvidin-3-glucoside [27]. Peonidin-3-glucoside amounts are similar and of low amounts (about 10% of the dominant pigments) in both red- and purple-flower type redbuds.
The lack of malvidin-3-glucoside in petioles of red-flowered redbuds may be indicative of temperature, light, or changes in enzyme activity on the anthocyanin biosynthesis between floral and vegetative tissues. The absence of detectable malvidin-3-glucoside in petioles is serendipitous for early selection of red-flowered redbud seedlings.
5. Conclusions
- Peonidin-3-glucoside and malvidin-3-glucoside content in petiole tissue could be used as a physiological marker predicting flower-color of eastern redbud.
- Both peonidin-3-glucoside and malvidin-3-glucoside were dominant in petioles from purple-type flower plants.
- Only peonidin-3-glucoside was detected in petioles from red-type flower plants.
- Neither peonidin-3-glucoside nor malvidin-3-glucoside was detected in petioles from white-type flower plants.
Author Contributions
Conceptualization, H.C. and P.P.-V.; methodology, P.P.-V., G.M. and J.S.; validation, H.C. and P.P.-V.; formal analysis, H.C.; investigation, H.C. and P.P.-V.; data curation, H.C., P.P.-V., G.M. and J.S.; writing—original draft preparation, H.C. and E.M.; writing—review and editing, H.C. and E.M.; visualization, H.C.; supervision, H.C. and P.P.-V.; project administration, H.C. and P.P.-V.; funding acquisition, H.C. and P.P-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially supported by funds from Hatch Research Capacity Funds USDA-02724 Improving and maintaining postharvest attributes of fresh produce for consumer health.
Acknowledgments
The authors thank Dennis Werner for developing the plant materials used in this study and Erin Deaton for sample preparation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Geneve, R.L. Eastern Redbud (Cercis canadensis L.) and Judas Tree (Cercis siliquastrum L.). In Trees III; Springer: Berlin/Heidelberg, Germany, 1991; pp. 142–151. [Google Scholar]
- Chen, H.; Werner, D.J. Inheritance of Compact Growth Habit, and Investigation of Linkage to Weeping Architecture and Purple Leaf Color in Eastern Redbud (Cercis canadensis L.). HortScience 2021, 1, 1513–1515. [Google Scholar] [CrossRef]
- Kidwell-Slak, D.L.; Pooler, M.R. A Checklist of Cercis (redbud) Cultivars. HortScience 2018, 53, 148–152. [Google Scholar] [CrossRef]
- Census of Horticultural Specialties for 2019. Available online: https://www.nass.usda.gov/Publications/AgCensus/2017/Online_Resources/Census_of_Horticulture_Specialties/hortic_1_0020_0021.pdf (accessed on 11 April 2019).
- Census of Horticultural Specialties for 2014. Available online: https://agcensus.library.cornell.edu/wp-content/uploads/2012-Census-of-Horticultural-Specialties-hortic_1_021_022.pdf (accessed on 29 May 2014).
- Census of Horticultural Specialties for 2007. Available online: https://agcensus.library.cornell.edu/wp-content/uploads/2007-Census-of-Horticultural-Specialties-hortic_1_020_021.pdf (accessed on 30 November 2007).
- Ony, M.A.; Nowicki, M.; Boggess, S.L.; Klingeman, W.E.; Zobel, J.M.; Trigiano, R.N.; Hadziabdic, D. Habitat Fragmentation Influences Genetic Diversity and Differentiation: Fine-scale Population Structure of Cercis canadensis (Eastern Redbud). Ecol. Evol. 2020, 10, 3655–3670. [Google Scholar] [CrossRef] [PubMed]
- Wadl, P.A.; Trigiano, R.N.; Werner, D.J.; Pooler, M.R.; Rinehart, T.A. Simple Sequence Repeat Markers from Cercis canadensis Show Wide Cross-Species Transfer and Use in Genetic Studies. J. Am. Soc. Hortic. Sci. 2012, 137, 189–201. [Google Scholar] [CrossRef]
- Roberts, D.J.; Werner, D.J. Genome Size and Ploidy Levels of Cercis (Redbud) Species, Cultivars, and Botanical Varieties. HortScience 2016, 51, 330–333. [Google Scholar] [CrossRef]
- Perkins-Veazie, P.; Ma, G.; Werner, D. Anthocyanin Pigments in Redbud (Cercis spp.) Flowers. J. Hortic. Sci. Res. 2017, 1, 13–18. [Google Scholar]
- Roberts, D.J.; Werner, D.J.; Wadl, P.A.; Trigiano, R.N. Inheritance and Allelism of Morphological Traits in Eastern Redbud (Cercis canadensis L.). Hortic. Res. 2015, 2, 15049. [Google Scholar] [CrossRef]
- Griffin, J.J.; Ranney, T.G.; Pharr, D.M. Heat and Drought Influence Photosynthesis, Water Relations, and Soluble Carbohydrates of Two Ecotypes of Redbud (Cercis canadensis). J. Am. Soc. Hortic. Sci. 2004, 129, 497–502. [Google Scholar] [CrossRef]
- Werner, D.J.; Snelling, L.K. ‘Ruby Falls’ and ‘Merlot’ Redbuds. HortScience 2010, 45, 146–147. [Google Scholar] [CrossRef]
- Bonner, V.; Stein, W.; Bonner, F.; Karrfalt, R.; Nisley, R.; Cercis, L. Woody-Plant Seed Manual: Redbud; USDA Forest Service: Washington, DC, USA, 2008; pp. 374–379.
- Grace, M.H.; Yousef, G.G.; Gustafson, S.J.; Truong, V.; Yencho, G.C.; Lila, M.A. Phytochemical Changes in Phenolics, Anthocyanins, Ascorbic acid, and Carotenoids Associated with Sweetpotato Storage and Impacts on Bioactive Properties. Food Chem. 2014, 145, 717–724. [Google Scholar] [CrossRef]
- Korkmaz, A.; Düver, E.; Szafrańska, K.; Karaca, A.; Ardıç, Ş.K.; Yakupoğlu, G. Feasibility of Using Melatonin Content in Pepper (Capsicum annuum) Seeds as a Physiological Marker of Chilling Stress Tolerance. Funct. Plant Biol. 2022, 49, 832–843. [Google Scholar] [CrossRef]
- Guellim, A.; Catterou, M.; Chabrerie, O.; Tetu, T.; Hirel, B.; Dubois, F.; Ben Ahmed, H.; Kichey, T. Identification of Phenotypic and Physiological Markers of Salt Stress Tolerance in Durum Wheat (Triticum durum Desf.) through Integrated Analyses. Agronomy 2019, 9, 844. [Google Scholar] [CrossRef]
- Chen, H.; Lattier, J.D.; Vining, K.; Contreras, R.N. Two SNP Markers Identified Using Genotyping-by-Sequencing are Associated with Remontancy in a Segregating F1 Population of Syringa meyeri ‘Palibin’× S. pubescens ‘Penda Bloomerang®’. J. Am. Soc. Hortic. Sci. 2020, 145, 104–109. [Google Scholar] [CrossRef]
- Wu, X.; Alexander, L.W. Genome-Wide Association Studies for Inflorescence Type and Remontancy in Hydrangea macrophylla. Hortic. Res. 2020, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Ünal, M.; Yentür, S.; Cevahir, G.; Sarsağ, M.; Kösesakal, T. Physiological and Anatomical Investigation of Flower Colors of Primula vulgaris L. Biotechnol. Biotechnol. Equip. 2003, 17, 102–108. [Google Scholar] [CrossRef]
- Paulsmeyer, M.N.; Vermillion, K.E.; Juvik, J.A. Assessing the Diversity of Anthocyanin Composition in Various Tissues of Purple Corn (Zea mays L.). Phytochemistry 2022, 201, 113263. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, A.I.; Gostin, A.I. Safety of Edible Flowers. Regulating Safety of Traditional and Ethnic Foods; Academic Press: Cambridge, MA, USA, 2016; pp. 395–419. [Google Scholar]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of Plant Pigments: Anthocyanins, Betalains and Carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Alappat, B.; Alappat, J. Anthocyanin Pigments: Beyond Aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef]
- Khammee, P.; Unpaprom, Y.; Whangchai, K.; Ramaraj, R. Comparative Studies of the Longan Leaf Pigment Extraction as a Photosensitizer for Dye-Sensitized Solar Cells’ Purpose. Biomass Convers. Biorefin. 2022, 2, 1619–1626. [Google Scholar] [CrossRef]
- Armbruster, W.S. Can Indirect Selection and Genetic Context Contribute to Trait Diversification? A Transition-Probability Study of Blossom-Colour Evolution in Two Genera. J. Evol. Biol. 2002, 15, 468–486. [Google Scholar] [CrossRef]
- Han, F.L.; Xu, Y. Effect of the Structure of Seven Anthocyanins on Self-Association and Colour in an Aqueous Alcohol Solution. S. Afr. J. Enol. Vitic. 2015, 36, 105–116. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).