Establishment of DNA Molecular Fingerprint of Caladium Core Collections
Abstract
1. Introduction
2. Results
2.1. Polymorphic Information of EST-SSR Markers
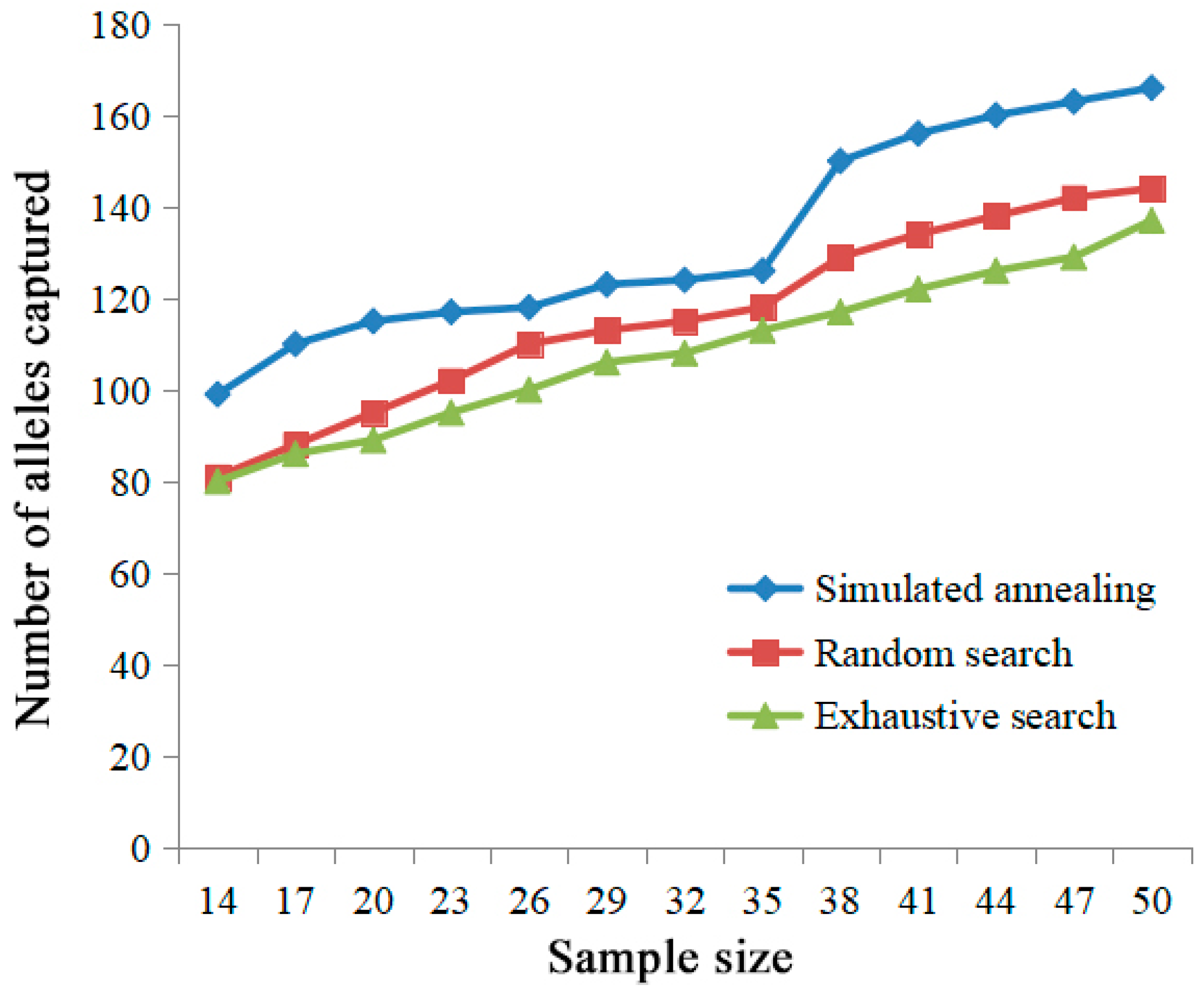
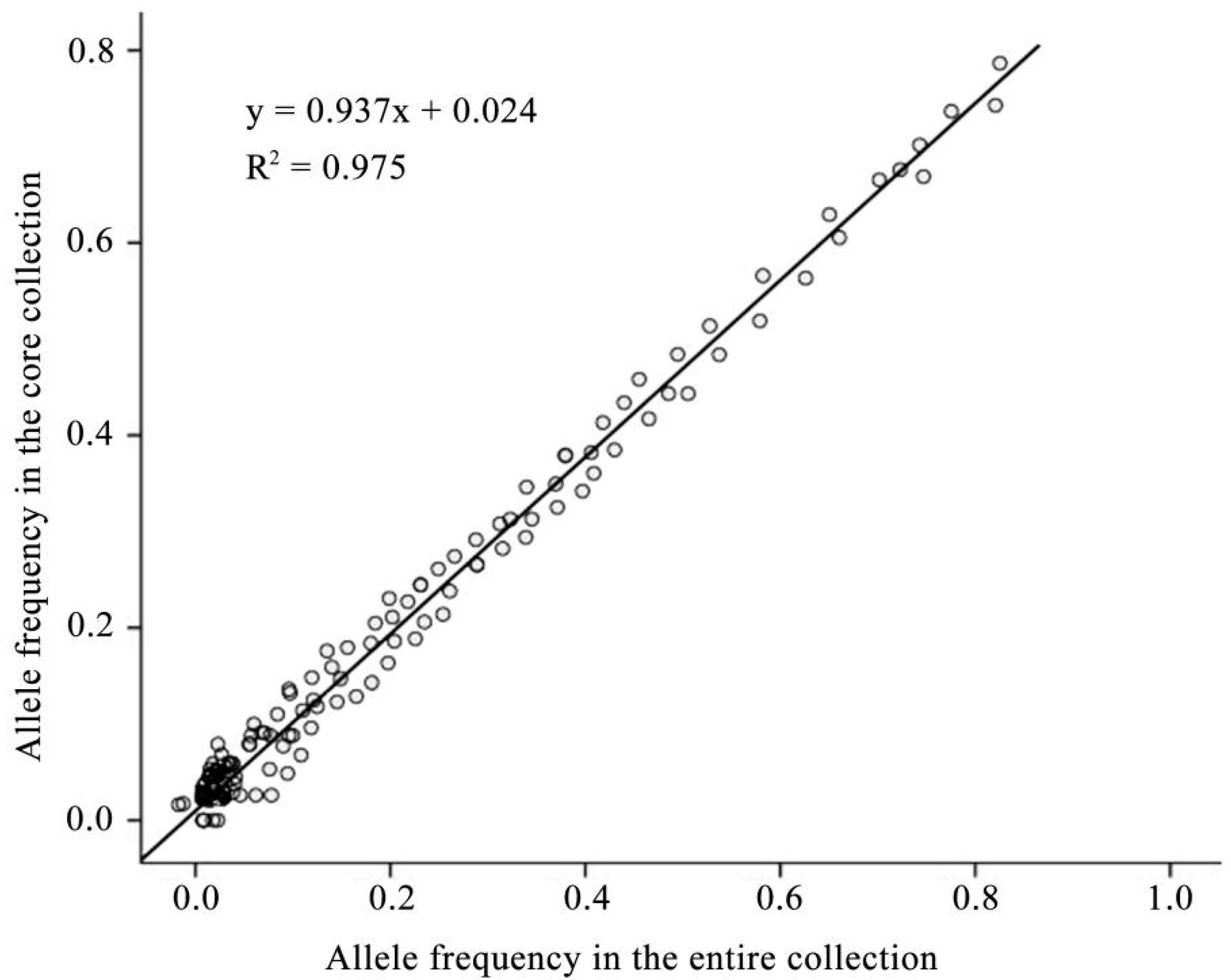
2.2. Confirmation and Evaluation of Core Collection Resources
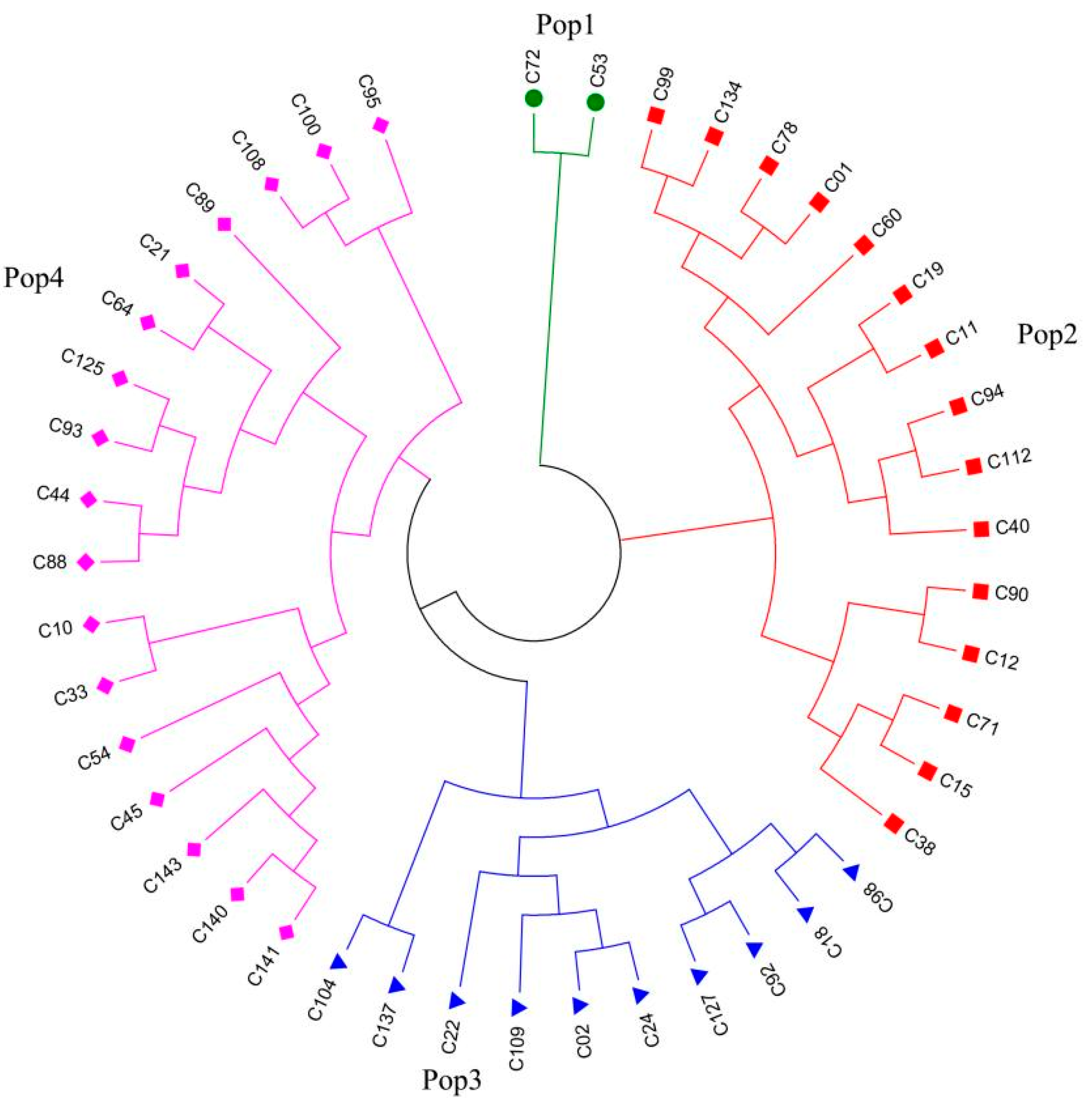
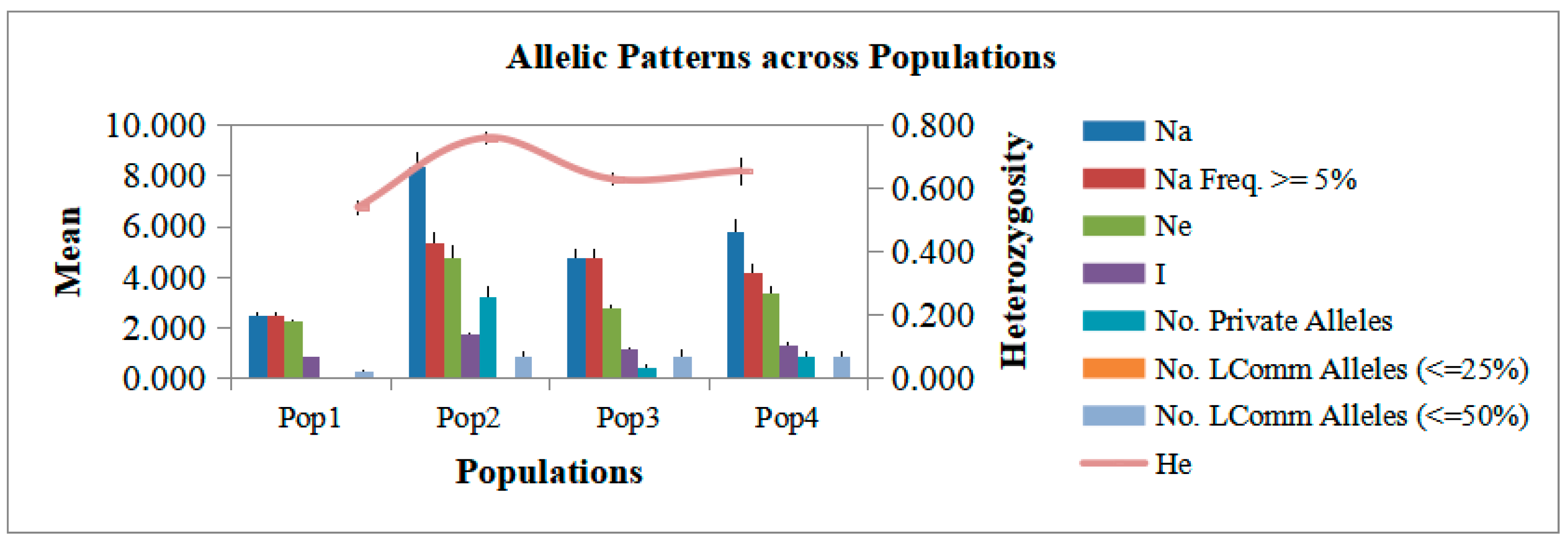
2.3. Cluster and Allelic Analyses of Core Collections
2.4. Establishment of Molecular ID for Core Collections
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. DNA Extraction and Detection
4.3. Expressed Sequence Tag (EST)-SSR Amplification and Sequencing
4.4. Genetic Diversity Analysis
4.5. Construction of Core Collections
4.6. Establishment of DNA Molecular Identification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Madison, M. Notes on Caladium (Araceae) and its allies. Selbyana 1981, 5, 342–377. [Google Scholar]
- Croat, T. Taxonomic status of neotropical Araceae. Aroideana 1994, 17, 33–60. [Google Scholar]
- Ahmed, E.U.; Hayashi, T.; Yazawa, S. Leaf color stability during plant development as an index of leaf color variation among micropropagated Caladium. HortScience 2004, 39, 328–332. [Google Scholar] [CrossRef]
- Yu, S.Y.; Zhao, X.Q.; Wang, Y.D.; Jiang, D.Z.; Zhang, Y.M.; Hu, L.; Liu, Y.Q.; Cai, X.D. Morphological, cytological, and molecular-based genetic stability analysis of in vitro-propagated plants from newly induced aneuploids in Caladium. Agriculture 2022, 12, 1708. [Google Scholar] [CrossRef]
- Isah, T. Changes in the biochemical parameters of albino, hyperhydric and normal green leaves of Caladium bicolor cv. "Bleeding hearts" in vitro long-term cultures. J. Photochem. Photobio. B 2019, 191, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.U.; Hayashi, T.; Yazawa, S. Auxins increase the occurrence of leaf-colour variants in Caladium regenerated from leaf explants. Sci. Hortic. 2004, 100, 153–159. [Google Scholar] [CrossRef]
- Deng, Z.A.; Harbaugh, B.K. Independent inheritance of leaf shape and main vein color in Caladium. J. Am. Soc. Hortic. Sci. 2006, 131, 53–58. [Google Scholar] [CrossRef]
- Maia, A.C.D.; Schlindwein, C. Caladium bicolor (Araceae) and Cyclocephala celata (Coleoptera, Dynastinae): A well-established pollination system in the northern atlantic rainforest of pernambuco, Brazil. Plant Biol. 2006, 8, 529–534. [Google Scholar] [CrossRef]
- Cao, Z.; Sui, S.Z.; Yang, Q.; Deng, Z.A. Inheritance of rugose leaf in Caladium and genetic relationships with leaf shape, main vein color, and leaf spotting. J. Am. Soc. Hortic. Sci. 2016, 141, 527–534. [Google Scholar] [CrossRef]
- Cao, Z.; Deng, Z.A. De novo assembly, annotation, and characterization of root transcriptomes of three Caladium cultivars with a focus on necrotrophic pathogen resistance/defense-related genes. Int. J. Mol. Sci. 2017, 18, 712. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.A.; Goktepe, F.; Harbaugh, B.K.; Hu, J.G. Assessment of genetic diversity and relationships among Caladium cultivars and species using molecular markers. J. Am. Soc. Hortic. Sci. 2007, 132, 219–229. [Google Scholar] [CrossRef]
- Loh, J.P.; Kiew, R.; Kee, A.; Gan, L.H.; Gan, Y.Y. Amplified fragment length polymorphism (AFLP) provides molecular markers for the identification of Caladium bicolor cultivars. Ann. Bot. 1999, 84, 155–161. [Google Scholar] [CrossRef]
- Cai, X.D.; Cao, Z.; Xu, S.X.; Deng, Z.A. Induction, regeneration and characterization of tetraploids and variants in ‘Tapestry’ Caladium. Plant Cell Tiss. Org. 2015, 120, 689–700. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Chen, J.J.; Cao, Y.M.; Duan, J.X.; Cai, X.D. Induction of tetraploids in ‘Red Flash’ Caladium using colchicine and oryzalin: Morphological, cytological, photosynthetic and chilling tolerance analysis. Sci. Hortic. 2020, 275, 109524. [Google Scholar] [CrossRef]
- Cao, Z.; Deng, Z.A. Interspecific genome size and chromosome number variation shed new light on species classification and evolution in Caladium. J. Am. Soc. Hortic. Sci. 2014, 139, 449–459. [Google Scholar] [CrossRef]
- Cao, Z.; Sui, S.Z.; Cai, X.D.; Yang, Q.; Deng, Z.A. Somaclonal variation in ‘Red Flash’ Caladium: Morphological, cytological and molecular characterization. Plant Cell Tiss. Org. 2016, 126, 269–279. [Google Scholar] [CrossRef]
- Deng, Z.A.; Peres, N.A. ‘Sea Foam Pink’ Caladium. HortScience 2019, 54, 1637–1640. [Google Scholar] [CrossRef]
- Yu, J.L.; Boyd, N.S. Tolerance of Caladium cultivars florida cardinal and florida fantasy to sulfonylurea herbicides. HortScience 2018, 53, 850–858. [Google Scholar] [CrossRef]
- Cao, Z.; Deng, Z.A. Morphological, cytological and molecular marker analyses of ‘Tapestry’ Caladium variants reveal diverse genetic changes and enable association of leaf coloration pattern loci with molecular markers. Plant Cell Tiss. Org. 2020, 143, 363–375. [Google Scholar] [CrossRef]
- Chen, J.J.; Zhang, Y.S.; Duan, J.X.; Cao, Y.M.; Cai, X.D. Morphological, cytological, and pigment analysis of leaf color variants regenerated from long-term subcultured Caladium callus. In Vitr. Cell. Dev. Biol. Plant 2021, 57, 60–71. [Google Scholar] [CrossRef]
- Deng, Z.A.; Goktepe, F.; Harbaugh, B.K. Inheritance of leaf Spots and their genetic relationships with leaf shape and vein color in Caladium. J. Am. Soc. Hortic. Sci. 2008, 133, 78–83. [Google Scholar] [CrossRef]
- Ekeke, C.; Agbagwa, I.O. Anatomical characteristics of Nigerian variants of Caladium bicolor (Aiton) Vent. (Araceae). Afr. J. Plant Sci. 2016, 10, 121–129. [Google Scholar]
- Croat, T.B.; Delannay, X.; Ortiz, O.O.; Jiménez, P.D. A review of the Aroid Tribe Caladieae with the description of three new species of Caladium and seven new species of Syngonium (Araceae). Novon J. Bot. Nom. 2019, 27, 38–64. [Google Scholar] [CrossRef]
- Hussain, R.; Younis, A.; Riaz, A.; Tariq, U.; Ali, S.; Ali, A.; Raza, S. Evaluating sustainable and environment friendly substrates for quality production of potted Caladium. Int. J. Recycl. Org. 2017, 6, 13–21. [Google Scholar] [CrossRef]
- Frankel, O.H. Genetic Perspectives of Germplasm Conservation; Cambridge University Press: Cambridge, UK, 1984; pp. 161–170. [Google Scholar]
- Brown, A. Core collections: A practical approach to genetic resources management. Genome 1989, 31, 818–824. [Google Scholar] [CrossRef]
- Song, J.M.; Arif, M.; Zi, Y.; Sze, S.H.; Zhang, M.P.; Zhang, H.B. Molecular and genetic dissection of the USDA rice mini-core collection using high-density SNP markers. Plant Sci. 2021, 308, 110910. [Google Scholar] [CrossRef]
- Razi, S.; Soleimani, A.; Zeinalabedini, M.; Vazifeshenas, M.R.; Martínez-Gómez, P.; Kermani, A.M.; Raiszadeh, A.R.; Tayari, M.; Martínez-García, P.J. Development of a multipurpose core collection of new promising Iranian pomegranate (Punica granatum L.) genotypes based on morphological and pomological traits. Horticulturae 2021, 7, 350. [Google Scholar] [CrossRef]
- Huang, F.Y.; Duan, J.H.; Lei, Y.; Liu, Z.; Kang, Y.K.; Luo, Y.; Chen, Y.Y.; Li, Y.Y.; Liu, S.Q.; Li, S.J.; et al. Genetic diversity, population structure and core collection analysis of Hunan tea plant germplasm through genotyping-by-sequencing. Beverage Plant Res. 2022, 2, 5. [Google Scholar] [CrossRef]
- Govindaraj, M.; Rai, K.N.; Kanatti, A.; Upadhyaya, H.D.; Shivade, H.; Rao, A.S. Publisher Correction: Exploring the genetic variability and diversity of pearl millet core collection germplasm for grain nutritional traits improvement. Sci. Rep. 2021, 11, 9757. [Google Scholar] [CrossRef]
- Balfourier, F.; Roussel, V.; Strelchenko, P.P.; Exbrayatvinson, F.; Spurdille, P.; Boutet, G.; Koening, J.L.; Ravel, C.; Mitrofanova, O.P.; Beckert, M.; et al. A worldwide bread wheat core collection arrayed in a 384-well plate. Theor. Appl. Genet. 2007, 114, 1265–1275. [Google Scholar] [CrossRef]
- Xu, C.Q.; Gao, J.; Du, Z.F.; Li, D.K.; Wang, Z.; Li, Y.Y.; Pang, X.M. Identifying the genetic diversity, genetic structure and a core colmection of Ziziphus jujuba Milm. Var. jujuba accessions using microsatelmite markers. Sci. Rep. 2016, 6, 31503. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.H.; Wang, Z.H.; Liu, N.W.; Song, L.; Yan, B.Q.; Xing, Y.; Zhang, Q.; Fang, K.F.; Zhao, Y.L.; Chen, X.; et al. Fingerprinting 146 Chinese chestnut (Castanea mollissima Blume) accessions and selecting a core collection using SSR markers. J. Integr. Agr. 2021, 20, 1277–1286. [Google Scholar] [CrossRef]
- Tian, H.L.; Wang, F.G.; Zhao, J.R.; Yi, H.M.; Wang, R.; Yang, Y.; Song, W. Development of maizeSNP3072, a high-throughput compatible SNP array, for DNA fingerprinting identification of Chinese maize varieties. Mol. Breed. 2015, 35, 136. [Google Scholar] [CrossRef] [PubMed]
- Natesan, S.; Venugopalan, G.; Selvamani, S.B.; Krishnamoorthy, M.; Chandran, S.; Angamuthu, N. Characterization of little millet (Panicum sumatrense) varieties using morphological descriptors and SSR based DNA fingerprinting. J. Phytol. 2020, 12, 29–34. [Google Scholar] [CrossRef]
- Ma, S.N.; Han, C.Y.; Zhou, J.; Hu, R.C.; Jiang, X.; Wu, F.F.; Tian, K.; Nie, G.; Zhang, X.Q. Fingerprint identification of white clover cultivars based on SSR molecular markers. Mol. Biol. Rep. 2020, 47, 8513–8521. [Google Scholar] [CrossRef]
- Liu, J.; Liao, K.; Nasir, M.; Sun, Q.; Liu, H.; Jia, Y. Analysis of genetic diversity and construction of DNA fingerprint database of Xinjiang apricot varieties (lines). Sci. Agric. Sin. 2015, 4, 134–144. (In Chinese) [Google Scholar]
- Xia, F.G.; Huang, J.X.; Ji, B.J.; Zhan, F.Y.; Xie, P.P.; Deng, B.Z.; Lin, H.; Zheng, J.G. Genetic diversity analysis and DNA fingerprint construction of Coix lacryma-jobi germplasm resources by SRAP marker. J. Plant Genet. Resour. 2017, 3, 36–43. (In Chinese) [Google Scholar]
- Guo, Y.C.; Zhang, L.L.; Chen, S.Y.; Qi, J.M.; Fang, P.P.; Tao, A.F.; Zhang, L.M.; Zhang, L.W. Establishment of DNA molecular fingerprint of applied core germplasm in jute (Corchorus spp.). Acta Agric. Sin. 2021, 47, 80–93. (In Chinese) [Google Scholar] [CrossRef]
- Freixas-Coutin, J.A.; An, S.; Postman, J.; Bassil, N.V.; Yates, B.; Shukla, M.; Saxena, P.K. Development of a reliable Corylus sp. reference database through the implementation of a DNA fingerprinting test. Planta 2019, 249, 1863–1874. [Google Scholar] [CrossRef]
- Yogi, R.; Kumar, N.; Kumar, R.; Jain, R.K. Genetic diversity analysis among important rice (Oryza sativa L.) genotypes using SSR markers. Adv. Biores. 2020, 11, 68–74. [Google Scholar]
- Anoumaa, M.; Yao, N.K.; Kouam, E.B.; Kanmegne, G.; Machuka, E.; Osama, S.K.; Nzuki, I.; Kamga, Y.B.; Fonkou, T.; Omokolo, D.N. Genetic diversity and core collection for potato (Solanum tuberosum L.) cultivars from cameroon as revealed by SSR markers. Am. J. Potato Res. 2017, 94, 449–463. [Google Scholar] [CrossRef]
- Nader, W.; Elsner, J.; Brendel, T.; Schubbert, R. The DNA fingerprint in food forensics: The Basmati rice case. Agro. Food Ind. Hi Tech. 2019, 30, 57–61. [Google Scholar]
- Di Guardo, M.; Scollo, F.; Ninot, A.; Rovira, M.; Hermoso, J.F.; Distefano, G.; La Malfa, S.; Batlle, I. Genetic structure analysis and selection of a core collection for carob tree germplasm conservation and management. Tree Genet. Genomes 2019, 15, 41. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Zhang, J.H.; Sun, H.Y.; Ning, N.; Yang, L. Construction and evaluation of a primary core colmection of apricot germplasm in China. Sci. Hortic. 2011, 128, 311–319. [Google Scholar] [CrossRef]
- Choi, Y.M.; Kim, K.M.; Lee, S.; Oh, S.; Lee, M.C. Development of a Core Collection Based on EST-SSR Markers and Phenotypic Traits in Foxtail Millet [Setaria italica (L.) P. Beauv.]. J. Crop Sci. Biotechnol. 2019, 21, 395–405. [Google Scholar] [CrossRef]
- Zhou, Y.W.; Ye, Y.J.; Zhu, G.F.; Xu, Y.C.; Tan, J.J.; Liu, J.M. Diversity, classification, and EST-SSR-based association analysis of caladium ornamental traits. Physiol. Plantarum 2022, 174, e13841. [Google Scholar] [CrossRef]
- Ye, Y.J.; Xu, Y.C.; Li, D.M.; Tan, J.J.; Liu, J.M. Characterization of EST-SSR markers in Curcuma kwangsiensis S. K. Lee & C. F. Liang based on RNA sequencing and its application for phylogenetic relationship analysis and core collection construction. Genet. Resour. Crop Evol. 2021, 68, 1503–1516. [Google Scholar]
- Ye, Y.J.; Feng, L.; Liang, X.H.; Liu, T.T.; Cai, M.; Cheng, T.R.; Wang, J.; Zhang, Q.X.; Pan, H.T. Characterization, validation, and cross-species transferability of newly developed EST-SSR markers and their application for genetic evaluation in crape myrtle (Lagerstroemia spp). Mol. Breed. 2019, 39, 26. [Google Scholar] [CrossRef]
- Duan, H.J.; Cao, S.; Zheng, H.Q.; Hu, D.H.; Lin, J.; Cui, B.B.; Lin, H.Z.; Hu, R.Y.; Wu, B.; Sun, Y.H.; et al. Genetic characterization of Chinese fifir from six provinces in southern China and construction of a core collection. Sci. Rep. 2017, 7, 13814. [Google Scholar] [CrossRef]
- Zhou, Q.; Mu, K.M.; Ni, Z.X.; Liu, X.H.; Li, Y.G.; Xu, L.A. Analysis of genetic diversity of ancient Ginkgo populations using SSR markers. Ind. Crop Prod. 2019, 145, 111942. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Xie, W.G.; Zhao, Y.Q.; Zhang, J.C.; Wang, N.; Ntakirutmana, F.; Yan, J.J.; Wang, Y.R. EST-SSR marker development based on RNA-sequencing of E. sibiricus and its application for phylogenetic relationships analysis of seventeen Elymus species. BMC Plant Biol. 2019, 19, 235. [Google Scholar] [CrossRef]
- Lu, X.Z.; Ni, J.L.; Li, L.; Wang, X.F.; Ma, H.; Zhang, X.J.; Yang, J.B. Construction of rice variety in dentity using SSR fingerprint and commodity information. Acta Agron. Sin. 2014, 40, 823–829. (In Chinese) [Google Scholar] [CrossRef]
- Gao, Y.; Liu, F.Z.; Wang, K.; Wang, D.J.; Gong, X.; Liu, L.J. Establishment of molecular ID for some apple germplasm resources. Sci. Agric. Sin. 2015, 48y, 3887–3898. (In Chinese) [Google Scholar]
- Zhang, L.W.; Cai, R.R.; Yuan, M.H.; Tao, A.F.; Xu, J.T.; Lin, L.H.; Fang, P.P.; Qi, J.M. Genetic diversity and DNA fingerprinting in jute (Corchorus spp.) based on SSR markers. Acta Agron. Sin. 2015, 3, 416–422. (In Chinese) [Google Scholar] [CrossRef]
- Schuelke, M. An economic method for the flfluorescent labeling of PCR fragments. Nat. Biotechnol. 2000, 18, 233–234. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic sofware for teaching and research-an update. Bioinformatics 2006, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Yeh, F.C.; Yang, R.C.; Boyle, T. POPGENE Version 1.31, University of Alberta: Edmonton, AB, Canada, 1999.
- Liu, K.; Muse, S.V. PowerMarker: An integrated analysis environment for genetic marker analysis. Bioinformatics 2005, 21, 2128–2129. [Google Scholar] [CrossRef]

| SSR Marker | Number of Identical Pairs of Genotypes | Probability of Identity | Cumulative Probability of Identity |
|---|---|---|---|
| CAL86 | 1123 | 4 × 10−2 | 4 × 10−2 |
| CAL106 | 674 | 4.2 × 10−2 | 1.6 × 10−3 |
| CAL16 | 535 | 6.8 × 10−2 | 1.1 × 10−4 |
| CAL81 | 398 | 9.8 × 10−2 | 1.1 × 10−5 |
| CAL180 | 242 | 9.9 × 10−2 | 1.1 × 10−6 |
| CAL181 | 136 | 1 × 10−1 | 1.1 × 10−7 |
| CAL135 | 120 | 1.2 × 10−1 | 1.3 × 10−8 |
| CAL77 | 90 | 1.3 × 10−1 | 1.7 × 10−9 |
| CAL90 | 46 | 1.3 × 10−1 | 2.2 × 10−10 |
| CAL96 | 33 | 1.6 × 10−1 | 3.6 × 10−11 |
| CAL156 | 21 | 1.6 × 10−1 | 5.6 × 10−12 |
| CAL143 | 15 | 1.8 × 10−1 | 1.0 × 10−12 |
| CAL52 | 2 | 1.8 × 10−1 | 1.8 × 10−13 |
| CAL188 | 2 | 2 × 10−1 | 3.6 × 10−14 |
| CAL162 | 2 | 2.3 × 10−1 | 8.3 × 10−15 |
| CAL79 | 1 | 2.4 × 10−1 | 2 × 10−15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Y.; Fu, H.; Zhou, Y.; Zhang, S.; Wang, Z.; Tian, L.; Tan, J.; Zhu, G.; Liu, J.; Xu, Y. Establishment of DNA Molecular Fingerprint of Caladium Core Collections. Agriculture 2023, 13, 200. https://doi.org/10.3390/agriculture13010200
Ye Y, Fu H, Zhou Y, Zhang S, Wang Z, Tian L, Tan J, Zhu G, Liu J, Xu Y. Establishment of DNA Molecular Fingerprint of Caladium Core Collections. Agriculture. 2023; 13(1):200. https://doi.org/10.3390/agriculture13010200
Chicago/Turabian StyleYe, Yuanjun, Haiping Fu, Yiwei Zhou, Shanxin Zhang, Zehuang Wang, Lihua Tian, Jianjun Tan, Genfa Zhu, Jinmei Liu, and Yechun Xu. 2023. "Establishment of DNA Molecular Fingerprint of Caladium Core Collections" Agriculture 13, no. 1: 200. https://doi.org/10.3390/agriculture13010200
APA StyleYe, Y., Fu, H., Zhou, Y., Zhang, S., Wang, Z., Tian, L., Tan, J., Zhu, G., Liu, J., & Xu, Y. (2023). Establishment of DNA Molecular Fingerprint of Caladium Core Collections. Agriculture, 13(1), 200. https://doi.org/10.3390/agriculture13010200








