Survival of Phytophthora cryptogea and Phytophthora cactorum in Commercial Potting Substrates for Eucalyptus globulus Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Media
2.2. Phytophthora Isolate Collection and Preparation
2.3. Preparation of Phytophthora Inoculum
2.4. Inoculation of PB and PF Substrates
2.5. Survival of P. cryptogea and P. cactorum
2.6. pH Measurement of Substrate
2.7. Mineral Nitrogen Content of Substrate
2.8. Pathogenicity Assay
2.9. Statistical Analyses
2.9.1. Survival Statistical Analysis: Colony Forming Units, Soil Properties, and Model Development and Validation for Each Pathogen
2.9.2. Pathogenicity Statistical Analysis: Model Development and Validation for Each Pathogen
3. Results
3.1. Survival of P. cryptogea and P. cactorum in PB and PF Substrates
3.2. Substrate pH Values, Ammonia (NH4) and Nitrogen (NO-x) Concentrations
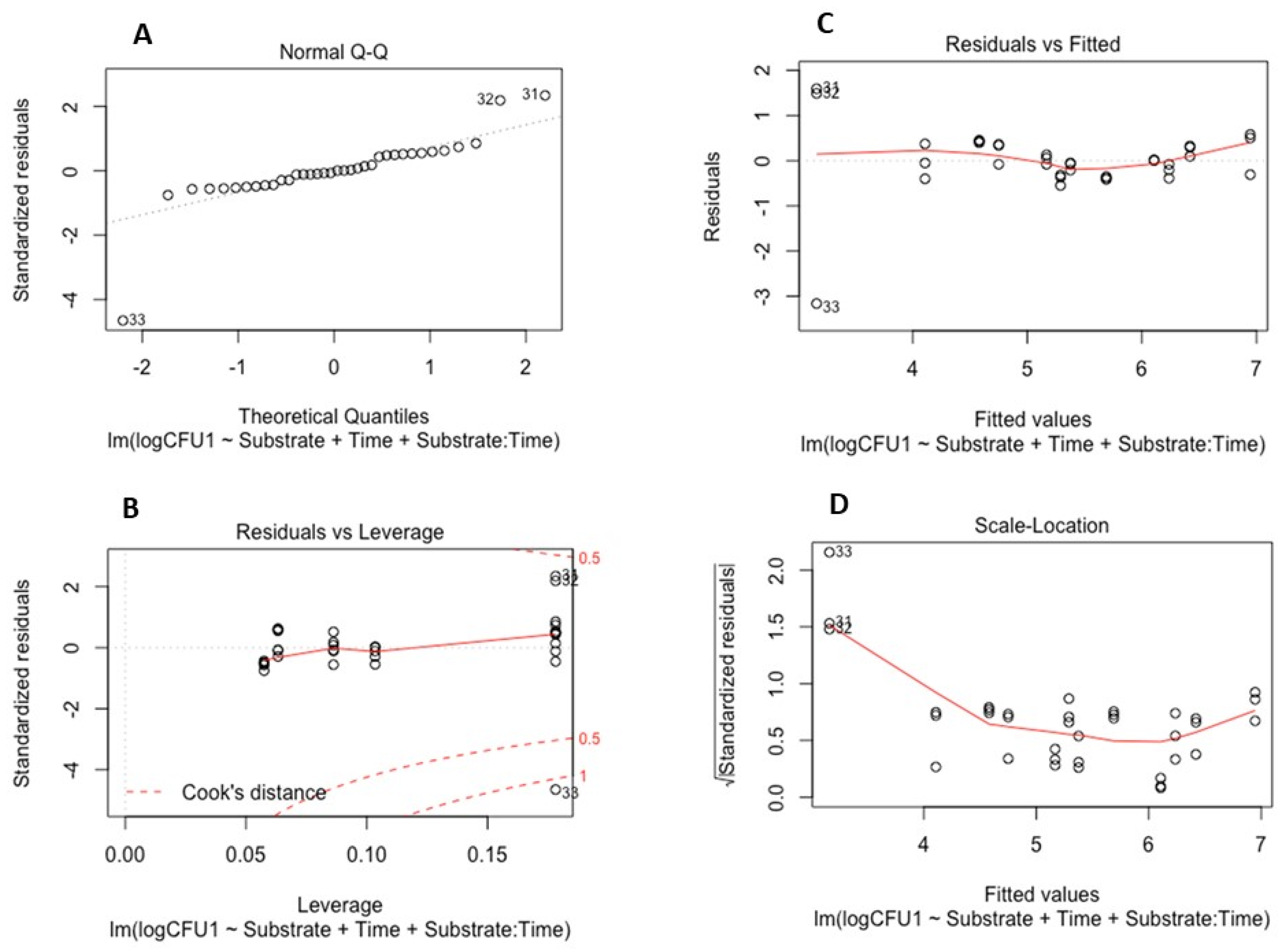
3.3. Statistical Model Development for Pathogen Survival Assay
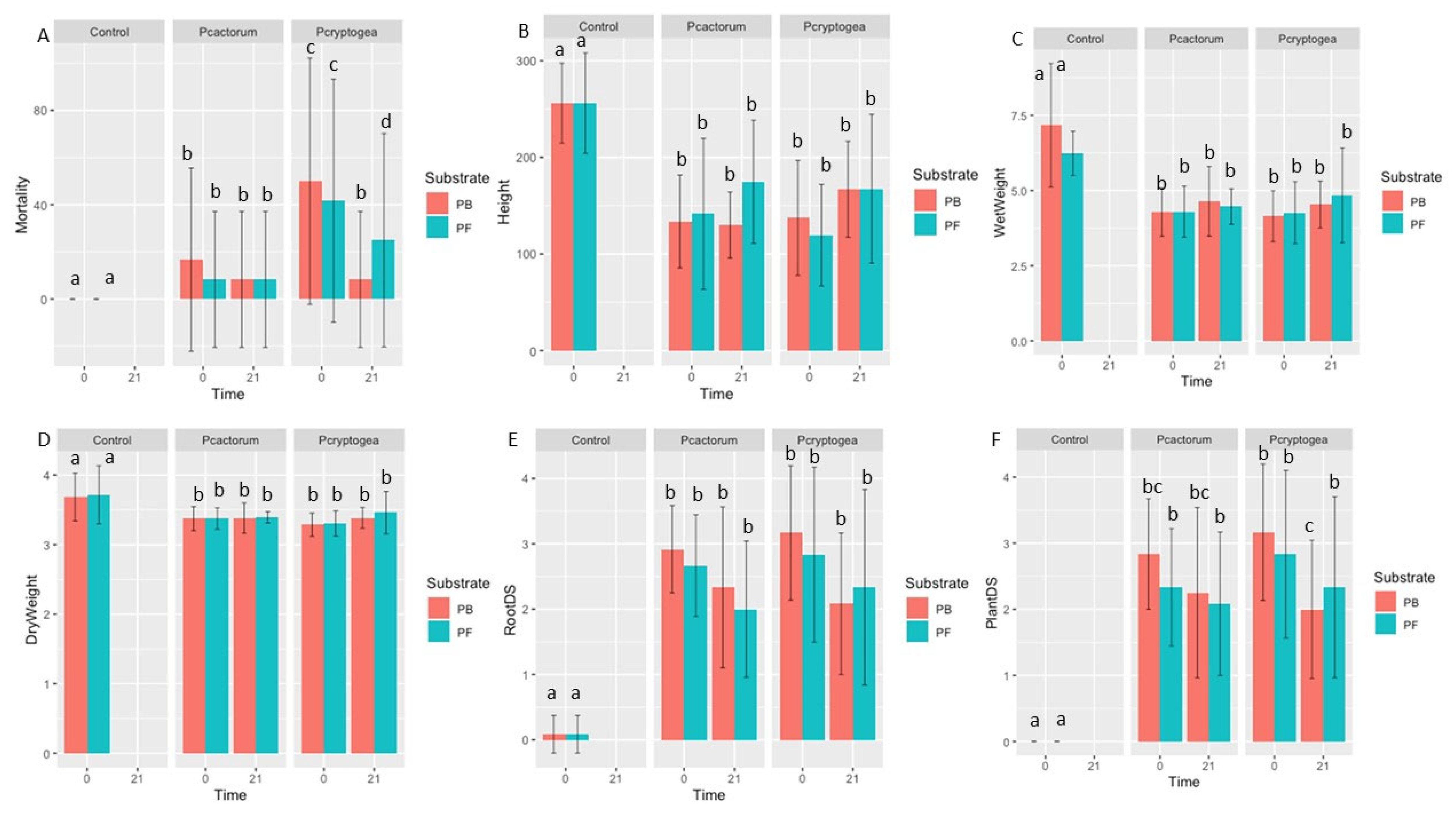
3.4. Pathogenicity Assay
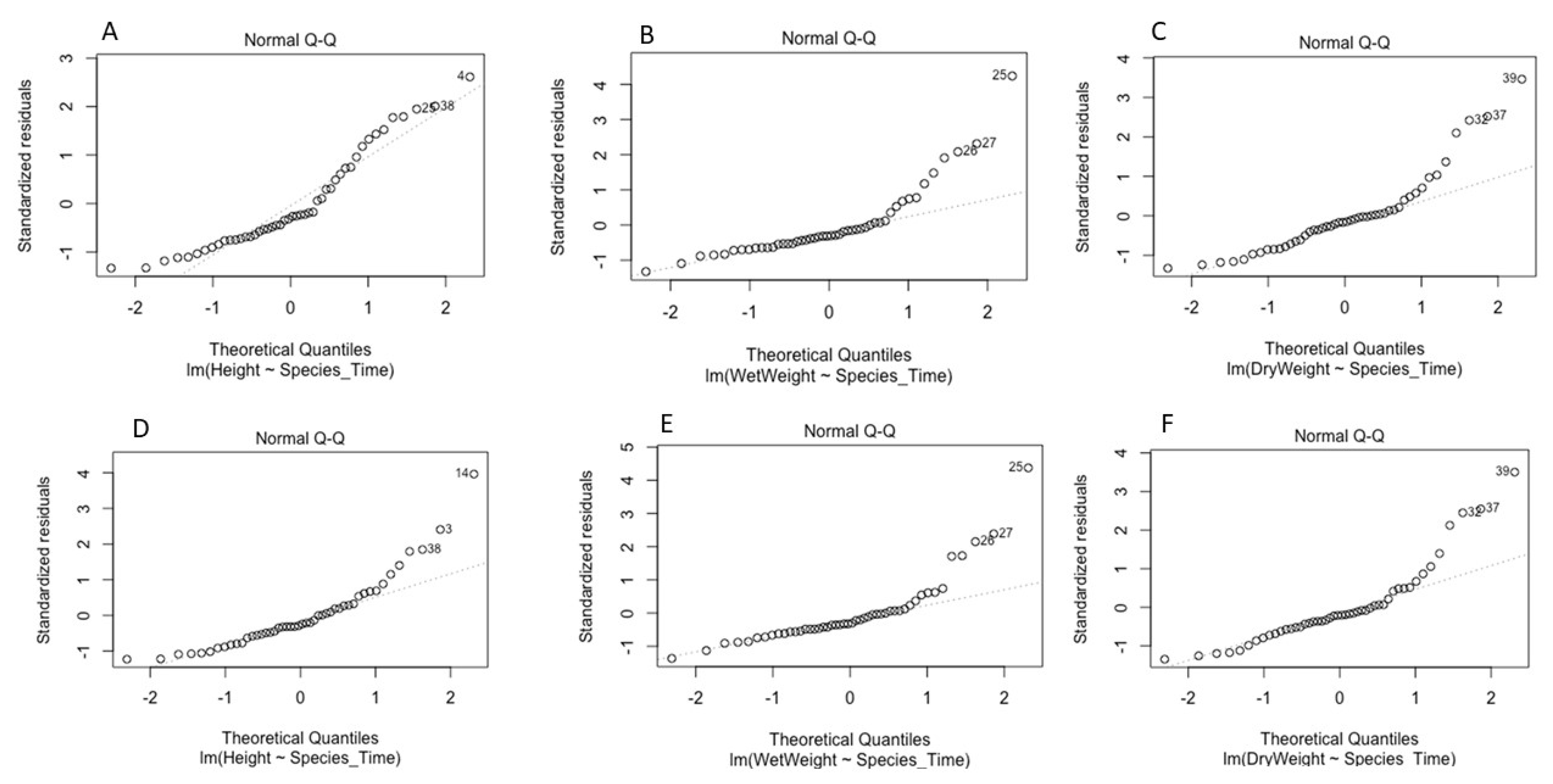
3.5. Statistical Model Development for Plant Pathogenicity Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, T.; Colquhoun, I.J.; Hardy, G.E.S.J. New Insights into the Survival Strategy of the Invasive Soilborne Pathogen Phytophthora Cinnamomi in Different Natural Ecosystems in Western Australia. For. Pathol. 2013, 43, 266–288. [Google Scholar] [CrossRef]
- Pérez-Sierra, A.; López-García, C.; León, M.; García-Jiménez, J.; Abad-Campos, P.; Jung, T. Previously Unrecorded Low-Temperature Phytophthora Species Associated with Quercus Decline in a Mediterranean Forest in Eastern Spain. For. Pathol. 2013, 43, 331–339. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Uematsu, S.; Suga, H.; Kageyama, K. Diversity of Phytophthora Species Newly Reported from Japanese Horticultural Production. Mycoscience 2015, 56, 443–459. [Google Scholar] [CrossRef]
- Benavent-Celma, C.; López-García, N.; Ruba, T.; Ściślak, M.E.; Street-Jones, D.; van West, P.; Woodward, S.; Witzell, J. Current Practices and Emerging Possibilities for Reducing the Spread of Oomycete Pathogens in Terrestrial and Aquatic Production Systems in the European Union. Fungal Biol. Rev. 2021, 40, 19–36. [Google Scholar] [CrossRef]
- Mircetich, S.M.; Zentmyer, G.A. Existence of Phytophthora Cinnamomi As Chlamydospores and Oospores in Roots and Soil. Phytopathology 1967, 51, 117–124. [Google Scholar]
- Van West, P.; Morris, B.M.; Reid, B.; Appiah, A.A.; Osborne, M.C.; Campbell, T.A.; Shepherd, S.J.; Gow, N.A.R. Oomycete Plant Pathogens Use Electric Fields to Target Roots. Mol. Plant-Microbe Interact. 2002, 15, 790–798. [Google Scholar] [CrossRef]
- Van West, P.; Appiah, A.A.; Gow, N.A.R. Advances in Research on Oomycete Root Pathogens. Physiol. Mol. Plant Pathol. 2003, 62, 99–113. [Google Scholar] [CrossRef]
- Crone, M.; McComb, J.A.; O’Brien, P.A.; Hardy, G.E.S.J. Survival of Phytophthora Cinnamomi as Oospores, Stromata, And Thick-Walled Chlamydospores in Roots of Symptomatic and Asymptomatic Annual and Herbaceous Perennial Plant Species. Fungal Biol. 2013, 117, 112–123. [Google Scholar] [CrossRef]
- Babadoost, M.; Pavon, C. Survival of Oospores of Phytophthora Capsici in Soil. Plant Dis. 2013, 97, 1478–1483. [Google Scholar] [CrossRef]
- Vercauteren, A.; Riedel, M.; Maes, M.; Werres, S.; Heungens, K. Survival of Phytophthora Ramorum in Rhododendron Root Balls and in Rootless Substrates. Plant Pathol. 2013, 62, 166–176. [Google Scholar] [CrossRef]
- Linderman, R.G.; Davis, E.A. Survival of Phytophthora Ramorum Compared to Other Species of Phytophthora in Potting Media Components, Compost, and Soil. Horttechnology 2006, 16, 502–507. [Google Scholar] [CrossRef]
- Vannini, A.; Breccia, M.; Bruni, N.; Tomassini, A.; Vettraino, A.M. Behaviour and Survival of Phytophthora Cambivora Inoculum in Soil-like Substrate under Different Water Regimes. For. Pathol. 2012, 42, 362–370. [Google Scholar] [CrossRef]
- Kuhlman, G.E. Survival and Pathogenicity of Phytophthora Cinnamomi in Several Western Oregon Soils. For. Sci. 1964, 10, 151–158. [Google Scholar] [CrossRef]
- Puértolas, A.; Boa, E.; Bonants, P.J.M.; Woodward, S. Survival of Phytophthora Cinnamomi and Fusarium Verticillioides in Commercial Potting Substrates for Ornamental Plants. J. Phytopathol. 2018, 166, 484–493. [Google Scholar] [CrossRef]
- Green, S.; Cooke, D.E.L.; Dunn, M.; Barwell, L.; Purse, B.; Chapman, D.S.; Valatin, G.; Schlenzig, A.; Barbrook, J.; Pettitt, T.; et al. Phyto-Threats: Addressing Threats to Uk Forests and Woodlands from Phytophthora; Identifying Risks of Spread in Trade and Methods for Mitigation. Forests 2021, 12, 1617. [Google Scholar] [CrossRef]
- Jung, T.; Pérez-Sierra, A.; Durán, A.; Jung, M.H.; Balci, Y.; Scanu, B. Canker and Decline Diseases Caused by Soil- and Airborne Phytophthora Species in Forests and Woodlands. Pers. Mol. Phylogeny Evol. Fungi 2018, 40, 182–220. [Google Scholar] [CrossRef]
- Paap, T.; Burgess, T.I.; Wingfield, M.J. Urban Trees: Bridge-Heads for Forest Pest Invasions and Sentinels for Early Detection. Biol. Invasions 2017, 19, 3515–3526. [Google Scholar] [CrossRef]
- Hulbert, J.M.; Paap, T.; Burgess, T.I.; Roets, F.; Wingfield, M.J. Botanical Gardens Provide Valuable Baseline Phytophthora Diversity Data. Urban For. Urban Green. 2019, 46, 126461. [Google Scholar] [CrossRef]
- Hulbert, J.M.; Agne, M.C.; Burgess, T.I.; Roets, F.; Wingfield, M.J. Urban Environments Provide Opportunities for Early Detections of Phytophthora Invasions. Biol. Invasions 2017, 19, 3629–3644. [Google Scholar] [CrossRef]
- Wu, E.J.; Wang, Y.P.; Yang, L.N.; Zhao, M.Z.; Zhan, J. Elevating Air Temperature May Enhance Future Epidemic Risk of the Plant Pathogen Phytophthora Infestans. J. Fungi 2022, 8, 808. [Google Scholar] [CrossRef]
- Gomes Marques, I.; Solla, A.; David, T.S.; Rodríguez-González, P.M.; Garbelotto, M. Response of Two Riparian Woody Plants to Phytophthora Species and Drought. For. Ecol. Manag. 2022, 518, 120281. [Google Scholar] [CrossRef]
- Brasier, C.M. The Biosecurity Threat to the UK and Global Environment from International Trade in Plants. Plant Pathol. 2008, 57, 792–808. [Google Scholar] [CrossRef]
- IPPC. Recommendation on: Internet Trade (e-Commerce) in Plants and Other Regulated Articles; IPPC: Rome, Italy, 2017. [Google Scholar]
- Bonanomi, G.; Antignani, V.; Capodilupo, M.; Scala, F. Identifying the Characteristics of Organic Soil Amendments That Suppress Soilborne Plant Diseases. Soil Biol. Biochem. 2010, 42, 136–144. [Google Scholar] [CrossRef]
- Aleandri, M.P.; Chilosi, G.; Bruni, N.; Tomassini, A.; Vettraino, A.M.; Vannini, A. Use of Nursery Potting Mixes Amended with Local Trichoderma Strains with Multiple Complementary Mechanisms to Control Soil-Borne Diseases. Crop Prot. 2015, 67, 269–278. [Google Scholar] [CrossRef]
- Chilosi, G.; Aleandri, M.P.; Bruni, N.; Tomassini, A.; Torresi, V.; Muganu, M.; Paolocci, M.; Vettraino, A.; Vannini, A. Assessment of Suitability and Suppressiveness of On-Farm Green Compost as a Substitute of Peat in the Production of Lavender Plants. Biocontrol Sci. Technol. 2017, 27, 539–555. [Google Scholar] [CrossRef]
- Morales, A.B.; Ros, M.; Ayuso, L.M.; Bustamante, M.d.l.A.; Moral, R.; Pascual, J.A. Agroindustrial Composts to Reduce the Use of Peat and Fungicides in the Cultivation of Muskmelon Seedlings. J. Sci. Food Agric. 2017, 97, 875–881. [Google Scholar] [CrossRef]
- Jeffers, S.N.; Martin, S.B. Comparison of Two Media Selective for Phytophthora and Pythium Species. Plant Dis. 1986, 70, 1038–1043. [Google Scholar] [CrossRef]
- Puertolas, A.; Bonants, P.J.M.; Boa, E.; Woodward, S. Application of Real-Time PCR for the Detection and Quantification of Oomycetes in Ornamental Nursery Stock. J. Fungi 2021, 7, 87. [Google Scholar] [CrossRef]
- Vettraino, A.M.; Natili, G.; Anselmi, N.; Vannini, A. Recovery and Pathogenicity of Phytophthora Species Associated with a Resurgence of Ink Disease in Castanea Sativa in Italy. Plant Pathol. 2001, 50, 90–96. [Google Scholar] [CrossRef]
- Johnson, L.F.; Curl, E.A. Burgess Methods for Research on the Ecology of Soil-Borne Plant Pathogens. In Methods for Research on the Ecology of Soil-Borne Plant Pathogens; Burgess Publishing: Minneapolis, MN, USA, 1972; p. 426. [Google Scholar]
- Gerlach, R.W.; Dobb, D.E.; Raab, G.A.; Nocerino, J.M. Gy Sampling Theory in Environmental Studies. 1. Assessing Soil Splitting Protocols. J. Chemom. 2002, 16, 321–328. [Google Scholar] [CrossRef]
- Allen, S.E. Chemical Analysis of Ecological Materials, 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 1989. [Google Scholar]
- Rojas, J.A.; Jacobs, J.L.; Napieralski, S.; Karaj, B.; Bradley, C.A.; Chase, T.; Esker, P.D.; Giesler, L.J.; Jardine, D.J.; Malvick, D.K.; et al. Oomycete Species Associated with Soybean Seedlings in North America-Part II: Diversity and Ecology in Relation to Environmental and Edaphic Factors. Phytopathology 2017, 107, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Strack, M.; Waddington, J.M.; Turetsky, M.; Roulet, N.T.; Byrne, K.A. Northern Peatland, Greenhouse Gas Exchange and Climate Change. In Peatlands and Climate Change; International Peat Socie: Jyväskylä, Finland, 2008. [Google Scholar]
- Bonanomi, G.; Antignani, V.; Pane, C.; Scala, F. Suppression of Soilborne Fungal Diseases with Organic Amendments. J. Plant Pathol. 2007, 89, 311–324. [Google Scholar]
- Mazzola, M.; Granatstein, D.M.; Elfving, D.C.; Mullinix, K. Suppression of Specific Apple Root Pathogens by Brassica Napus Seed Meal Amendment Regardless of Glucosinolate Content. Phytopathology 2001, 91, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Tilston, E.L.; Pitt, D.; Groenhof, A.C. Composted Recycled Organic Matter Suppresses Soil-Borne Diseases of Field Crops. New Phytol. 2002, 154, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Judelson, H.S.; Ah-Fong, A.M.V. Exchanges at the Plant-Oomycete Interface That Influence Disease. Plant Physiol. 2019, 179, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Moreau, D.; Bardgett, R.D.; Finlay, R.D.; Jones, D.L.; Philippot, L. A Plant Perspective on Nitrogen Cycling in the Rhizosphere. Funct. Ecol. 2019, 33, 540–552. [Google Scholar] [CrossRef]
- Sparks, D.L. Methods of Soil Analysis. Chemical Methods; Sparks, D.K., Page, A.L., Helmke, P.A., Loeppert, R.H., Eds.; Soil Science Society of America: Madison, WI, USA, 1996. [Google Scholar]
- Jackson, B.E.; Wright, R.D.; Alley, M.M. Comparison of Fertilizer Nitrogen, Nitrogen Immobilization, Carbon Dioxide Efflux, and Leaching in Peat-Lite, Pine, and Pine Tree Substrates. HortScience 2009, 44, 781–790. [Google Scholar] [CrossRef]
- Boyer, C.R.; Torbert, H.A.; Gilliam, C.H.; Fain, G.B.; Gallagher, T.V.; Sibley, J.L. Nitrogen Immobilization in Plant Growth Substrates: Clean Chip Residual, Pine Bark, and Peatmoss. Int. J. Agron. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Khdiar, M.Y.; Barber, P.A.; Hardy, G.E.S.; Shaw, C.; Steel, E.J.; McMains, C.; Burgess, T.I. Association of Phytophthora with Declining Vegetation in an Urban Forest Environment. Microorganisms 2020, 8, 973. [Google Scholar] [CrossRef]
- Stewart-Wade, S.M. Plant Pathogens in Recycled Irrigation Water in Commercial Plant Nurseries and Greenhouses: Their Detection and Management. Irrig. Sci. 2011, 29, 267–297. [Google Scholar] [CrossRef]
- Turbelin, A.J.; Diagne, C.; Hudgins, E.J.; Moodley, D.; Kourantidou, M.; Novoa, A.; Haubrock, P.J.; Bernery, C.; Gozlan, R.E.; Francis, R.A.; et al. Introduction Pathways of Economically Costly Invasive Alien Species. Biol. Invasions 2022, 24, 2061–2079. [Google Scholar] [CrossRef]
- Jung, T.; Orlikowski, L.; Henricot, B.; Abad-Campos, P.; Aday, A.G.; Aguín Casal, O.; Bakonyi, J.; Cacciola, S.O.; Cech, T.; Chavarriaga, D.; et al. Widespread Phytophthora Infestations in European Nurseries Put Forest, Semi-Natural and Horticultural Ecosystems at High Risk of Phytophthora Diseases. For. Pathol. 2016, 46, 134–163. [Google Scholar] [CrossRef]
- Rodríguez-Romero, M.; Cardillo, E.; Santiago, R.; Pulido, F. Susceptibility to Phytophthora cinnamomi of Six Holm Oak (Quercus Ilex) Provenances: Are Results under Controlled vs. Natural Conditions Consistent? For. Syst. 2022, 31, e011. [Google Scholar] [CrossRef]
- Ireland, K.B.; Hardy, G.E.S.J.; Kriticos, D.J. Combining Inferential and Deductive Approaches to Estimate the Potential Geographical Range of the Invasive Plant Pathogen, Phytophthora Ramorum. PLoS ONE 2013, 8, e0063508. [Google Scholar] [CrossRef]
- Duque-Lazo, J.; Navarro-Cerrillo, R.M.; van Gils, H.; Groen, T.A. Forecasting Oak Decline Caused by Phytophthora cinnamomi in Andalusia: Identification of Priority Areas for Intervention. For. Ecol. Manag. 2018, 417, 122–136. [Google Scholar] [CrossRef]
- Serrano, M.S.; Romero, M.Á.; Homet, P.; Gómez-Aparicio, L. Climate Change Impact on the Population Dynamics of Exotic Pathogens: The Case of the Worldwide Pathogen Phytophthora cinnamomi. Agric. For. Meteorol. 2022, 322, 109002. [Google Scholar] [CrossRef]
- Stenlid, J.; Oliva, J. Phenotypic Interactions between Tree Hosts and Invasive Forest Pathogens in the Light of Globalization and Climate Change. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150455. [Google Scholar] [CrossRef]
- Jung, T.; La Spada, F.; Pane, A.; Aloi, F.; Evoli, M.; Jung, M.H.; Scanu, B.; Faedda, R.; Rizza, C.; Puglisi, I.; et al. Diversity and Distribution of Phytophthora Species in Protected Natural Areas in Sicily. Forests 2019, 10, 259. [Google Scholar] [CrossRef]
- Matsiakh, I.; Kramarets, V.; Cleary, M. Occurrence and Diversity of Phytophthora Species in Declining Broadleaf Forests in Western Ukraine. For. Pathol. 2021, 51, e12662. [Google Scholar] [CrossRef]
- Erktan, A.; Or, D.; Scheu, S. The Physical Structure of Soil: Determinant and Consequence of Trophic Interactions. Soil Biol. Biochem. 2020, 148, 107876. [Google Scholar] [CrossRef]
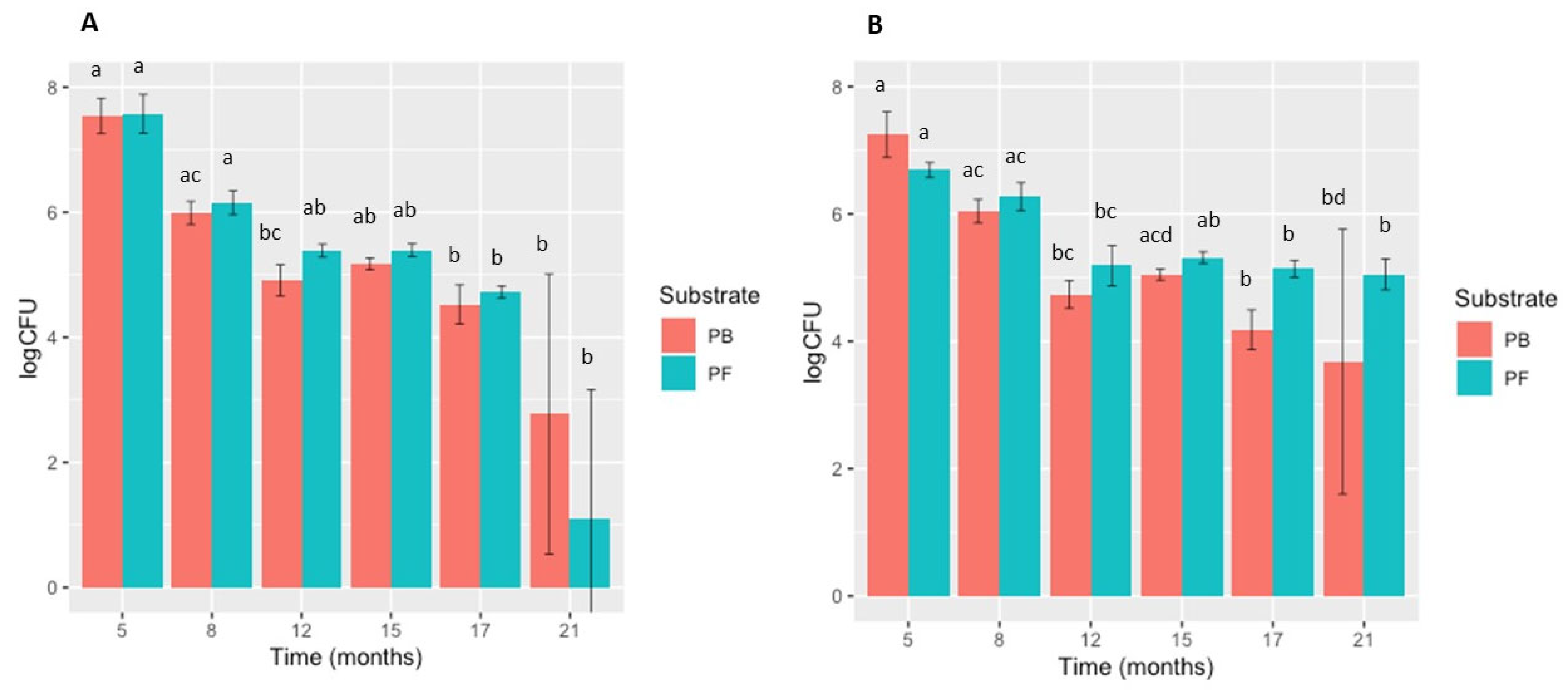



| Time of Sampling after Inoculation (A) P. cryptogea | Substrate | Mean CFUs g−1 Dry Substrate ± SD Statistical Test: Kruskal-Wallis Dunn | Mean pH ± SD Statistical Test: Welch Test | Mean NH4 (mg L−1) ± SD Statistical Test: PB: ANOVA PF: Kruskal-Wallis Dunn | Mean NO-x (mg L−1) ± SD Statistical Test: Welch Test |
|---|---|---|---|---|---|
| 5 months | PB | 4.06 × 107 ± 2.35 × 107 (a) | 4.71 ± 0.24 (a) | 0.29 ± 0.08 (b) | 6.68 ± 7.47 (a) |
| PF | 4.61 × 107 ± 3.26 × 107 (a) | 5.66 ± 0.31 (a) | 0.31 ± 0.07 (a) | 15.43 ± 1.00 (a) | |
| 8 months | PB | 1.04 × 106 ± 3.63 × 105 (ac) | 4.65 ± 0.34 (a) | 0.76 ± 0.29 (a) | 0.40 ± 0.35 (b) |
| PF | 1.54 × 106 ± 6.81 × 105 (a) | 5.94 ± 0.13 (a) | 0.79 ± 0.04 (ab) | 1.16 ± 0.49 (b) | |
| 12 months | PB | 9.24 × 104 ± 4.92 × 104 (bc) | 4.89 ± 0.43 (a) | 0.29 ± 0.07 (b) | 8.96 ± 6.06 (a) |
| PF | 2.49 × 105 ± 5.94 × 104 (ab) | 5.89 ± 0.16 (a) | 0.27 ± 0.07 (a) | 15.32 ± 1.80 (a) | |
| 15 months | PB | 1.52 × 105 ± 3.78 × 104 (ab) | 4.90 ± 0.17 (a) | 0.62 ± 0.10 (ab) | 0.26 ± 0.27 (b) |
| PF | 2.54 × 105 ± 6.03 × 104 (ab) | 6.18 ± 0.11 (a) | 0.61 ± 0.08 (ab) | 0.38 ± 0.30 (b) | |
| 17 months | PB | 4.18 × 104 ± 3.30 × 104 (b) | 4.68 ± 0.08 (a) | 0.60 ± 0.15 (ab) | 0.156 ± 0.11 (b) |
| PF | 5.42 × 104 ± 1.17 × 104 (b) | 6.18 ± 0.11 (a) | 0.72 ± 0.18 (ab) | 1.073 ± 0.18 (b) | |
| 21 months | PB | 1.78 × 104 ± 2.30 × 104 (b) | 5.07 ± 0.16 (a) | 0.29 ± 0.08 (ab) | 0.04 ± 0.04 (b) |
| PF | 1.15 × 104 ± 2.57 × 104 (b) | 6.07 ± 0.01 (a) | 0.31 ± 0.07 (b) | 0.44 ± 0.07 (b) | |
| Time of Sampling after Inoculation (B) P. cactorum | Substrate | Mean CFUs g−1 Dry Substrate ± SD Statistical Test: PB: ANOVA PF: Kruskal-Wallis Dunn | Mean pH ± SD Statistical Test: PB: Welch Test PF: Kruskal-Wallis Dunn | Mean NH4 (mg L−1) ± SD Statistical Test: ANOVA | Mean NO-x (mg L−1) ± SD Statistical Test: Welch Test |
| 5 months | PB | 2.17 × 107 ± 1.16 × 107 (a) | 4.46 ± 0.15 (a) | 0.37 ± 0.10 (b) | 11.33 ± 6.79 (ab) |
| PF | 5.03 × 106 ± 1.30 × 106 (a) | 5.82 ± 0.26 (a) | 0.27 ± 0.05 (c) | 14.87 ± 2.02 (ab) | |
| 8 months | PB | 1.19 × 105 ± 5.15 × 105 (ac) | 5.12 ± 0.52 (a) | 0.71 ± 0.15 (a) | 0.57 ± 0.57 (a) |
| PF | 2.08 × 106 ± 1.19 × 106 (ac) | 6.26 ± 0.07 (a) | 0.77 ± 0.15 (a) | 1.47 ± 0.20 (a) | |
| 12 months | PB | 6.02 × 104 ± 2.83 × 104 (bc) | 4.73 ± 0.18 (a) | 0.37 ± 0.03 (b) | 16.53 ± 10.68 (ab) |
| PF | 1.79 × 105 ± 7.91 × 104 (bc) | 5.88 ± 0.31 (a) | 0.38 ± 0.05 (bc) | 23.65 ± 12.80 (ab) | |
| 15 months | PB | 1.13 × 105 ± 2.46 × 104 (acd) | 5.13 ± 1.02 (a) | 0.66 ± 0.11 (ab) | 0.14 ± 0.19 (ab) |
| PF | 2.11 × 105 ± 4.10 × 104 (ab) | 5.97 ± 0.05 (a) | 0.59 ± 0.10 (ab) | 0.71 ± 0.38 (ab) | |
| 17 months | PB | 1.81 × 104 ± 1.01 × 104 (b) | 4.89 ± 0.39 (a) | 0.49 ± 0.09 (ab) | 0.11 ± 0.10 (ab) |
| PF | 1.43 × 105 ± 4.16 × 104 (b) | 6.28 ± 0.12 (a) | 0.62 ± 0.05 (a) | 0.44 ± 0.14 (ab) | |
| 21 months | PB | 3.88 × 104 ± 3.28 × 104 (bd) | 5.02 ± 0.11 (a) | 0.65 ± 0.18 (ab) | 0.01 ± 0.06 (ab) |
| PF | 1.26 × 105 ± 6.32 × 104 (b) | 6.11 ± 0.12 (a) | 0.71 ± 0.02 (a) | 0.38 ± 0.05 (ab) |
| Mortality (%) | RootDS (0–4) | PlantDS (0–4) | Fresh Weight (g) | Dry Weight (g) | Height (mm) | |
|---|---|---|---|---|---|---|
| P. cryptogea 0 PB N = 12 | 6 0 Fisher’s Test p = 0.008254 (**) | 3.17 ± 1.03 0.08 ± 0.29 Kruskal-Wallis Dunn p = 3.3 ×10−6 (****) | 3.17 ± 1.03 0.00 ± 0.00 Kruskal-Wallis Dunn p = 2 × 10−6 (****) | 4.15 ± 0.85 7.17 ± 2.05 Kruskal-Wallis Dunn p = 0.00282 (**) | 3.29 ± 0.17 3.67 ± 0.34 Kruskal-Wallis Dunn p = 0.0014 (**) | 137.30 ± 59.39 256.05 ± 41.32 Kruskal-Wallis Dunn p = 0.00013 (***) |
| P. cryptogea 21 PB N = 12 | 1 0 Fisher’s Test p = 0.008254 (**) | 2.08 ± 1.08 0.08 ± 0.29 Kruskal-Wallis Dunn p = 0.0079 (**) | 2.00 ± 1.04 0.00 ± 0.00 Kruskal-Wallis Dunn p = 0.0079 (**) | 4.54 ± 0.78 7.17 ± 2.05 Kruskal-Wallis Dunn p = 2.1 × 10−5 (****) | 3.39 ± 0.15 3.69 ± 0.34 Kruskal-Wallis Dunn p = 0.0245 (*) | 167.03 ± 49.71 256.06 ± 41.32 Kruskal-Wallis Dunn p = 0.01169 (*) |
| P. cryptogea 0 PF N = 12 | 5 0 Fisher’s Test p = 0.06176 (NS) | 2.83 ± 1.34 0.08 ± 0.29 Kruskal-Wallis Dunn p = 4.7× 10−5 (****) | 2.83 ± 1.2673045 0.00 ± 0.00 Kruskal-Wallis Dunn p = 1 × 10−5 (****) | 4.27 ± 1.03 6.23 ± 0.73 Kruskal-Wallis Dunn p = 0.00026 (***) | 3.30 ± 0.18 3.72 ± 0.42 Kruskal-Wallis Dunn p = 0.00088 (***) | 119.54 ± 52.61 256.09 ± 52.04 Kruskal-Wallis Dunn p = 6.1 × 10−5 (****) |
| P. cryptogea 21 PF N = 12 | 3 0 Fisher’s Test p = 0.06176 (NS) | 2.33 ± 1.50 0.08 ± 0.29 Kruskal-Wallis Dun p = 0.00062 (***) | 2.33 ± 1.37 0.00 ± 0.00 Kruskal-Wallis Dunn p = 0.00021 (***) | 4.84 ± 1.58 6.23 ± 0.73 Kruskal-Wallis Dunn p = 0.00594 (**) | 3.46 ± 0.30 3.72 ± 0.42 Kruskal-Wallis Dunn p = 0.04551 (*) | 167.50 ± 76.91 256.09 ± 52.04 Kruskal-Wallis Dunn p = 0.01770 (*) |
| P. cactorum 0 PB N = 12 | 2 0 Fisher’s Test p = 0.758 (NS) | 2.92 ± 0.67 0.08 ± 0.29 Kruskal-Wallis Dunn p = 3.3 × 10−5 (****) | 2.83 ± 0.83 0.00 ± 0.00 Kruskal-Wallis Dunn p = 3 × 10−5 (****) | 4.28 ± 0.80 7.17 ± 2.05 Kruskal-Wallis Dunn p = 0.00027 (***) | 3.37 ± 0.17 3.69 ± 0.34 Kruskal-Wallis Dunn p = 0.0171 (*) | 133.58 ± 47.97 256.06 ± 41.32 Kruskal-Wallis Dunn p = 0.00013 (***) |
| P. cactorum 21 PB N = 12 | 1 0Fisher’s Test p = 0.758 (NS) | 2.33 ± 1.23 0.08 ± 0.29 Kruskal-Wallis Dunn p = 0.0014 (***) | 2.25 ± 1.29 0.00 ± 0.00 Kruskal-Wallis Dunn p = 0.0013 (**) | 4.64 ± 1.16 7.17 ± 2.05 Kruskal-Wallis Dunn p = 0.00289 (**) | 3.38 ± 0.22 3.69 ± 0.34 Kruskal-Wallis Dunn p = 0.0171 (*) | 129.86 ± 34.14 256.06 ± 41.32 Kruskal-Wallis Dunn p = 0.00013 (***) |
| P. cactorum 0 PF N = 12 | 1 0 Fisher’s Test p = 1 (NS) | 2.67 ± 0.78 0.08 ± 0.291 Kruskal- Wallis Dunn p = 0.00013 (***) | 2.33 ± 0.89 0.00 ± 0.00 Kruskal-Wallis Dunn p = 0.00021 (***) | 4.30 ± 0.85 6.23 ± 0.73 Kruskal-Wallis Dunn p = 0.00026 (***) | 3.37 ± 0.15 3.72 ± 0.42 Kruskal-Wallis Dunn p = 0.01258 (*) | 141.51 ± 78.17 256.09 ± 52.04 Kruskal-Wallis Dunn p = 0.00048 (***) |
| P. cactorum 21 PF N = 12 | 1 0 Fisher’s Test p = 1 (NS) | 2.00 ± 1.04 0.08 ± 0.29 Kruskal-Wallis Dunn p = 0.00671 (**) | 2.08 ± 1.08 0.00 ± 0.00 Kruskal-Wallis Dunn p = 0.00089 (***) | 4.47 ± 0.59 6.23 ± 0.73 Kruskal-Wallis Dunn p = 0.00594 (**) | 3.39 ± 0.08 3.72 ± 0.42 Kruskal-Wallis Dunn p = 0.10943 (NS) | 174.89 ± 63.66 256.09 ± 52.04 Kruskal-Wallis Dunn p = 0.03414 (*) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benavent-Celma, C.; McLaggan, D.; van West, P.; Woodward, S. Survival of Phytophthora cryptogea and Phytophthora cactorum in Commercial Potting Substrates for Eucalyptus globulus Plants. Agriculture 2023, 13, 581. https://doi.org/10.3390/agriculture13030581
Benavent-Celma C, McLaggan D, van West P, Woodward S. Survival of Phytophthora cryptogea and Phytophthora cactorum in Commercial Potting Substrates for Eucalyptus globulus Plants. Agriculture. 2023; 13(3):581. https://doi.org/10.3390/agriculture13030581
Chicago/Turabian StyleBenavent-Celma, Clara, Debbie McLaggan, Pieter van West, and Steve Woodward. 2023. "Survival of Phytophthora cryptogea and Phytophthora cactorum in Commercial Potting Substrates for Eucalyptus globulus Plants" Agriculture 13, no. 3: 581. https://doi.org/10.3390/agriculture13030581
APA StyleBenavent-Celma, C., McLaggan, D., van West, P., & Woodward, S. (2023). Survival of Phytophthora cryptogea and Phytophthora cactorum in Commercial Potting Substrates for Eucalyptus globulus Plants. Agriculture, 13(3), 581. https://doi.org/10.3390/agriculture13030581







