Abstract
Lupine seeds are a valuable nutritive source for animal feeding, but for poultry nutrition, the content in crude fiber and non-starch polysaccharides (NSP) have an antinutritional factor. The aim of this research was to highlight the effect of partial soybean meal replacement with L. albus seeds and enzyme addition in the laying quail diets on productive performance, digestion, blood biochemical indices and egg quality. A total of 210 homogenous female Japanese quails (Coturnix japonica) at 24 week of age were randomly assigned to 6 dietary treatments, with the standard diet based on soybean meal unsupplemented (−) and supplemented with enzyme (+) (S−/S+) and the experimental diets on which the soybean meal was based partially substituted by including lupine in the amount of 200 g/kg and 250 g/kg, unsupplemented and supplemented with enzymes (L20−/L20+; L25−/L25+).The use of enzymes in the lupine-based diets allowed increasing the proportion of lupine in the diet of laying quails by up to 25% (% of feed) without changing egg production, egg weight, feed conversion rate and physical–chemical quality parameters of the eggs. In addition, the use of lupine (−/+) improved (p < 0.001) the carotenoid content of the egg yolk, as well as the quality of the yolk fats by decreasing the cholesterol content and the level of fatty acids (FA) with an atherogenic effect, in favor of omega-3 FA. Enzyme supplementation of the lupine-based diets had a negative effect on the health lipid indices of the fats in the yolk (ratio of the hypocholesterolemic/Hypercholesterolemic FA—h/H, atherogenic index—AI, thrombogenic index—TI and health promotion index—HPI). The use of exogenous enzymes increased the nutrients’ efficiency of the quails’ feed, which is supported by the improvement of the blood metabolic indices and a decrease of intestinal digesta viscosity and feces moisture. In conclusion, white lupine can be used up to 25% in the laying quail feed in association with specific enzymes without affecting the productive performance and egg quality; moreover, lupine use has improved the quality of the eggs, increasing humans’ health.
1. Introduction
At the present time, the high protein requirements for human and animal nutrition determine the protein sources which have become progressively more limited and expensive [1]. In the field of animal feeding, in the past 10 years, research attention has focused years on the detection and validation of alternative protein sources for soybean meal, considered the most important vegetable protein source. In this context, the seed of Lupinus spp. represent a potential alternative for soybean meal [2,3].
L. albus from the low-alkaloid varieties is important for monogastric animals feeding, due to their high content in crude protein (CP, 35–43%) and crude fat (EE, 8–12%) [4,5]. The amino acid content of proteins is also appreciable, and the FA profile of fats is well represented in unsaturated fatty acids (UFA), at approximately 70–75% of FAME [6,7,8]. However, the high content in crude fiber (12–15%), oligosaccharides from the raffinose family and non-starch polysaccharides (arabinose, xylose, rhamnose, mannose, galactose) reduce the potential of white lupine to substitute for soybean meal in high proportions in the feed structure of monogastrics, especially for poultry [9,10]. Among NSP compounds, attention is directed toward soluble compounds, whose antinutritional effect in poultry consists in the binding of high amounts of water giving a gelatinous consistency to the digesta, which contributes to the increase of intestinal tract viscosity and to the reduction of nutrients’ absorption [11,12]. It is well known that poultry do not possess specific endogenous enzymes for the efficient use of these compounds [13,14].
A common practice worldwide is the use of carbohydrase enzymes to improve feed energy [15]. Supplementing the poultry diets with multi-carbohydrase can improve nutrient digestibility and growth rates [16,17,18]. The main mechanism behind this consists of improving nutrient availability through the NSP degradation [19,20]. Moreover, it can degrade the cell walls, enhancing the access of endogenous enzymes [20,21,22].
Available research in the field has revealed that the use of exogenous multi-enzymes combinations containing cellulase, β-glucanase, xylanase, hemicellulase, pentosanase and pectinase in broiler feeds has allowed an increase of L. albus seeds’ inclusion by up to 35% in the diet, without influencing productive performance, because the digestibility of proteins, fats and NSP from feed has improved, while the viscosity of the intestinal digesta has decreased [23,24,25,26]. Moreover, L. luteus seeds were efficiently used up to 40% in the diet of broilers from 1 to 25 days age old when a mix of enzymes (β-glucanase, hemicellulase, pectinase, endoglucanase, cellulase, β-xylanase and protease) was used, due to the feed conversion improvement as a result of a better NSP valorization [11,27].
To our knowledge, research about the addition of specific enzymes in the lupine-based diets for laying hens is limited and has only partially examined the effects on productive performance or egg quality. In this regard, the use of enzymes derived from a natural culture of Aspergillus Niger in the laying hens’ diet which contains 150 g/kg of blue lupine has led to obtaining similar productive performances to the use of only soybean meal [28]. Research regarding the effects of exogenous enzyme use in poultry diets containing lupine seeds is relatively limited.
Therefore, considering this background, as well as a lack of information related to improving lupine use efficiency in the diets of poultry for egg production, we aimed to investigate the possibility of increasing the use of lupine in quail feeding, by the addition of specific exogenous enzymes in relation to productive performance, quality of the eggs and physiological status of the birds.
In this research, the following hypotheses were evaluated: (1) an increase proportion of soybean meal substitution with lupine seeds by supplementing the laying quail diets with exogenous enzyme, without affecting the productive performance and health status; (2) substantial changes in egg quality are expected (egg weight, fatty acid profile and health lipid indices of yolk, and yolk content in carotenoids) after the inclusion of lupine and exogenous enzyme in the diets.
2. Materials and Methods
2.1. Animal Ethics
The experimental procedures were reviewed and approved by the ethical committee of the University of Agricultural Science and Veterinary Medicine of Cluj-Napoca, number 291/22/11/2021.
2.2. Birds and Experimental Design
A total of 210 homogenous laying Japanese quails at 24 weeks (wk) of age were distributed in a completely randomized design of a 3 × 2 factorial arrangement. The dietary factors were: (1) three sources for ensuring the protein requirements (soybean meal and two levels of lupine seed: 20% and 25% in feed); (2) the enzymes addition (commercial product Hostazym® X: 0 and 0.02%—in feed). Hostazym® X is a non-GMO enzyme complex consisting of endo-1.4-β-xylanase (EC 3.2.1.8), endo-1.4-β-glucanase (cellulase; EC 3.2.1.4), 1.3(4)-α-glucanase (EC 3.2.1.59), α-amylase (EC 3.2.1.1) and protease. The primary enzymatic activity is endo-1.4-β-xylanase (minimum enzyme activity: 6000 EPU/g produs) and the secondary is that of endo-1.4-β-glucanase (200 units/g produs), 1.3(4)-α-glucanase (60 units/g), α-amylase (120 units/g) and protease (traces). The additive Hostazym® X micro-granulate was introduced into quail feed in a quantity of 0.2 g/kg, ensuring an enzyme activity of 1200 EPU/kg feed for β-xylanase; 40 units/kg feed for β-glucanase; 12 units/kg feed for α-glucanase and 24 units/kg feed for α-amylase. The quails were randomly assigned to 6 dietary treatments (experimental groups), with each treatment consisting of 5 replicates with 7 birds/unit (35/group). The quails were kept in cages with an equal surface area of 337.5 cm2/bird. The trial period lasted for 8 wk (age of the birds: 24–32 weeks) after an initial adaptation period of 2 wk. During the trial, all technological parameters specific to laying quails were ensured (temperature 22 °C, humidity 65%, ventilation 0.2 m/s and 18 h light/day).
2.3. Experimental Diets
All groups of birds were randomly assigned to the tested diets. The ingredients and nutrient levels of laying quail diets are listed in Table 1.

Table 1.
Ingredients and nutritional characteristics of diets used in the laying quails’ feeding experimental procedure (as-fed basis, %).
All diets ensured the nutritional requirements of quails [29]. The main protein source (soybean meal) of standard diet (S) was partially substituted by including the lupine without enzymes (−): L20− = group with lupine (200 g/kg of feed) and L25− =group with lupine (250 g/kg). The treatments with enzymes were marked with + (C+, L20+, L25+). The quails feeding was ad libitum.
2.4. Ouails’ Performance Evaluation
The birds were weighed individually at the start and end of the trial. The following parameters were determined on a weekly basis: feed intake, laying rate, egg weight and feed conversion rate. The birds’ health and behavior were observed daily.
2.5. Egg Collection and Evaluation
The egg morphological structure (albumen, yolk and eggshell-% of the whole egg) from a number of 25 eggs/treatment (5 eggs/replicate) were assessed bi-weekly (at 2, 4, 6, and 8 wk).
The physical characteristics of the eggs were assessed based on: AI (albumen index = equatorial diameter ÷ height × 100), YI (yolk index = equatorial diameter ÷ height × 100), and HU (albumen Haugh Unit = 100 log (height − 1.7) × egg weight0.37 + 7.57) [30].
The eggshell thickness was measured at three different points (sharp end, equator and air cell) without internal membrane and after they were dried in the oven [14].
The values of yolk color were performed by the ”La Roché scale”, with color samples corresponding to values from 1 to 15. Egg quality evaluations (chemical content of the albumen and yolk, egg yolk fatty acids, cholesterol, egg yolk carotenoids and health lipid indices) were performed only for the eggs provided from groups with best performance responses: the birds fed with standard diets (S− and S+) and of which the lupine seed were used at a level of 20% in feed (L20− and L20+).
2.6. Feed and Egg Chemical Analyses
2.6.1. Feed and Egg Composition
The white lupine seeds (cv. Amiga; low-alkaloid variety) were provided by a local farmer from Romania (Transylvanian area). From the collected seeds, the following analyses of samples were performed in the laboratory: raw chemical composition, amino acids (AA) and fatty acid (FA) content (n = 5).
The raw chemical composition was performed according to AOAC International [31] and the nitrogen free extract (N-FE) by difference (100% − CP% + CA% + EE% + CF%). Nitrogen-corrected metabolizable energy (AMEN) of lupine was calculated according to Sibbald [32]:
where MG = crude fat, CB = crude fiber and Ce = crude ash.
AMEN = 3951 + 54.4 MG − 88.7 CB − 40.8 Ce,
The AA determination from lupine proteins (n = 5) was performed by HPLC (high performance liquid chromatography) according to the SR EN ISO 13903: 2005 standard and the method performed by Struți et al. [14]. All amino acids (AA) were determinated in the presence of ninhydrin and the AA identification was performed by comparing the retention times of each AA resulting from lupine samples with those of the standard (Santa Cruz Biotechnology standard).
Fatty acids from lupine fats were identified as FAME (methyl esters of fatty acids; g/100 g of total) using the technique of GC-MS (gas chromatography with mass spectrometry detection), according to the standards: SR EN ISO/TS 17764-2: 2008 and ISO 5508: 2002. The steps of the method followed are described by Struți at al. [14]. In the lupine fats, the major fatty acids identified were: palmitic (C16:0); stearic (C18:0); oleic (C18:1 n-9); eicosenoic acid (C20:1 n-9); linoleic acid (C18:2 n-6); α-linolenic acid (C18:3 n-3). The identification of FA peaks was realized by comparing the FAME relative retention time with that of the standard (Mix FAME Supelco 37).
The raw chemical content of albumen and yolk was analyzed according to the methods of AOAC International [31] (albumen: CP and CA and yolk: EE, CP and CA).
2.6.2. Egg Yolk Fatty Acid Analysis
The FA profile of egg yolk was performed from a number of 5 eggs randomly selected from each replicate and group: miristic (C14:0); miristoleic (C14:1); pentadecanoic (C15:0); pentadecenoic (C15:1); palmitic (C16:0); palmitoleic (C16:1); heptadecanoic (C17:0); heptadecenoic (C17:1); stearic (C18:0); oleic (C18:1 n-9); linoleic (C18:2 n-6); γ-linolenic (C18:3 n-6); α-linolenic (C18:3 n-3); eicosadienoic (C20:2 n-6); eicosatrienoic (C20:3 n-6); eicosapentaenoic (C20:3 n-3); erucic (C22:1 n-9); arachidonic (C20:4 n-6); nervonic (C24:1 n-9); docosatetraenoic (C22:4 n-6); docosapentaenoic (C22:5 n-3) and docosahexaenoic (C22:6 n-3). The fatty acids analysis was carried out in three steps: extraction with chloroform–methanol [33], methylation [34] and identification of fatty acids by gas chromatography analysis according to Struți et al. [14].
2.6.3. Egg Yolk Cholesterol Analysis
The cholesterol content from egg yolk was performed according to AOAC International [35] and Method no. 994.10 and 976.26. The principle consists of the saponification of lupine samples, followed by petroleum ether extraction. The concentration extract was resumed in chloroform and standard solution of cholesterol in chloroform 10 mg/mL was used [36,37]. The Perkin Elmer-Clarus 500 GC used was set up according to Struți et al. [14].
2.6.4. Egg Yolk Carotenoid Analysis
The carotenoids were extracted with a mixture of methanol/ethyl acetate/petroleum ether (1:1:1) according to the procedure described by Schlatterer et al. [38]. A total of 5 g of yolk/sample was used (5 samples/group). The LGC Standards (UK) were used for the identification of zeaxanthin, lutein and β-cryptoxanthin.
2.6.5. Nutritional Indices and FA Ratios
In order to highlight the influence of the dietary treatments on the nutritional qualities of yolk fats, it was considered optimal to calculate some health lipid indices (n = 5), as follows:
Ratio of n-6/n-3 FA.
Polyunsaturation index [39]:
PI = C18: 2 n-6 + (C18: 3 n-3 × 2)
Atherogenic index [40]:
AI = (C12:0 + C16:0 + 4 × C14:0) ÷ [ΣMUFA + Σ(n − 6) + Σ(n − 3)]
Thrombogenic index [40]:
TI = (C14:0 + C16:0 + C18:0) ÷ [0.5 × ΣMUFA + 0.5 × Σ(n − 6) + 3 × Σ(n − 3) + Σ(n − 3) ÷ Σ(n − 6)]
Hypocholesterolemic/Hypercholesterolemic FA ratio [41]:
h/H = (C18:1 + PUFA) ÷ (C12:0 + C14:0 + C16:0)
Health promotion index [42]:
HPI = UFA ÷ [C12:0 + (4 × C14:0) + C16:0)]
2.7. Measurements of the Excreta Dry Matter and Digesta Viscosity
The dry matter of excreta was evaluated every 7 days, from the fresh feces that resulted in 60 min (n = 5/group). The samples were cleaned of impurities and dried in an oven (105.5 °C).
The intestinal content viscosity was determined on the last day of the experimental period (n = 5/group).
The samples were collected from the digestive content of ileon and prepared according to Konieczka and Smulikowska [43]. The Brookfield viscometer (model LVDV E, Brookfield Engineering Laboratories, Middleboro, MA, USA) was used to determine the viscosity in cP (centipoise).
2.8. Blood Parameters
Samples (n = 5) of fresh blood were collected in vacutainers on lithium–heparin medium after 5 h of no feed. At the laboratory, the samples were centrifuged for 30 min at 3000 rotations/min to separate the blood plasma and were then stored at −20 °C. The following were determined: hemoglobin (Hb, g/dL); hematocrit (Hct, %); erythrocytes (Ery, mL/mm3); total proteins (Prot, g/dL); total lipids (Lip, mg/dL); albumins (Alb, g/dL); γ-globulins (g/dL); aspartate aminotransferase (AST, U/L); alanine aminotransferase (ALAT, U/L); urea (mg/dL); creatinine (Creat., mg/dL); glutathione peroxidase (GPx, U/gHb); superoxide dismutase (SOD, U/gHb); cholesterol (Chol, mg/dL); triglycerides (Try, mg/dL) [44].
2.9. Statistical Analysis
All data were analyzed using the GLM procedure, software system ver. 10.0 (StatSoft Inc., Tulsa, OK, USA, 2011). The experiment was a completely randomized 3 × 2 factorial design, with a two-way ANOVA performed to assess the main effects of dietary lupine seed level (20 and 25%) compared with the standard diet, without and with enzymes supplementation (−/+), as well as the interaction with these factors (lupine level and enzymes). The two-way ANOVA was performed to assess the effects of dietary treatments on the excreta dry matter content and intestinal viscosity, blood biochemical parameters and egg quality (raw chemical composition, fatty acids, nutritional indices, carotenoids, cholesterol). The Tukey multiple-range test was used to compare the differences between the mean values of applied treatments. Differences were considered significant when p < 0.05. All data were presented as means with a pooled standard error of the mean estimates.
3. Results
3.1. Chemical Composition of the Lupine Seeds
In addition to the high content in crude protein (CP, 43.11% of DM) and fat (CF, 10.55% of DM), lupine seeds are also characterized by a high level of essential amino acids (e.g., lysine—4.98 g/16 g N; arginine—9.42 g/16 g N) but also in UFA, of which the oleic acid (C18:1 cis-9; OA), linoleic acid (C18:2 n-6; LA) and α-linolenic acid (C18:3 n-3; ALA) are well represented (Table 2).

Table 2.
Chemical composition, major amino acids and fatty acids of white lupine from low-alkloid varieties (cv. Amiga).
3.2. Quails’ Performance
Feeding quails with diets containing 20% and 25% of white lupine with the enzymes did not influence the weight of the birds (p > 0.05) (Table 3).

Table 3.
Influence of enzymes addition in lupine-based diets on the productive performance of quails (mean ± std. error).
The laying rate (%) was influenced by the use of lupine in the diets, with the lowest laying intensity recorded when the lupine was added in an amount of 250 g/kg in the feed (L25−) without enzymes (p ˂ 0.001) (Table 3). The use of exogenous enzymes in lupine-based diets led to an increase in egg production (p < 0.001). The use of lupine in the amount of 200 g/kg in the diet with enzymes (L20+) resulted in the highest egg production.
The feed intake (g feed/bird/day) was not influenced (p > 0.05) by the applied treatments (Table 3). The quails fed with lupine in the amount of 250 g/kg in their diets (L25− and L25+) had similar feed intakes to quails with standard diets (S− and S+) (32.78 and 32.34 vs. 32.52 and 32.34 g of feed/quail/day) (Table 3).
The feed conversion ratio (FCR) was influenced by the applied treatments (Table 3). The use of lupine in the amount of 250 g/kg in the diet without enzymes led to the FCR depreciation (p < 0.05). Enzyme inclusion in the diet did not affect the FCR (Table 3).
In the trial period, no specific symptoms of a pathological condition or a nutritional deficiency were observed.
3.3. Excreta Dry Matter Content and Intestinal Viscosity
The feces moisture of the quails fed with lupine based-diets was higher, while the enzymes addition had a positive effect, as it ensured a level of moisture close to that obtained by quails fed with standard diets (Table 4). Large amounts of lupine in the feed, without enzymes (L25−), led to the highest level of excreta moisture.

Table 4.
Influence of enzymes addition in lupine-based diets on the excreta moisture and intestinal viscosity of the quails (mean ± std. error).
The use of lupine without enzymes (L20−; L25−) in the diets of laying quails increased the viscosity of the intestinal digesta (p ˂ 0.001), compared to the standard diet (S−) and also with the diets with enzymes. When the enzymes were used (L20+ and L25+), a decrease (p ˂ 0.001) in the intestinal digesta viscosity of the quails was achieved (Table 4).
3.4. Blood Hematological and Biochemical Parameters
The use of lupine and the enzymes in the laying quail feeding did not affect (p > 0.05) the hematological parameters: Hct (hematocrit %); Hb (hemoglobin g/dL); Ery (erythrocytes mL/mm3); Alb (albumins g/dL); γ-globulins (g/dL) (Table 5).

Table 5.
Influence of enzymes addition in lupine-based diets on the blood biochemical parameters of quails.
The highest value of plasmatic urea and creatinine was recorded when the lupine was used at 250 g/kg in feed, but the use of enzymes led to lower values (p < 0.05) at a level similar to that of quails fed with standard diets (Table 5).
The indice values of the lipid profile have a decreasing trend as a result of the enzymes addition, as well as with the lupine dose increase in the feed (Table 5). Compared to the groups without lupine, the birds from L25+ recorded the lowest values (p < 0.05) of plasmatic Lip, Try and Chol.
Moreover, the presence of lupine in the diets led to an increase (p < 0.05) in the SOD and GPx values (Table 5).
3.5. Physico-Chemical Traits of the Eggs
The lupine-based diets without enzymes (L20− and L25−) led to obtaining eggs with a lower weight (p < 0.05) (Table 6). An egg weight improvement was achieved when the lupine-based diets were supplemented with specific enzymes (L20+ and L25+), with the values similar to the weight of eggs obtained from the birds fed with standard diets (S− and S+) (Table 6).

Table 6.
Influence of enzymes addition in lupine-based diets on the physical quality indices of fresh eggs (mean ± std. error).
The proportion of the egg’s morphological components, namely the albumen (%), yolk (%) and shell (%), were influenced by the applied treatments (Table 6). The use of lupine in the quail diets (200 and 250 g/kg) led to an increase (p < 0.05) in the albumen weight.
The proportion of yolk and eggshell decreased (p < 0.001) when the lupine was included in the diets (without enzymes) (Table 6). The presence of enzymes in diets led to an increase in yolk weight, but the weight of the eggshell did not change (Table 6). The decrease in eggshell weight at the same time as the lupine inclusion in diets is associated with the reduction in eggshell thickness (p < 0.05) (Table 6).
The use of lupine in the diets (−/+) did not influence (p > 0.05) the physical quality indices of the eggs, namely, AI, YI and HU (Table 6).
The partial substitution of soybean meal with lupine (−/+) did not affect (p > 0.05) the CP and CA in albumen, nor the CF, CP and CA of yolk (Table 7).

Table 7.
Influence of enzymes addition in lupine-based diets on the chemical composition of quail eggs (% of DM).
3.6. Egg Yolk Fatty Acids and Cholesterol Content
The fats from the yolk are rich in several fatty acids such as oleic acid (31.67–34.00% of FAME), palmitic acid (19.04–23.21% of FAME), linoleic acid (16.92–18.52% of FAME) and stearic acid 13.85–14.38% of FAME) (Table 8).

Table 8.
Influence of enzymes addition in lupine-based diets on FAs’ profile of fats in the yolk of quail eggs (% of FAME).
The use of lupine white in the laying quail diets led to an improvement in the fatty acid profile of yolk fats, as a result of the decrease in the SFA proportion (mainly palmitic acid; p < 0.01) and an increase in the PUFA proportion (p < 0.001) (mainly α-linolenic acid—ALA, eicosapentaenoic acid—EPA and docosahexaenoic acid—DHA) (Table 8).The use of enzymes in quail diets did not influence the SFA and cholesterol level in the yolk fats (p > 0.05) (Figure 1). The addition of enzymes in the lupin-based diets of quails led to a decrease in the PUFA level (27.98 vs. 26.39% of FAME) (p < 0.05) of yolk (Table 8).
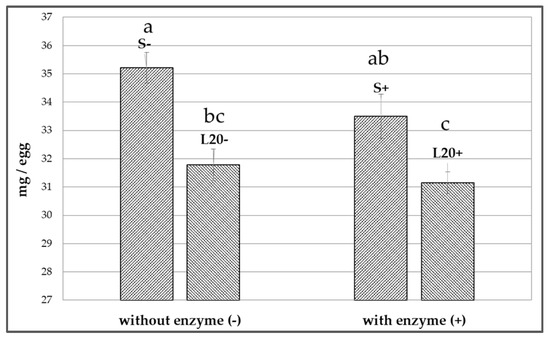
Figure 1.
Influence of enzymes addition in lupine-based diets on the cholesterol content of quail eggs. a–c: bars with no common letters differ significantly (p < 0.05). S−: standard diet without enzymes; S+: standard diet with enzymes; L20−: experimental diet (200 g/kg lupine) without enzymes; L20+: experimental diet (200 g/kg lupine) with enzymes.
3.7. Egg Yolk Carotenoids Content
The carotenoids content of egg yolk increased (p < 0.001) after lupine and also enzymes were used in the laying quail diets (Table 9).

Table 9.
Influence of enzymes addition in lupine-based diets on the carotenoids content (µg/g yolk) and the color of the yolk.
Lupine use in the diets of laying quails led to an increase (p < 0.05) in the yolk color intensity, but the enzymes addition did not influence this parameter (p > 0.05).
3.8. Nutritional Indices for Assessing the Fatty Acids of Egg Yolks
The use of lupine in quail diets led to an improvement in the health lipid indices, except for n-6 FA and PUFA/SFA (Table 10). The enzymes addition in the quail diets had a negative effect on the value of the health lipid indices of yolk fats (Table 10).

Table 10.
Influence of enzymes addition in lupine-based diets on the lipid quality indices of the fats from egg yolk.
The use of lupine in the quail diets led to obtaining eggs with health lipid indices favorable to the consumer’s health (Table 10). As a result, compared to the eggs provided from quails fed with standard diets, the proportion of FA with a hypercholesterolemic (HPA) effect decreased and the content of FA with a hypocholesterolemic (hFA) effect increased (p < 0.05) (Table 10).
Moreover, the n-6/n-3 ratio decreased (p < 0.001), but the n-3 FA content and h/H FA ratio increased (p < 0.05) (Table 10). In addition, the presence of quality fats in lupine-based diets caused a decrease of the atherogenic (AI) and thrombogenic index (TI) values, as well as an increase of the health promotion index (HPI) (p < 0.05) values of the fats (Table 10).
The addition of enzymes in the diets containing lupine had a negative effect on the quality of the fats in the egg yolk, producing an increase in the HFA proportion and a decrease in the hFA content, which had a negative effect on the value of the health lipid indices (n-6/n-3 FA, h/H, AI, TI and HPI) (Table 10).
4. Discussion
The purpose of this research was to increase the efficiency of lupine use in quail nutrition by adding enzymes to improve the digestion and nutrients utilization, especially non-starch polysaccharides. As a result, the productive performances of the quails fed with lupine-based diets and enzymes were similar to those fed with standard diets. The egg quality was improved by introducing lupine into the diets, due to the increase in the n-3 FA proportion in yolk fats.
4.1. Productive Performance
The use of enzymes in the lupine-based diets allowed a higher nutrients’ utilization in quails due to the minimization of the antinutritional effect of NSP from lupine; therefore, the body weight was not affected. Similarly, to our findings, Lee et al. [28] showed that the weight of laying hens was not influenced by the use of enzymes (Allzyme–Aspergillus niger) in a diet which contained 150 g/ kg blue lupine.
The enzymes addition in lupine-based diets led to an egg production comparable to the group without lupine, where the main protein source was soybean meal. These findings can be justified by the efficient activity of the enzymes which diminished the negative effects of NSP in the gastrointestinal tract of birds and improved the digestion processes and nutrients absorption from feed [27]. Similar findings were previously reported by Lee et al. [28], with laying hens fed a lower content of blue lupine (150 g/kg) in their diet.
In our research, a trend of improving the daily feed intake of quails was observed when the enzymes were included in the lupine-based diets. Similar findings have been reported by Brenes et al. [45], who revealed that the inclusion of enzymes (β-glucanases, hemicellulases, pectinases, endoglucanases, proteases and α-galactosidases) in the lupine-based diets (350 g/kg white lupine in feed) of broilers improved their daily feed intake (by 3.8%), being close to that of a standard diet. Quails fed with lupine based-diets with enzymes have a similar FCR (kg feed/kg egg mass) to those of standard diets (p > 0.05). Based on these results, the addition of enzymes improved the feed efficiency for quails and contributed to the successful partial substitution of soybean meal with lupine in diets. Similar findings with broilers highlight an improvement in feed utilization by 9% as a result of enzymes addition in the lupine-based diets, due to the increase of digestion and nutrient absorption [11,23,24].
The soluble NSP from lupine seeds bind large amounts of water and increases the viscosity of the intestinal digesta, thus reducing the nutrient digestion and absorption [13,27]. In our research, the antinutritional effects of NSP were reduced when the exogenous enzymes were included in the diets of quails, causing an increase in the feed energy availability and increasing the efficiency of nitrogen assimilation, which led to the improvement of productive performances [13,18,20].
4.2. Excreta Moisture and Intestinal Viscosity
The specific enzymatic activity of endo-1.4 β-xylanases, endo-1.3(4) β-glucanases and galactosidases contributed to the degradation of soluble NSP from lupine, which has the ability to retain high amounts of water [9,46]. This led to a decrease in the excreta moisture of quails from enzyme-supplemented diets (p < 0.01).
The role of the soluble NSP to increase the intestinal transit time at the area of the small intestine is well known, due to the modification of useful microbiota and the resultant viscous consistency [43,47]. The addition of enzymes into lupine-based diets reduced the viscosity of the quails’ intestinal chyle, contributing to the increase in digestibility and absorption of nutrients [48,49]. The decrease of intestinal viscosity of broiler chickens was reported by Kocher et al. [23], when the exogenous enzymes (endo-1.4-β-xylanase, endo-1.3-β-glucanase, pectinases, cellulases, hemicellulases) were included in the lupine-based diets (350 g/kg).
4.3. Biochemical Blood Parameters
The enzymes addition into quail diets improved the NSP digestion which led to an increase in the available energy in the organism, ensuring a better use of proteins from feed in the synthesis processes. Therefore, there was a resultant decrease in plasmatic urea and creatinine levels, which are the final compounds of proteins catabolism (Table 5). The use of multi-enzymatic complex for NSP degradation in broilers was previously shown to decrease uric acid, which suggests an improvement in nutrients’ use from feed [50].
The use of white lupine in quail diets and the addition of enzymes had a beneficial effect on lipid metabolism, which consisted of a decrease in the total level of Lip, Chol and plasma Try (p < 0.05). This is due to a higher PUFA content from lupine and to the improvement in nutrients’ digestibility realized by enzymes. In this way, Konca et al. [51] concluded that raw materials rich in PUFA can reduce blood cholesterol levels of quails. Similar to our results, Straková et al. [52] showed that plasma triglycerides and cholesterol levels in laying hens decreased significantly when 50% of soybean meal from the diet was substituted with white lupine.
The lower values of ALT and AST in the blood of quails fed with diets supplemented with enzymes may suppose a higher level of protein use from the feed. This may be due to the protease content of the enzymes complex; therefore, a lower quantity of AA was deaminated [53].
The use of lupine up to 250 g/kg in the quail’s diet (without enzymes) led to increased values of SOD and GPx, being consistent with our previous findings in quails [44]. These two enzymatic biomarkers of antioxidant activity in the organism increase when oxidative stress is induced by the increase of UFA in the blood [53,54].
It can be considered that enzymes addition improves the use efficiency of lupine and ensures a successfully inclusion at a level of 25% in the laying quails’ feeding without affecting their physiological and health status.
4.4. Egg Quality
The use of white lupine without enzymes in the diet of quails led to obtaining eggs with a lower weight compared to the standard diet. Previous studies have reported a reduction in egg weight when L. albus seeds in amounts of 180–300 g/kg were included in the laying hens’ feed [55], or L. luteus seeds in amounts of 250 g/kg [56]. Previously, Hammershøj and Steenfeldt [57] reported a significant decrease in egg weight when lupine was used in feed at a level of 250 g/kg. However, some of the research revealed that supplementation of up to 22% lupine did not exert a deleterious effect on egg weight [58,59].
The addition of exogenous enzymes contributed to an increase in egg weight (p < 0.05) (case of L20+), respectively, to obtaining eggs with a similar weight (p > 0.05) to those of quails fed with soybean meal. These findings are in agreement with research conducted by Lee et al. [28], which used an enzyme complex in the diet of hens with blue lupine (150 g/kg in feed) and obtained eggs with a similar weight (p > 0.05) to those of hens fed a standard diet.
Similar to our results, a reduction in hens’ eggshell weight (p < 0.05) was also found by Lee et al. [28], even though the lupine-based diet was supplemented with enzymes. However, Nguyen et al. [13] revealed that xylanase inclusion in the wheat-based diets (higher in soluble NSP) improved the shell thickness of eggs, due to increased digestion and absorption of feed minerals, but this effect was not confirmed in our research.
The yolk index is an indicator of the spherical nature of the egg yolk and can be used to reflect freshness [60]. A yolk index of 48.9–51.7% is considered optimal for a fresh egg and the decrease occurs over time as a result of the perivitelline membrane alteration, following the hydrolytic processes [61]. In the present research, there were no differences regarding the yolk index, which means that lupine do not influence this physical parameter of egg quality.
The Haugh Unit (HU) is frequently used as a standard indicator for assessing the quality of albumen proteins. The HU values are influenced by the albumen content in ovomucin, which is a glycoprotein that gives the albumen thick its viscous property [61,62]. The ovomucin is composed of α-ovomucin and β-ovomucin, and the α part is composed of acidic amino acids such as glutamic acid and aspartic acid, while the β part is predominantly composed of hydroxyl amino acids such as threonine and serine [63,64]. Therefore, the content of the diets in these amino acids influences the ovomucin composition, and the lupine seeds are rich in glutamine and aspartic acid, which can explain the slightly higher values of HU values obtained for the eggs provided from quails fed with lupine-based diets. A high value (HU: 85–98%) corresponds to a very good quality of egg albumen [65].
The applied treatments in quail’s feed did not influence the chemical composition of the albumen and yolk (p > 0.05). Similar findings have been reported by Lee et al. [28] in a study conducted on laying hens.
The differences between the FAs’ content of the fats from the egg yolk of quails fed with lupine-based diets and those fed with standard diets are due to the high amount of UFA in the lupine seeds which are rich in oleic, linoleic and α-linolenic acid. Thus, the fatty acid profile of yolk fats has improved (p < 0.05) as a result of the increase in the FA proportion from omega-3 series (ALA, EPA, DHA), considered important for human health [66,67,68]. Some researchers consider the current Western diets as being generally deficient in n-3 FA compared to the diets of their human ancestors [69]. Therefore, one way to increase the intake of n-3 FA is by consuming natural sources rich in these FA, such as fish or linseed [70], or by consuming functional foods enriched in n-3 FA such as eggs [71,72,73].
The enzymes addition in the diets did not affect the fatty acid profile of fats from the egg yolk, with the exception of PUFA which decreased, being in line with some previous reports [74,75]. These studies reveal that no significant differences were found regarding the FA profile of egg yolk after feeding laying hens with diets based on different proportions of corn–wheat–soybean meal and multienzymes consisting of xylanase, protease and amylase. In another study, Westbrook et al. [76] reported a decrease in PUFA content (especially linoleic and arachidonic acid) after the addition of xylanase in diets based on corn and flaxseed. In the current research, the enzymes addition did not affect the levels of n-3 FA. Contrary to our findings, research carried out by Jia et al. [77] showed that the use of multicarbohydrase in the diets of hens which contain flaxseed can result in an increase (p < 0.05) in the n-3 FA content of yolk fats. The authors attribute these findings to a depolymerization of the polysaccharides cell wall due to the enzymatic action, which increase the availability of fats in the intestine and favor a better action of digestive enzymes.
The lower cholesterol content in the eggs of quails fed with lupine is due to the high quality of lupine fats, which is characterized by a high level in PUFA [8]. Our results are not confirmed by the data reported by Krawczyk et al. [78], where the cholesterol content did not change in the eggs of laying hens fed with diets containing yellow lupine in amounts of 300 g/kg compared with a standard diet.
It is known that carotenoids are fat-soluble pigments which are absorbed by passive diffusion together with dietary lipids by forming mixed micelles. Papadopoulos et al. [79] claimed the possibility that an improvement in the nutrients’ digestion and absorption by xylanase supplementation could be reflected in the carotenoid content of egg yolks. Their research showed that the properties of xylanase NSP enzyme improved the digestibility and absorption of specific dietary nutrients such as carotenoids from wheat-based diets in laying hens [79]. In our research, the high content of carotenoids in the yolk of quail eggs is because of the presence of lutein, zeaxanthin and canthaxanthin in the lupine seeds [80]. Increasing the concentration of carotenoids in egg yolk by using lupine in the diet of birds has also been reported in other studies [56,80].
Egg yolk color is influenced by the natural pigments from the feed [81,82]. The yolk enrichment in carotenoids led to a higher yolk color intensity (p < 0.05) in quail groups fed lupine-based diets. Canthaxanthin is a red carotenoid which convert the typical yellow–orange color of the yolk into an orange–red color [79]. In our research, canthaxanthin has the highest level in the egg yolk from quails fed with lupine-based diets. The final yolk color is given by the content in yellow and red carotenoids, because the yellow base is necessary to establish a good saturation, while the red carotenoids act additively to establish the final orange–reddish color [79]. The addition of enzymes in the quail diets did not influence (p > 0.05) the yolk color intensity, being in agreement with the report by Lee et al. [28] that showed a similar color intensity of the yolks, even if the enzymes were added in the lupine-based diet of laying hens. However, recently, Nguyen et al. [13] showed an improvement in the egg yolk color following xylanase supplementation of the wheat-based diets in laying hens.
5. Conclusions
This research reveals that lupine seeds can be used in laying quail diets up to 20% (% of feed), as an alternative source to proteins of soybean meal, without any negative effect on productive performance. The addition of commercial enzymes (Hostazyme®) in diets containing white lupine has proved to be a feasible nutritional strategy, which allows an increase in the lupine proportion in quail diets by up to 25% (% of feed) without negatively influencing egg production, egg weight and FCR.
White lupine can be used as part of a global strategy to improve the nutritional quality of egg yolk fats (by increasing the omega-3 FA and carotenoid content and decreasing cholesterol concentration), although data have shown a negative effect on eggshell thickness. The FA profile and health lipid indices of the yolk fats were negatively influenced by the presence of enzymes in the laying quail diets.
It is important to continue the research in order to improve the use efficiency of lupine in quails feed by using enzymes or a mix of enzymes adapted to the chemical components of lupine, but also by associating with a source of polyunsaturated FA (e.g., hemp seeds, flax seeds, camelina seeds) to contribute to the yolk enrichment in FA with a health effect and bioactive compounds with an antioxidant role.
Author Contributions
Conceptualization, D.I.S. and D.M.; methodology, D.I.S. and D.M.; software, D.I.S.; validation, D.I.S., D.M. and A.B.; formal analysis, D.I.S., D.M. and A.B.; investigation, D.I.S., D.M. and A.B.; resources, D.I.S., D.M. and A.B.; data curation, D.I.S., D.M. and A.B.; writing—original draft preparation, D.I.S. and D.M.; writing—review and editing, D.M. and A.B.; visualization, D.I.S., D.M. and A.B.; supervision, D.M. and A.B.; project administration, D.M.; funding acquisition, D.I.S. and D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Oradea, Romania.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Ethics Committee of the University of Agricultural Science and Veterinary Medicine of Cluj-Napoca, number 291/22/11/2021.
Data Availability Statement
The data supporting the reported results are in the possession of the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Henchion, M.; Hayes, M.; Mullen, A.; Fenelon, M.; Tiwari, B. Future protein supply and demand: Strategies and factors influencing a sustainable equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- De Visser, M.; Schreuder, R.; Stoddard, F. The EU’s dependency on soya bean import for the animal feed industry and potential for EU produced alternatives. OCL 2014, 21, D407. [Google Scholar] [CrossRef]
- Lucas, M.M.; Stoddard, F.L.; Annicchiarico, P.; Frías, J.; Martínez-Villaluenga, C.; Sussmann, D.; Duranti, M.; Seger, A.; Peter, M.Z.; Pueyo, J.J. The future of lupin as a protein crop in Europe. Front. Plant Sci. 2015, 6, 705. [Google Scholar] [CrossRef]
- Chiofalo, B.B.; Lo Presti, V.; Chiofalo, V.; Gresta, F. The productive traits, fatty acid profile and nutritional indices of three lupin (Lupinus spp.) species cultivated in a Mediterranean environment for the livestock. Anim. Feed Sci. Technol. 2012, 171, 230–239. [Google Scholar] [CrossRef]
- Musco, N.; Cutrignelli, M.I.; Calabrò, S.; Tudisco, R.; Infascelli, F.; Grazioli, R.; Lo Presti, V.; Gresta, F.; Chiofalo, B. Comparison of nutritional and antinutritional traits among different species (Lupinus albus L., Lupinus luteus L., Lupinus angustifolius L.) and varieties of lupin seeds. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1227–1241. [Google Scholar] [CrossRef] [PubMed]
- Sujak, A.; Kotlarz, A.; Strobel, W. Compositional and nutritional evaluation of several lupin seeds. Food Chem. 2006, 98, 711–719. [Google Scholar] [CrossRef]
- Boschin, G.; D’Agostina, A.; Annicchiarico, P.; Arnoldi, A. Effect of genotype and environment on fatty acid composition of Lupinus albus L. seed. Food Chem. 2008, 108, 600–606. [Google Scholar] [CrossRef]
- Mierliță, D.; Simeanu, D.; Pop, I.M.; Criste, F.; Pop, C.; Simeanu, C.; Lup, F. Chemical composition and nutritional evaluation of the lupine seeds (Lupinus albus L.) from low-alkaloid varieties. Rev. Chim. 2018, 69, 453–458. [Google Scholar] [CrossRef]
- Knudsen, K.E.B. Fiber and nonstarch polysaccharide content and variation in common crops used in broiler diets. Poult. Sci. 2014, 93, 2380–2393. [Google Scholar] [CrossRef]
- Jeroch, H.; Kozłowski, K.; Schöne, F.; Zdunczyk, Z. Lupines (Lupinus spp.) as a protein feedstuff for poultry. Varieties, composition and nutritional values for poultry. Eur. Poult. Sci. 2016, 80, 125. [Google Scholar] [CrossRef]
- Olkowski, I.; Janiuk, I.; Jakubczak, A. Effect of enzyme preparation with activity directed towards degradation of non starch polysaccharides on yellow lupine seed based diet for young broilers. Acta. Vet. Brno. 2010, 79, 395–402. [Google Scholar] [CrossRef]
- Smulikowska, S.; Konieczka, P.; Czerwinski, J.; Mieczkowska, A.; Jankowiak, J. Feeding broiler chickens with practical diets containing lupin seeds (L. angustifolius or L. luteus): Effects of incorporation level and mannanase supplementation on growth performance, digesta viscosity, microbial fermentation and gut morphology. J. Anim. Feed. Sci. 2014, 23, 64–72. [Google Scholar] [CrossRef]
- Nguyen, X.H.; Nguyen, H.T.; Morgan, N.K. Dietary soluble non-starch polysaccharide level and xylanase supplementation influence performance, egg quality and nutrient utilization in laying hens fed wheat-based diets. Anim. Nutr. 2021, 7, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Struţi, D.I.; Bunea, A.; Pop, I.M.; Păpuc, T.; Mierliţă, D. The influence of dehulling on the nutritional quality of lupine seeds (Lupinus albus L.) and the effect of their use in the feed of laying quails on the live performance and quality of eggs. Animals 2021, 11, 2898. [Google Scholar] [CrossRef] [PubMed]
- Cowieson, A.; Adeola, O. Carbohydrases, protease, and phytase have an additive beneficial effect in nutritionally marginal diets for broiler chicks. Poult. Sci. 2005, 84, 1860–1867. [Google Scholar] [CrossRef]
- Hafeez, A.; Iqbal, S.; Sikandar, A.; Din, S.; Khan, I.; Ashraf, S.; Khan, R.U.; Tufarelli, V.; Laudadio, V. Feeding of phytobiotics and exogenous protease in broilers: Comparative effect on nutrient digestibility, bone strength and gut morphology. Agriculture 2021, 11, 228. [Google Scholar] [CrossRef]
- Cozannet, P.; Kidd, M.; Neto, R.; Geraert, A. Next-generation non-starch polysaccharide-degrading, multi-carbohydrase complex rich in xylanase and arabinofuranosidase to enhance broiler feed digestibility. Poult. Sci. 2017, 96, 2743–2750. [Google Scholar] [CrossRef]
- Saleh, A.A.; Kirrella, A.A.; Abdo, S.E.; Mousa, M.M.; Badwi, N.A.; Ebeid, T.A.; Nada, A.L.; Mohamed, M.A. Effects of dietary xylanase and arabinofuranosidase combination on the growth performance, lipid peroxidation, blood constituents, and immune response of broilers fed low-energy diets. Animals 2019, 9, 467. [Google Scholar] [CrossRef]
- Meng, X.; Slominski, A.; Nyachoti, M.; Campbell, D.; Guenter, W. Degradation of cell wall polysaccharides by combinations of carbohydrase enzymes and their effect on nutrient utilization and broiler chicken performance. Poult. Sci. 2005, 84, 37–47. [Google Scholar] [CrossRef]
- Musigwa, S.; Morgan, N.; Swick, R.A.; Cozannet, P.; Kheravii, S.K.; Wu, S.B. Multi-carbohydrase enzymes improve feed energy in broiler diets containing standard or low crude protein. Anim. Nutr. 2021, 7, 496–505. [Google Scholar] [CrossRef]
- Tahir, M.; Saleh, F.; Ohtsuka, A.; Hayashi, K. An effective combination of carbohydrases that enables reduction of dietary protein in broilers: Importance of hemicellulase. Poult. Sci. 2008, 87, 713–718. [Google Scholar] [CrossRef]
- Slominski, A. Recent advances in research on enzymes for poultry diets. Poult. Sci. 2011, 90, 2013–2023. [Google Scholar] [CrossRef]
- Kocher, A.; Choct, M.; Hughes, J.; Broz, J. Effect of food enzymes on utilisation of lupin carbohydrates by broilers. Br. Poult. Sci. 2000, 41, 75–82. [Google Scholar] [CrossRef]
- Brenes, A.; Slominski, B.; Marquardt, R.; Guenter, W.; Viveros, A. Effect of enzyme addition on the digestibilities of cell wall polysaccharides and oligosaccharides from whole, dehulled, and ethanol-extracted white lupins in chickens. Poult. Sci. 2003, 82, 1716–1725. [Google Scholar] [CrossRef]
- Mieczkowska, A.; Smulikowska, S.; Nguyen, V. Effect of enzyme supplementation of white lupin (Lupinus albus var. Butan)-containing diets on performance, nutrient digestibility, viscosity, pH, and passage rate of digesta in broiler chickens. J. Anim. Feed. Sci. 2004, 13, 475–486. [Google Scholar] [CrossRef]
- Mieczkowska, A.; Jansman, A.J.M.; Kwakkel, R.P.; Smulikowska, S. Effect of dehulling and α-galactosidase supplement on the ileal digestibility of yellow lupin based diets in broiler chickens and adult roosters. J. Anim. Feed Sci. 2005, 14, 297–304. [Google Scholar] [CrossRef]
- Olkowski, B. Lupin as primary protein source in young broiler chicken diets: Effect of enzymes preparations catalyzing degradation of non-starch polysaccharides or phytates. World. J. Microbiol. Biotechnol. 2011, 27, 341–347. [Google Scholar] [CrossRef]
- Lee, M.R.; Parkinson, S.; Fleming, H.R.; Theobald, V.J.; Leemans, D.K.; Burgess, T. The potential of blue lupins as a protein source, in the diets of laying hens. Vet. Anim. Sci. 2016, 1–2, 29–35. [Google Scholar] [CrossRef]
- National Research Council (NRC). Subcommittee on Poultry Nutrition 1994. Nutrient requirements of ring-necked pheasants, Japanese quail, and bobwhite quail. In Nutrient Requirements of Poultry, 9th ed.; National Academy of Sciences: Washington, DC, USA, 1994; pp. 44–45. [Google Scholar]
- Williams, K.C. Some factors affecting albumen quality with particular reference to Haugh units score. Worlds Poult. Sci. J. 1992, 48, 5–16. [Google Scholar] [CrossRef]
- Official Method of Analysis, 18th ed.; The Association of Official Analytical Chemists: Washington, DC, USA, 2005.
- Sibbald, I.R.; Morse, P.M. Effects of the nitrogen correction and of feed intake on true metabolizable energy values. Poult. Sci. 1983, 62, 138–142. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Christie, W.W. Preparation of methyl ester and other derivatives. In Gas-Chromatography and Lipids. A Practical Guide, 1st ed.; Christie, W.W., Ed.; Oily Press: Glasgow, UK, 1989; pp. 36–47. [Google Scholar]
- Official Method of Analysis of the Association of Official Analytical Chemists, 16th ed.; The Association of Official Analytical Chemists, Inc.: Arlington, VA, USA, 1996.
- Horwitz, W. AOAC Guidelines for Single Laboratory Validation of Chemical Methods for Dietary Supplements and Botanicals; AOAC International: Gaithersburg, MD, USA, 2002; pp. 1–38. [Google Scholar]
- Panaite, T.; Criste, R.D.; Ropota, M.; Cornescu, G.M.; Alexandrescu, D.C.; Criste, V.; Vasile, G.; Olteanu, M.; Untea, A. Effect of layer diets enriched in omega-3 fatty acids supplemented with Cu on the nutritive value of the eggs. Rom. Biotechnol. Lett. 2016, 21, 11652–11660. [Google Scholar]
- Schlatterer, J.; Breithaupt, E. Xanthophylls in commercial egg yolks: Quantification and identification by HPLC and LC-(APCI) MS using a C30 phase. J. Agric. Food. Chem. 2006, 54, 2267–2273. [Google Scholar] [CrossRef]
- Timmons, J.S.; Weiss, W.P.; Palmquist, D.L.; Harper, W.J. Relationships among dietary roasted soybeans, milk components, and spontaneous oxidized flavor of milk. J. Dairy Sci. 2001, 84, 2440–2449. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Fernandez, M.; Ordóñez, J.A.; Cambero, I.; Santos, C.; Pin, C.; de la Hoz, L. Fatty acid compositions of selected varieties of Spanish dry ham related to their nutritional implications. Food Chem. 2007, 101, 107–112. [Google Scholar] [CrossRef]
- Chen, S.; Bobe, G.; Zimmerman, S.; Hammond, E.G.; Luhman, C.M.; Boylston, T.D.; Freeman, A.E.; Beitz, D.C. Physical and sensory properties of dairy products from cows with various milk fatty acid compositions. J. Agric. Food Chem. 2004, 52, 3422–3428. [Google Scholar] [CrossRef]
- Konieczka, P.; Smulikowska, S. Viscosity negatively affects the nutritional value of blue lupin seeds for broilers. Animal 2017, 12, 1144–1153. [Google Scholar] [CrossRef]
- Struţi, D.I.; Mierliţă, D. Influence of dehulling Lupinus albus seeds used in the laying quail (Coturnix coturnix) diets on the intestinal viscosity, faecal humidity and blood biochemical parameters. Anim. Biol. Anim. Husb. 2021, 13, 48–57. [Google Scholar]
- Brenes, R.; Marquardt, R.; Guenter, W.; Viveros, A. Effect of enzyme addition on the performance and gastrointestinal tract size of chicks fed lupin seed and their fractions. Poult. Sci. 2002, 81, 670–678. [Google Scholar] [CrossRef]
- Písaříková, B.; Zralý, Z. Dietary fibre content in lupine (Lupinus albus L.) and soya (Glycine max L.) seeds. Acta Vet. Brno 2010, 79, 211–216. [Google Scholar] [CrossRef]
- Smits, M.; Annison, G. Non-starch plant polysaccharides in broiler nutrition–towards a physiologically valid approach to their determination. Worlds Poult. Sci. J. 1996, 52, 203–221. [Google Scholar] [CrossRef]
- Pandit, R.J.; Hinsu, A.T.; Patel, N.V.; Koringa, P.G.; Jakhesara, S.J.; Thakkar, J.R.; Joshi, C.G. Microbial diversity and community composition of caecal microbiota in commercial and indigenous indian chickens determined using 16s rDNA amplicon sequencing. Microbiome 2018, 6, 115e21. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, S.; Qi, G.; Fu, Y.; Wang, W.; Zhang, H.; Wang, J. Dietary supplemental xylooligosaccharide modulates nutrient digestibility, intestinal morphology, and gut microbiota in laying hens. Anim. Nutr. 2021, 7, 152–162. [Google Scholar] [CrossRef]
- Saleh, A.; El-Far, A.H.; Abdel-Latif, M.A.; Emam, A.; Ghanem, R.; Abd, E.H.S. Exogenous dietary enzyme formulations improve growth performance of broiler chickens fed a low-energy diet targeting the intestinal nutrient transporter genes. PLoS ONE 2018, 13, e0198085. [Google Scholar] [CrossRef]
- Konca, Y.; Yalcin, H.; Karabacak, M.; Kaliber, M.; Durmuscelebi, F.Z. Effect of hempseed (Cannabis sativa L.) on performance, egg traits and blood biochemical parameters and antioxidant activity in laying Japanese Quail (Coturnix coturnix japonica). Br. Poult. Sci. 2014, 55, 785–794. [Google Scholar] [CrossRef]
- Straková, E.; Všetičková, L.; Kutlvašr, M.; Timová, I.; Suchý, P. Beneficial effects of substituting soybean meal for white lupin (Lupinus albus, cv. Zulika) meal on the biochemical blood parameters of laying hens. Ital. J. Anim. Sci. 2021, 20, 352–358. [Google Scholar] [CrossRef]
- Mierliță, D. Effect of diets containing essential fatty acids-rich oil calcium soaps on functional lipid components of lamb tissues. Rom. Biotechnol. Lett. 2017, 23, 13205–13213. [Google Scholar]
- Surai, F. Antioxidant systems in poultry biology: Superoxide dismutase. J. Anim. Physiol. Anim. Nutr. 2016, 1, 8. [Google Scholar] [CrossRef]
- Kubiś, M.; Kaczmarek, S.A.; Nowaczewski, S.; Adamski, M.; Hejdysz, M.; Rutkowski, A. Influence of graded inclusion of white lupin (Lupinus albus) meal on performance, nutrient digestibility and ileal viscosity of laying hens. Br. Poult. Sci. 2018, 59, 477–484. [Google Scholar] [CrossRef]
- Rutkowski, A.; Hejdysz, M.; Kaczmarek, S.; Adamski, M.; Nowaczewski, S.; Jamroz, D. The effect of addition of yellow lupin seeds (Lupinus luteus L.) to laying hen diets on performance and egg quality parameters. J. Anim. Feed Sci. 2017, 26, 247–256. [Google Scholar] [CrossRef]
- Hammershøj, M.; Steenfeldt, S. Effects of blue lupin (Lupinus angustifolius) in organic layer diets and supplementation with foraging material on egg production and some egg quality parameters. Poult. Sci. 2005, 84, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Drażbo, A.; Mikulski, D.; Zdu’nczyk, Z.; Szmatowicz, B.; Rutkowski, A.; Jankowski, J. Fatty acid composition, physicochemical and sensory properties of eggs from laying hens fed diets containing blue lupine seeds. Eur. Poult. Sci. 2014, 78, 245–252. [Google Scholar]
- Park, J.H.; Lee, S.I.; Kim, I.H. Effects of lupin seed supplementation on egg production performance, and qualitative egg traits in laying hens. Vet. Med. 2016, 61, 701–709. [Google Scholar] [CrossRef]
- Jones, R.; Musgrove, T. Effects of extended storage on egg quality factors. Poult. Sci. 2005, 84, 1774–1777. [Google Scholar] [CrossRef]
- Wang, X.; Wu, S.; Zhang, H.; Yue, H.; Qi, G.; Li, J. Effect of dietary protein sources and storage temperatures on egg internal quality of stored shell eggs. Anim. Nutr. 2015, 1, 299–304. [Google Scholar] [CrossRef]
- Attia, Y.A.; Al-Harthi, M.A.; Sagan, A.A.; Abdulsalam, N.M.; Hussein, E.O.S.; Olal, M.J. Egg production and quality, lipid metabolites, antioxidant status and immune response of laying hens fed diets with various levels of soaked flax seed meal. Agriculture 2022, 12, 1402. [Google Scholar] [CrossRef]
- Robinson, S.; Monsey, B. Studies on the composition of egg-white ovomucin. Biochem. J. 1971, 121, 537–547. [Google Scholar] [CrossRef]
- Omana, A.; Wang, J.; Wu, J. Ovomucin–a glycoprotein with promising potential. Trends Food Sci. Technol. 2010, 21, 455–463. [Google Scholar] [CrossRef]
- Eisen, J.; Bohren, B.; McKean, E. The Haugh Unit as a measure of egg albumen quality. Poult. Sci. 1962, 41, 1461–1468. [Google Scholar] [CrossRef]
- Aguillón, P.J.; Romero, A.; Diaz, J. Effect of full-fat sunflower or flaxseed seeds dietary inclusion on performance, egg yolk fatty acid profile and egg quality in laying hens. Anim. Nutr. 2020, 6, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, M.; O’Reilly, J.; Heitmann, L.; Pereira, A.; Balter, K.; Fraser, G.E. Major types of dietary fat and risk of coronary heart disease: A pooled analysis of 11 cohort studies. Am. J. Clin. Nutr. 2009, 89, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Wu, H. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Gonzalez, E.R.; Leeson, S. Effect of feeding hens regular or deodorized men-haden oil on production parameters, yolk fatty acid profile, and sensory quality of eggs. Poult. Sci. 2000, 79, 1597–1602. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.; Seburg, S.; Flanagan, L. Enriched eggs as a source of n-3 polyunsaturated fatty acids for humans. Poult. Sci. 2000, 79, 971–974. [Google Scholar] [CrossRef]
- Attia, Y.A.; Al-Harthi, M.A.; Al-Sagan, A.A.; Alqurashi, A.D.; Korish, M.A.; Abdulsalam, N.M.; Olal, M.J.; Bovera, F. Dietary supplementation with different ω-6 to ω-3 fatty acid ratios affects the sustainability of performance, egg quality, fatty acid profile, immunity and egg health indices of laying hens. Agriculture 2022, 12, 1712. [Google Scholar] [CrossRef]
- Criste, F.; Mierliță, D.; Simeanu, D.; Boisteanu, P.; Pop, I.M.; Georgescu, B.; Nacu, G. Study of fatty acids profile and oxidative stability of egg yolk from hens fed a diet containing white lupine seeds meal. Rev. Chim. 2018, 69, 2454–2460. [Google Scholar] [CrossRef]
- Świątkiewicz, S.; Arczewska, A.; Krawczyk, J.; Puchała, M.; Józefiak, D. Effects of selected feed additives on the performance of laying hens given a diet rich in maize dried distiller’s grains with solubles (DDGS). Br. Poult. Sci. 2013, 54, 478–485. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Chaudhry, T.; Mahrose, M.; Noreldin, A.; Emam, M.; Alagawany, M. The efficacy of using exogenous enzymes cocktail on production, egg quality, egg nutrients and blood metabolites of laying hens fed distiller’s dried grains with solubles. J. Anim. Physiol. Anim. Nutr. 2018, 102, e726–e735. [Google Scholar] [CrossRef]
- Westbrook, A.; Cherian, G. Egg quality, fatty-acid composition and gastrointestinal morphology of layer hens fed whole flaxseed with enzyme supplementation. Br. Poult. Sci. 2019, 60, 146–153. [Google Scholar] [CrossRef]
- Jia, W.; Slominski, A.; Guenter, W.; Humphreys, A.; Jones, O. The effect of enzyme supplementation on egg production parameters and omega-3 fatty acid deposition in laying hens fed flaxseed and canola seed. Poult. Sci. 2008, 87, 2005–2014. [Google Scholar] [CrossRef]
- Krawczy, M.; Przywitowski, M.; Mikulski, D. Effect of yellow lupine (L. luteus) on the egg yolk fatty acid profile, the physicochemical and sensory properties of eggs, and laying hen performance. Poult. Sci. 2015, 94, 1360–1367. [Google Scholar] [CrossRef]
- Papadopoulos, G.A.; Lioliopoulou, S.; Ordoudi, S.A.; Giannenas, I.; Van Hoeck, V.; Morisset, D.; Arsenos, G.; Fortomaris, P.; Mantzouridou, F.T. Xylanase supplementation in wheat-based diets of laying hens affects the egg yolk color, carotenoid and fatty acid profiles. Foods 2022, 11, 2209. [Google Scholar] [CrossRef]
- Wang, S.; Errington, S.; Yap, H.H. Studies on carotenoids from lupin seeds. In Lupins for Health and Wealth’Proceedings of the 12th International Lupin Conference, Fremantle, Western Australia, 14–18 September 2008; Palta, J.A., Berger, J.D., Eds.; International Lupin Association: Canterbury, New Zealand, 2008; pp. 14–18. [Google Scholar]
- Akdemir, F.; Orhan, C.; Sahin, N.; Sahin, K.; Hayirli, A. Tomato powder in laying hen diets: Effects on concentrations of yolk carotenoids and lipid peroxidation. Br. Poult. Sci. 2012, 53, 675–680. [Google Scholar] [CrossRef]
- Toomer, O.T.; Vu, T.C.; Sanders, E.; Redhead, A.K.; Malheiros, R.; Anderson, K.E. Feeding laying hens a diet containing high-oleic peanuts or oleic acid enriches yolk color and beta-carotene while reducing the saturated fatty acid content in eggs. Agriculture 2021, 11, 771. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).