Abstract
Volatile organic compounds emanating from plant surfaces serve as a sustainable natural solution to combat biotic stresses in plants. Leaf methanol is the simplest and second major volatile organic compound after isoprene emitted through the leaf surface. Methanol has been neglected as a by-product of other secondary metabolites for a long time, but recent studies have suggested its importance in development and stress responses. In our previous findings, we had revealed that transgenic plants over-expressing PME, enhanced methanol production providing resistance against a broad spectrum of insects. In the current study, we extended our previous work to provide new insights by performing differential transcriptomics of high-methanol-producing insect-resistant transgenic lines. We found that 2262 genes were differentially expressed in the transgenics plants, including transcription factors, cell wall modulating, phytohormones signaling and development-related genes. Our results demonstrated that the expression levels of transcription factors associated with development and biotic stress were altered in the transgenic lines. In addition, phytohormones ABA and gibberellin signalling genes were upregulated, whereas ethylene and auxin signalling genes were downregulated. Moreover, biochemical characteristics of cell walls in both transgenic tobacco plants were comparable to the control plants.
1. Introduction
Plant evolution has led to the development of advanced strategies of defense against arthropod herbivores [1]. Secondary metabolites are major contributors to plant immunity. These act as bioactive toxic compounds or as volatiles that elicit defensive signal transduction resulting in the collective regulation of downstream stress-responsive genes [2]. Methanol is one of the simplest organic products of plant metabolism [3,4]. Endogenous methanol produced in plants serves as a signalling molecule in inter/intra plant communication, as well as in plant defence, growth, and development [4]. A major source of methanol production in plants is the de-esterification of cell wall pectin. Pectin methylesterase (PME), a cell wall enzyme, plays a key role in methanol production [5,6]. It works on a methylated backbone of pectin and produces methanol during de-esterification. They are broadly divided into two groups based on their domain architecture. Group I PMEs consist only of the catalytic domain (PME domain) along with N-terminal signal peptide and transmembrane domains, whereas Group II PMEs consists of all group I domains along with an extra inhibitory domain (PMEI) [5,6]. Plants possess both group II PMEs along with group I [5,6].
Since PME directly uses the cell wall as a substrate, it plays an important role in plant growth and development. Earlier, it was reported that over-expressed PME tobacco lines showed dwarfism phenotypes [7]. On the other hand, PME knock-down lines of tomato and tobacco did not affect the yield or vegetative growth [8,9]. However, accumulating evidence suggests that foliar sprays of aqueous methanol increased the growth and yield of crop plants [10,11,12]. Thus, a detailed biochemical characterization of cell wall may provide a deeper insight of cell wall remodelling in high-methanol-producing plants.
Methanol has a vital role in the defence against herbivores; therefore, enhancing methanol production by overexpression of PME has been a recently explored strategy for improved insect protection in many plant species [13,14,15]. We had also successfully demonstrated that enhanced methanol production in transgenic plants over-expressing PME provides resistance against a broad spectrum of insects without compromising growth and yield [13]. However, little effort has been made to unravel the molecular mechanism by which methanol functions under biotic stress in plants. Recently, Tran et al. demonstrated that methanol signalling in plant cells is mediated through cytosolic calcium influx leading to plant responses against biotic stress [16]. PME-generated methanol release has been shown to upregulate methanol inducible genes (MIGs) and bacterial resistance in neighbouring plants, but global transcription changes in high-methanol-producing plants is less understood [2,16,17].
Therefore, in the present study, we extended our work in which we had developed high-methanol-producing insect-resistant transgenic tobacco plants. The current manuscript is focused on the comprehensive analysis of transcriptional changes in these transgenic plants. Our findings revealed modulation in the expression of genes for biological processes commonly associated with cell wall homeostasis, phytohormones signalling, and development-related processes in the insect-resistant transgenic plants. Furthermore, we also biochemically characterized the pectin in transgenic plants and later validated by confocal microscopy.
2. Materials and Methods
2.1. Plant Material, Molecular Characterization and Insect Bioassay
We previously developed two separate types of high-methanol-producing transgenic plants by overexpressing PME from Arabidopsis thaliana, and Aspergillus niger and named them AtPMEs and AnPMEs, respectively [13]. Briefly, anPME and atPME genes were separately cloned in plant expression vector pBI121 by replacing GUS under the control of CaMV35S promoter and transformed in Agrobacterium tumefaciens LBA4404. Transgenic plants were developed by Agrobacterium-mediated genetic transformation using the direct organogenesis method following standard procedure [13,18]. In total, 25 transgenic lines were developed for each gene; among them, the fifth and fourth line (At-5 and An-4) showed the highest levels of PME activity, methanol emission, and resistance against all tested insects [13]. These lines were continuously selected on kanamycin (300 μg/mL), and the T4 generation named An-4.4 and At-5.4 was used in this study. All the molecular analysis such as cDNA PCR, PME activity, and methanol emission assays in An-4.4 and At-5.4 were performed as per previously optimized protocols [13]. In brief, RNA was isolated by transgenic plants and cDNA was synthesized; cDNA PCR was performed with gene-specific primers [13]. PME activity was performed using the titration method by measuring the amount of free carboxyl groups of methylated pectin in the reaction. The reaction mixture (30 mL) contained 0.125% citrus pectin (95% methylated, Sigma) solution, 0.15 M NaCl and 0.2 mL total soluble protein, with the pH adjusted to 8. The reaction (at 30 °C for 45 min) was stopped by incubating at 100 °C for 5 min followed by cooling in running water. The reaction mixture was titrated against 0.1 M NaOH by taking phenolphthalein as an indicator. The mixture without total soluble protein was taken as the control. One unit of PME was defined as the number of enzymes, which released 1 μmole of carboxyl groups/min. PME activity was calculated using the following formula:
For methanol emission assays, methanol content was estimated in water emitted by leaf surfaces through transpiration. Leaves from transgenic and control plant were cut and placed individually in a polybag without touching the surface of the polybag. The polybag was sealed and hanged in sunlight by thread for two hours. The polybags were opened carefully and transpirated water was collected in a fresh tube. Methanol was quantified in this transpirated water by using the Purpald/Alcohol Oxidase (AO) method. Both PME activity and methanol emission assay were performed in two independent trials with 3–5 biological replicates per trial.
A bioassay for Bemisia tabaci Asia 1 was performed in 100 mL perforated specimen tubes in which 20–25 freshly hatched adult whiteflies were released. Agar (1%) was poured in the caps of the bioassay tubes (2/3 level) and leaf discs of the transgenic and control plants were placed separately on the solidified agar. Bioassay tubes containing insects were closed with caps containing leaf discs. A fresh leaf disc was provided on each alternate day. Mortality data were recorded after six days by counting whitefly cadavers at the bottom of the tube. For the bioassay with Spodoptera litura Fabricius, 10 neonate larvae were released in the bioassay vial containing detached leaves. Larval mortality was recorded at regular intervals. Fresh leaves were provided each alternate day.
2.2. Microarray Analysis
RNA was isolated from six independent transgenic plants of each line (An-4.4 and At-5.4) and control plants. RNA from three plants were mixed and considered as one biological replicate. The quality of the RNA was checked using Bioanalyzer (Agilent 2100); samples with an RNA Integrity Number of more than 8.0 were used for further experiment. Microarray analysis was outsourced to Genotypic technology Pvt. Ltd., Bangalore, India, and was performed using Agilent Platform with the Agilent tobacco whole genome microarray having 44,000 probe sets as per their standard protocol. Data normalization and statistical analysis were carried out using Agilent’s Gene Spring GX version 10.0 software. Genes having 2-fold or greater change at p < 0.05 in the t-tests were considered as significantly differentially expressed genes. The sequence was obtained from the sequence information file of the tobacco genome array and reannotated by BLASTx at National Center for Biotechnology Information. Microarray probe identifiers of differentially expressed genes were used as input data for Gene Ontology annotation using the agriGO: GO Analysis Toolkit (https://systemsbiology.cpolar.cn/agriGOv2/, accessed on 4 April 2021). Fisher’s method was used for statistical tests, whereas Yekutieli’s (FDR) method was used for multi-test adjustment. Agilent tobacco Genome Array (ID: A_95_P311588) was selected as the reference background for all GO analyses.
2.3. Pectinase and Cellulase Activity Assay
Pectinase and cellulase activity were measured by the DNS (3,5-dinitrosalicylic acid) method using a spectrophotometer. For the cellulase assay, the reaction mixture consisted of total soluble protein (100 µL), 1 mL citrate buffer pH 5.0 (1 mL), and carboxymethyl cellulose (1 mL). The reaction mixture was incubated at 45 °C for 30 min, and then DNS was added to stop the reaction. The reaction mixture was boiled for 10 min, followed by cooling for color stabilization. Absorbance was measured by a UV-visible spectrophotometer at 575 nm. One unit was considered as release of 1 µmole glucose in 1 min of reaction during the hydrolysis of carboxymethyl cellulose. For the Pectinase assay, total soluble protein (100 µL) was added to 0.5% polygalacturonic acid prepared in a citrate buffer (0.05 M, pH 4.4) and incubated at 50 °C for 30 min. Thereafter, DNS was added to stop the reaction, and the reaction mixture was boiled followed by cooling to develop the colour. Absorbance was measured at 575 nm in spectrophotometer. One unit was considered as the release of 1 µmole of D-galacturonic acid per minute. For both pectinase and cellulase activity, the experiment was performed in one trial with six biological replicates.
2.4. Biochemical Characterization of Cell Wall
Pectin from the leaves of transgenic and control plants was isolated by the acidified water hydrolysis method. Samples (50 g) were boiled separately in 1 L of extraction solvent (1 M HCl) for 2 h on heating plate with continuous stirring to avoid burning. After heating, samples were filtered with double layered cheese cloth. For pectin precipitation, absolute ethanol in the ratio of 1:2 was added to the filtrate and kept at room temperature overnight. Pectin was recovered by centrifugation (10,000× g for 10 min) and washed serially with 75% ethanol (v/v), 85% ethanol (v/v) and absolute ethanol to remove the soluble impurities. The pectin was dried at 40 °C and stored at room temperature in a glass vial.
Equivalent weight (EW) was determined by the titration method. The reaction mixture was prepared in a 250 mL conical flask by adding 0.5 g purified pectin, 5 mL ethanol, and 1 g of sodium chloride, and the total volume was maintained at 100 mL in milliQ. Finally, 6 drops of phenol red were added and titrated against 0.1 N NaOH. The itration point was indicated by purple color. Equivalent weight was calculated by the following formula: EW = [(Weight of sample × 100)/(mL of alkali × Normality of alkali)].
A neutral solution (light purple), which was obtained after the determination of equivalent weight, was further used to determine the methoxyl content (MeO%). In total, 25 mL of sodium hydroxide (0.25 N) was added to this neutral solution. The mixture was stirred thoroughly and kept at room temperature for 30 min. After incubation, 25 mL of 0.25 N hydrochloric acid was added and titrated against 0.1 N NaOH. Methoxyl content was calculated using the formula: %MeO = [(mL of alkali × Normality of alkali × 3.1)/(Weight of sample)].
The total anhydrouronic acid (AUA) content of the pectin was obtained by the following formula: %AUA = [(176 × 0.1z × 100)/(w × 1000) + (176 × 0.1y × 100)/(w × 1000)] formula, where z = mL (titre) of NaOH from equivalent weight determination, and y = mL (titre) of NaOH from methoxyl content determination, w = weight of sample.
The degree of esterification (DE) of pectin was measured by applying the value of methoxyl and AUA content to the formula: %DE = [(176 × %MeO)/(31 × %AUA)].
2.5. Confocal Microscopy for Analyzing Degree of Esterification in Cell Wall
Commercially available pectin-specific Leeds Monoclonal Antibody (LM 7), John Innes Monoclonal Antibodies 5 and 7 (JIM 5 and JIM 7) were purchased from PlantProbes (http://www.plantprobes.net/index.php, accessed on 4 April 2021). LM 7 is specific to the homogalacturonan domain of pectin, whereas JIM 5 and JIM 7 are specific to un-esterified and esterified homogalacturonan, respectively. Leaves from transgenic and control plants were cut into 1 cm2 and fixed in 2.5% paraformaldehyde prepared in 0.1 M phosphate buffer at pH 7. Samples were incubated at 40C overnight. Samples were washed thrice in phosphate buffer for 20 min each. Thereafter, samples were dehydrated with a series of alcohol at 15%, 30%, 60%, 90%, and, finally, 100% (3 times) for 20 min each. Dehydrated samples were embedded in paraffin wax and, subsequently, 5 μM sections were made by using microtome (Leica). Sections were transferred on poly-l-lysine-coated slides and incubated with supplied antibody solutions (1:36) at 4 °C for 16 h, followed by washing with buffer. Afterwards, sections were incubated with 2 μg/μL Alexa Fluor™ 488 labelled anti-rat IgG secondary antibodies at 20 °C for 1 h. followed by washing. Sections were analysed under a confocal microscope (Zeiss LSM 510 Meta) at 40× optical and 2× digital zoom with excitation at 488 nm and emission at filter BP500-550 IR. Five representative images per sample were used for the quantification of average fluorescence intensity by ImageJ software for comparing the degree of esterification between the control and transgenic plants.
3. Results and Discussion
3.1. Molecular Characterization of High-Methanol-Producing Transgenic Tobacco Plants
PME-directed pectin demethylation is the main source of methanol release in plants [13,16]. In our previous study, we developed two different types of high-methanol-producing transgenic plants AtPMEs and AnPMEs overexpressing PMEs [13]. AtPME because of its plant origin, was selected to be easily expressed in transgenic plants, whereas AnPME is of fungal origin; therefore, it cannot be inhibited by plant PME inhibitor (PMEI) due to structural incompatibility. Hence, it is suitable for the development of transgenic plants. High PME activity correlates with increased methanol production, which results in insect virulence and disease resistance in plants. Hence, the fifth and fourth lines (At-5 and An-4) of AtPMEs and AnPMEs showing the highest PME activity, methanol emission, and resistance against all tested insects, were selected for generation advancement. The T4 generation of these transgenic lines (An-4.4 and At-5.4) was used in this study for the analysis of transcriptional changes. It has been reported that during generation advancement, the transgenic plants sometimes fail to generate active recombinant proteins [19]. To make sure that the T4 generation of both transgenics (An-4.4 and At-5.4) has the same characteristics as the ancestral lines, we characterized them for different parameters. The expression analysis confirmed the active transcription of atpme and anpme genes in their respective transgenic lines (Figure 1A). Titration assay confirmed that both An-4.4 and At-5.4 have significantly high PME activity than the control plants (Figure 1B). The rate of methanol emission from both An-4.4 and At-5.4 transgenic plants was significantly higher than for control plants (Figure 1C). This shows that the PME expressed in transgenic plants had high activity, which resulted in increased methanol production than in control plants. An-4.4 and At-5.4 also showed resistance against Spodoptera litura Fabricius and Bemisia tabaci Asia 1 (Figure 1D) than control plants, which is consistent with the finding that increased methanol production leads to active defensive reactions in plants and show that these transgenic plants have similar functional characteristics to the ancestral lines [16]. Collectively, these results suggest that the T4 generation of both transgenics with active PME expression has high methanol emission, which resulted in insect resistance.
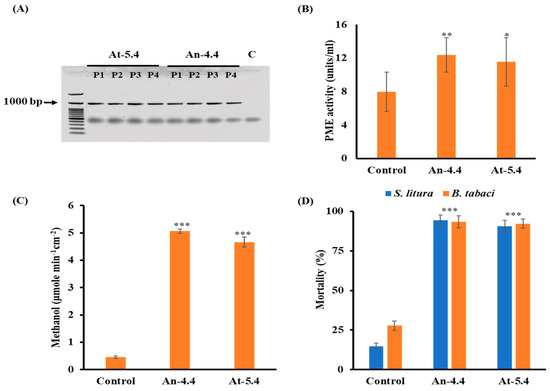
Figure 1.
Molecular analysis of T4 generation transgenics. (A) Gel image showing cDNA PCR from At-5.4, An-4.4, and control. Arrow indicates band corresponding to atpme and anpme from four independent plants (P1-P4) of both transgenic lines. Graph showing (B) PME activity, (C) quantification of methanol emission in transpirated water through the stomata on leaves surface, in both transgenics and control plants. (D) Mortality of S. litura and whiteflies on At-5.4, An-4.4, and control plants after 6 days. * p < 0.05; ** p < 0.01; *** p < 0.001.
3.2. Differential Transcriptome Analysis of Transgenic Tobacco Line
The success of the plant response on signal perception depends on the regulatory network that connects to the downstream response [1]. Global analysis of gene expression profiles can improve our overall knowledge of the molecular basis of the plant response. Therefore, Microarray analysis was performed to understand transcriptional changes in both types of high methanol-producing transgenic lines. A Total of 1311 and 951 genes were found differentially expressed (2-fold or greater change at p value < 0.05) in the transgenics of An-4.4 and At-5.4, respectively (Figure 2A, Supplementary Datasets S1 and S2). An-4.4 transgenic lines had a greater number of differentially expressed genes than At-5.4. This might be due to their interkingdom origin. Out of these, 332 and 979 genes were upregulated and downregulated in An-4.4 transgenics, whereas 245 and 703 were upregulated and downregulated in At-5.4 transgenics over the control (Figure 2A). This was consistent with previous findings where wounding and direct methanol spraying in plants showed alteration in gene expression [16,20]. We explored common genes in both transgenic lines and, interestingly, 114 and 445 genes were commonly up- and downregulated in both types of transgenics, respectively (Figure 2B,C). These commonly shared differentially expressed genes were further annotated by Gene Ontology (GO) on agriGO under biological process, cellular component, and molecular function categories. Total 96 GO terms were significantly (p value < 0.05) identified for all differentially expressed gene (Supplementary Dataset S3). Among them, the cell (GO:0005623) and cell parts (GO:0044464); transcription factor (GO:0003700) and regulator activity (GO:0030528); and response to stimulus (GO:0050896) were highest in their respective categories (Figure 3). It is plausible to have changes in cell and cell part categories because PME is a cell wall re-modelling enzyme which was overexpressed to develop these transgenic lines [5,6]. We also recorded responses to stimulus GO, as these transgenic plants have high rates of methanol emission, which has a major role in biotic interactions [13,20]. As many genes were differentially expressed in both transgenic lines, this may be due to changes in the expression of transcription factors.
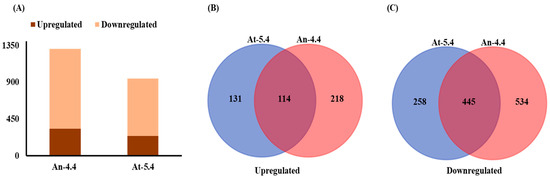
Figure 2.
Microarray analysis of transgenic plants. (A) Number of significantly up and down regulated genes in An-4.4 and At-5.4; number of commonly up- (B) and downregulated (C) genes between An-4.4 and At-5.4.
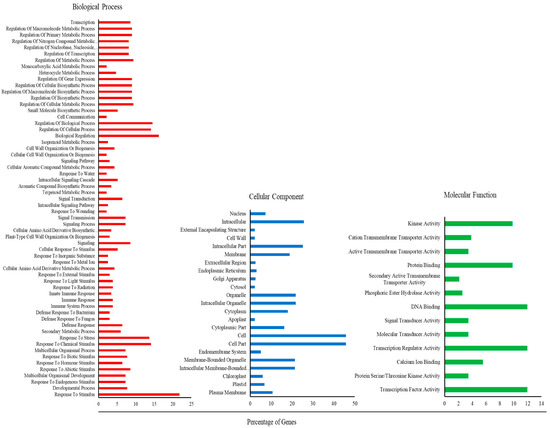
Figure 3.
GO enrichment analysis of significantly differentially expressed genes.
Scatter plot analysis suggested that all the significant GO terms were clustered in five major zones (Supplementary Figure S1). An interactive model of all significant GO terms showed that the biological process, immune system process (GO:0002376), response to stimulus (GO:0050896), multicellular organismal process (GO:0032501), and developmental process (GO:0032502) were the highest significant nodes (Figure 4). All these nodes finally converged to the defense response to the bacterium (GO:0042742) and fungus (GO:0050832) (Figure 4). It is expected because previous reports have suggested that overexpression of PMEs may lead to enhanced susceptibility for fungal infection [21]. Interestingly, we also found a significant node related to cell wall organization or biogenesis (GO:0071554) under the same category. This might be as the cell wall acts as the first line of defense against biotic stresses [22]. Under the metabolic process; secondary metabolic process (GO:0019748), and amino acid derivative metabolic process (GO:0005575) were the highest significant nodes which eventually terminated on terpenoid metabolic process (GO:0006721) node. This indicates that methanol may directly or indirectly affect terpenoid production in transgenic plants. This is line with prior findings, where terpenoids were shown to be associated with plant–biotic interactions [23]. Thus, collectively, our data demonstrate that both transgenic lines overexpressing PME showed varied gene expression profiles with commonalities.
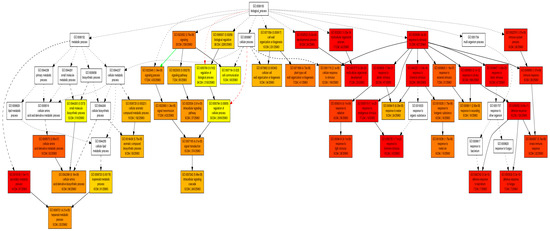
Figure 4.
An interactive model of significantly enriched GO in transgenic plants.
3.3. Expression Profiling of Selected Genes
Microarray analysis revealed that many genes were differentially expressed in transgenic lines. Most of them were involved in cell wall development, phytohormone signaling, and secondary metabolite production (Figure 5). In our data set, we identified a total of 10 transcription factors that were significantly differentially expressed in both transgenic plants; out of these, 40% were upregulated, whereas 60% were downregulated (Figure 5B). Transcription factors such as WRKY2, MYB13, MYB20, and HAT4, whose roles are pre-established as plant development regulators, were also present in the data set [24,25,26,27]. TFs of the MYC and MYB families are also known to regulate herbivore-responsive gene expression, including terpenoids and flavonoids [28]. Furthermore, BZIP59 or POSF21, which participates in plant-biotic interaction was also found to be differently expressed in the transgenic lines [29]. A study by Teeples et al. reported that MYB13 is associated with the gene regulatory network for the secondary cell wall synthesis gene in Arabidopsis [25], which may be the reason we identified 10 cell-wall-related genes in the dataset (Figure 5A). Although the expressions of the 10 cell-wall-related genes were downregulated, we did not see any dwarfism phenotypes in our transgenic plants which were, however, observed in the PME transgenic line developed by Hasunuma et al. [30]. Xyloglucanendotransglucosylase/hydrolase, UDP-arabinose 4-epimerase 1, and cellulose synthase-like protein D3 were amongst the main cell-wall-related genes found downregulated in the dataset. Interestingly, we also found downregulation of pectinesterase/pectinesterase inhibitor 12, which is involved in resistance to Botrytis cinerea [21]. Plant hormones such as cytokinin and abscisic acid (ABA) directly correlated with the expression levels of certain PMEs, which regulated methanol effects [31]. We also observed that many phytohormone signaling genes were differentially expressed in transgenic plants suggesting possible crosstalk between methanol and phytohormone signaling (Figure 5C). It is also notable that auxin and ethylene signaling genes were found to be downregulated, whereas ABA and gibberellin signaling were upregulated in transgenic plants. In a recent study methanol was notably shown to induce a calcium-dependent ethylene production in cultured Arabidopsis thaliana L. (Col-0) suspension cells and Nicotiana tabacum (BY-2) cell suspensions [16]. However, we observed downregulation of ethylene signaling genes in our transgenic lines. In line with GO analysis results, we found that nine genes involved in secondary metabolism were differentially expressed, including multiple isoforms of Caffeoyl-CoA O-methyltransferase (Figure 5D). Caffeoyl-CoA O-methyltransferases have been reported to play a role in the regulation of phenylpropanoid enzymes, lignin-like phenolic compounds and lignin content. Alteration in Caffeoyl-CoA O-methyltransferases expression has been shown to regulate lignin content in transgenic tobacco plants and modulate defense responses [32,33]. Methanol has been known to induce a MAPK-dependent response [34]; however, we found reduced MAPK3 transcripts in our transgenic lines. This may be due to increased gibberellin signaling genes found in the transgenic lines. Gibberellins treatment has been reported to downregulate MAPK3 transcripts in Brassica rapa seedling leaves [35]. Thus, our findings provide a comprehensive overview of expression patterns of different gene networks in the high-methanol-producing transgenic lines.
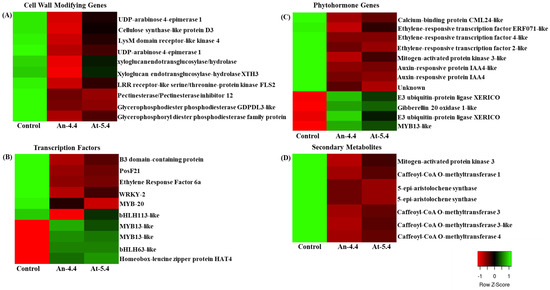
Figure 5.
Expression analysis of cell-wall-related genes (A), transcription factors (B), phytohormone signaling genes (C), and secondary metabolite pathway genes (D) in control as well as in both (An-4.4 and At-5.4) transgenic lines.
3.4. Biochemical Characterization of Cell Wall
The expression analysis suggested that several cell walls remodelling related genes were downregulated in transgenic lines, however we did not observe any structural deformities in these transgenic lines, which was previously observed by Hasunuma et al. (20). Thus, we decided to analyse the cell wall enzyme activities in the transgenic lines. We already know that these transgenic lines have high PME activity (de-esterification of pectin), which promotes the formation of egg-box structure (a gel-like structure) in cell wall. This egg-box structure has low pH which activates other cell wall degrading enzymes, such as pectinases and cellulases [5,6]. Therefore, we analyse the enzyme activity of cellulase and pectinase by spectrophotometer using DNS (3,5-dinitrosalicylic acid) method. No significant difference was observed in the activity of both the enzymes in An-4.4 and At-5.4 transgenic lines compared with the control plant (Figure 6A); this indicates that cell wall integrity was intact and not degraded by cell wall enzymes. These results also support our previous finding, where we observed well integrated hexagonal cell network without any deformities in propidium iodide-stained transverse section of transgenic leaves [13].
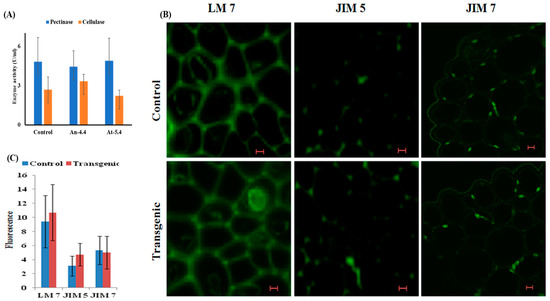
Figure 6.
Biochemical characterization of cell wall. (A) Graph showing average values (n = 6) of pectinase and cellulase enzyme activity in control and both transgenic lines (An-4.4 and At-5.4). (B) Representative confocal images showing transverse section of transgenic (An-4.4) and control leaves at 40× magnification. Leaf sections were stained with fluorescence labelled pectin specific monoclonal antibodies (LM 7, JIM 5, JIM 7). (C) Graph representing average fluorescence values (n = 5) in the transgenic (An-4.4) and control leaves.
Cell wall pectin is a direct substrate of PMEs; thus, the biochemical status of pectin was analysed in transgenic lines using the titration assay method. Commercial pectin (Sigma) was taken as a reference control whose biochemical parameters are already reported (degree of esterification: 75%). We found that the equivalent weight (EW), methoxyl content and anhydrouronic acid content of the control plant is marginally higher than both types of transgenic line, but not statistically significant (Table 1). The most interesting result observed in this experiment is that the DE of both types of transgenics is only ~5% less than control plant, despite the fact that esterified pectin is a direct substrate of PME. Therefore, this result was further validated very critically with a confocal microscope by using three pectin-specific monoclonal antibodies. LM 7 was specific to the homogalacturonan domain (HG) of pectin, whereas JIM 5 and JIM 7 were specific to un-esterified and methylesterified backbone of HG. All three used monoclonal antibodies did not have any cross reactivity. Equal fluorescence in LM 7 panel was observed, suggesting that both control and transgenics have same pectin content because HG domain represent more than 80% of the pectin in cell wall and only methylated domain in pectin (Figure 6B). Similar fluorescence in JIM 7 panel further confirmed that transgenics have similar DE patterns in comparison to the control plant (Figure 6B). Collectively, these results indicate that higher levels of methanol production did not cause any structural deformities despite a reduction in the expression of cell wall remodelling genes.

Table 1.
Characterization of pectin at different biochemical parameters isolated from transgenic and control plants.
4. Conclusions
Methanol is naturally produced by all plants and is not toxic to the host; had it been, plants would have to discard its production during evolution. Instead, methanol protects photosynthetic machinery from photo-inhibition, stimulates plant growth, and involved in plant–biotic interactions. We previously established that methanol emissions from host plant can be utilized as a tool to confer a broad range of insect resistance. In this study we had provided the insight of its molecular mechanism. We found that several important biological processes were altered in high-methanol-producing plants due to the differential expression of several genes, including transcription factors. It would be interesting to see how leaf methanol will modulate the expression of these transcription factors. It would be also very important to find how plants perceive this inter-/intra-leaf methanol. Furthermore, cross talk/modulation of phytohormone with leaf methanol is another aspect to be studied in detail. As methanol is being enhanced through overexpression of PME, a cell wall enzyme, it is extremely important to analyse structural and biochemical parameters of transgenic plants to make field-viable technology. Our current results, along with previous findings, confirmed that transgenic plants did not have any major structural and biochemical deformities. However, it will be very intriguing to see how plants re-esterify cell wall pectin despite high levels of PME activity. Overall, achieving broad-spectrum insect resistance by enhancing methanol production is a field-viable technology and could be further explored in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture13030521/s1, Figure S1: Scatter plot analysis of significant GO terms; Dataset S1: List of significantly differentially expressed genes in An-4.4 transgenic; Dataset S2: List of significantly differentially expressed genes in At-5.4 transgenic; Dataset S3: Significantly Enriched GO categories in transgenic lines.
Author Contributions
K.C. and P.C.V. conceptualized the idea of this study. P.C.V., S.K.U. and K.C. designed the experiments. S.D. performed all the experiments in P.C.V. except Biochemical characterization of pectin, which is carried out in S.K.U., S.D., S.K.U. and P.C.V. analysed the results, and prepared the manuscript. All authors have read and approved the manuscript for publication. All authors have read and agreed to the published version of the manuscript.
Funding
SERB, Department of Science & Technology (DST), Govt. of India for the financial support (CRG/2021/005998).
Institutional Review Board Statement
The institutional manuscript ID No is CSIR-NBRI_MS/2023/01/03.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be provided by corresponding author on request.
Acknowledgments
S.D. is thanks full to DBT for M.K. Bhan-YRFP fellowships. P.C.V. is thankful to the SERB, Department of Science & Technology (DST), Govt. of India for the financial support (CRG/2021/005998). S.K.U. is grateful to Department of Science and Technology, Government of India for partial financial support under Promotion of University Research and Scientific Excellence (PURSE) grant scheme. The institutional manuscript ID No is CSIR-NBRI_MS/2023/01/03.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Garcia, A.; Santamaria, M.E.; Diaz, I.; Martinez, M. Disentangling transcriptional responses in plant defense against arthropod herbivores. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef]
- Erb, M.; Reymond, P. Molecular Interactions between Plants and Insect Herbivores. Annu. Rev. Plant Biol. 2019, 70, 527–557. [Google Scholar] [CrossRef]
- Vivaldo, G.; Masi, E.; Taiti, C.; Caldarelli, G.; Mancuso, S. The network of plants volatile organic compounds. Sci. Rep. 2017, 7, 11050. [Google Scholar] [CrossRef]
- Dorokhov, Y.L.; Sheshukova, E.V.; Komarova, T.V. Methanol in plant life. Front. Plant Sci. 2018, 9, 1623. [Google Scholar] [CrossRef]
- Guo, X.; Chang, S.; Hu, J.; Wang, Y.; Zhang, D.; Huang, L.; Zhang, Z.; Gao, J.; Liu, W.; He, G. Research progress of pectin methylesterase and its inhibitors. Curr. Protein Pept. Sci. 2022, 23, 684–696. [Google Scholar] [CrossRef]
- Pelloux, J.; Rustérucci, C.; Mellerowicz, E.J. New insights into pectin methylesterase structure and function. Trends Plant Sci. 2007, 12, 267–277. [Google Scholar] [CrossRef]
- Hasunuma, T.; Fukusaki, E.-I.; Kobayashi, A. Methanol production is enhanced by expression of an Aspergillus niger pectin methylesterase in tobacco cells. J. Biotechnol. 2003, 106, 45–52. [Google Scholar] [CrossRef]
- Frenkel, C.; Peters, J.S.; Tieman, D.M.; Tiznado, M.E.; Handa, A.K. Pectin methylesterase regulates methanol and ethanol accumu-lation in ripening tomato (Lycopersicon esculentum) fruit. J. Biol. Chem. 1998, 273, 4293–4295. [Google Scholar] [CrossRef]
- Körner, E.; Von Dahl, C.C.; Bonaventure, G.; Baldwin, I.T. Pectin methylesterase NaPME1 contributes to the emission of methanol during insect herbivory and to the elicitation of defence responses in Nicotiana attenuata. J. Exp. Bot. 2009, 60, 2631–2640. [Google Scholar] [CrossRef]
- Nonomura, A.M.; Benson, A.A. The path of carbon in photosynthesis: Improved crop yields with methanol. Proc. Natl. Acad. Sci. USA 1992, 89, 9794–9798. [Google Scholar] [CrossRef]
- Ramadan, T.; Omran, Y.A. The effect of foliar application of methanol on productivity and fruit quality of grapevine cv. Flame Seedless. Vitis 2005, 44, 11–16. [Google Scholar]
- Rowe, R.N.; Farr, D.J.; Richards, B.A.J. Effects of foliar and root applications of methanol or ethanol on the growth of tomato plants (Lycopersicon esculentum Mill). New Zealand J. Crop. Hortic. Sci. 1994, 22, 335–337. [Google Scholar] [CrossRef]
- Dixit, S.; Upadhyay, S.K.; Singh, H.; Sidhu, O.P.; Verma, P.C. Enhanced Methanol Production in Plants Provides Broad Spectrum Insect Resistance. PLoS ONE 2013, 8, e79664. [Google Scholar] [CrossRef] [PubMed]
- Razzaq, A.; Ali, A.; Zafar, M.M.; Nawaz, A.; Xiaoying, D.; Pengtao, L.; Qun, G.; Ashraf, M.; Ren, M.; Gong, W.; et al. Pyramiding of cry toxins and methanol producing genes to increase insect resistance in cotton. GM Crop. Food 2021, 12, 382–395. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Latif, A.; Rao, A.Q.; Azam, S.; Shahid, N.; Samiullah, T.R.; Yasmeen, A.; Shahid, A.A.; Nasir, I.A.; Husnain, T. A Combinational Approach of Enhanced Methanol Production and Double Bt Genes for Broad Spectrum Insect Resistance in Transgenic Cotton. Mol. Biotechnol. 2019, 61, 663–673. [Google Scholar] [CrossRef]
- Tran, D.; Dauphin, A.; Meimoun, P.; Kadono, T.; Nguyen, H.T.H.; Arbelet-Bonnin, D.; Zhao, T.; Errakhi, R.; Lehner, A.; Kawano, T.; et al. Methanol induces cytosolic calcium variations, membrane depolarization and ethylene production in arabidopsis and tobacco. Ann. Bot. 2018, 122, 849–860. [Google Scholar] [CrossRef]
- Downie, A.; Miyazaki, S.; Bohnert, H.; John, P.; Coleman, J.; Parry, M.; Haslam, R. Expression profiling of the response of Arabidopsis thaliana to methanol stimulation. Phytochemistry 2004, 65, 2305–2316. [Google Scholar] [CrossRef]
- Cangelosi, G.A.; Martinetti, G.; Leigh, J.A.; Lee, C.C.; Theines, C.; Nester, E.W. Role for Agrobacterium tumefaciens ChvA protein in export of beta-1, 2-glucan. J. Bacteriol. 1989, 171, 1609–1615. [Google Scholar] [CrossRef]
- Kumar, S.R.; Anunanthini, P.; Sathishkumar, R. Epigenetic silencing in transgenic plants. Front. Plant Sci. 2015, 6, 693. [Google Scholar] [CrossRef]
- Dorokhov, Y.L.; Komarova, T.V.; Petrunia, I.V.; Frolova, O.Y.; Pozdyshev, D.V.; Gleba, Y.Y. Airborne signals from a wounded leaf facilitate viral spreading and induce antibacterial resistance in neighboring plants. PLoS Pathog. 2012, 8, e1002640. [Google Scholar] [CrossRef]
- Lionetti, V.; Fabri, E.; De Caroli, M.; Hansen, A.R.; Willats, W.G.; Piro, G.; Bellincampi, D. Three Pectin Methylesterase Inhibitors Protect Cell Wall Integrity for Arabidopsis Immunity to Botrytis. Plant Physiol. 2017, 173, 1844–1863. [Google Scholar] [CrossRef]
- Hamann, T. Plant cell wall integrity maintenance as an essential component of biotic stress response mechanisms. Front. Plant Sci. 2012, 3, 77. [Google Scholar] [CrossRef]
- Boncan, D.A.; Tsang, S.S.; Li, C.; Lee, I.H.; Lam, H.M.; Chan, T.F.; Hui, J.H. Terpenes and terpenoids in plants: Interactions with en-vironment and insects. Int. J. Mol. Sci. 2020, 21, 7382. [Google Scholar] [CrossRef]
- Lei, R.; Ma, Z.; Yu, D. WRKY2/34–VQ20 modules in Arabidopsis thaliana negatively regulate expression of a trio of related MYB transcription factors during pollen development. Front. Plant Sci. 2018, 9, 331. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Teeples, M.; Lin, L.; De Lucas, M.; Turco, G.; Toal, T.W.; Gaudinier, A.; Young, N.F.; Trabucco, G.M.; Veling, M.T.; Lamothe, R.; et al. An Arabidopsis gene regulatory network for secondary cell wall synthesis. Nature 2015, 517, 571–575. [Google Scholar] [CrossRef]
- Geng, P.; Zhang, S.; Liu, J.; Zhao, C.; Wu, J.; Cao, Y.; Fu, C.; Han, X.; He, H.; Zhao, Q. MYB20, MYB42, MYB43, and MYB85 Regulate Phenylalanine and Lignin Biosynthesis during Secondary Cell Wall Formation. Plant Physiol. 2019, 182, 1272–1283. [Google Scholar] [CrossRef]
- He, G.; Liu, P.; Zhao, H.; Sun, J. The HD-ZIP II Transcription Factors Regulate Plant Architecture through the Auxin Pathway. Int. J. Mol. Sci. 2020, 21, 3250. [Google Scholar] [CrossRef]
- Onkokesung, N.; Reichelt, M.; van Doorn, A.; Schuurink, R.C.; van Loon, J.J.; Dicke, M. Modulation of flavonoid metabolites in Arabidopsis thaliana through overexpression of the MYB75 transcription factor: Role of kaempferol-3,7-dirhamnoside in resistance to the specialist insect herbivore Pieris brassicae. J. Exp. Bot. 2014, 65, 2203–2217. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Zhang, C.; Jin, P.; Tetteh, C.; Dong, X.; Luo, S.; Zhang, S.; Li, X.; Liu, Y.; Zhang, H. The cell-type specific role of Arabidopsis bZIP59 transcription factor in plant immunity. Plant Cell Environ. 2022, 45, 1843–1861. [Google Scholar] [CrossRef]
- Hasunuma, T.; Fukusaki, E.-I.; Kobayashi, A. Expression of fungal pectin methylesterase in transgenic tobacco leads to alteration in cell wall metabolism and a dwarf phenotype. J. Biotechnol. 2004, 111, 241–251. [Google Scholar] [CrossRef]
- Kang, K.; Park, S.; Natsagdorj, U.; Kim, Y.S.; Back, K. Methanol is an endogenous elicitor molecule for the synthesis of tryptophan and tryptophan-derived secondary metabolites upon senescence of detached rice leaves. Plant J. 2011, 66, 247–257. [Google Scholar] [CrossRef]
- Maury, S.; Geoffroy, P.; Legrand, M. Tobacco O-methyltransferases involved in phenylpropanoid metabolism. The different caffeoyl-coenzyme A/5-hydroxyferuloyl-coenzyme A 3/5-O-methyltransferase and caffeic acid/5-hydroxyferulic acid 3/5-O-methyltransferase classes have distinct substrate specificities and expression patterns. Plant Physiol. 1999, 121, 215–224. [Google Scholar]
- Wang, G.-F.; Balint-Kurti, P.J. Maize Homologs of CCoAOMT and HCT, Two Key Enzymes in Lignin Biosynthesis, Form Complexes with the NLR Rp1 Protein to Modulate the Defense Response. Plant Physiol. 2016, 171, 2166–2177. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Liu, Z.; Shen, H.; Wu, D. Damage-Associated Molecular Pattern-Triggered Immunity in Plants. Front. Plant Sci. 2019, 10, 646. [Google Scholar] [CrossRef]
- Lu, K.; Guo, W.; Lu, J.; Yu, H.; Qu, C.; Tang, Z.; Li, J.; Chai, Y.; Liang, Y. Genome-Wide Survey and Expression Profile Analysis of the Mitogen-Activated Protein Kinase (MAPK) Gene Family in Brassica rapa. PLoS ONE 2015, 10, e0132051. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).