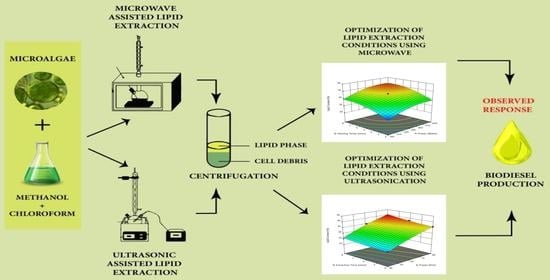
Prospects for Biodiesel Production from Emerging Algal Resource: Process Optimization and Characterization of Biodiesel Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Algae Collections
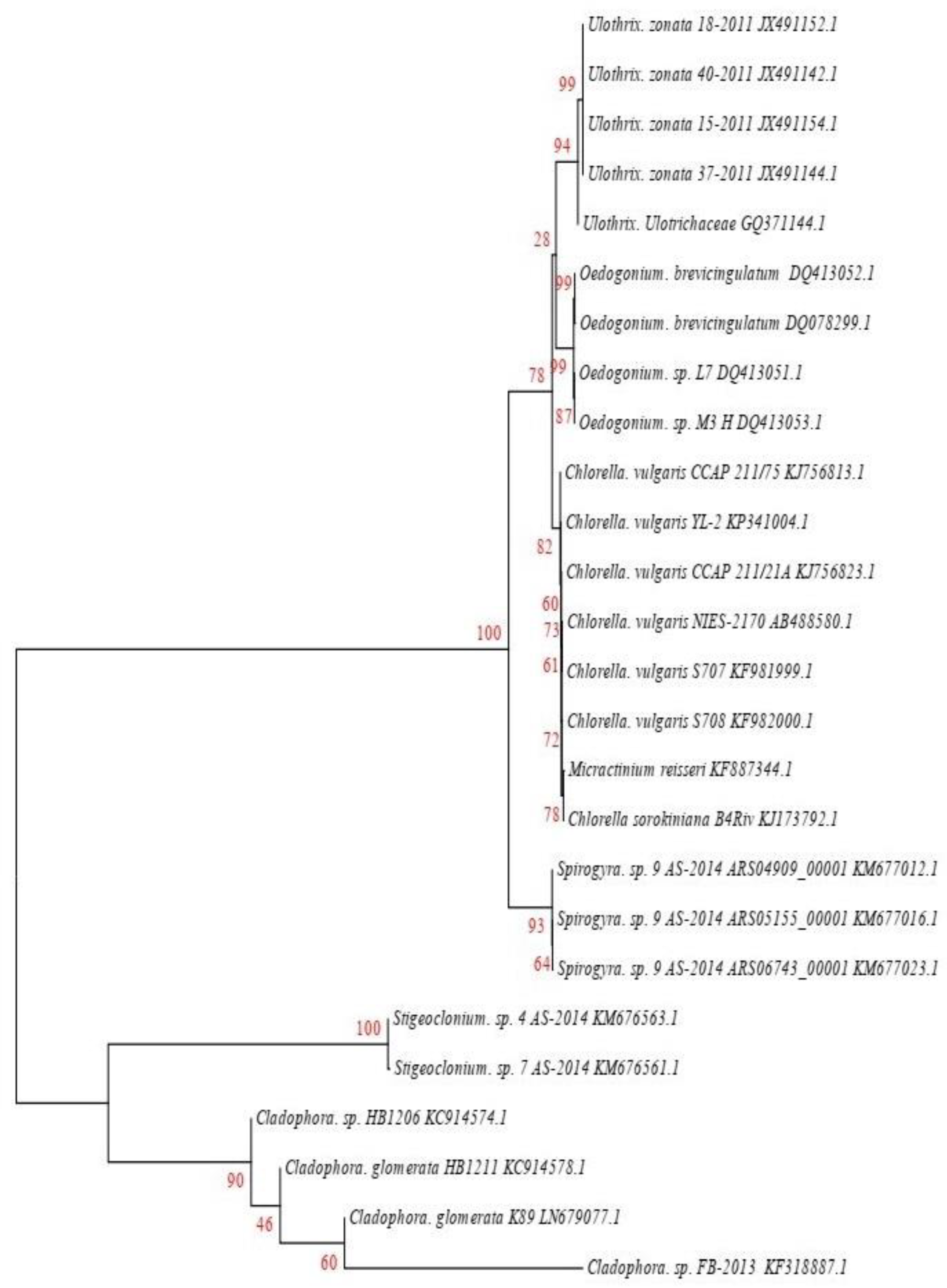
2.2. Molecular Identification
2.3. Cultivation and Harvesting
2.4. Microwave Assisted Lipid Extraction
2.5. Ultrasonic Assisted Lipid Extraction
2.6. Response Surface Optimization Designs of Two Extraction Processes
2.7. Statistical Analysis
2.8. Transesterification and Fatty Acid Methyl Esters (FAMEs) Analysis
2.9. Fuel Properties
3. Results and Discussions
3.1. Molecular Identification of Algae
3.2. Experimental Design and Statistical Analysis
3.3. Equations in Terms of Coded Factors of Quadratic Models
3.4. Analysis of Variance (ANOVA)
3.5. Validation of Models
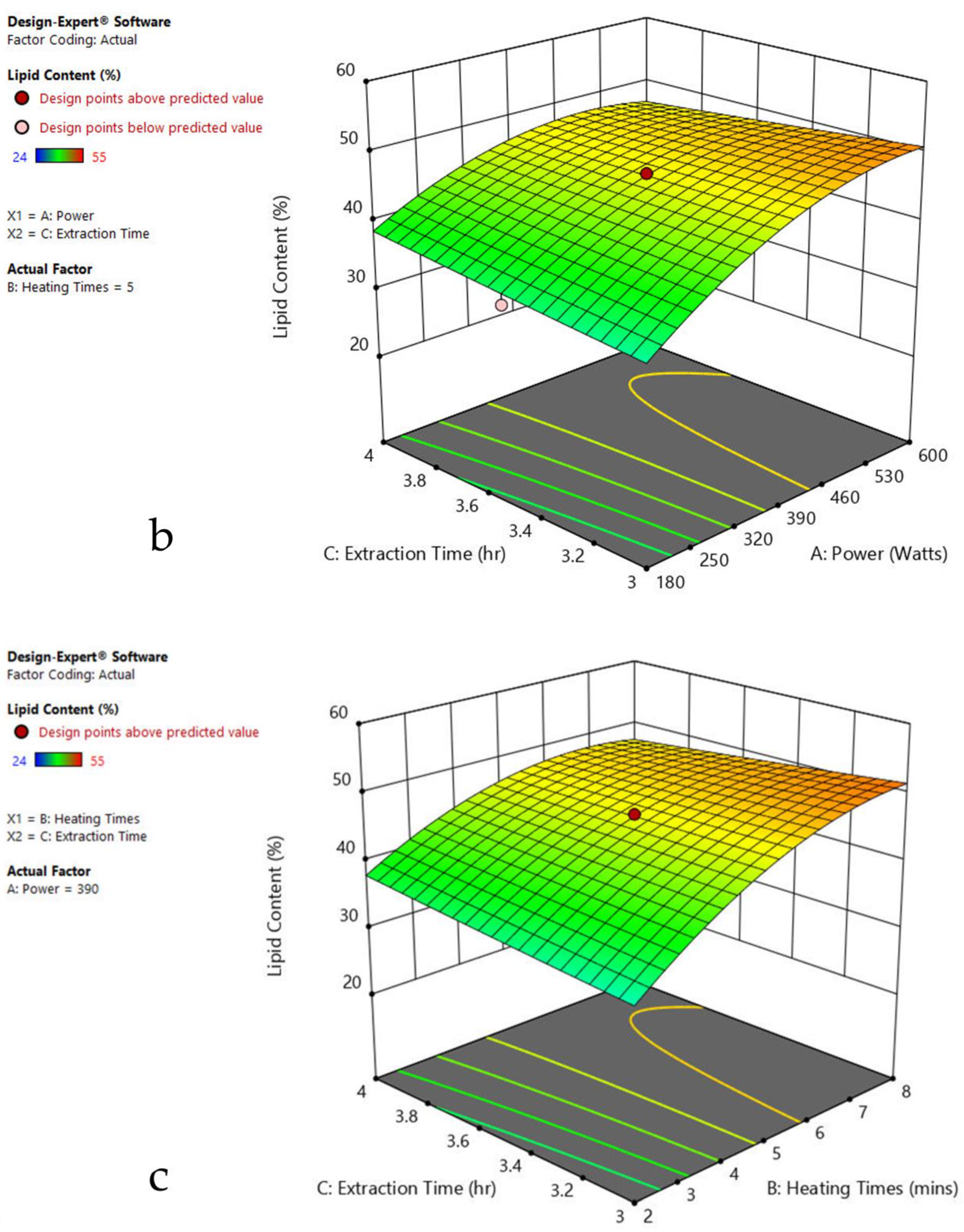
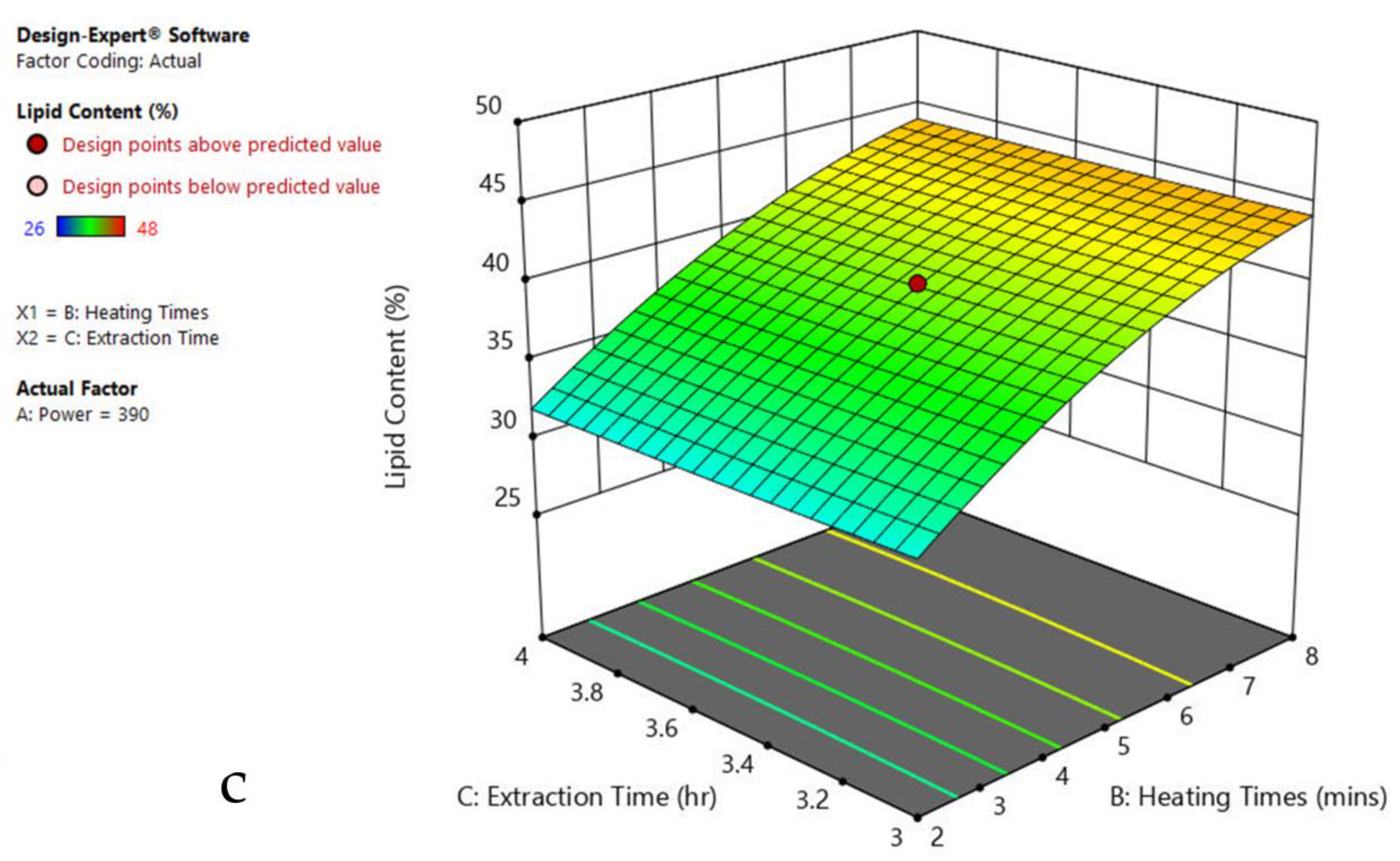
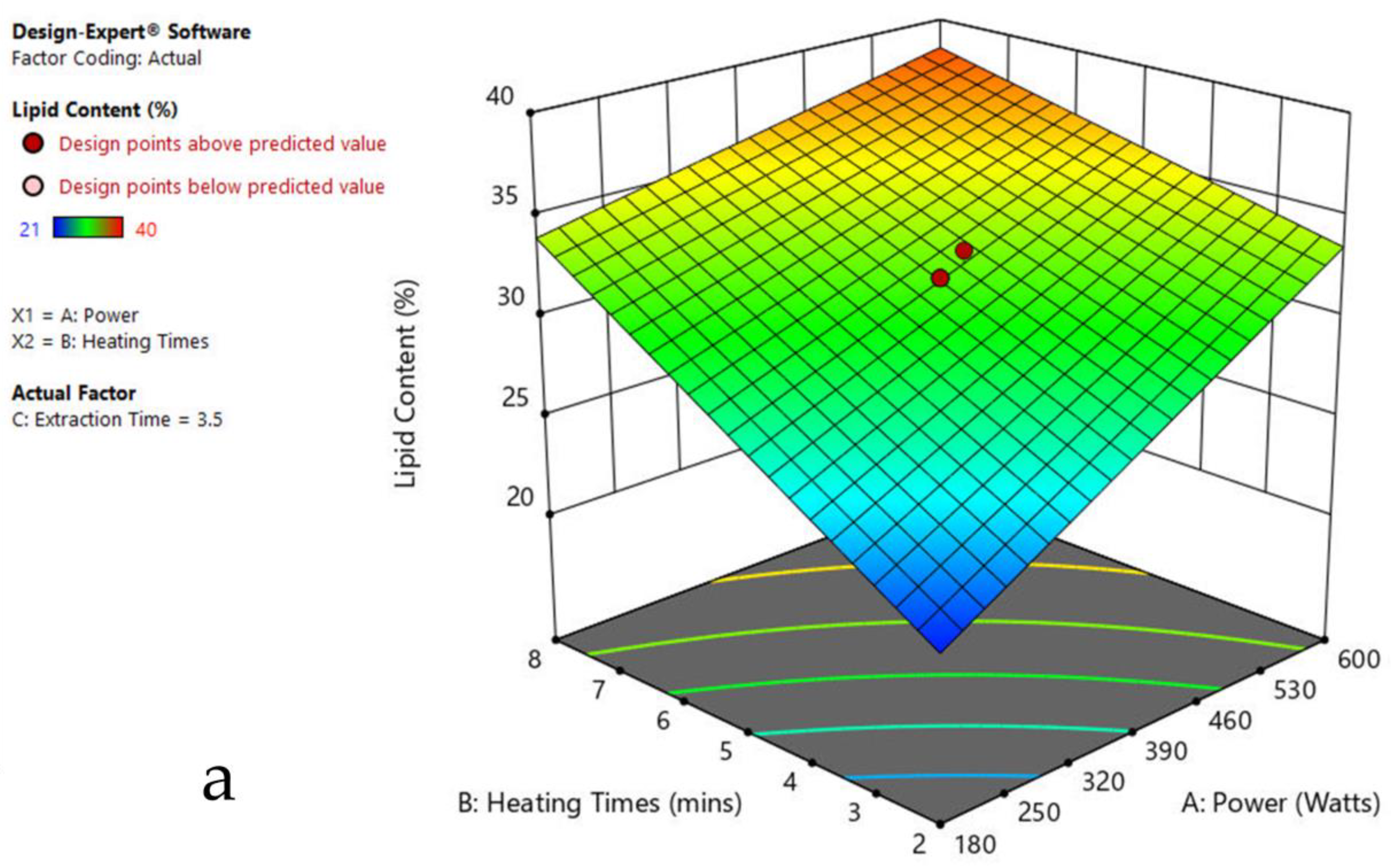
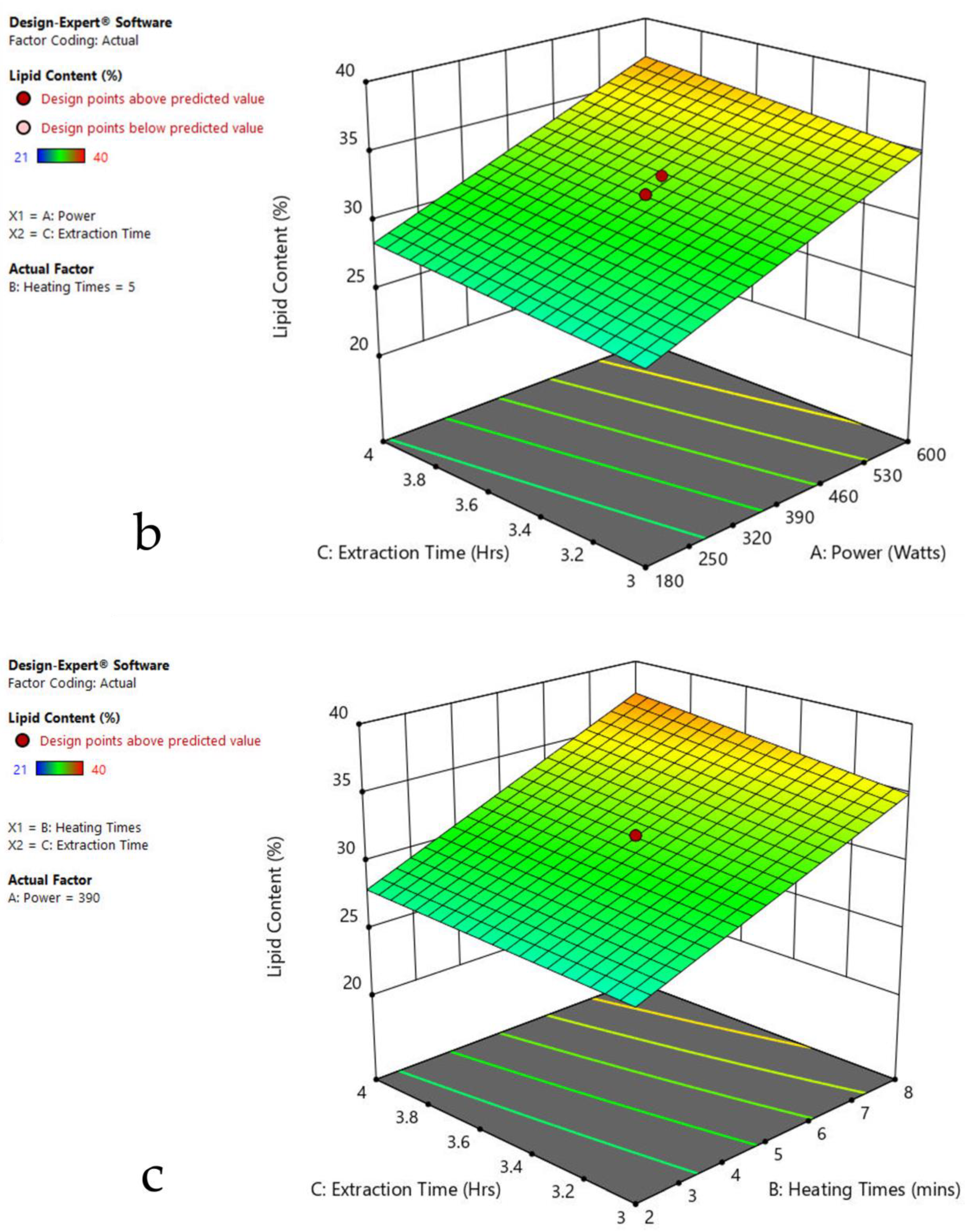
3.6. Interaction of Factors
3.7. Fatty Acid Composition
3.8. Fuel Properties
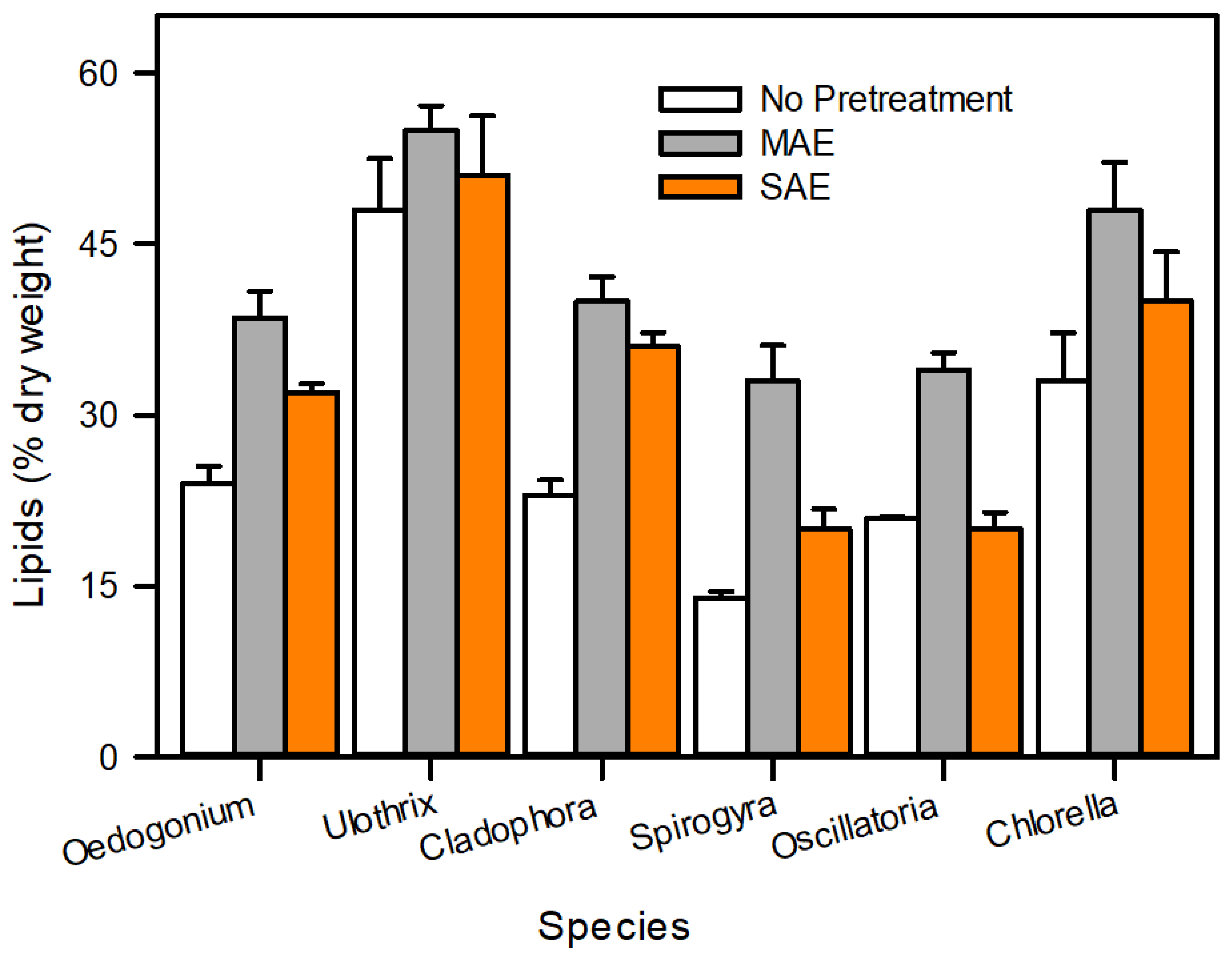
3.9. Comparative Analysis of Lipid Extraction Pretreatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Munir, N.; Hasnain, M.; Roessner, U.; Abideen, Z. Strategies in Improving Plant Salinity Resistance and Use of Salinity Resistant Plants for Economic Sustainability. Crit. Rev. Environ. Sci. Technol. 2022, 52, 2150–2196. [Google Scholar]
- Qadri, H.; Bhat, R.A. The Concerns for Global Sustainability of Freshwater Ecosystems. In Fresh Water Pollution Dynamics and Remediation; Springer: Singapore, 2020; pp. 1–13. [Google Scholar]
- Mann, A.; Kumar, A.; Sanwal, S.K.; Sharma, P.C. Sustainable Production of Pulses under Saline Lands in India. In Legume Crops-Prospects, Production and Uses; InTech Open: London, UK, 2020. [Google Scholar]
- Menegazzo, M.L.; Fonseca, G.G. Biomass Recovery and Lipid Extraction Processes for Microalgae Biofuels Production: A Review. Renew. Sustain. Energy Rev. 2019, 107, 87–107. [Google Scholar]
- Munir, N.; Abideen, Z.; Sharif, N. Development of Halophytes as Energy Feedstock by Applying Genetic Manipulations. All Life 2020, 13, 1–10. [Google Scholar]
- Hasnain, M.; Abideen, Z.; Naz, S.; Roessner, U.; Munir, N. Biodiesel Production from New Algal Sources Using Response Surface Methodology and Microwave Application. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Reed, O. Global Warming: Modeling Surface Structures for the Capture of Carbon Dioxide; University of Tennessee at Chattanooga: Chattanooga, TN, USA, 2020. [Google Scholar]
- Muhammad, G.; Alam, M.A.; Mofijur, M.; Jahirul, M.I.; Lv, Y.; Xiong, W.; Ong, H.C.; Xu, J. Modern Developmental Aspects in the Field of Economical Harvesting and Biodiesel Production from Microalgae Biomass. Renew. Sustain. Energy Rev. 2021, 135, 110209. [Google Scholar]
- Gnansounou, E.; Raman, J.K. Life Cycle Assessment of Algae Biodiesel and Its Co-Products. Appl. Energy 2016, 161, 300–308. [Google Scholar] [CrossRef]
- Hasnain, M.; Abideen, Z.; Anthony Dias, D.; Naz, S.; Munir, N. Utilization of Saline Water Enhances Lipid Accumulation in Green Microalgae for the Sustainable Production of Biodiesel. BioEnergy Res. 2022, 1–14. [Google Scholar] [CrossRef]
- Patil, S.; Lali, A.M.; Prakash, G. An Efficient Algae Cell Wall Disruption Methodology for Recovery of Intact Chloroplasts from Microalgae. J. Appl. Biol. Biotechnol. 2020, 8, 23–28. [Google Scholar]
- Teuling, E.; Wierenga, P.A.; Agboola, J.O.; Gruppen, H.; Schrama, J.W. Cell Wall Disruption Increases Bioavailability of Nannochloropsis Gaditana Nutrients for Juvenile Nile Tilapia (Oreochromis Niloticus). Aquaculture 2019, 499, 269–282. [Google Scholar]
- Patel, A.; Mikes, F.; Matsakas, L. An Overview of Current Pretreatment Methods Used to Improve Lipid Extraction from Oleaginous Microorganisms. Molecules 2018, 23, 1562. [Google Scholar]
- Hara, A.; Radin, N.S. Lipid Extraction of Tissues with a Low-Toxicity Solvent. Anal. Biochem. 1978, 90, 420–426. [Google Scholar] [CrossRef]
- Low, L.K. Analysis of Oils: Extraction of Lipids (Modified Folch’s Method). In Laboratory Manual on Analytical Methods and Procedures for Fish and Fish Products; Marine Fisheries Research Department, Southeast Asian Fisheries Development Center: Singapore, 1992; p. C–2. ISBN 9971883295. [Google Scholar]
- Chen, W.; Zhang, C.; Song, L.; Sommerfeld, M.; Hu, Q. A High Throughput Nile Red Method for Quantitative Measurement of Neutral Lipids in Microalgae. J. Microbiol. Methods 2009, 77, 41–47. [Google Scholar] [PubMed]
- Jensen, S.K. Improved Bligh and Dyer Extraction Procedure. Lipid Technol. 2008, 20, 280–281. [Google Scholar] [CrossRef]
- Ali, M.; Watson, I.A. Microwave Treatment of Wet Algal Paste for Enhanced Solvent Extraction of Lipids for Biodiesel Production. Renew. Energy 2015, 76, 470–477. [Google Scholar] [CrossRef]
- Katare, A.K.; Singh, B.; Shukla, P.; Gupta, S.; Singh, B.; Yalamanchili, K.; Kulshrestha, N.; Bhanwaria, R.; Sharma, A.K.; Sharma, S. Rapid Determination and Optimisation of Berberine from Himalayan Berberis Lycium by Soxhlet Apparatus Using CCD-RSM and Its Quality Control as a Potential Candidate for COVID-19. Nat. Prod. Res. 2022, 36, 868–873. [Google Scholar] [PubMed]
- Dehghani, M.H.; Karri, R.R.; Yeganeh, Z.T.; Mahvi, A.H.; Nourmoradi, H.; Salari, M.; Zarei, A.; Sillanpää, M. Statistical Modelling of Endocrine Disrupting Compounds Adsorption onto Activated Carbon Prepared from Wood Using CCD-RSM and DE Hybrid Evolutionary Optimization Framework: Comparison of Linear vs Non-Linear Isotherm and Kinetic Parameters. J. Mol. Liq. 2020, 302, 112526. [Google Scholar]
- Pashaei, H.; Ghaemi, A.; Nasiri, M.; Karami, B. Experimental Modeling and Optimization of CO2 Absorption into Piperazine Solutions Using RSM-CCD Methodology. ACS Omega 2020, 5, 8432–8448. [Google Scholar]
- Munir, N.; Sharif, N.; Naz, S.; Saleem, F.; Manzoor, F. Harvesting and Processing of Microalgae Biomass Fractions for Biodiesel Production (a Review). Sci. Technol. Dev. 2016, 32, 235–243. [Google Scholar]
- Reddy, A.; Majumder, A.B. Use of a Combined Technology of Ultrasonication, Three-Phase Partitioning, and Aqueous Enzymatic Oil Extraction for the Extraction of Oil from Spirogyra sp. J. Eng. 2014, 2014, 740631. [Google Scholar]
- Sharif, N.; Munir, N.; Saleem, F.; Aslam, F.; Naz, S. Mass Cultivation of Various Algal Species and Their Evaluation as a Potential Candidate for Lipid Production. Nat. Prod. Res. 2015, 29, 1938–1941. [Google Scholar]
- Hasnain, M.; Abideen, Z.; Hashmi, S.; Naz, S.; Munir, N. Assessing the Potential of Nutrient Deficiency for Enhancement of Biodiesel Production in Algal Resources. Biofuels 2022, 14, 1–34. [Google Scholar]
- Hasnain, M.; Munir, N.; Siddiqui, Z.S.; Ali, F.; El-Keblawy, A.; Abideen, Z. Integral Approach for the Evaluation of Sugar Cane Bio-Waste Molasses and Effects on Algal Lipids and Biodiesel Production. Waste Biomass Valorization 2023, 14, 23–42. [Google Scholar] [CrossRef]
- Singh, A.; Nigam, P.S.; Murphy, J.D. Mechanism and Challenges in Commercialisation of Algal Biofuels. Bioresour. Technol. 2011, 102, 26–34. [Google Scholar] [PubMed]
- Gupta, S.; Sharma, R.; Soni, S.K.; Sharma, S. Biomass Utilization of Waste Algal Consortium for Extraction of Algal Oil. J. Algal Biomass Util. 2012, 3, 34–38. [Google Scholar]
- Munir, N.; Imtiaz, A.; Sharif, N.; Haq, R.; Naz, S. Screening of Lipid Contents of Microalgae by Optimization of Oil Extraction Processes. Pak. J. Sci. 2016, 68, 1. [Google Scholar]
- Kumar, M.; Sun, Y.; Rathour, R.; Pandey, A.; Thakur, I.S.; Tsang, D.C.W. Algae as Potential Feedstock for the Production of Biofuels and Value-Added Products: Opportunities and Challenges. Sci. Total Environ. 2020, 716, 137116. [Google Scholar] [CrossRef]
- Qv, X.; Zhou, Q.; Jiang, J. Ultrasound-enhanced and Microwave-assisted Extraction of Lipid from Dunaliella tertiolecta and Fatty Acid Profile Analysis. J. Sep. Sci. 2014, 37, 2991–2999. [Google Scholar]
- Martínez, N.; Callejas, N.; Morais, E.G.; Costa, J.A.V.; Jachmanián, I.; Vieitez, I. Obtaining Biodiesel from Microalgae Oil Using Ultrasound-Assisted in-Situ Alkaline Transesterification. Fuel 2017, 202, 512–519. [Google Scholar]
- Koberg, M.; Cohen, M.; Ben-Amotz, A.; Gedanken, A. Bio-Diesel Production Directly from the Microalgae Biomass of Nannochloropsis by Microwave and Ultrasound Radiation. Bioresour. Technol. 2011, 102, 4265–4269. [Google Scholar] [CrossRef]
- Bermúdez Menéndez, J.M.; Arenillas, A.; Menéndez Díaz, J.Á.; Boffa, L.; Mantegna, S.; Binello, A.; Cravotto, G. Optimization of Microalgae Oil Extraction under Ultrasound and Microwave Irradiation. J. Chem. Technol. Biotechnol. 2014, 89, 1779–1784. [Google Scholar]
- Garoma, T.; Janda, D. Investigation of the Effects of Microalgal Cell Concentration and Electroporation, Microwave and Ultrasonication on Lipid Extraction Efficiency. Renew. Energy 2016, 86, 117–123. [Google Scholar]
- Jakhar, A.M.; Aziz, I.; Kaleri, A.R.; Hasnain, M.; Haider, G.; Ma, J.; Abideen, Z. Nano-Fertilizers: A Sustainable Technology for Improving Crop Nutrition and Food Security. NanoImpact 2022, 27, 100411. [Google Scholar] [PubMed]
- Nowacka, M.; Wiktor, A.; Dadan, M.; Rybak, K.; Anuszewska, A.; Materek, L.; Witrowa-Rajchert, D. The Application of Combined Pre-Treatment with Utilization of Sonication and Reduced Pressure to Accelerate the Osmotic Dehydration Process and Modify the Selected Properties of Cranberries. Foods 2019, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- Abd El Fatah, H.M.; El-Baghdady, K.Z.; Zakaria, A.E.; Sadek, H.N. Improved Lipid Productivity of Chlamydomonas globosa and Oscillatoria pseudogeminata as a Biodiesel Feedstock in Artificial Media and Wastewater. Biocatal. Agric. Biotechnol. 2020, 25, 101588. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Yoo, C.; Jun, S.-Y.; Ahn, C.-Y.; Oh, H.-M. Comparison of Several Methods for Effective Lipid Extraction from Microalgae. Bioresour. Technol. 2010, 101, S75–S77. [Google Scholar]
- Ferreira, A.F.; Dias, A.P.S.; Silva, C.M.; Costa, M. Effect of Low Frequency Ultrasound on Microalgae Solvent Extraction: Analysis of Products, Energy Consumption and Emissions. Algal Res. 2016, 14, 9–16. [Google Scholar] [CrossRef]
- De Souza Silva, A.P.F.; Costa, M.C.; Lopes, A.C.; Neto, E.F.A.; Leitão, R.C.; Mota, C.R.; dos Santos, A.B. Comparison of Pretreatment Methods for Total Lipids Extraction from Mixed Microalgae. Renew. Energy 2014, 63, 762–766. [Google Scholar]
- Zheng, H.; Yin, J.; Gao, Z.; Huang, H.; Ji, X.; Dou, C. Disruption of Chlorella vulgaris Cells for the Release of Biodiesel-Producing Lipids: A Comparison of Grinding, Ultrasonication, Bead Milling, Enzymatic Lysis, and Microwaves. Appl. Biochem. Biotechnol. 2011, 164, 1215–1224. [Google Scholar]
- Gouveia, L.; Oliveira, A.C. Microalgae as a Raw Material for Biofuels Production. J. Ind. Microbiol. Biotechnol. 2009, 36, 269–274. [Google Scholar]
- Fulke, A.B.; Mudliar, S.N.; Yadav, R.; Shekh, A.; Srinivasan, N.; Ramanan, R.; Krishnamurthi, K.; Devi, S.S.; Chakrabarti, T. Bio-Mitigation of CO2, Calcite Formation and Simultaneous Biodiesel Precursors Production Using Chlorella sp. Bioresour. Technol. 2010, 101, 8473–8476. [Google Scholar] [CrossRef]
- Maheshwari, N.; Krishna, P.K.; Thakur, I.S.; Srivastava, S. Biological Fixation of Carbon Dioxide and Biodiesel Production Using Microalgae Isolated from Sewage Waste Water. Environ. Sci. Pollut. Res. 2020, 27, 27319–27329. [Google Scholar] [CrossRef] [PubMed]
- Bagul, S.Y.; Bharti, R.K.; Dhar, D.W. Assessing Biodiesel Quality Parameters for Wastewater Grown Chlorella sp. Water Sci. Technol. 2017, 76, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Benayache, N.-Y.; Nguyen-Quang, T.; Hushchyna, K.; McLellan, K.; Afri-Mehennaoui, F.-Z.; Bouaïcha, N. An Overview of Cyanobacteria Harmful Algal Bloom (CyanoHAB) Issues in Freshwater Ecosystems. Limnology: Some New Aspects of Inland Water Ecology; InTech Open: London, UK, 2019; pp. 1–25. [Google Scholar]
- Selvarajan, R.; Felföldi, T.; Tauber, T.; Sanniyasi, E.; Sibanda, T.; Tekere, M. Screening and Evaluation of Some Green Algal Strains (Chlorophyceae) Isolated from Freshwater and Soda Lakes for Biofuel Production. Energies 2015, 8, 7502–7521. [Google Scholar] [CrossRef]
- Rizwan, M.; Mujtaba, G.; Memon, S.A.; Lee, K. Influence of Salinity and Nitrogen in Dark on Dunaliella tertiolecta’s Lipid and Carbohydrate Productivity. Biofuels 2022, 13, 475–481. [Google Scholar]
- Rokicka, M.; Zieliński, M.; Dudek, M.; Dębowski, M. Effects of Ultrasonic and Microwave Pretreatment on Lipid Extraction of Microalgae and Methane Production from the Residual Extracted Biomass. BioEnergy Res. 2021, 14, 752–760. [Google Scholar]
- Almutairi, A.W.; Toulibah, H.E. Effect of Salinity and Ph on Fatty Acid Profile of the Green Algae Tetraselmis suecica. J. Pet. Environ. Biotechnol. 2017, 8, 3–8. [Google Scholar] [CrossRef]
- Chi, N.T.L.; Anto, S.; Ahamed, T.S.; Kumar, S.S.; Shanmugam, S.; Samuel, M.S.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A. A Review on Biochar Production Techniques and Biochar Based Catalyst for Biofuel Production from Algae. Fuel 2021, 287, 119411. [Google Scholar]
- Sathish, T.; Singaravelu, D.K. Diesel Engine Emission Characteristics Study Using Algae Biofuel. J. Sci. Ind. Res. 2020, 79, 547–551. [Google Scholar]
- Karthikeyan, S.; Periyasamy, M.; Prathima, A. Performance Characteristics of CI Engine Using Chlorella vulgaris Microalgae Oil as a Pilot Dual Fuel Blends. Mater. Today Proc. 2020, 33, 3277–3282. [Google Scholar] [CrossRef]
- Banerji, A.; Deshpande, R.; Elk, M.; Shoemaker, J.A.; Tettenhorst, D.R.; Bagley, M.; Santo Domingo, J.W. Highlighting the Promise of QPCR-Based Environmental Monitoring: Response of the Ribosomal RNA: DNA Ratio of Calanoid Copepods to Toxic Cyanobacteria. Ecotoxicology 2021, 30, 411–420. [Google Scholar]
- Carter, S.K.; Pilliod, D.S.; Haby, T.; Prentice, K.L.; Aldridge, C.L.; Anderson, P.J.; Bowen, Z.H.; Bradford, J.B.; Cushman, S.A.; DeVivo, J.C. Bridging the Research-Management Gap: Landscape Science in Practice on Public Lands in the Western United States. Landsc. Ecol. 2020, 35, 545–560. [Google Scholar]
- Javed, F.; Aslam, M.; Rashid, N.; Shamair, Z.; Khan, A.L.; Yasin, M.; Fazal, T.; Hafeez, A.; Rehman, F.; Rehman, M.S.U. Microalgae-Based Biofuels, Resource Recovery and Wastewater Treatment: A Pathway towards Sustainable Biorefinery. Fuel 2019, 255, 115826. [Google Scholar]
- Rahman, A.; Agrawal, S.; Nawaz, T.; Pan, S.; Selvaratnam, T. A Review of Algae-Based Produced Water Treatment for Biomass and Biofuel Production. Water 2020, 12, 2351. [Google Scholar] [CrossRef]
- Li, P.; Du, Z.; Chang, C.; Zhao, S.; Xu, G.; Xu, C.C. Efficient Catalytic Conversion of Waste Peanut Shells into Liquid Biofuel: An Artificial Intelligence Approach. Energy Fuels 2020, 34, 1791–1801. [Google Scholar] [CrossRef]
- Joshi, M.P.; Thipse, S.S. Combustion Analysis of a Compression-Ignition Engine Fuelled with an Algae Biofuel Blend and Diethyl Ether as an Additive by Using an Artificial Neural Network. Biofuels 2021, 12, 429–438. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, H.; Li, J.; Wang, Y.; Leng, L.; Li, J.; Yao, Y.; Lu, Q.; Yuan, W.; Zhou, W. Algal Biorefinery to Value-Added Products by Using Combined Processes Based on Thermochemical Conversion: A Review. Algal Res. 2020, 47, 101819. [Google Scholar] [CrossRef]
- Bai, X.; Naghdi, F.G.; Ye, L.; Lant, P.; Pratt, S. Enhanced Lipid Extraction from Algae Using Free Nitrous Acid Pretreatment. Bioresour. Technol. 2014, 159, 36–40. [Google Scholar]



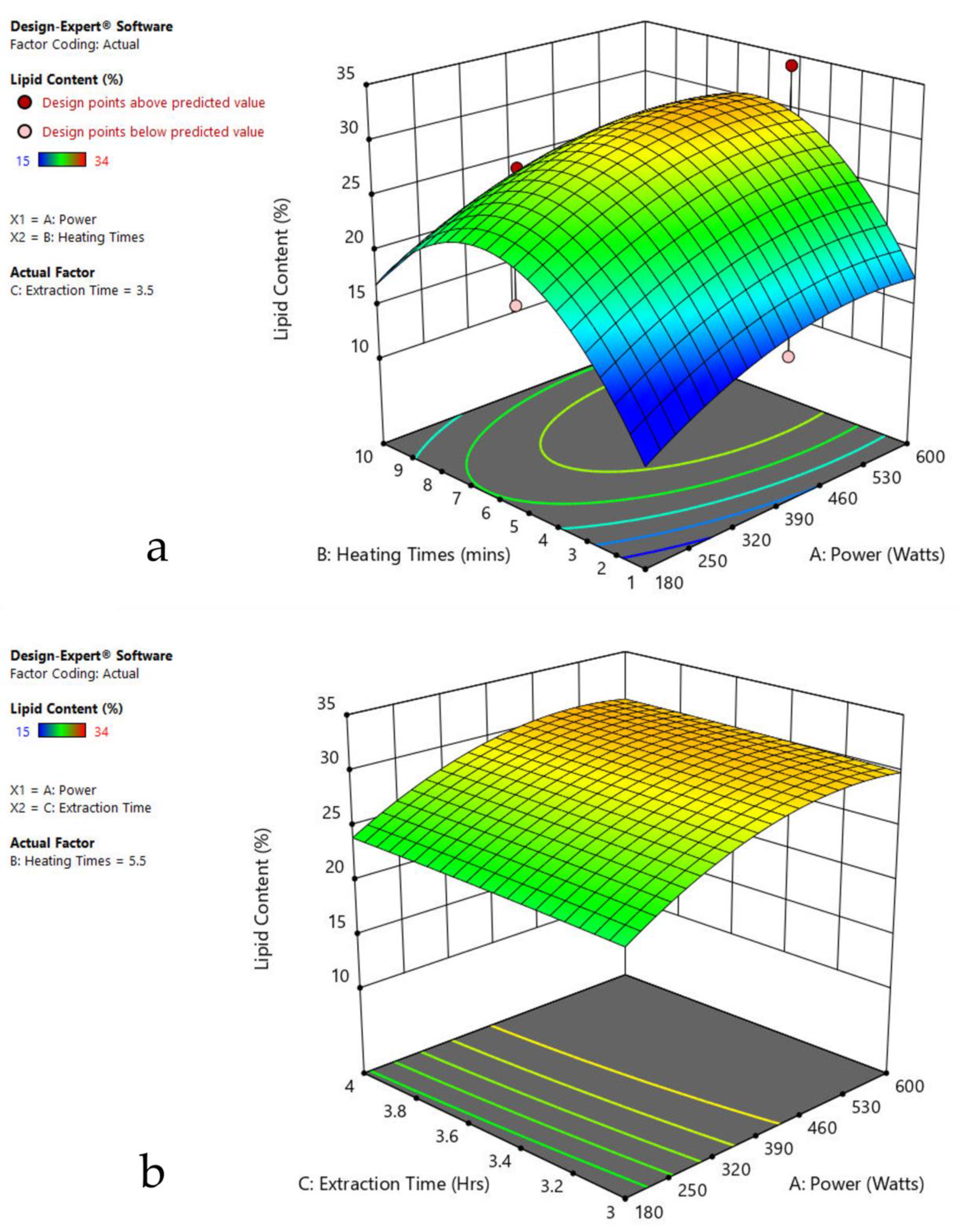
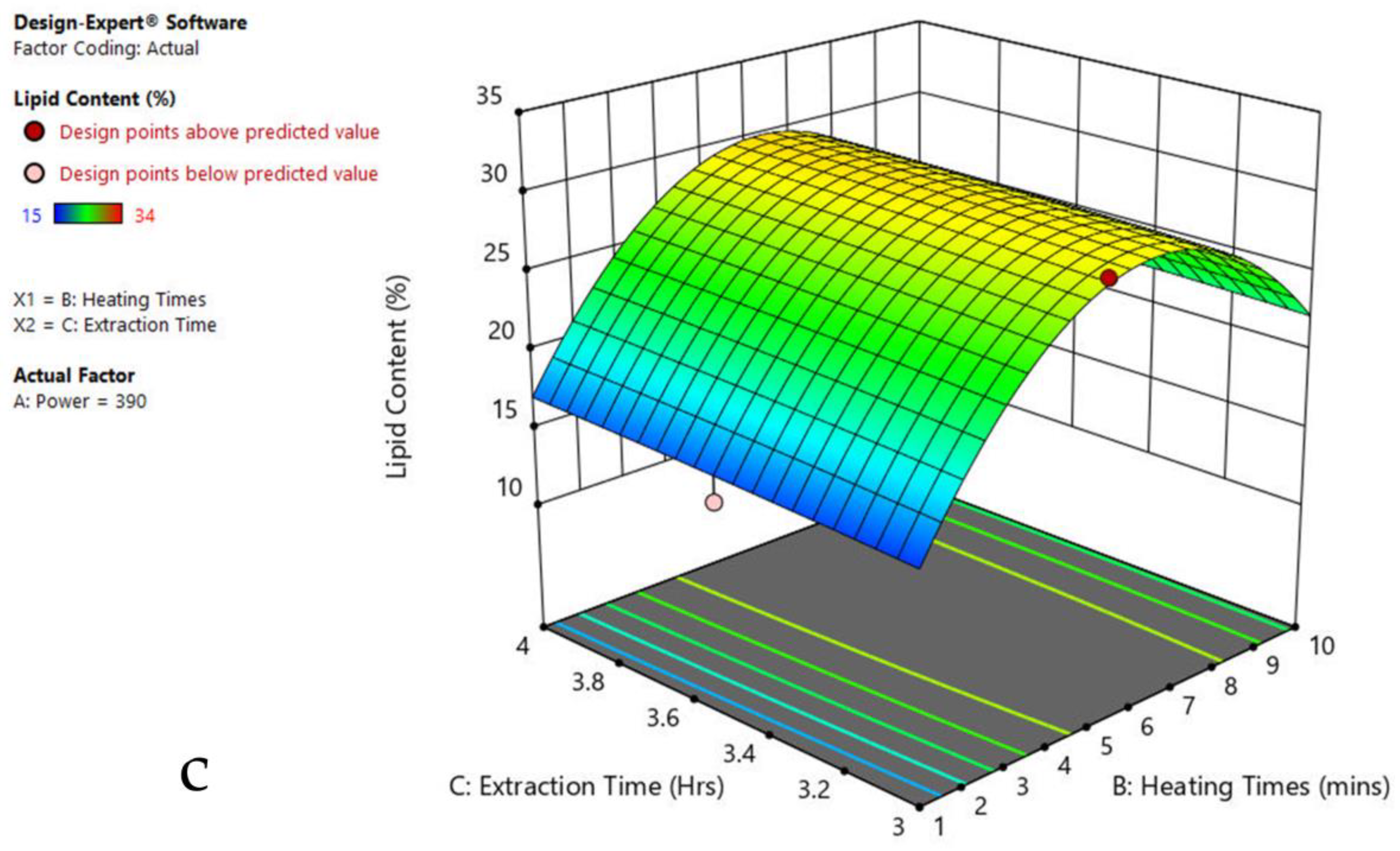



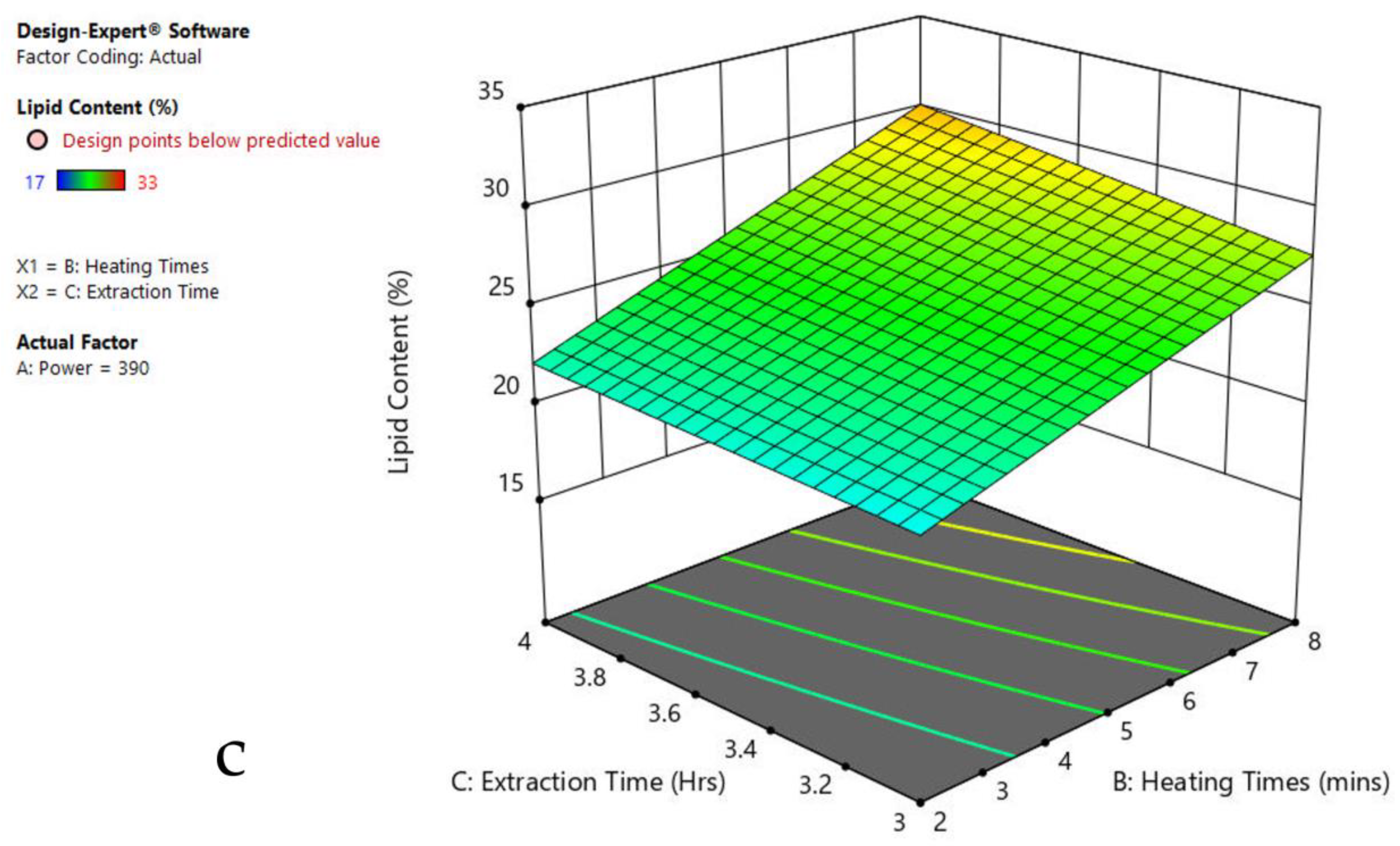
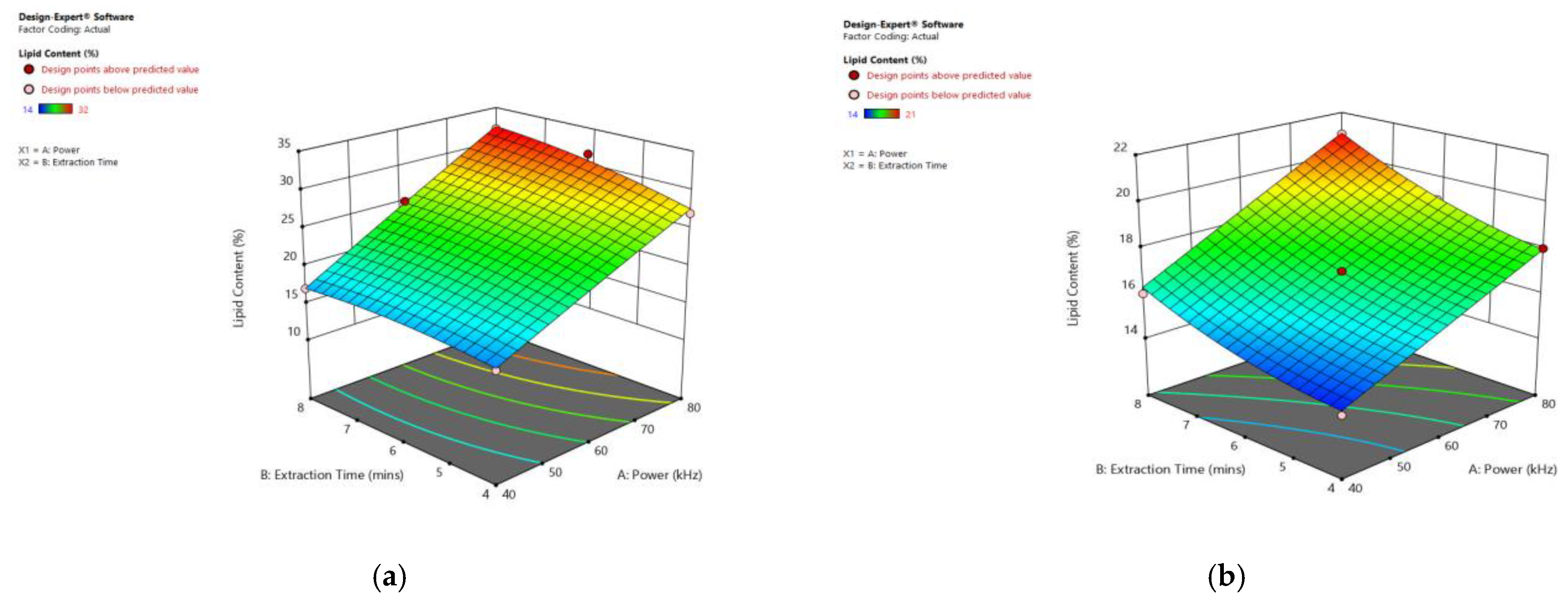

| Factor 1 | Factor 2 | Factor 3 | Response 1 | ||
|---|---|---|---|---|---|
| Std | Run | A:Power | B:Heating Times | C:Extraction Time | Lipid Content |
| Watts | Mins | Hr | % | ||
| 13 | 1 | 390 | 5 | 3.5 | 31 |
| 2 | 2 | 180 | 8 | 3 | 26 |
| 9 | 3 | 390 | 5 | 3.5 | 31 |
| 19 | 4 | 390 | 5 | 2.5 | 30 |
| 5 | 5 | 390 | 5 | 4.5 | 32 |
| 3 | 6 | 390 | 5 | 3.5 | 31 |
| 17 | 7 | 600 | 8 | 4 | 38.5 |
| 18 | 8 | 600 | 2 | 4 | 28 |
| 11 | 9 | 390 | 5 | 3.5 | 31 |
| 16 | 10 | 390 | 1 | 3.5 | 22 |
| 7 | 11 | 390 | 5 | 3.5 | 31 |
| 4 | 12 | 180 | 2 | 3 | 19 |
| 6 | 13 | 390 | 10 | 3.5 | 35 |
| 14 | 14 | 180 | 2 | 4 | 20 |
| 8 | 15 | 600 | 8 | 3 | 38 |
| 12 | 16 | 750 | 5 | 3.5 | 37 |
| 10 | 17 | 600 | 2 | 3 | 27.5 |
| 15 | 18 | 180 | 8 | 4 | 27 |
| 1 | 19 | 390 | 5 | 3.5 | 31 |
| 20 | 20 | 360 | 5 | 3.5 | 31 |
| Factor 1 | Factor 2 | Factor 3 | Response 1 | ||
|---|---|---|---|---|---|
| Std | Run | A:Power | B:Heating Times | C:Extraction Time | Lipid Content |
| Watts | Mins | Hrs | % | ||
| 7 | 1 | 180 | 8 | 4 | 28 |
| 3 | 2 | 180 | 8 | 3 | 26 |
| 16 | 3 | 390 | 5 | 3.5 | 29 |
| 14 | 4 | 390 | 5 | 4.5 | 32 |
| 9 | 5 | 180 | 5 | 3.5 | 24 |
| 8 | 6 | 600 | 8 | 4 | 32 |
| 1 | 7 | 180 | 2 | 3 | 17 |
| 15 | 8 | 390 | 5 | 3.5 | 29 |
| 11 | 9 | 390 | 1 | 3.5 | 16 |
| 2 | 10 | 600 | 2 | 3 | 31 |
| 5 | 11 | 180 | 2 | 4 | 20 |
| 10 | 12 | 600 | 5 | 3.5 | 34 |
| 20 | 13 | 390 | 5 | 3.5 | 29 |
| 12 | 14 | 390 | 10 | 3.5 | 33.5 |
| 19 | 15 | 390 | 5 | 3.5 | 29 |
| 4 | 16 | 600 | 8 | 3 | 32 |
| 13 | 17 | 390 | 5 | 3 | 31 |
| 6 | 18 | 600 | 2 | 4 | 32 |
| 17 | 19 | 390 | 5 | 3.5 | 29 |
| 18 | 20 | 390 | 5 | 3.5 | 29 |
| Factor 1 | Factor 2 | Factor 3 | Response 1 | ||
|---|---|---|---|---|---|
| Std | Run | A:Power | B:Heating Times | C:Extraction Time | Lipid Content |
| Watts | Mins | Hr | % | ||
| 12 | 1 | 600 | 2 | 4 | 38 |
| 13 | 2 | 390 | 5 | 3.5 | 47 |
| 1 | 3 | 180 | 2 | 3 | 24 |
| 15 | 4 | 390 | 10 | 3.5 | 49 |
| 16 | 5 | 390 | 5 | 2.5 | 47 |
| 5 | 6 | 390 | 5 | 4.5 | 48 |
| 20 | 7 | 180 | 2 | 4 | 30 |
| 9 | 8 | 180 | 5 | 3.5 | 35 |
| 3 | 9 | 390 | 5 | 3.5 | 47 |
| 10 | 10 | 180 | 8 | 3 | 38 |
| 11 | 11 | 390 | 1 | 3.5 | 29 |
| 8 | 12 | 600 | 8 | 4 | 45 |
| 4 | 13 | 750 | 5 | 3.5 | 47 |
| 17 | 14 | 180 | 8 | 4 | 39 |
| 2 | 15 | 600 | 8 | 3 | 55 |
| 7 | 16 | 390 | 5 | 3.5 | 47 |
| 6 | 17 | 390 | 5 | 3.5 | 47 |
| 14 | 18 | 600 | 2 | 3 | 37 |
| 18 | 19 | 390 | 5 | 3.5 | 47 |
| 19 | 20 | 390 | 5 | 3.5 | 47 |
| Factor 1 | Factor 2 | Factor 3 | Response 1 | ||
|---|---|---|---|---|---|
| Std | Run | A:Power | B:Heating Times | C:Extraction Time | Lipid Content |
| Watts | Mins | Hr | % | ||
| 16 | 1 | 390 | 5 | 3.5 | 40 |
| 17 | 2 | 390 | 5 | 4.5 | 39 |
| 14 | 3 | 390 | 5 | 3.5 | 40 |
| 15 | 4 | 600 | 8 | 3 | 47 |
| 2 | 5 | 600 | 2 | 4 | 37 |
| 7 | 6 | 180 | 2 | 3 | 26 |
| 1 | 7 | 180 | 8 | 3 | 39 |
| 6 | 8 | 390 | 5 | 3.5 | 40 |
| 11 | 9 | 390 | 5 | 3.5 | 40 |
| 4 | 10 | 600 | 8 | 4 | 48 |
| 12 | 11 | 390 | 5 | 2.5 | 40 |
| 20 | 12 | 390 | 1 | 3.5 | 27 |
| 8 | 13 | 390 | 8 | 3.5 | 42 |
| 18 | 14 | 390 | 5 | 3.5 | 40 |
| 19 | 15 | 600 | 2 | 3 | 37 |
| 3 | 16 | 700 | 5 | 3.5 | 41 |
| 5 | 17 | 180 | 2 | 4 | 26 |
| 10 | 18 | 390 | 5 | 3.5 | 40 |
| 9 | 19 | 600 | 5 | 3.5 | 45 |
| 13 | 20 | 180 | 8 | 4 | 39 |
| Factor 1 | Factor 2 | Factor 3 | Response 1 | ||
|---|---|---|---|---|---|
| Std | Run | A:Power | B:Heating Times | C:Extraction Time | Lipid Content |
| Watts | mins | Hrs | % | ||
| 14 | 1 | 390 | 5 | 3.5 | 32 |
| 5 | 2 | 180 | 2 | 4 | 22 |
| 6 | 3 | 600 | 2 | 4 | 34 |
| 2 | 4 | 600 | 2 | 3 | 33 |
| 7 | 5 | 180 | 8 | 4 | 35 |
| 3 | 6 | 180 | 8 | 3 | 33 |
| 9 | 7 | 365 | 5 | 3.5 | 30 |
| 10 | 8 | 415 | 5 | 3.5 | 33 |
| 8 | 9 | 600 | 8 | 4 | 40 |
| 1 | 10 | 180 | 2 | 3 | 21 |
| 11 | 11 | 390 | 5 | 3.5 | 32 |
| 4 | 12 | 600 | 8 | 3 | 37 |
| 12 | 13 | 390 | 5 | 3.5 | 32 |
| 13 | 14 | 390 | 5 | 3.5 | 32 |
| 19 | 15 | 390 | 5 | 4.5 | 34 |
| 16 | 16 | 390 | 5 | 2.5 | 32 |
| 20 | 17 | 390 | 1 | 3.5 | 24 |
| 15 | 18 | 390 | 8 | 3.5 | 37 |
| 18 | 19 | 700 | 5 | 3.5 | 37.5 |
| 17 | 20 | 600 | 5 | 3.5 | 38 |
| Factor 1 | Factor 2 | Factor 3 | Response 1 | ||
|---|---|---|---|---|---|
| Std | Run | A:Power | B:Heating Times | C:Extraction Time | Lipid Content |
| Watts | Mins | Hrs | % | ||
| 9 | 1 | 365 | 5 | 3.5 | 23 |
| 1 | 2 | 180 | 2 | 3 | 17 |
| 10 | 3 | 415 | 5 | 3.5 | 26 |
| 13 | 4 | 390 | 5 | 3.5 | 25 |
| 12 | 5 | 390 | 5 | 3.5 | 25 |
| 6 | 6 | 600 | 2 | 4 | 27 |
| 7 | 7 | 180 | 8 | 4 | 28 |
| 14 | 8 | 390 | 5 | 3.5 | 25 |
| 4 | 9 | 600 | 8 | 3 | 30 |
| 2 | 10 | 600 | 2 | 3 | 26 |
| 3 | 11 | 180 | 8 | 3 | 26 |
| 5 | 12 | 180 | 2 | 4 | 18 |
| 8 | 13 | 600 | 8 | 4 | 33 |
| 11 | 14 | 390 | 5 | 3.5 | 25 |
| 20 | 15 | 390 | 5 | 4.5 | 26 |
| 17 | 16 | 390 | 5 | 2.5 | 24 |
| 19 | 17 | 390 | 1 | 3.5 | 18 |
| 15 | 18 | 390 | 8 | 3.5 | 27 |
| 18 | 19 | 700 | 5 | 3.5 | 32 |
| 16 | 20 | 600 | 5 | 3.5 | 31.5 |
| Factor 1 | Factor 2 | Lipid Content | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Std | Run | A:Power | B:Extraction Time | Oedogonium sp. | Oscillatoria sp. | Ulothrix sp. | Chlorella sp. | Cladophora sp. | Spirogyra sp. |
| kHz | Mins | % | % | % | % | % | % | ||
| 8 | 1 | 60 | 8 | 25 | 20 | 45 | 35 | 29 | 19 |
| 12 | 2 | 60 | 6 | 24 | 17 | 44 | 32 | 26 | 16 |
| 9 | 3 | 60 | 6 | 24 | 17 | 44 | 32 | 26 | 16 |
| 3 | 4 | 40 | 8 | 17 | 16 | 36 | 26 | 21 | 15 |
| 1 | 5 | 40 | 4 | 16 | 14 | 35 | 24 | 15 | 13 |
| 4 | 6 | 80 | 8 | 32 | 21 | 51 | 40 | 36 | 20 |
| 10 | 7 | 60 | 6 | 24 | 17 | 44 | 32 | 26 | 16 |
| 7 | 8 | 60 | 3 | 21 | 16 | 38 | 30 | 25 | 15 |
| 2 | 9 | 80 | 4 | 27 | 18 | 46 | 36 | 29 | 17 |
| 11 | 10 | 60 | 6 | 24 | 17 | 44 | 32 | 26 | 16 |
| 13 | 11 | 60 | 6 | 24 | 17 | 44 | 32 | 26 | 16 |
| 6 | 12 | 80 | 6 | 31.5 | 19 | 49 | 38 | 31.5 | 18 |
| 5 | 13 | 30 | 6 | 14 | 14 | 32 | 20 | 14 | 13 |
| Lipid Parameter Contents | Correlation | |
|---|---|---|
| Oedogonium sp. | (2) | |
| Oscillatoria sp. | (3) | |
| Ulothrix sp. | (4) | |
| Chlorella sp. | (5) | |
| Cladophora sp. | (6) | |
| Spirogyra sp. | (7) | |
| Equations in terms of coded factors of quadratic model of SAE | ||
| Oedogonium sp. | (8) | |
| Oscillatoria sp. | (9) | |
| Ulothrix sp. | (10) | |
| Chlorella sp. | (11) | |
| Cladophora sp. | (12) | |
| Spirogyra sp. | (13) | |
| Fatty Acids | Oedogonium sp. | Oscillatoria sp. | Ulothrix sp. | Chlorella sp. | Cladophora sp. | Spirogyra sp. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | MAE | UAE | Control | MAE | UAE | Control | MAE | UAE | Control | MAE | UAE | Control | MAE | UAE | Control | MAE | UAE | |
| Myristoleic acid (14:1) | 0.15 | - | - | - | - | - | - | - | - | - | - | - | 0.32 | - | - | 0.18 | 0.12 | 0.15 |
| Myristic acid (14:0) | 0.37 | 0.25 | 0.28 | 0.34 | 0.26 | 0.30 | 0.19 | 0.12 | 0.16 | 0.20 | 0.10 | 0.15 | 0.18 | 0.11 | 0.15 | 0.05 | - | 0.01 |
| Hexadecadienoic (16:2) | 0.46 | 0.29 | 0.34 | - | - | - | - | - | - | - | - | - | - | - | - | 2.28 | 2.11 | 2.18 |
| Palmitoleic acid (16:1) | 2.92 | 3.5 | 3.2 | 0.38 | 1.12 | 0.81 | - | - | - | 0.10 | 0.31 | 0.22 | - | - | - | 0.37 | 0.56 | 0.41 |
| Palmatic acid (16:0) | 1.19 | 1.38 | 1.26 | 3.12 | 3.21 | 3.19 | 0.28 | 1.10 | 0.57 | 1.0 | 1.28 | 1.19 | 0.44 | 1.22 | 0.98 | 0.19 | 0.31 | 0.29 |
| Linolenic acid (18:3) | 0.44 | 0.39 | 0.40 | - | - | - | - | - | - | - | - | - | - | - | - | 0.68 | 0.45 | 0.51 |
| Linoleic acid (18:2) | 0.38 | 0.23 | 0.28 | 2.18 | 1.91 | 2.1 | 0.6 | 0.4 | 0.5 | 0.50 | 0.41 | 0.46 | 2.13 | 1.80 | 1.90 | 1.56 | 1.11 | 1.28 |
| Oleic acid (18:1) | 3.12 | 3.52 | 3.31 | 2.15 | 3.11 | 2.25 | 0.46 | 1.91 | 1.51 | 0.10 | 0.25 | 0.15 | 0.15 | 0.19 | 0.17 | 0.35 | 1.23 | 1.17 |
| Stearic acid (18:0) | 1.32 | 1.23 | 1.27 | 3.14 | 2.72 | 2.98 | - | - | - | - | - | - | - | - | - | 0.06 | - | - |
| Gondoic acid (20:1) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.18 | - | - |
| Arachidic acid (20:0) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.14 | - | - |
| Erucic acid (22:1) | 0.46 | 0.29 | 0.31 | - | - | - | - | - | - | 0.12 | 0.11 | 0.10 | 0.12 | 0.12 | 0.12 | - | - | - |
| Caprylate (8:0) | - | - | - | 0.72 | 0.71 | 0.70 | 0.34 | 0.33 | 0.34 | - | - | - | 0.34 | 0.33 | 0.34 | - | - | - |
| Caprate (10:0) | - | - | - | 1.38 | 1.36 | 1.38 | 0.67 | 0.51 | 0.61 | - | - | - | 0.13 | 0.13 | 0.12 | - | - | - |
| Laurate (12:0) | - | - | - | 8.57 | 8.51 | 8.52 | 0.28 | 0.28 | 0.28 | - | - | - | 0.62 | 0.60 | 0.61 | - | - | - |
| Methyl myristate (14:0) | 4.51 | 3.52 | 3.81 | - | - | - | 3.35 | 3.21 | 3.29 | 2.21 | 1.91 | 1.89 | 3.34 | 3.29 | 3.31 | 2.16 | 1.90 | 1.95 |
| Methyl palmitoleate (16:1) | 3.16 | 3.01 | 3.11 | 10.4 | 9.51 | 9.81 | 5.13 | 4.13 | 5.10 | 3.13 | 2.7 | 2.9 | 3.27 | 2.93 | 3.12 | 5.90 | 4.21 | 4.34 |
| Methyl palmitate (16:0) | 46.41 | 39.10 | 40.1 | 12.5 | 10.01 | 11.1 | 24.41 | 21.14 | 22.2 | 40.0 | 34 | 37 | 34.3 | 29.1 | 30.0 | 49.7 | 43.2 | 44.5 |
| Linolenate (18:3) | 2.35 | 2.12 | 2.24 | 9.17 | 8.51 | 8.81 | 2.64 | 2.26 | 2.43 | 0.81 | 0.49 | 0.62 | 7.62 | 6.21 | 6.91 | 1.43 | 1.21 | 1.32 |
| Linoleate (18:2) | 2.58 | 2.23 | 2.4 | 3.13 | 2.69 | 2.81 | 9.16 | 7.81 | 8.12 | 2.12 | 1.72 | 1.91 | 5.46 | 4.41 | 4.90 | 0.54 | 0.35 | 0.43 |
| Methyl oleate (18:1) | 28.06 | 25.24 | 26.1 | 16.19 | 14.01 | 15.6 | 44.24 | 40.12 | 41.1 | 42.5 | 39.1 | 41.8 | 42.8 | 38.1 | 39.0 | 29.1 | 27.0 | 28.3 |
| Methyl stearate (18:0) | 2.17 | 2.25 | 2.18 | 27.14 | 27.21 | 27.1 | 3.87 | 4.15 | 4.10 | 3.92 | 3.25 | 3.51 | - | - | - | 5.2 | 5.8 | 5.5 |
| Arachidate (21:0) | - | - | - | 0.65 | 0.62 | 0.64 | 2.44 | 2.21 | 2.36 | 1.0 | - | - | - | - | - | 1.1 | 1.1 | 1.1 |
| Erucate (22:1) | - | - | - | - | 2.23 | 2.19 | 2.21 | 1.0 | - | - | - | - | - | 0.5 | 0.5 | 0.5 | ||
| Behenic acid (22:0) | - | 0.10 | - | - | - | - | - | 0.15 | - | - | 0.12 | - | - | 0.13 | - | - | - | - |
| Algae | IV (g I2100/g fat) | SV (mg KOH/g) | CN | LCSF | CFPP (°C) | HHVi (MJ/kg) | ρ (g cm−3) | ʋ (mm2 /s) | Y (Hour) at 110 °C | |
|---|---|---|---|---|---|---|---|---|---|---|
| Biodiesel Standard EN 14214 | ≤120 | - | ≥51 | - | ≤5/≤−20 | NA | 0.86–0.9 | 3.5–5.0 | ≥6 | |
| Biodiesel Standard ASTM D6751-02 | NA | - | ≥47 | - | NA | ≥35 | NA | 1.9–6.0 | - | |
| Oedogonium sp. | Control | 91.6 | 202 | 50 | 0.85 | −13.8 | 38.18 | 0.87 | 4.0 | 171 |
| MAE | 81 | 178 | 56 | 0.75 | −14.7 | 38.5 | 0.87 | 3.8 | 192 | |
| UAE | 84 | 182 | 54 | 0.76 | −14.0 | 38.4 | 0.87 | 3.6 | 176 | |
| Oscillatoria sp. | Control | 93 | 206 | 49 | 1.60 | −11.4 | 38 | 0.88 | 3.9 | 56 |
| MAE | 88.3 | 194 | 52 | 1.4 | −11.8 | 38.3 | 0.87 | 4.0 | 64 | |
| UAE | 90.7 | 199 | 50 | 1.8 | −10.7 | 38.1 | 0.87 | 4.1 | 58 | |
| Ulothrix sp. | Control | 88.8 | 195 | 51 | 0.028 | −16.3 | 38.12 | 0.88 | 3.6 | 199 |
| MAE | 81.6 | 180 | 54 | 0.11 | −16.1 | 38.6 | 0.87 | 3.7 | 297 | |
| UAE | 85 | 185 | 53 | 0.057 | −16.2 | 38.3 | 0.87 | 3.6 | 238 | |
| Chlorella sp. | Control | 88 | 195 | 52 | 2.05 | −14.21 | 39.1 | 0.87 | 3.7 | 237 |
| MAE | 77 | 170 | 59 | 1.7 | −11.13 | 41 | 0.87 | 3.9 | 290 | |
| UAE | 82 | 182 | 55 | 1.8 | −10.61 | 39.1 | 0.87 | 3.8 | 258 | |
| Cladophora sp. | Control | 91 | 201 | 50 | 0.04 | −16.3 | 38.40 | 0.87 | 3.6 | 58.6 |
| MAE | 80 | 176 | 57 | 0.12 | −16.0 | 39.5 | 0.87 | 3.7 | 68 | |
| UAE | 82 | 182 | 56 | 0.09 | −16.1 | 39 | 0.87 | 3.7 | 62 | |
| Spirogyra sp. | Control | 92 | 204 | 50 | 0.09 | −13.6 | 40 | 0.88 | 3.9 | 55 |
| MAE | 82 | 182 | 55 | 0.03 | −16.3 | 39.5 | 0.87 | 4 | 78 | |
| UAE | 85 | 188 | 53 | 0.02 | −16.3 | 39 | 0.87 | 3.9 | 58 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasnain, M.; Munir, N.; Abideen, Z.; Macdonald, H.; Hamid, M.; Abbas, Z.; El-Keblawy, A.; Mancinelli, R.; Radicetti, E. Prospects for Biodiesel Production from Emerging Algal Resource: Process Optimization and Characterization of Biodiesel Properties. Agriculture 2023, 13, 407. https://doi.org/10.3390/agriculture13020407
Hasnain M, Munir N, Abideen Z, Macdonald H, Hamid M, Abbas Z, El-Keblawy A, Mancinelli R, Radicetti E. Prospects for Biodiesel Production from Emerging Algal Resource: Process Optimization and Characterization of Biodiesel Properties. Agriculture. 2023; 13(2):407. https://doi.org/10.3390/agriculture13020407
Chicago/Turabian StyleHasnain, Maria, Neelma Munir, Zainul Abideen, Heather Macdonald, Maria Hamid, Zaheer Abbas, Ali El-Keblawy, Roberto Mancinelli, and Emanuele Radicetti. 2023. "Prospects for Biodiesel Production from Emerging Algal Resource: Process Optimization and Characterization of Biodiesel Properties" Agriculture 13, no. 2: 407. https://doi.org/10.3390/agriculture13020407
APA StyleHasnain, M., Munir, N., Abideen, Z., Macdonald, H., Hamid, M., Abbas, Z., El-Keblawy, A., Mancinelli, R., & Radicetti, E. (2023). Prospects for Biodiesel Production from Emerging Algal Resource: Process Optimization and Characterization of Biodiesel Properties. Agriculture, 13(2), 407. https://doi.org/10.3390/agriculture13020407