Abstract
A significant part of marker-assisted backcross breeding (MABB) is recurrent parent genome recovery (RPGR). The purpose of this study was to introduce MABB-mediated resistance genes against numerous bacterial leaf blight (BLB) pathogens. Here, we examine the introgression of four Xoo resistance genes to the high-yielding Malaysian MR297 lineage Xa21, xa13, xa5, and Xa4 from the IRBB60 lineage. To accomplish both foreground and background selection, we employed polymorphic functional linked markers to target genes and simple sequence repeat (SSR) markers. We used 83 of the 475 authorized polymorphic microsatellite markers to determine the proportion of RPGR in the chosen lines. According to the data, the RPGR at BC1F1, BC2F1, and BC2F2 are 81.94, 92.30, and 95.04%, respectively. Incorporating the four BLB resistance genes into the newly created lines would result in long-lasting and comprehensive protection against BLB. Therefore, a resistant gene may be introduced into a population more quickly with MABB than through conventional breeding. All ten lines tested showed a significant level of resistance to BLB, with three lines displaying very high levels of resistance. Consequently, it was recommended that new lines should be used to produce commercially viable rice varieties.
1. Introduction
Throughout the world, rice is a basic diet for billions of people. As the world’s largest producer and consumer of grain, Asia cultivates rice on more than 150 million hectares of its lush land. When circumstances are satisfactory, rice crops may develop rapidly and deliver abundant harvests. Moreover, crops benefit from a light fertilization and thrive in salty water as long as they receive an adequate number of micronutrients. The rice harvest in many agricultural regions may increase due to several variables. The provision of equipment, fertilizers, seeds, irrigation, and new technology subsidies; the availability of and continued development of irrigation infrastructure; and the pace at which the human population is growing are quite concerning [1]. More food will be needed since the world’s population is expected to increase to over 8 billion in 2030 and 9 billion in 2050 [2].
As a result, in order to keep people from hunger, we need a 40% increase in rice output. Nevertheless, several diseases have an impact on rice cultivation. One example is blight, a bacterial infectious disease that has a significant impact on rice output in several regions. These diseases seriously affect global rice production. The bacterium Xanthomonas oryzae pv oryzae (Xoo) causes a devastating disease in rice known as bacterial leaf blight (BLB). There are currently 42 R genes known to protect rice from Xoo, and this number is rising. These R genes are selective in their effectiveness against particular Xoo strains or races, which are collections of strains that share incompatibility with designated sets of R genes due to the co-evolution and selection pressure between Xoo and rice. However, in order to prevent the disease from spreading, we must have successful yield generation as well as resistance to BLB-induced biotic stress [2]. Significant progress in producing viable rice varieties that can survive different forms of biotic and abiotic stress has led to a dramatic rise in rice output. Due to the consistent success of traditional breeding, its acquisition was achievable. Farmers were compelled to pyramid many resistance genes into high-performing, agronomically sound cultivars as novel biotypes evolved. This increases the cultivars’ resistance to diseases and unfavorable growing environments.
Backcrossing is a common way to produce new and interesting varieties by combining two parents, one as a donor and the other as a receiver, in order that a single gene that controls a certain trait can be passed on. If the receiver parent is used several times in the crossing scheme, it is called the repeating parent. Disease-resistant genes may now be traded across normally inferior cultivars. Utilizing marker-assisted backcross breeding (MABB) to reliably and effectively maintain the essential qualities of the recurring “parent”, such as a high-yielding feature, is one example. This strategy combines the donor’s focused gene with the recurrent parent’s recognized location. It aims to restore the lost parent’s genome by decreasing the donor parent’s genome, and this, in turn, decreases linkage drag. Molecular markers with a functional role, such as simple sequence repeats (SSR) or microsatellite markers, are also an option [3].
To integrate disease resistance into rice via backcross breeding without destroying its genetic background, the marker approach is essential. Multi-generational recombination with the repeating parent is another viable option. Background marker selection allowed us to return to the original parent genome, which kept appearing. Marker-assisted background selection has some problems, such as the high cost of molecular markers and sometimes the fact that SSR cannot find polymorphisms, which is an important part of making the whole system work well. Limitations in natural BLB screening due to variances in the severity of typical infections need a lot of manpower and time [4]. Artificial inoculation of BLB has been shown to be the most efficient screening approach. Artificial inoculation of the disease with BLB may be the most efficient screening method. Prick inoculation of the leaves, spraying the plants with a bacterial solution, immersing the seedlings before transplanting, and clipping the leaves and spraying them with bacterial suspension are all successful methods. For marker-assisted backcrossing, markers are used to focus on the desired locus. This allows for the recurring parent genome to be recovered faster.
Foreground selection, background selection, and recombinant selection are the three stages that make up the MABB process [5]. In addition, the ratio of recurrent parent genome recovery (RPGR) may be accelerated with the help of background selection, which, in turn, reduces the number of breeder selection cycles needed [6]. In each generation of backcrossing, the ratio of donor to parent genomes decreases by about half. Therefore, the RPGR fraction is given as a percentage of the donor parent’s genome recovery fraction [7]. Furthermore, during background selection, it is possible to identify all genomic areas using marker alleles for a recurrent parent, and the target locus may be selected by means of phenotypic screening. Background selection helps with RPGR when performing MABB. Moreover, additional backcrossing causes more varietal advancement and total line conversion [8,9], but this is not the case with less backcrossing. Therefore, we embarked on finding the RPGR of the new introgression lines produced by crossing MR297 and IRBB60 using both functional and SSR markers.
2. Materials and Methods
2.1. Breeding Procedure and Germplasm Source
The F1 hybrid was produced by crossing MR297 and IRBB60. The parent in this research was MR297 developed at the Malaysian Agricultural Research and Development Institute (MARDI) in 2016; MR297 is Padi MARDI (MRQ76 P446)/P446 [10]. The MR297 variety has a high yield. In addition, IRBB60 was gathered from the Philippines-based International Rice Research Institute (IRRI). The rice leaf blight caused by bacteria is not harmful to the IRBB60 cultivar. It is a collection of “Xoo R-genes Xa21, xa13, xa5, and Xa4” developed by the IRRI [2]. On the other hand, the yield from MR297 is rather high. Through backcrossing and hybridization, MR297 performed the roles of female recipient and recurrent parent, whereas IRBB60 performed the role of sole donor, namely, the male parent, in producing F1 plants. Heterozygous F1 plants were verified using a front-focused set of SSR and functional markers. To re-establish its high-producing trait, MR297 was employed as a recurrent parent in all backcross generations. Throughout the completely backcrossing procedure, the foreground selection of target genes was performed using markers that included both SSR and functional markers. The breeding program selected progeny with a high rate of genome recovery from the recurrent parent and a short genome section from the donor parent. BC2F2 plants were engaging in self-fertilization, a process that allowed for the breeding program. Figure 1 displays the studied MABB breeding system that was authorized.
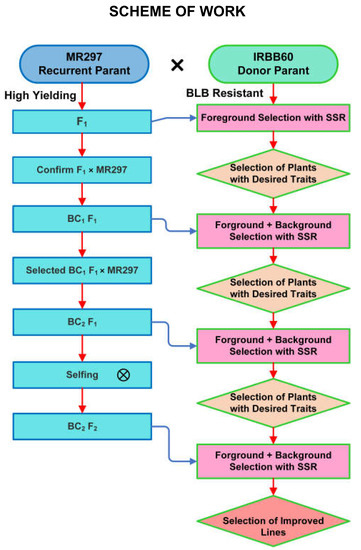
Figure 1.
A schematic showing how marker-assisted backcross breeding was used to create high-yielding, BLB-resistant rice varieties (MABB).
2.2. Molecular Markers: Screening and DNA Extraction
After 2 weeks of transplantation in a greenhouse using young, fresh leaves (0.5 g), genomic DNA was extracted from the plants. A CTAB DNA extraction technique developed by Doyle [11] was refined by another researcher [12] and found to be acceptable in this study. The concentration, accuracy, as well as purity of the DNA were measured using a Nanodrop spectrophotometry device (ND1000 Spectrophotometer, NH 03842, Hampton, USA). Protein pollution in DNA may be determined from the A260 or A280 ratio, whereas the existence of organic pollutants in the nucleotides can be determined from the A260 or A230 proportion. “Pure” DNA is defined as having a 260/280 ratio of 1.8 or above, whereas “pure” DNA at 230 nm absorbance may often pass with a 260/230 ratio from 2.0 to 2.2. Nevertheless, the finest suited samples for DNA nominated for polymerase chain reaction have an A260/280 purity extent from 1.8 to 2.0 (PCR). The extracted material was carried out on gel electrophoresis to verify that DNA was present. Image doc results showing a single, high-molecular-weight DNA band were considered as good DNA, whereas those showing numerous, low-quality DNA bands were ruled as unfit for PCR. As shown in Table 1, we first analyzed the polymorphism of both foreground and background markers associated with BLB resistance to identify the most potential avenues for further research. The DNA master mix, nuclease-free water, DNA, backward primer, and forward primer were all mixed together in a smaller rotating device for 15 s. The total volume was 7.5 μL. For 3.5 h, we used the PCR tools [1].

Table 1.
Background selection with simple sequence repeating markers.
2.3. The Scoring for DNA
The progeny was graded according to the two parental banding patterns as seen in the Gel Imager® (GelDocTM XR, Bio-Rad Lab. Inc., Hercules, CA, USA). Genotypic match to the MR297 variety was graded as an “A” for progeny that replicated the characteristics of the homozygous recurrent parent, although progeny that replicated those of the homozygous donor parent was scored as a “B” for progeny that replicated those of the IRBB60 variety. Moreover, the progeny that had a heterozygous banding pattern was given a score of “H”, indicating a high level of genetic identity for both parents.
2.4. The Selection for the Background and Foreground
The foreground markers were used to select only the progeny carrying the Xoo resistance genes. Ultimately, molecular screening of 475 SSR markers was performed to find polymorphisms (differences in alleles) throughout the rice’s 12 chromosomes. Of the 475 SSR markers, 83 were polymorphic and used for background selection.
2.5. Analysis of Data for Phenotyping Agro-Morphological Traits
At each backcrossing step, phenotypic selection was applied to the whole population, following foreground and background choices. Only plants with observable phenotypic expression for resistance genes Xoo were subjected to phenotypic selection. Through the use of the IRRI-defined standard procedure [13,14], we were able to accurately record the agro-morphological qualities of suitable plants in terms of yield and yield component traits. After genotyping the foreground markers, the data were analyzed and the chi-square test in SAS version 9.4 was used. The results were compared to those obtained using Mendelian genetics. With the mostly quantitative data we obtained through yield and yield components, we used the same program to perform descriptive statistical analysis. Background selection involving 83 polymorphic markers yielded genotypic data, which were then evaluated with a genetic application called Graphical Genotyper to determine the highest RPGR (GGT 2.0).
3. Results
3.1. Polymorphisms in Molecular Markers
The selection of suitable polymorphic markers is important in a MABB for maximum recovery of the recurrent parent genome and reduction in the donor parent genome. Moreover, it leads to a faster recovery of the recurrent parent genome. Four hundred and seventy-five rice SSR markers were screened for polymorphisms between MR297 and IRBB60 parental varieties, and polymorphic markers were selected while considering the number of repeat motifs of markers and their locations across the 12 chromosomes of rice. Following effective PCR amplification and the cycling conditions used in this investigation, 83 polymorphic markers between the two parents were found as shown in Figure 2. The 475 markers examined revealed a total amount of polymorphisms spread across the 12 chromosomes of 17.47%. The outcome revealed that there were between four (on chromosomes 1 and 10) and ten verified polymorphic markers (on chromosomes 2 and 8). Chromosomes 5 and 12 had nine polymorphic markers, while chromosome 4 had seven polymorphic markers, and chromosomes 3, 6, 7, 9, and 11 had six polymorphic markers each (Table 1). Background selection was performed using the MABB program’s 83 polymorphic background markers to ensure that an approximately equal amount of the recurrent parent genome remained present in the BC1–BC2 lineage offspring that had multiple BLB resistance genes added.
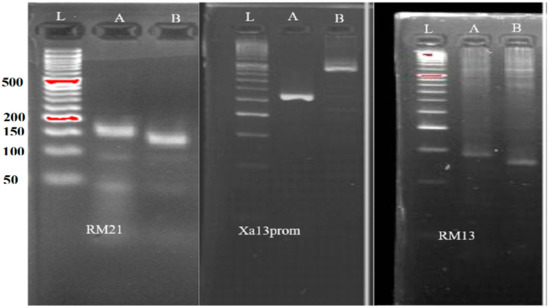
Figure 2.
Molecular markers of foreground screening polymorphism amongst the two parents MR297 (A) and IRBB60 (B) for the various target genes: RM21 (xa5), xa13-prom (xa13), and RM13 (xa5); L: 50 bp DNA ladder.
3.2. F1 Individuals and Foreground Selection
Of the 70 plants F1 generated, the practical marker pTA248 with heterozygous alleles was found in 64 real F1 plants (Xa21). However, in 60 plants, all four BLB R genes “Xa21, xa13, xa5, and Xa4” were verified but only five hybrid plants F1 were chosen for the backcrossing process as shown in Figure 3. Of the 290 plants grown at BC1F1, 158 were found to be Xa21 BLB R gene carriers. In addition, 157, 156, and 159 plants, respectively, had the BLB R genes inserted in xa13, xa5 and Xa4. Foreground markers at BC1F1 showed no significant deviation based on a scenario in which a single gene is inherited with a Mendel’s segregation ratio of 1:1, as determined by the chi-square test shown in Table 2. Nine plants were confirmed to have all of the BLB resistance genes tested, and these were the plants utilized for the selection at BC1F1. From the 270 plants grown at BC2F1, 140 were discovered to be heterozygous for the dominant Xa21 allele, and 138 were found to be heterozygous for the recessive xa13 allele, as determined by testing with the pTA248 and xa13prom functional markers, respectively. Similarly, xa5 and Xa4 were detected in 136 and 142 plants. At BC2F1, BLB R genes were integrated into nine offspring. Only five out of the original nine plants had adequately recovered their recurrent parent genomes by background selection, and these were the ones chosen for the next crossing. Table 3 shows the findings of a chi-square test confirming that the model is statistically significant close to a 1:1 Mendel’s ratio for a single gene.
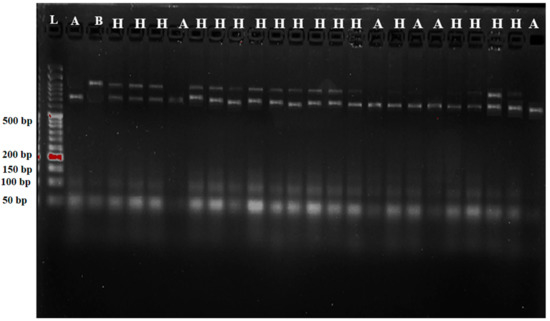
Figure 3.
Genotyping and foreground marker-assisted selection of F1 hybrids using pTA248 tightly linked marker. F1 developed from a cross between A: MR297 and B: IRBB60. H represents heterozygotes; L: 50 bp DNA ladder.

Table 2.
The BC1F1 progenies: Foreground marker segregation analysis.

Table 3.
The BC2F1 progenies: Foreground marker segregation analysis.
From the nine BC2F1 lines, the recurrent parent genome was recovered and 220 BC2F2 plants were successfully produced. The 42 plants had their Xa21 mutation fixed, according to the results of the molecular genotyping analysis. In addition, 34, 42, and 40 plants were identified to be homozygous for the xa13, xa5, and Xa4 BLB resistance genes, respectively. Those with a high RPGR percentage, as shown in Table 4, were selected as the final homozygotes for the donor IRBB60 parent allele in Table 4.

Table 4.
The BC2F2 progenies: Foreground marker segregation analysis.
3.3. Marker-Assisted Background Selection
However, the BC1F1-4, BC1F1-35, BC1F1-17, BC1F1-26, BC1F1-41, BC1F1-8, BC1F1-22, BC1F1-10, and BC1F1-11 are nine of the plants that were evaluated at progenies of BC1F1 for RPGR. In addition, they were found to have RPGR values of 80% or above. Therefore, several individuals were suggested for the BC2F1 crossover [15,16]. With 84.20% RPGR, a 10.90% heterozygous ratio and 4.90% donor genomes were discovered, and the BC1F1-4 was found to be the greatest progeny of the BC1F1 generation. The discovery of RPGR through marker-assisted perspective selection of BC2F1 led to the selection of nine exceptional individual plants for genotyping and then quantitative trait validation. The nine BC2F1 progenies with the highest RPGR (mean observed) of 95.31% were selected as a prerequisite. Figure 4 and Figure 5 show that the nine most promising BC2F1 plants were chosen as recombinant parental seeds. These plants were self-pollinated to produce BC2F2 plants.
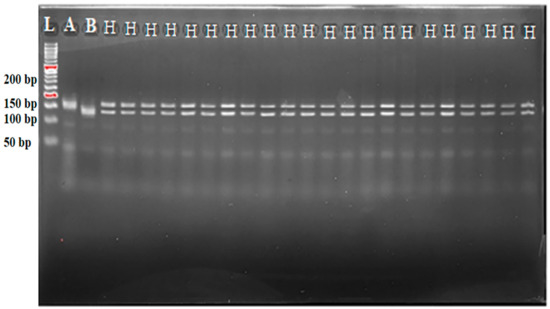
Figure 4.
Genotyping and foreground marker-assisted selection of BC1F1 progenies using RM21 tightly linked marker for (xa5) Xoo resistance gene. A: MR297; B: IRBB60. H represents heterozygotes; L: 50 bp DNA ladder.
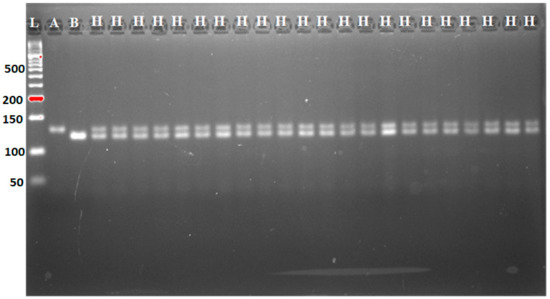
Figure 5.
Genotyping and foreground marker-assisted selection of BC2F1 progenies using MP tightly linked marker for (Xa4) Xoo resistance gene. A: MR297; B: IRBB60. H represents heterozygotes; L: 50 bp DNA ladder.
3.4. Selected Improved BC2F2 Lines with Recurrent Parent Genome Recovery
Analyses using RPGR showed a spread of 92.90–98.10% at BC2F2, as shown in Figure 6. At location BC2F2-21, we found the highest RPGR. Across the 12 chromosomes examined, the 10 chosen lines showed an average of 95.04% RPGR. Donor parent genome averaged 1.98%, reaching from 0.60% of the BC2F2-11 toward 2.90% of the BC2F2-10. As for the heterozygous genome, its frequency varied from 0.7% in BC2F2-21 to 6.2% in BC2F2-11. These data reveal that RPGR increased by 2.74% due to the crossover after self-fertilization of one generation between BC2F1 and BC2F2. The proportion of heterozygotes is reduced by 0.04%, and the donor parent genome is reduced by 2.70% as a result of this process. Figure 7 depicts the highest RPGR by chromosome for the produced nominated lines of BC2F2-21.
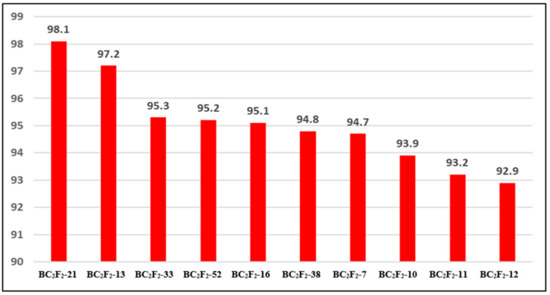
Figure 6.
The 10 selected BC2F2 progenies of recurrent parent genome recovery percentage.
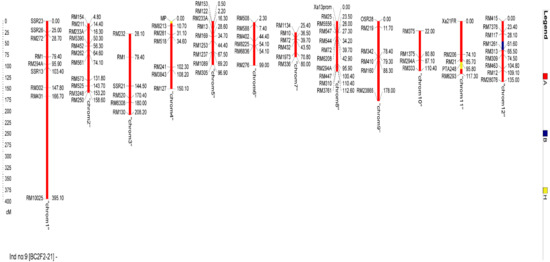
Figure 7.
The best BC2F2 progeny (BC2F2-21) chromosome-by-chromosome recurrent parent genome recovery (RPGR). Yellow lines denote the heterozygous region, the homozygous region for the MR297 allele is represented by the red lines, and the homozygous region for the IRBB60 allele is shown by the blue line.
3.5. Increase the Size of the Recurrent Parent Genome
The BC1F1 was 1202.3 and 1259.1 cM in length in the recurrent parent genome, but the BC2F1 generation was 1365.2 and 1432.5 cM in length. However, in BC1F1 as well as BC2F1, the mean of the recurrent parent genome varied from 1225.3 to 1382.72 cM. This indicates the growth of 157.42 cM after the two backcross generations. In addition, the individual BC1F1 generation with the greatest RPGR at BC1F1-4 has a recurrent parent genome size of 1259.04 cM. On the other hand, the progeny with the greatest RPGR at BC2F1 generation (BC2F1-20) has a recurring parent genome size of 1432.5 cM. This showed that the 173.4 cM recurrent parent genome size had grown genetically. The results indicate that within backcross generations, molecular MABB might progressively increase the proportion of the recurrent parent genome [16].
3.6. Donor Parent Genome Size in Backcross Generations of the Genetic Reduction
The BC1F1 generation has a heterozygous segment with a genome size from 127.10 cM (BC1F1-22) to 180.9 cM (BC1F1-10), whereas the BC2F1 generation has a heterozygous segment with a genome size from 23.9 cM (BC2F1-10) to 77.7 cM (BC2F1-32). BC1F1 and BC2F1 generations had an average heterozygous genome size of 147.19 and 44.78 cM, respectively. The same downward trend was observed. Donor parent genome sizes varied from 98.69 to 161.5 cM in the BC1F1 strain and from 31.4 to 91.2 cM in the BC2F1 strain. Average donor parent genome sizes decreased from 122.78 cm in the BC1F1 generation to 67.8 cM in the BC2F2 generation. BC1F1 had the best offspring, with a mean height of 73.27 cM, whereas BC2F2 had the best individual, with a mean height of 31 cM. Through demonstrating that the heterozygous segment and donor parent genome size reduced as the inquiry moved from BC1F1 to BC2F2, the results demonstrate that backcrossing serves to minimize the genome of the donor parent while simultaneously increasing or recovering the genome of the recurrent parent [6].
3.7. Agronomic Performance for the Selected Backcross Lines
Table 5 shows the results on the agro-morphological traits of the BC2F2 population. The outcome presented that BC2F2-21, which recorded the highest recurrent parent genome recovery of 98.1%, had the following phenotypic characteristics: Number of panicles per hill (17), days to flowering (80.00), days to maturity (117.00), leaf area (85.76 cm2), number of panicles (20), number of tillers (55.00), panicle length (28 cm), spikelet per panicle (328), spikelet fertility (85.33%), total number of grains per panicle (182.00), 1000 grain weight (46 g), total grain weight per plant (101 g), seed length (12 mm), and seed width (2.4 m).

Table 5.
The 10 selected BC2F2 lines for agro-morphological characteristics.
However, the ten BC2F2 selected had the following mean agro-morphological traits: Generations of plant height (118.90 cm), flag leaf length-to-width ratio (12.40 cm), number of panicles per hill (15.50 cm), days to flowering (82.50 cm), days to maturity (122.30 cm), number of tillers (49.80 cm), number of panicles (20.30 cm), number of tillers per panicle (327.8 cm), panicle length (31.40 cm), and spikelet fertility (89.63 cm) are some of the plant characteristics. Nevertheless, the seed has an 11 mm length, a 2.78 mm width, and a length-to-width ratio of 4.08.
The means comparison of the morphological traits of the advanced rice lines and their two parents were presented in Table 5. The two parents showed significant differences for most traits except for FLWR and SLWR. In this study, IRBB60 flowered significantly earlier than MR297. The advanced rice lines had similar plant height, days to flowering, days to maturity, panicle length, total spikelets per panicle, spikelet fertility, 1000 grain weight, and grain yield per plant as those of MR297. Most of the morphological traits of the advanced rice lines closely resembled MR297, as supported by a genetic background recovery of the recurrent parent genome of 95.04%. In addition, compared to certain recently released varieties, the advanced rice lines displayed higher yield-related features as shown in Table 5. The advanced rice lines had a higher grain output per plant than MR297 as shown in Figure 8 and Figure 9, respectively. This Malaysian high-yielding variety has been widely employed by local farmers since 2001, with a grain yield of 42.4 g per plant [17].

Figure 8.
MR297, IRBB60, and the selected BC2F2 plants.

Figure 9.
The selected BC2F2 lines during the vegetative phase.
Moreover, compared to the most current high-yielding rice variety recently issued by MARDI, MR284, the advanced rice lines displayed more total spikelets per panicle (154 spikelets per panicle) [18]. Advanced rice lines matched Liangyoupeijiu, a hybrid rice produced in China in 2000 with a maximum grain output of 12.11 t/ha, in terms of plant height, panicle length, total spikelets per panicle, and seed-setting rate [19]. In addition, compared to IRRI inbreds (IR72, PSBRc28, and IR72903-121-2-1-2) and new plant type (NPT) lines (IR72967-12-2-3), the advanced rice lines had more total spikelets per panicle.
Furthermore, they had a seed-setting rate comparable to IRRI inbred but higher than IR72967-12-2-3 [20]. The verification of yield potential and stability of the advanced rice lines created as a result of this study is advised in subsequent studies at yield performance trials across many locations and seasons before releasing them as varieties, in order to recognize the yield potential of those lines. In general, the advanced rice lines had intermediate plant heights, a large amount of tillers and panicles, and a good yield, and they grew their flowers at the same time as MR297.
4. Discussion
For more information regarding RPGR, background selection with marker guidance is an excellent place to start. Donor and heterozygous genomic segments may be uncovered by performing a background check. When breeding plants, breeders select individuals with high RPGR and work to introduce these genes into the crop without compromising the recurring parental genes [21,22]. A small number of progenies from both BC1F1 and BC2F2 generations were found to have a lower rate of RPGR than the theorized average for all generations of backcrossing. Other researchers have reached similar conclusions [23,24]. Moreover, several studies showed that the gene of interest in a study imposed a “pull” that caused a lower proportion of RPGR than the theoretical mean since it favors the transfer of extra loci from the donor gene [25].
However, at BC1F1 and BC2F2, the vast majority of offspring met the theoretical MABB RPGR of 81.94% and 95.04%, respectively [6,7]. This finding is in line with previous research [17,26], which demonstrated that there is no measurable difference in progeny and their parents. Moreover, the percentage of productive tillers in a rice crop is directly proportional to the yield in panicles [27]. This study found that both panicle length and the average amount of grains per panicle had grown considerably, which are factors contributing to the increase in line grain production recovery. There is a correlation between rice grain production and the ratio of tillers to grains in a panicle [21,22]. Conversely, grain length and breadth are essential quantitative attributes that correlate strongly with objective physical quality [28,29]. On the other hand, grain/seed grain shape has been shown to be simultaneously controlled by maternal, cytoplasmic, as well as triploid endosperm genes by previous studies [30,31]. BC1F1 had the highest RPGR (80.5%) and the most heterozygous genome segments (12.1%); (BC1F1-10). A 1.1% discrepancy was found between the actual number of background markers and the 79% predicted by the MABB in the current study. This shows that there was a shift in marker distribution toward the heterozygous region of the genome. Previous research [15] suggests that preferential inheritance of IRBB60 alleles at certain locations may be to blame for the larger heterozygous section in some descendants. The proportion of heterozygous genome segments was greater than the mean (9.84%) in four of the nine BC1F1 examined progenies, leading to a more prevalent occurrence. All BC2F1 offspring reached the predicted MABB RPGR of 90.0%, with 5.2% heterozygous genome segments being the highest detected. The results matched those from the literature [32], which revealed that RPGR ranged from 75.40% to 91.30% in BC1F1 generations and from 80.40% to 96.70% in BC2F2 generations. Previous research [33] indicates that the F1 paternity status may affect the way in which alleles are passed down to the offspring.
In many places, BC1F1 hybrids that were developed by crossing an indica and a japonica F1 hybrid back and forth showed a change in the transmission ratio. Since F1 was the female, the japonica alleles completed segregation at a specific site during F1 meiosis. However, in backcrosses to indica, the japonica embryo sac was very likely to be fertilized by the indica pollen. As a result, marker segregation skewed toward the region of the genome where both alleles were present. The female plants in this experiment were descendants of the F1 and BC1F1 generations, whereas the males were MR297 plants employed to provide pollen. Transmission ratio distortion likely occurred during meiosis, as shown by the presence of a significant heterozygous genome segment in numerous BC1F1 progenies. For both F1 and BC1F1, the IRBB60 allele was favorably expressed throughout fertilization, increasing the likelihood that MR297 pollen will indeed fertilize an embryonic containing the IRBB60 allele.
5. Conclusions
The transferring of resistance genes from a donor parent to a recipient is a simple technique to perform with the assistance of the MABB method. This proved that foreground selection could be used to screen for BLB resistance target genes. Moreover, our results proved that background selection could recover the recurrent parent genome. The breeding experiment further highlighted the ability of the backcross breeding strategy to both incorporate resistance genes and decrease the donor parent genome. Furthermore, to introduce BLB resistance, this study is significant for recovering the recipient parent genome in MR297 and increasing the crop’s output. Adopting MABB accelerated the introduction of the Xoo R genes into the genome. The high RPGR percentage in this investigation illustrates the MABB’s promise for recovering RPGR in rice and other cereal crops. According to accessible data, this represents the first effort to update the genome of MR297, a superior Malaysian rice variety, and increase BLB resistance without affecting the variety’s high yield. Consequently, it is recommended that farmers in rice-growing areas should apply the new improved lines as new varieties.
Author Contributions
The study concept was developed by S.J.A. and M.Y.R.; S.J.A. conducted the research and prepared the first draft; S.J.A., M.Y.R., S.Z.S., K.A. and A.A.-J. provided the resources. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the grants from the Higher Institution Centre of Excellence (HiCoE), Institute of Tropical Agriculture and Food Security (Grant No. 6369105) and the research grant title evaluation and selection of crop varieties for utilization to increase yield and quality (Grant No. 6383900), Universiti Putra Malaysia.
Data Availability Statement
According to copyright law, researchers must keep their data private.
Acknowledgments
The authors would like to thank management and staff members of the Institute of Tropical Agriculture and Food Security, Universiti Putra Malaysia for creating a conducive setting for academic inquiry and development.
Conflicts of Interest
The authors declare no conflict of interest. The sponsors had no input on the study’s conception or execution, data acquisition, analysis, or interpretation, article preparation, or final publication decision. Concerning potential bias, we can say that there is no conflict of interest on the part of the writers. Funders had no input on the study’s conception or execution, nor did they contribute to data acquisition, analysis, or interpretation, article drafting, or the final publication decision.
References
- Jasim Aljumaili, S.; Rafii, M.; Latif, M.; Sakimin, S.Z.; Arolu, I.W.; Miah, G. Genetic diversity of aromatic rice germplasm revealed by SSR markers. BioMed Res. Int. 2018, 2018, 7658032. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sarma, B.; Singh, P.; Nandan, R. Screening of rice (Oryza sativa L.) germplasms against Xanthomonas oryzae pv. oryzae. J. Eco-Friendly Agric. 2013, 8, 86–88. [Google Scholar]
- Akos, I.S.; Yusop, M.R.; Ismail, M.R.; Ramlee, S.I.; Shamsudin, N.A.A.; Ramli, A.B.; Haliru, B.S.; Ismai’la, M.; Chukwu, S.C. A review on gene pyramiding of agronomic, biotic and abiotic traits in rice variety development. Int. J. Appl. Biol. 2019, 3, 65–96. [Google Scholar]
- Yugander, A.; Sundaram, R.M.; Singh, K.; Ladhalakshmi, D.; Subba Rao, L.V.; Madhav, M.S.; Badri, J.; Prasad, M.S.; Laha, G.S. Incorporation of the novel bacterial blight resistance gene Xa38 into the genetic background of elite rice variety Improved Samba Mahsuri. PLoS ONE 2018, 13, e0198260. [Google Scholar] [CrossRef] [PubMed]
- Collard, B.C.; Mackill, D.J. Marker-assisted selection: An approach for precision plant breeding in the twenty-first century. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 557–572. [Google Scholar] [CrossRef]
- Hasan, M.M.; Rafii, M.Y.; Ismail, M.R.; Mahmood, M.; Rahim, H.A.; Alam, M.A.; Ashkani, S.; Malek, M.A.; Latif, M.A. Marker-assisted backcrossing: A useful method for rice improvement. Biotechnol. Biotechnol. Equip. 2015, 29, 237–254. [Google Scholar] [CrossRef]
- Matthew, R. Backcrossing, Backcross (BC) population and backcross breeding. Plant Breeding and Genomics. Ohio State Univ. 2012, 1. [Google Scholar]
- Hospital, F. Size of donor chromosome segments around introgressed loci and reduction of linkage drag in marker-assisted backcross programs. Genetics 2001, 158, 1363–1379. [Google Scholar] [CrossRef]
- Mackill, D. From genes to farmer’s fields. Rice Today 2006, 5, 28–31. [Google Scholar]
- Razak, S.A.; Azman, N.H.E.N.; Ismail, S.N.; Yusof, M.F.M.; Ariffin, M.A.T.; Sabdin, Z.H.M.; Hassan, M.H.M.; Nasir, K.H.; Sani, M.A.; Abdullah, N. Assessment of diversity and population structure of mango (‘Mangifera indica’L.) germplasm based on microsatellite (SSR) markers. Aust. J. Crop Sci. 2019, 13, 315–320. [Google Scholar] [CrossRef]
- Doyle, J.J. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Ashkani, S.; Rafii, M.Y.; Rusli, I.; Sariah, M.; Abdullah, S.N.A.; Rahim, H.A.; Latif, M. SSRs for marker-assisted selection for blast resistance in rice (Oryza sativa L.). Plant Mol. Biol. Report. 2012, 30, 79–86. [Google Scholar] [CrossRef]
- International Rice Research Institute. Standard Evaluation System for Rice; International Rice Research Institute: Los Baños, Philippines, 1996. [Google Scholar]
- Zuki, Z.M.; Rafii, M.Y.; Ramli, A.; Oladosu, Y.; Latif, M.A.; Sijam, K.; Ismail, M.R.; Sarif, H.M. Segregation analysis for bacterial leaf blight disease resistance genes in rice ‘MR219’using SSR marker. Chil. J. Agric. Res. 2020, 80, 227–233. [Google Scholar] [CrossRef]
- Lau, W.C.P.; Rafii, M.Y.; Ismail, M.R.; Puteh, A.; Latif, M.A.; Asfaliza, R.; Miah, G. Development of advanced fragrant rice lines from MR269× Basmati 370 through marker-assisted backcrossing. Euphytica 2017, 213, 1–15. [Google Scholar] [CrossRef]
- Olalekan, K.K.; Rafii, M.Y.; Salleh, A.M.; Mohamed, M.T.; Ahmad, K.; Misran, A.; Abro, T.F.; Oladosu, Y.; Arolu, I.W.; Samuel, C. Analysis of recurrent parent genome recovery in marker-assisted backcross breeding programme in Watermelon. Int. J. Sci. Technol. Res. 2019, 8, 945–955. [Google Scholar]
- Sabu, K.K.; Abdullah, M.Z.; Lim, L.S.; Wickneswari, R. Development and evaluation of advanced backcross families of rice for agronomically important traits. Commun. Biometry Crop Sci. 2006, 1, 111–123. [Google Scholar]
- Saidon, S.A.; Yusob, S.M.; Jack, A.; Masarrudin, M.F.; Hussain, Z.P.; Haron, I.C.; Omar, O.; Shamsudin, N. Penilaian prestasi hasil dan komponen hasil varieti padi terpilih di Seberang Perai. J. Teknol. 2014, 70. [Google Scholar] [CrossRef]
- Peng, S.; Khush, G.S.; Virk, P.; Tang, Q.; Zou, Y. Progress in ideotype breeding to increase rice yield potential. Field Crops Res. 2008, 108, 32–38. [Google Scholar] [CrossRef]
- Islam, M.S.; Peng, S.; Visperas, R.M.; Bhuiya, M.S.U.; Hossain, S.A.; Julfiquar, A. Comparative study on yield and yield attributes of hybrid, inbred, and NPT rice genotypes in a tropical irrigated ecosystem. Bangladesh J. Agric. Res. 2010, 35, 343–353. [Google Scholar] [CrossRef][Green Version]
- Dutta, R.; Mia, M.B.; Khanam, S. Plant architecture and growth characteristics of fine grain and aromatic rices and their relation with grain yield. Int. Rice Comm. Newsl. 2002, 51, 51–55. [Google Scholar]
- Kusutani, A.; Tovata, M.; Asanuma, K.; Cui, J. Studies on the varietal differences of harvest index and morphological characteristics of rice. Jpn. J. Crop Sci. 2000, 69, 359–364. [Google Scholar]
- Neeraja, C.N.; Maghirang-Rodriguez, R.; Pamplona, A.; Heuer, S.; Collard, B.C.; Septiningsih, E.M.; Vergara, G.; Sanchez, D.; Xu, K.; Ismail, A.M. A marker-assisted backcross approach for developing submergence-tolerant rice cultivars. Theor. Appl. Genet. 2007, 115, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Nwe, K.T.; Vanavichit, A.; Chai-arree, W.; Toojinda, T. Marker assisted backcross breeding to improve cooking quality traits in Myanmar rice cultivar Manawthukha. Field Crops Res. 2009, 113, 178–186. [Google Scholar] [CrossRef]
- Sundaram, R.M.; Vishnupriya, M.R.; Biradar, S.K.; Laha, G.S.; Reddy, G.A.; Rani, N.S.; Sarma, N.P.; Sonti, R.V. Marker assisted introgression of bacterial blight resistance in Samba Mahsuri, an elite indica rice variety. Euphytica 2008, 160, 411–422. [Google Scholar] [CrossRef]
- Martínez, C.; Tohme, J.; López, J.; Borrero, J.; McCouch, S.; Almeida, A. Identification and utilization of genes from wild rice germplasm for the improvement of yield and stress resistance. In Proceedings of the 27th Rice Technical Working Group, Reno, NV, USA, 1–4 March 1998. [Google Scholar]
- Hossain, M.; Islam, M.; Hasanuzzaman, M. Influence of different nitrogen levels on the performance of four aromatic rice varieties. Int. J. Agric. Biol. 2008, 10, 693–696. [Google Scholar]
- Shi, C.; Zhu, J.; Wu, J.; Fan, L. Genetic and genotype× environment interaction effects from embryo, endosperm, cytoplasm and maternal plant for rice grain shape traits of indica rice. Field Crops Res. 2000, 68, 191–198. [Google Scholar] [CrossRef]
- Sarif, H.M.; Rafii, M.Y.; Ramli, A.; Oladosu, Y.; Musa, H.M.; Rahim, H.A.; Zuki, Z.M.; Chukwu, S.C. Genetic diversity and variability among pigmented rice germplasm using molecular marker and morphological traits. Biotechnol. Biotechnol. Equip. 2020, 34, 747–762. [Google Scholar] [CrossRef]
- Juliano, B.O. Rice in Human Nutrition; International Rice Research Institute: Los Baños, Philippines, 1993. [Google Scholar]
- Huang, S.; Wang, P.; Yamaji, N.; Ma, J.F. Plant nutrition for human nutrition: Hints from rice research and future perspectives. Mol. Plant 2020, 13, 825–835. [Google Scholar] [CrossRef]
- Miah, G.; Rafii, M.Y.; Ismail, M.R.; Puteh, A.B.; Rahim, H.A.; Latif, M.A. Recurrent parent genome recovery analysis in a marker-assisted backcrossing program of rice (Oryza sativa L.). Comptes Rendus Biol. 2015, 338, 83–94. [Google Scholar] [CrossRef]
- Fatimah; Reflinur; Prasetiyono, J.; Wartono; Nugroho, K.; Kirana, R.; Satyawan, D.; Terryana, R.T.; Polosoro, A.; Lestari, P. Hybrid purity assessment in F1 hybrids segregating for phytophthora root rot resistance genes of chili pepper (Capsicum annuum L.). AIP Conf. Proc. 2022, 2462, 030007. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).