Control of Spodoptera frugiperda on Fresh Corn via Pesticide Application before Transplanting
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Plants
2.1.2. Insects
2.1.3. Pesticides
2.2. Methods
2.2.1. Determination of Insecticide Toxicity to Newly Hatched or Third Instar S. frugiperda Larvae
2.2.2. Toxicity of Pesticides to S. frugiperda
2.2.3. Control Effect of S. frugiperda in the Field
2.3. Statistical Analysis
3. Results
3.1. Determination of Insecticide Toxicity in Newly Hatched and Third Instar Larvae of S. frugiperda
3.2. Toxicity of Corn Leaves in S. frugiperda Larvae with Different Pesticides Treatments
3.3. Pesticide Residues in Corn Leaves with Different Pesticide Treatments
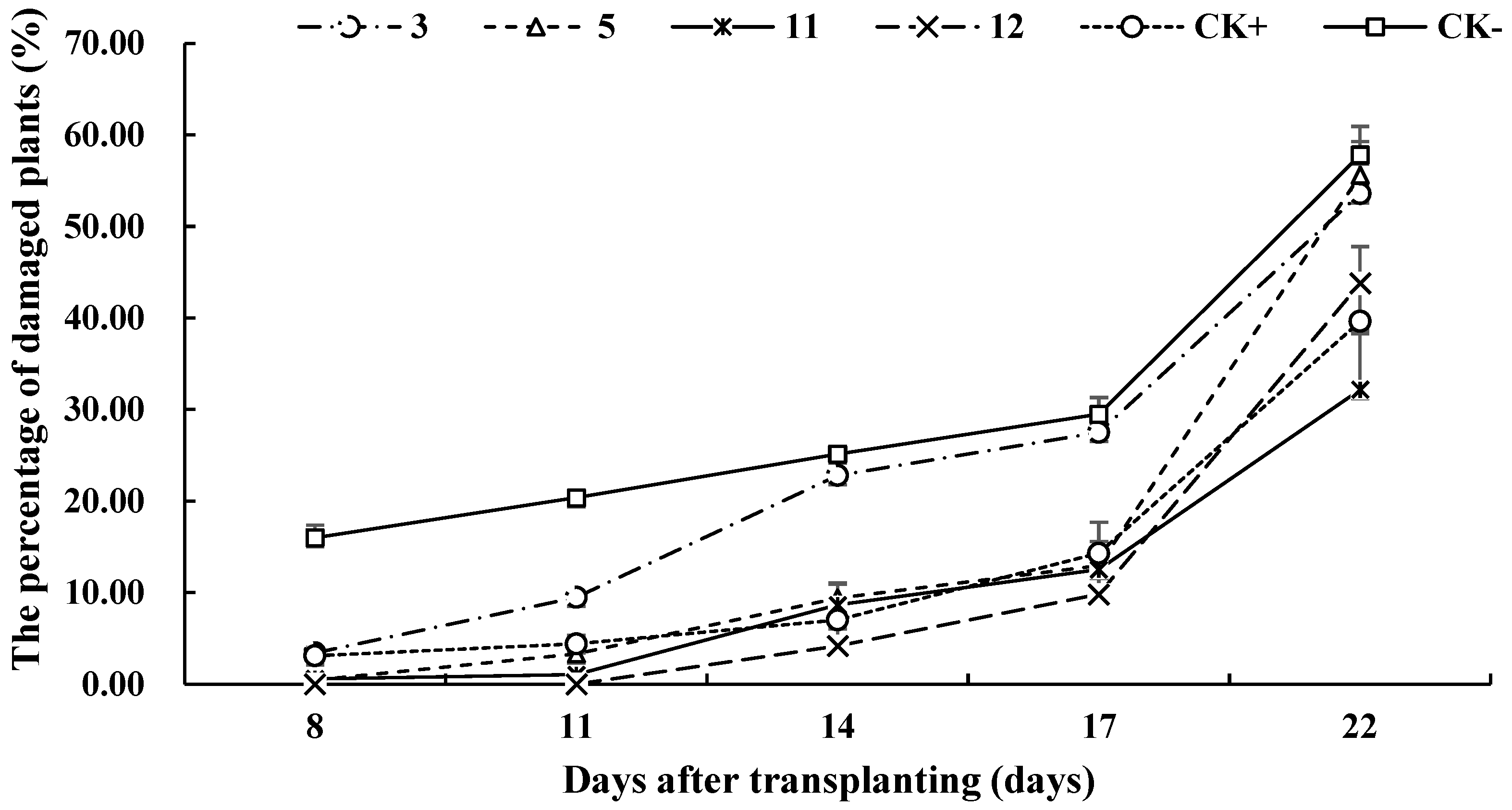
3.4. Field Control Effects of Different Pesticide Treatments on S. frugiperda
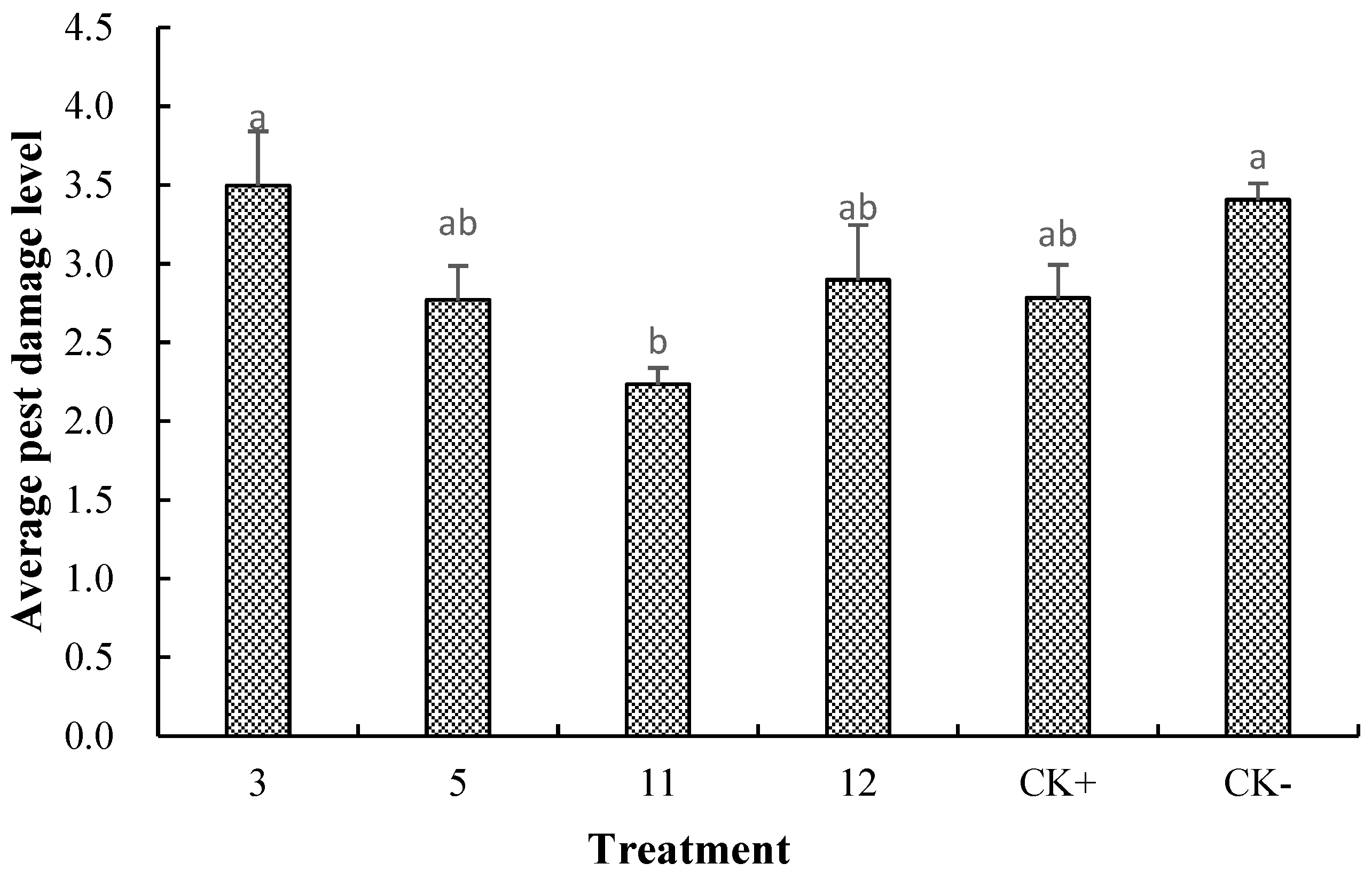
3.5. Average Pest Damage Level
3.6. Economic Estimates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ge, S.S.; He, L.M.; He, W.; Yan, R.; Wyckhuys Kris, A.G.; Wu, K.M. Laboratory-based flight performance of the fall armyworm, Spodoptera frugiperda. J. Integr. Agric. 2021, 20, 707–714. [Google Scholar] [CrossRef]
- Sun, X.X.; Hu, C.X.; Jia, H.R.; Wu, Q.L.; Shen, X.J.; Zhao, S.Y.; Jiang, Y.Y.; Wu, K.M. Case study on the first immigration of fall armyworm, Spodoptera frugiperda invading into China. J. Integr. Agric. 2021, 20, 664–672. [Google Scholar] [CrossRef]
- Montezano, D.G.; Specht, A.; Sosa-Gomez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–301. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Liu, J.; Xie, M.C.; Li, Y.H.; Yang, J.J.; Zhang, M.L.; Qiu, K. Observation on law of diffusion damage of Spodoptera frugiperda in China in 2019. Plant Prot. 2019, 45, 10–19, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Liu, J.; Jiang, Y.Y.; Huang, C.; Wu, Q.L.; Zhang, T.; Zeng, J. Forecast of occurrence trend of major diseases and insect pests of grain crops in 2021. China Plant Prot. 2021, 41, 37–39+42, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Altinel, B.; Tonk, F.A.; Pazir, F.; Istipliler, D.; Tosun, M. Improving sweet corn x Dent corn hybrids based on kernel color, size, and quality properties. Fresenius Environ. Bull. 2019, 28, 2368–2374. [Google Scholar]
- Oktem, A.; Oktem, A.G.; Emeklier, H.Y. Effect of nitrogen on yield and some quality parameters of sweet corn. Commun. Soil Sci. Plant Anal. 2010, 41, 832–847. [Google Scholar] [CrossRef]
- Dai, Q.X.; Li, Z.Y.; Tian, Y.J.; Zhang, Z.F.; Wang, L.; Lu, Y.Y.; Li, Y.Z.; Chen, K.W. Effects of different corn varieties on development and reproduction of Spodoptera frugiperda. Chin. J. Appl. Ecol. 2020, 31, 3273–3281, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Yang, J.W.; Wen, S.H.; Li, Y.L.; Jia, X.; Wang, J.J.; Zhao, B.P.; Du, Y.C.; Wang, F.R. Study on the suitability of Spodoptera frugiperda on different traps of corn ears and analysis of potential transmission risks. J. Hebei Agric. Sci. 2022, 26, 64–67, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Li, X.J.; Wu, J.Y.Z.; Dai, X.C.; Wang, Y.Q.; Wang, R.F.; Zhang, Z.X.; Xu, H.H. Study on the damage of Spodoptera frugiperda to corn ears of different cultivars. J. South China Agric. Univ. 2021, 42, 71–79, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhang, K.X.; Ma, Y.; Zhang, Q.Y.; Liu, W.H.; Jiang, H.X.; Yang, L.K.; Liu, C.Z. Relationship between the feeding selectivity of Spodoptera frugiperda to maize varieties and the chemical substances in the leaves. China Plant Prot. 2021, 41, 10–16+39. (In Chinese) [Google Scholar] [CrossRef]
- Zhou, X.J.; Lu, B.Q.; Lu, H.; Tang, J.H.; Lin, N.F. Comparison of damage grades in laboratory and population fitness of Spodoptera frugiperda (Lepidoptera: Noctuidae) on different maize varieties. Chin. J. Trop. Crops 2022, 43, 862–869, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Liu, Y.L.; Lu, B.Q.; Lu, H.; Tang, J.H.; Zhang, Y.J.; Zhu, X.M. Effect of different population density of Spodoptera frugiperda on the yield of corn and its economic threshold. Chin. J. Tropical Crops 2021, 42, 3394–3401, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Han, H.L.; Zhang, J.M.; Xu, H.X.; Bao, F.; Liu, M.; Zhao, F.C.; Lu, Y.B.; Lu, Z.X.; Wang, G.Y. Occurrence and control strategy of Spodoptera frugiperda (Lepidoptera: Noctuidae) on fresh-eating maize. Acta Entomol. Sin. 2020, 63, 613–623, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Lu, H.; Tang, J.H.; Lu, B.Q.; Zhang, Y.J.; Liu, W.C.; Su, H. Effects on control period and frequency of Spodoptera frugiperda on Corn yield. Chin. J. Trop. Crops 2021, 42, 3388–3393, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Rocha, I.; Ma, Y.; Soua-Alonso, P.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Seed coating: A tool for delivering beneficial microbes to agricultural crops. Front. Plant Sci. 2019, 10, 1357. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, Z.G.; Wang, S.P.; Dou, R.M. Efficacy evaluation of 14 suspension seed coating agents on controlling corn underground pests, aphids and stalk rot. Chin. Agric. Sci. Bull. 2019, 35, 128–132. [Google Scholar]
- Gan, L.; Zhang, Y.; Zou, C.J.; Qiu, L.M.; Liao, C.J.; Li, X.; He, Y.X.; Lu, H.D.; Yang, X.J. Efficacies of nine seed coating agents for disease control and safety for fresh-eating consumption of Maize. Fujian J. Agric. Sci. 2021, 36, 564–571, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Han, H.L.; Guo, J.F.; Chen, B.; Tan, H.P.; Bao, F.; Wang, G.Y.; Zhao, F.C. Control effect of different seed coating on Spodoptera frugiperda at seedling stage of waxy maize. J. Zhejiang Agric. Sci. 2022, 63, 131–133. (In Chinese) [Google Scholar] [CrossRef]
- Meng, J.Z.; Shen, Y.F.; Yang, Z.B.; Xie, X.B.; Yang, Y.Y.; Sai, Z.Z.; Luo, G.J. Field experiment of controlling Spodoptera frugiperda by seed coating with Fortenza Duo (Syngenta). Yunnan Agric. Sci. Technol. 2022, S1, 67–68. (In Chinese) [Google Scholar]
- Feng, L.; Liu, F.; Tang, S.S.; Dai, C.G.; Hu, Y.; Xing, J.C.; Li, H.B. Preliminary evaluation on the effect of seed coating on controlling Spodoptera frugiperda in maize seedling stage. China Plant Prot. 2022, 42, 63–65+54. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, X.D.; Wu, Y.R.; Mao, R.L.; Zheng, T.F.; Zhao, X.X. Difference of vigor and physiology between coating seeds and non-coating seeds of maize stored under low temperature and low humidity environment. J. Maize Sci. 2020, 28, 105–110, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Lu, W.J.; Tan, M.X.; Shu, X.; Li, Z.J. Screening of sweet corn seed coating agent and optimization of coating technical parameters. China Seed Ind. 2014, 11, 56–58. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, F.C.; Cai, R.X.; Zhou, Z.F.; Tan, H.P.; Han, H.L.; Bao, F.; Wang, G.Y. Innovative farming system in Zhejiang: High-value cultivation of rotation between sweet corn and late rice. Mol. Plant Breed. 2020, 18, 7953–7958, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Li, Y.C.; Lu, X.Z.; Xu, L.L.; Wu, X.J.; Zhou, W.H. Discussion on the whole process mechanization technology and operation mode of fresh corn in Shanghai. Bull. Agric. Sci. Technol. 2021, 12, 270–272. (In Chinese) [Google Scholar] [CrossRef]
- Bao, K.Q. Study on the field efficacy of high dose 6% Avermectin Chlorantraniliprole delivery drug to prevent the 1st generation Chilo suppressalis in middle rice seedling fields. Xiandai Nonfyr Keji 2015, 11, 139–142. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, S.; Shu, K.Y.; Huang, X.Y.; Liu, X.Y. Control Effect of insecticide treated before transplantation on Chilo suppressalis(Walker) in early rice. China Plant Prot. 2018, 38, 68–70. (In Chinese) [Google Scholar] [CrossRef]
- Dai, B.F.; Chen, J.; Chen, W. Effect of Armistar Top (Syngenta) on disease control and yield increase of single cropping rice. J. Zhejiang Agric. Sci. 2017, 58, 1204–1205+1209. (In Chinese) [Google Scholar] [CrossRef]
- Chen, J.H.; Ren, S.P.; Chen, R.X.; Wang, Q.S. Application experiment of high dose pesticide application before transplanting on Yongyou rice. J. Zhejiang Agric. Sci. 2021, 62, 1813–1815. (In Chinese) [Google Scholar] [CrossRef]
- Chen, B.; Zheng, L.S.; Zhou, S.K.; Zhang, T.; Yang, X.; Zhou, G.H. Integrated demonstration of prevention and control techniques foe rice virus in South China. China Plant Prot. 2021, 41, 55–61. (In Chinese) [Google Scholar] [CrossRef]
- Wang, H.H.; Lu, S.L.; Zhao, R.; Liang, P.; Zhang, S.; Gao, X.W.; Zhang, L.; Gu, S.H. Establishment of the relative susceptible baselines of Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae to commonly used insecticides. Acta Entomol. Sin. 2021, 64, 1427–1432, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Davis, F.M.; Ng, S.S.; Williams, W.P. Visual rating scales for screening whorl-stage corn for resistance to fall armyworm. Tech. Bull. 1992, 186, 1–9. [Google Scholar]
- IRAC. Integrated Pest Management (IPM) & Insect Resistance Management (IRM) for Fall Armyworm in South African Maize (R/OL). Available online: www.irac-online.org (accessed on 1 May 2018).
- Zhao, S.Y.; Yang, X.M.; Yang, X.L.; Song, Y.F.; Wang, W.H.; Wu, K.M. Field sfficacy of eight insecticides on fall armyworm, Spodoptera frugiperda. Plant Prot. 2019, 45, 74–78. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, Y.X.; Huang, J.M.; Ni, H.; Guo, D.; Yang, F.X.; Wang, X.; Wu, S.F.; Gao, C.F. Susceptibility of fall armyworm, Spodoptera frugiperda (J.E. Smith), to eight insecticides in China, with special reference to lambda-cyhalothrin. Pestic. Biochem. Physiol. 2020, 168, 104623. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Wang, Y.Q.; Tan, Y.T.; Ma, Q.L.; Yan, W.J.; Yang, S.; Xu, H.H.; Zhang, Z.X. Bioactivity of spinetoram and its field efficiency against Spodoptera frugiperda. J. Environ. Entomol. 2019, 41, 1169–1174, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Lu, Y.H.; Tian, J.C.; Zheng, X.S.; Xu, H.X.; Yang, Y.J.; Yang, T.Y.; Shi, Z.Y.; Lu, Z.X. Laboratory toxicity test of 6 chemical insecticides against Spodoptera frugiperda. J. Environ. Entomol. 2020, 42, 329–334, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Chen, J.C.; Cao, L.J.; Ma, Z.Z.; Yuan, X.X.; Gong, Y.J.; Shen, X.J.; Wei, S.J. Susceptibility of different instar larvae of Spodoptera frugiperda to commonly used insecticides. Guangdong Agric. Sci. 2022, 49, 81–86, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Ma, Y.J.; Zhen, S.; Sun, J.; YU, M.; Zhao, R.; Guo, X.Y.; Xu, Y.; Wu, X.M. Refinement and functionalization of pesticide formulation research and development and efficient utilization in agricultural production. Chin. J. Pestic. Sci. 2022, 24, 1080–1098, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Liu, T.T.; Liu, S.K.; Li, B.X.; Liu, F.; Mu, W.; Pan, C.P.; Zou, N. Research progress on internal absorption and conduction behavior and application techniques of pesticides in plants. J. Agric. Pharmacol. 2021, 23, 607–616. [Google Scholar] [CrossRef]
- Hirooka, T.; Nihimastu, T.; Kodama, H.; Reckmann, U.; Nauen, R. The biological profile of flubendiamide, a new benzenedicarboxamide insecticide. Pflanzenschutz-Nachr. Bayer 2007, 60, 183–202. [Google Scholar]
- Zhang, S.; Shao, Z.R. Guidelines for the Scientific Use of Diamide and Neonicotinic Insecticides; China Agriculture Press: Beijing, China, 2014. (In Chinese) [Google Scholar]
- Jiang, J.L.; Shan, Z.J.; Cheng, Y.; Zhou, J.Y.; Bu, Y.Q. Advances in ecotoxicological effects of common pesticide adjuvants on aquatic organisms. Asian J. Ecotoxicol. 2017, 12, 45–58, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Xu, G.C.; Xu, D.J.; Xu, L.; Wang, C.B.; Cao, A.C.; Gu, Z.Y. Study on the synergistic effect of organosilicon adjuvant on chlorantraniliprole in the control of rice leaf folder, Cnaphalocrocis medinalis Guenée. Chin. J. Pestic. Sci. 2020, 22, 285–292, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Yang, S.Y.; Zhang, R.; Lu, B.Q. Synergistic effect of organosilicon adjuvant silwet408 on chlorantraniliprole in the control of Spodoptera frugiperda. J. Maize Sci. 2021, 29, 151–156, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Shan, T.S.; Xu, G.S.; Wang, C.C.; Zhang, H.H.; Shi, X.Y. Synergism of two organosilicon additives to tetrachlorantraniliprole against Pieris rapae. Plant Prot. 2019, 45, 241–244, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
| Treatment Number | Treatment | Times of Conventional Spray | Application Concentration |
|---|---|---|---|
| 1 | LCB | 5 | 330 mg/L |
| 2 | LCB + HTY-A8 | 5 | 330 mg/L + 0.02% HTY-A8 |
| 3 | LCB + MF | 5 | 330 mg/L + 0.02% MF |
| 4 | LCB | 25 | 1650 mg/L |
| 5 | LCB + HTY-A8 | 25 | 1650 mg/L + 0.02%HTY-A8 |
| 6 | LCB + MF | 25 | 1650 mg/L + 0.02% MF |
| 7 | YJD | 5 | 400 mg/L |
| 8 | YJD + HTY-A8 | 5 | 400 mg/L + 0.02%HTY-A8 |
| 9 | YJD + MF | 5 | 400 mg/L + 0.02% MF |
| 10 | YJD | 25 | 2000 mg/L |
| 11 | YJD + HTY-A8 | 25 | 2000 mg/L + 0.02%HTY-A8 |
| 12 | YJD + MF | 25 | 2000 mg/L + 0.02% MF |
| 13 | JWY | 5 | 50 mg/L |
| 14 | JWY + HTY-A8 | 5 | 50 mg/L + 0.02%HTY-A8 |
| 15 | JWY + MF | 5 | 50 mg/L + 0.02% MF |
| 16 | JWY | 25 | 250 mg/L |
| 17 | JWY + HTY-A8 | 25 | 250 mg/L + 0.02%HTY-A8 |
| 18 | JWY + MF | 25 | 250 mg/L + 0.02% MF |
| CK+ | LMW | / | 6.8 g/kg seed coating |
| CK− | water | / | 0.0 |
| Tested Insects | Insecticides | Number of Tested Insects | Treatment Time | Slope ± SE | LC50 (95% CL) (mg/L) | χ2 | df |
|---|---|---|---|---|---|---|---|
| Newly hatched larvae | 3% Emamectin Benzoate WDG | 432 | 48 h | 2.108 ± 0.302 | 0.011 (0.005–0.018) | 59.822 | 13 |
| 6% Spinetoram SC | 504 | 48 h | 1.220 ± 0.127 | 0.040 (0.026–0.057) | 22.673 | 16 | |
| 35% Chlorantraniliprole WDG | 432 | 48 h | 1.483 ± 0.181 | 0.065 (0.035–0.102) | 32.329 | 13 | |
| Third instar larvae | 3% Emamectin Benzoate WDG | 432 | 72 h | 1.002 ± 0.121 | 0.014 (0.009–0.020) | 7.085 | 13 |
| 6% Spinetoram SC | 504 | 72 h | 1.210 ± 0.111 | 0.089 (0.061–0.124) | 19.253 | 16 | |
| 35% Chlorantraniliprole WDG | 432 | 72 h | 1.174 ± 0.100 | 0.144 (0.081–0.235) | 43.928 | 16 |
| Treatment Number | Corrected Mortality % | |||||
|---|---|---|---|---|---|---|
| 2nd Day | 10th Day | 20th Day | ||||
| Newly Hatched Larvae | Third Instar Larvae | Newly Hatched Larvae | Third Instar Larvae | Newly Hatched Larvae | Third Instar Larvae | |
| 1 | 100.00 | 100.00 | 100.00 a | 95.45 ± 4.55 ab | 42.46 ± 7.09 cde | 11.14 ± 4.50 f |
| 2 | 100.00 | 100.00 | 100.00 a | 70.78 ± 1.35 cdef | 47.84 ± 4.07 cde | 41.81 ± 5.87 bc |
| 3 | 100.00 | 100.00 | 100.00 a | 83.12 ± 2.93 bcde | 58.75 ± 5.58 bc | 28.87 ± 4.52 cde |
| 4 | 100.00 | 100.00 | 100.00 a | 96.97 ± 3.03 a | 45.888 ± 4.77 cde | 39.56 ± 8.53 bcd |
| 5 | 100.00 | 100.00 | 100.00 a | 92.35 ± 1.44 ab | 95.38 ± 2.62 a | 73.08 ± 9.46 a |
| 6 | 100.00 | 100.00 | 100.00 a | 98.48 ± 1.52 a | 87.51 ± 3.90 a | 29.34 ± 5.57 cde |
| 7 | 100.00 | 100.00 | 95.52 ± 2.51 bc | 90.84 ± 2.56 abc | 37.71 ± 8.43 cde | 30.33 ± 8.96 cd |
| 8 | 100.00 | 100.00 | 100.00 a | 87.81 ± 3.94 abcd | 28.32 ± 9.05 efg | 27.64 ± 2.11 cde |
| 9 | 100.00 | 100.00 | 100.00 a | 86.22 ± 2.50 abcde | 31.64 ± 2.40 def | 12.22 ± 1.12 ef |
| 10 | 100.00 | 100.00 | 98.51 ± 1.45 ab | 92.35 ± 2.99 ab | 28.09 ± 9.05 efg | 19.69 ± 5.39 def |
| 11 | 100.00 | 100.00 | 100.00 a | 96.97 ± 3.03 a | 52.22 ± 7.78 cd | 19.89 ± 3.76 edf |
| 12 | 100.00 | 100.00 | 100.00 a | 95.45 ± 2.62 ab | 14.46 ± 3.15 gh | 35.08 ± 5.91 bcd |
| 13 | 100.00 | 100.00 | 80.60 ± 1.66 fg | 63.35 ± 10.25 efgh | 30.05 ± 3.62 def | 22.92 ± 6.76 cdef |
| 14 | 100.00 | 100.00 | 89.55 ± 1.58 de | 58.51 ± 6.78 fgh | 25.28 ± 2.67 efg | 28.67 ± 9.59 cde |
| 15 | 100.00 | 100.00 | 94.03 ± 1.59 cd | 41.78 ± 14.09 hi | 15.69 ± 10.78 gh | 24.41 ± 8.68 cdef |
| 16 | 100.00 | 100.00 | 98.51 ± 1.51 ab | 43.15 ± 10.73 hi | 15.36 ± 10.23 gh | 19.25 ± 10.91 def |
| 17 | 100.00 | 100.00 | 74.63 ± 5.65 g | 44.23 ± 19.15 ghi | 31.80 ± 1.86 def | 27.48 ± 4.78 cde |
| 18 | 100.00 | 100.00 | 85.07 ± 2.91 ef | 26.19 ± 1.77 i | 7.94 ± 3.32 h | 25.97 ± 3.41 cde |
| CK+ | 100.00 | 100.00 | 100.00 a | 66.31 ± 6.32 defg | 81.08 ± 2.23 ab | 66.82 ± 14.24 a |
| Treatment Number | Pesticide Active Ingredient | Pesticide Content in Leaves (mg/kg) | ||
|---|---|---|---|---|
| 2nd Day | 10th Day | 20th Day | ||
| 1 | Chlorantraniliprole | 47.4 (5.44) | 8.71 (13.83) | 0.63 |
| 2 | 126 (5.94) | 21.2 (52.29) | 0.37 | |
| 3 | 88.6 (3.32) | 26.7 (51.35) | 0.52 | |
| 4 | 394 (12.75) | 30.9 (34.33) | 0.9 | |
| 5 | 700 (5.15) | 136 (53.98) | 2.52 | |
| 6 | 394 (6.46) | 60.9 (52.95) | 1.15 | |
| 7 | Spinetoram | 2.27 (24.67) | 0.092 (/) | <0.01 |
| 8 | 2.8 (21.54) | 0.13 (10.83) | 0.012 | |
| 9 | 1.25 (1.95) | 0.64 (/) | <0.01 | |
| 10 | 10 (12.66) | 0.79 (/) | <0.01 | |
| 11 | 6.32 (4.05) | 1.56 (53.79) | 0.029 | |
| 12 | 7.1 (2.42) | 2.93 (244.17) | 0.012 | |
| 13 | Emamectin Benzoate | 5.35 (232.61) | 0.023 (/) | <0.005 |
| 14 | 1.46 (66.36) | 0.022 (/) | <0.005 | |
| 15 | 3.13 (69.56) | 0.045 (/) | <0.005 | |
| 16 | 26.1 (483.33) | 0.054 (/) | <0.005 | |
| 17 | 12.5 (208.33) | 0.06 (6.00) | 0.01 | |
| 18 | 12.5 (156.25) | 0.08 (/) | <0.005 | |
| CK+ | Chlorantraniliprole | 0.25 (3.01) | 0.083 (1.00) | 0.083 |
| Treatment | Relative Control Effect % | ||||
|---|---|---|---|---|---|
| 8th Day | 11th Day | 14th Day | 17th Day | 22nd day | |
| 3 | 60.32 ± 28.20 b | 49.10 ± 13.63 c | 8.81 ± 18.33 b | 4.83 ± 8.11 b | 3.17 ± 12.10 a |
| 5 | 92.01 ± 7.99 ab | 79.25 ± 14.20 abc | 62.47 ± 8.49 a | 53.01 ± 15.68 a | 7.71 ± 13.05 a |
| 11 | 90.08 ± 9.91 ab | 94.24 ± 3.44 ab | 65.66 ± 11.08a | 55.27 ± 15.42 a | 37.42 ± 27.70 a |
| 12 | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 83.31 ± 9.14 a | 62.34 ± 15.83 a | 16.67 ± 22.11 a |
| CK+ | 74.05 ± 11.00 ab | 75.72 ± 9.46 bc | 71.81 ± 11.50 a | 47.25 ± 19.82 ab | 28.08 ± 14.23 a |
| Method of Application | Number of Field Sprays | Single Dose | Active Ingredient Required for Single Control/Hectare | Labor Cost of Application (RMB) |
|---|---|---|---|---|
| 35% Chlorantraniliprole field spray | 1 | 150 g/hectare | 52.5 g | 300 |
| 50% Chlorantraniliprole FSC seed coating | 0 | 6.8 g/kg seed | 38.25 g | 50 |
| 35% Chlorantraniliprole application before transplanting (25 times that of field spray concentration) | 0 | 3750 g/ hectare | 9.84 g | 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, H.; Chen, B.; Xu, H.; Qin, Y.; Wang, G.; Lv, Z.; Wang, X.; Zhao, F. Control of Spodoptera frugiperda on Fresh Corn via Pesticide Application before Transplanting. Agriculture 2023, 13, 342. https://doi.org/10.3390/agriculture13020342
Han H, Chen B, Xu H, Qin Y, Wang G, Lv Z, Wang X, Zhao F. Control of Spodoptera frugiperda on Fresh Corn via Pesticide Application before Transplanting. Agriculture. 2023; 13(2):342. https://doi.org/10.3390/agriculture13020342
Chicago/Turabian StyleHan, Hailiang, Bin Chen, Hongxing Xu, Yan Qin, Guiyue Wang, Zhongxian Lv, Xingliang Wang, and Fucheng Zhao. 2023. "Control of Spodoptera frugiperda on Fresh Corn via Pesticide Application before Transplanting" Agriculture 13, no. 2: 342. https://doi.org/10.3390/agriculture13020342
APA StyleHan, H., Chen, B., Xu, H., Qin, Y., Wang, G., Lv, Z., Wang, X., & Zhao, F. (2023). Control of Spodoptera frugiperda on Fresh Corn via Pesticide Application before Transplanting. Agriculture, 13(2), 342. https://doi.org/10.3390/agriculture13020342









