Pyrolysis of Amaranth Inflorescence Wastes: Bioenergy Potential, Biochar and Hydrocarbon Rich Bio-Oil Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Amaranth Inflorescence Wastes
2.2. Physicochemical Characterization
2.3. Pyrolysis Experimental Procedure
2.4. Thermogravimetric Analysis (TGA)
2.5. Kinetic Analysis
2.6. Model-Free Methods
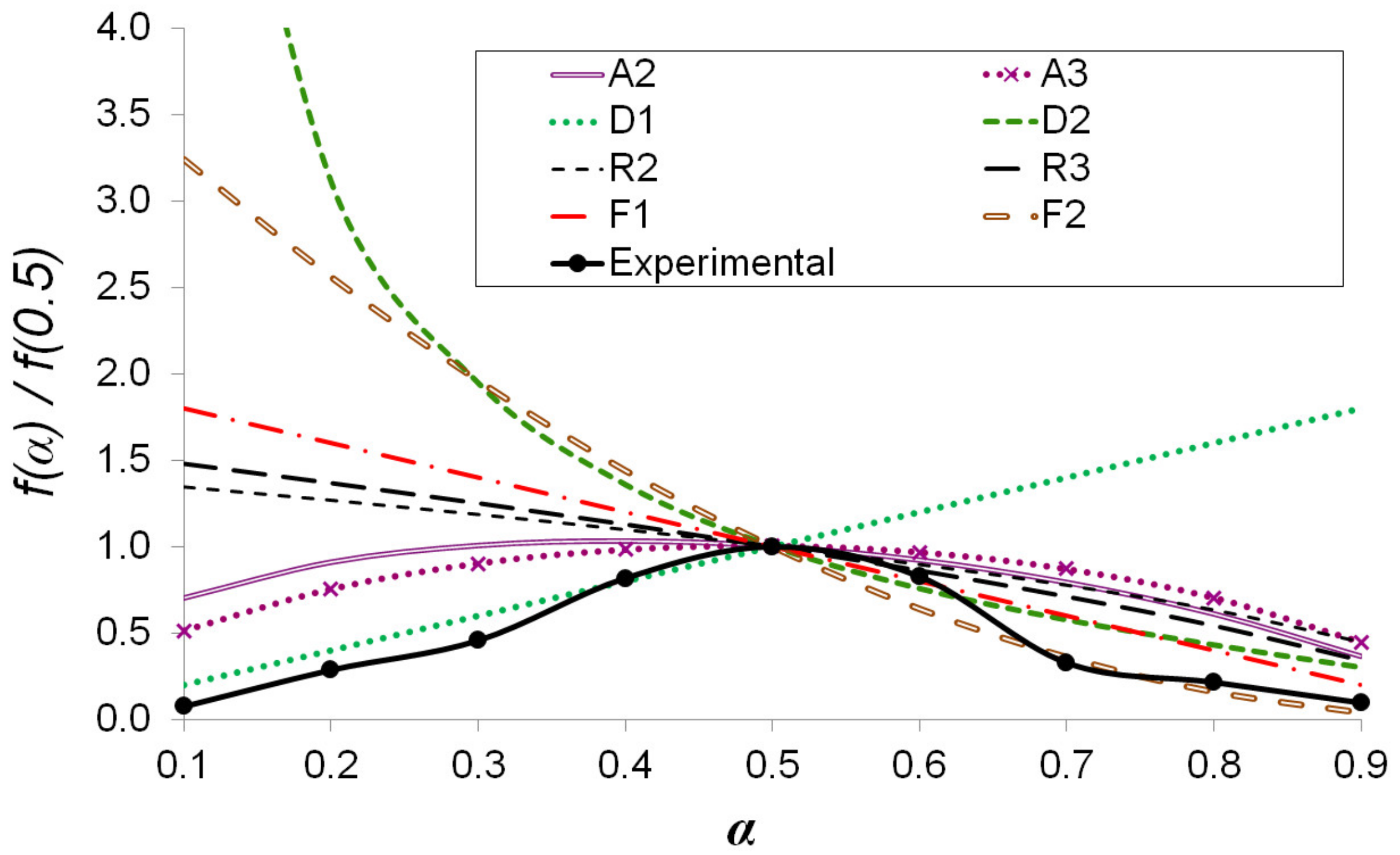
2.7. Reaction Model Determination for AIW Pyrolysis
2.8. Thermodynamic Parameters
3. Results and Discussion
3.1. Results of Proximate and Ultimate Analyses
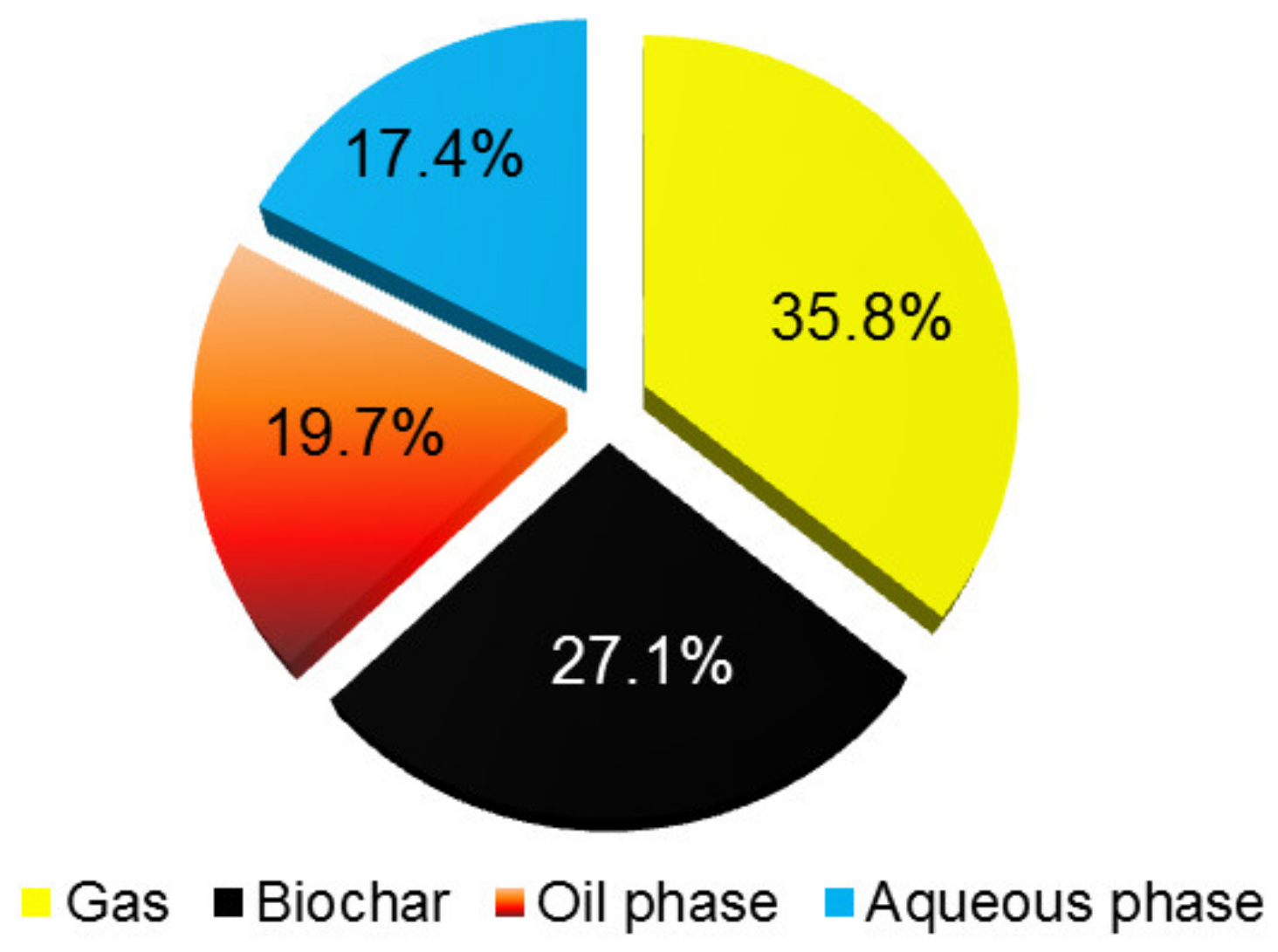
3.2. Pyrolysis Products Yields and Their Quality
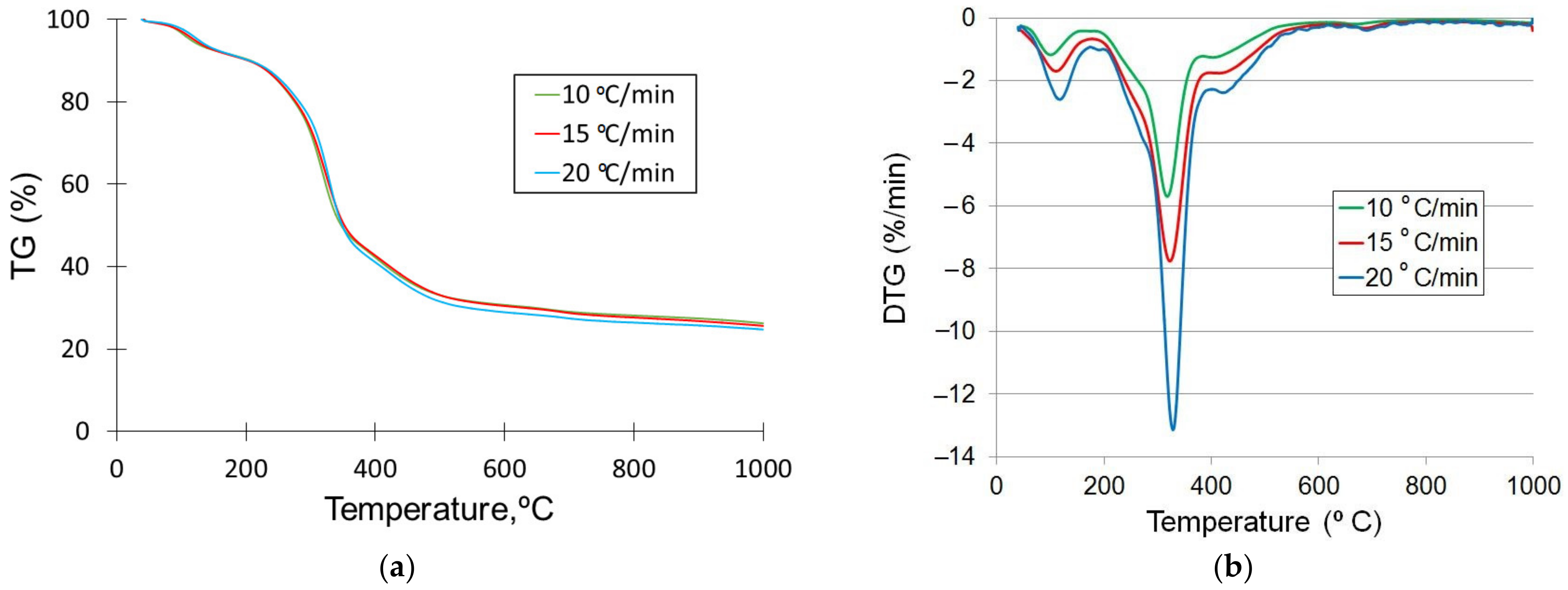
3.3. Thermal Degradation Analysis
3.4. Kinetic Analysis
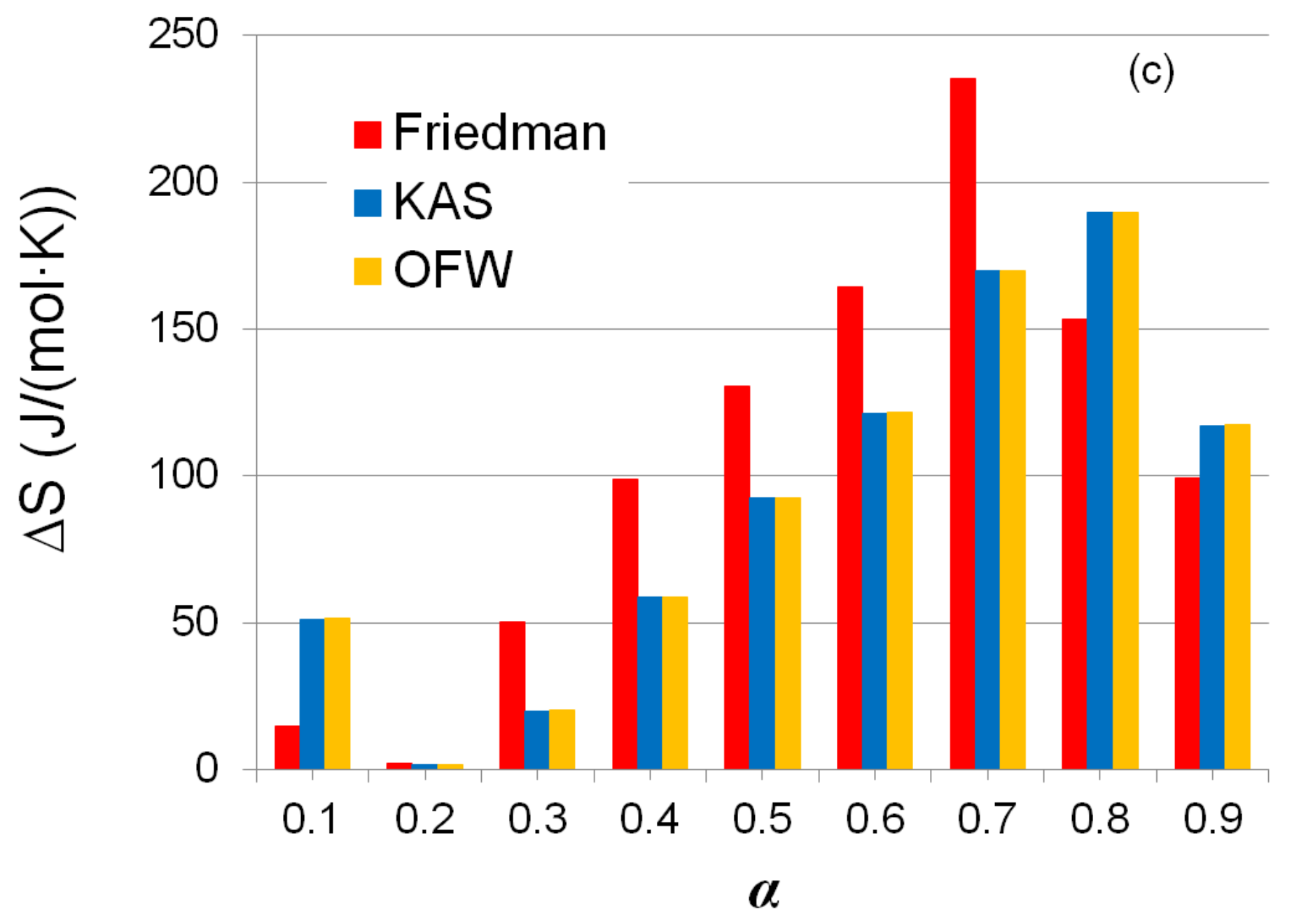
3.5. Thermodynamic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Emmanuel, O.C.; Babalola, O.O. Amaranth production and consumption in South Africa: The challenges of sustainability for food and nutrition security. Int. J. Agric. Sustain. 2022, 20, 449–460. [Google Scholar] [CrossRef]
- Sulaiman, M.I.; Andini, R. Potential of Amaranth in Alleviating Malnutrition in Indonesia. In Nutritional Value of Amaranth; Waisundara, Y., Ed.; IntechOpen: London, UK, 2020. [Google Scholar]
- Schmidt, D.; Verruma-Bernardi, M.R.; Forti, V.A.; Borges, M.T.M.R. Quinoa and Amaranth as Functional Foods: A Review. Food Rev. Int. 2021, 37, 1–20. [Google Scholar] [CrossRef]
- Adhikary, D.; Khatri-Chhetri, U.; Slaski, J. Amaranth: An Ancient and High-Quality Wholesome Crop. In Nutritional Value of Amaranth; Waisundara, Y., Ed.; IntechOpen: London, UK, 2020. [Google Scholar]
- Aderibigbe, O.R.; Ezekiel, O.O.; Owolade, S.O.; Korese, J.K.; Sturm, B.; Hensel, O. Exploring the potentials of underutilized grain amaranth (Amaranthus spp.) along the value chain for food and nutrition security: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Shi, Z.; Wang, S.; Mu, W.; Jönsson, P.G.; Yang, W. Pyrolysis of raw and anaerobically digested organic fractions of municipal solid waste: Kinetics, thermodynamics, and product characterization. Chem. Eng. J. 2021, 415, 129064. [Google Scholar] [CrossRef]
- Farooq, A.; Ashraf, M.; Aslam, Z.; Anwar, A.; Jiang, S.; Farooq, A.; Liu, L. Pyrolytic conversion of a novel biomass Ficus na-talensis barkcloth: Physiochemical and thermo-kinetic analysis. Biomass Conv. Bioref. 2021, 11. [Google Scholar] [CrossRef]
- Karaeva, J.V.; Timofeeva, S.S.; Islamova, S.I.; Gerasimov, A.V. Pyrolysis kinetics of new bioenergy feedstock from anaerobic digestate of agro-waste by thermogravimetric analysis. J. Environ. Chem. Eng. 2022, 10, 107850. [Google Scholar] [CrossRef]
- Figueirêdo, M.B.; Hita, I.; Deuss, P.J.; Venderbosch, R.H.; Heeres, H.J. Pyrolytic lignin: A promising biorefinery feedstock for the production of fuels and valuable chemicals. Green Chem. 2022, 24, 4680–4702. [Google Scholar] [CrossRef]
- Yang, Q.; Zhou, H.; Bartocci, P.; Fantozzi, F.; Mašek, O.; Agblevor, F.A.; Wei, Z.; Yang, H.; Chen, H.; Lu, X.; et al. Prospective contributions of biomass pyrolysis to China’s 2050 carbon reduction and renewable energy goals. Nat. Commun. 2021, 12, 1698. [Google Scholar]
- Mlonka-Mędrala, A.; Evangelopoulos, P.; Sieradzka, M.; Zajemska, M.; Magdziarz, A. Pyrolysis of agricultural waste biomass towards production of gas fuel and high-quality char: Experimental and numerical investigations. Fuel 2021, 296, 120611. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Vuppaladadiyam, S.S.V.; Sahoo, A.; Murugavelh, S.; Anthony, E.; Bhaskar, T.; Zheng, Y.; Zhao, M.; Duan, H.; Zhao, Y.; et al. Bio-oil and biochar from the pyrolytic conversion of biomass: A current and future perspective on the trade-off between economic, environmental, and technical indicators. Sci. Total. Environ. 2023, 857, 159155. [Google Scholar] [CrossRef]
- Lobzenko, I.; Burachevskaya, M.; Zamulina, I.; Barakhov, A.; Bauer, T.; Mandzhieva, S.; Sushkova, S.; Minkina, T.; Tereschenko, A.; Kalinichenko, V.; et al. Development of a Unique Technology for the Pyrolysis of Rice Husk Biochar for Promising Heavy Metal Remediation. Agriculture 2022, 12, 1689. [Google Scholar] [CrossRef]
- Ansari, K.B.; Gaikar, V.G. Investigating production of hydrocarbon rich bio-oil from grassy biomass using vacuum pyrolysis coupled with online deoxygenation of volatile products over metallic iron. Renew. Energy 2019, 130, 305–318. [Google Scholar] [CrossRef]
- Giorcelli, M.; Das, O.; Sas, G.; Försth, M.; Bartoli, M. A Review of Bio-Oil Production through Microwave-Assisted Pyrolysis. Processes 2021, 9, 561. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Z.; Chen, X.; Chen, Y.; Dong, Z.; Wang, X.; Yang, H. Comparative pyrolysis behaviors of stalk, wood and shell biomass: Correlation of cellulose crystallinity and reaction kinetics. Bioresour. Technol. 2020, 310, 123498. [Google Scholar] [CrossRef] [PubMed]
- Nour, M.; Amer, M.; Elwardany, A.; Attia, A.; Li, X.; Nada, S. Pyrolysis, kinetics, and structural analyses of agricultural residues in Egypt: For future assessment of their energy potential. Clean. Eng. Technol. 2021, 2, 100080. [Google Scholar] [CrossRef]
- Pu, X.; Wei, M.; Chen, X.; Wang, L.; Deng, L. Thermal Decomposition Characteristics and Kinetic Analysis of Chicken Manure in Various Atmospheres. Agriculture 2022, 12, 607. [Google Scholar] [CrossRef]
- Tian, B.; Wang, X.; Zhao, W.; Xu, L.; Bai, L. Pyrolysis behaviors, kinetics and gaseous product evolutions of two typical bi-omass wastes. Catal. Today 2021, 374, 77–85. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Mehmood, M.A.; Taqvi, S.T.H.; Elkamel, A.; Liu, C.-G.; Xu, J.; Rahimuddin, S.A.; Gull, M. Pyrolysis, kinetics analysis, thermodynamics parameters and reaction mechanism of Typha latifolia to evaluate its bioenergy potential. Bioresour. Technol. 2017, 245, 491–501. [Google Scholar] [CrossRef]
- Kumar, M.; Sabbarwal, S.; Mishra, P.; Upadhyay, S. Thermal degradation kinetics of sugarcane leaves (Saccharum officinarum L) using thermo-gravimetric and differential scanning calorimetric studies. Bioresour. Technol. 2019, 279, 262–270. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; Ramachandran, S.; Subbiah, S. Determination of kinetic parameters in the pyrolysis operation and thermal behavior of Prosopis juliflora using thermogravimetric analysis. Bioresour. Technol. 2017, 233, 413–422. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K. Kinetic analysis and pyrolysis behaviour of waste biomass towards its bioenergy potential. Bioresour. Technol. 2020, 311, 123480. [Google Scholar] [CrossRef] [PubMed]
- Soria-Verdugo, A.; Goos, E.; García-Hernando, N.; Riedel, U. Analyzing the pyrolysis kinetics of several microalgae species by various differential and integral isoconversional kinetic methods and the Distributed Activation Energy Model. Algal Res. 2018, 32, 11–29. [Google Scholar] [CrossRef]
- Ermolaev, D.V.; Timofeeva, S.S.; Islamova, S.I.; Bulygina, K.S.; Gilfanov, M.F. A comprehensive study of thermotechnical and thermogravimetric properties of peat for power generation. Biomass Conv. Bioref. 2019, 9, 767–774. [Google Scholar] [CrossRef]
- Karaeva, J.V.; Timofeeva, S.S.; Bashkirov, V.N.; Bulygina, K.S. Thermochemical processing of digestate from biogas plant for recycling dairy manure and biomass. Biomass Conv. Bioref. 2021, 13, 685–695. [Google Scholar] [CrossRef]
- Wang, G.; Dai, Y.; Yang, H.; Xiong, Q.; Wang, K.; Zhou, J.; Li, Y.; Wang, S. A Review of Recent Advances in Biomass Pyrolysis. Energy Fuels 2020, 34, 15557–15578. [Google Scholar] [CrossRef]
- Lewandowski, W.M.; Ryms, M.; Kosakowski, W. Thermal Biomass Conversion: A Review. Processes 2020, 8, 516. [Google Scholar] [CrossRef]
- Karaeva, J.V.; Timofeeva, S.S.; Kovalev, A.A.; Kovalev, D.A.; Gilfanov, M.F.; Grigoriev, V.S.; Litti, Y.V. Co-pyrolysis of agri-cultural waste and estimation of the applicability of pyrolysis in the integrated technology of biorenewable hydrogen produc-tion. Int. J. Hydrogen Energy 2022, 47, 11787–11798. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, K.; Kun, L.; Hou, C.; Liu, J.; Huang, R.; Cao, C.; Song, W. Nitrogen, Sulfur Co-doped Carbon Materials Derived from the Leaf, Stem and Root of Amaranth as Metal-free Catalysts for Selective Oxidation of Aromatic Hydrocarbons. ChemCatChem 2019, 11, 1010–1016. [Google Scholar] [CrossRef]
- Gao, S.; Geng, K.; Liu, H.; Wei, X.; Zhang, M.; Wang, P.; Wang, J. Transforming organic-rich amaranthus waste into nitro-gen-doped carbon with superior performance of the oxygen reduction reaction. Energy Environ. Sci. 2015, 8, 221–229. [Google Scholar] [CrossRef]
- Tinwala, F.; Mohanty, P.; Parmar, S.; Patel, A.; Pant, K.K. Intermediate pyrolysis of agro-industrial biomasses in bench-scale pyrolyser: Product yields and its characterization. Bioresour. Technol. 2015, 188, 258–264. [Google Scholar] [CrossRef]
- Biswas, B.; Pandey, N.; Bisht, Y.; Singh, R.; Kumar, J.; Bhaskar, T. Pyrolysis of agricultural biomass residues: Comparative study of corn cob, wheat straw, rice straw and rice husk. Bioresour. Technol. 2017, 237, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Rego, F.; Dias, A.P.S.; Casquilho, M.; Rosa, F.C.; Rodrigues, A. Pyrolysis kinetics of short rotation coppice poplar biomass. Energy 2020, 207, 118191. [Google Scholar] [CrossRef]
- Bhardwaj, G.; Kumar, M.; Mishra, P.K.; Upadhyay, S.N. Kinetic analysis of the slow pyrolysis of paper wastes. Biomass Conv. Bioref. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Shahbeig, H.; Nosrati, M. Pyrolysis of biological wastes for bioenergy production: Thermo-kinetic studies with machine-learning method and Py-GC/MS analysis. Fuel 2020, 269, 117238. [Google Scholar] [CrossRef]
- Singh, R.K.; Pandey, D.; Patil, T.; Sawarkar, A.N. Pyrolysis of banana leaves biomass: Physico-chemical characterization, thermal decomposition behavior, kinetic and thermodynamic analyses. Bioresour. Technol. 2020, 310, 123464. [Google Scholar] [CrossRef]
- Jiang, G.; Wei, L. Analysis of pyrolysis kinetic model for processing of thermogravimetric analysis data. In Phase Change Materials and Their Applications; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Yuan, X.; He, T.; Cao, H.; Yuan, Q. Cattle manure pyrolysis process: Kinetic and thermodynamic analysis with isoconversional methods. Renew. Energy 2017, 107, 489–496. [Google Scholar] [CrossRef]
- Khawam, A. Application of Solid-State Kinetics to Desolvation Reactions. Ph.D. Thesis, University of Iowa, Iowa, IA, USA, 2007. [Google Scholar]
- Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A.; Perejón, A.; Criado, J.M. Generalized master plots as a straightforward approach for determining the kinetic model: The case of cellulose pyrolysis. Thermochim. Acta 2013, 552, 54–59. [Google Scholar] [CrossRef]
- Gotor, F.J.; Criado, J.M.; Malek, J.; Koga, N. Kinetic Analysis of Solid-State Reactions: The Universality of Master Plots for Analyzing Isothermal and Nonisothermal Experiments. J. Phys. Chem. A 2000, 104, 10777–10782. [Google Scholar] [CrossRef]
- Brown, M.E. Handbook of Thermal Analysis and Calorimetry; Principles and Practice; Elsevier Science: Amsterdam, The Netherlands, 1998; Volume 1, p. 722. [Google Scholar]
- Alves, J.L.F.; Da Silva, J.C.G.; Filho, V.F.S.; Alves, R.F.; Ahmad, M.S.; Ahmad, M.S.; Galdino, W.V.A.; De Sena, R.F. Bioenergy potential of red macroalgaeGelidium floridanumby pyrolysis: Evaluation of kinetic triplet and thermodynamics parameters. Bioresour. Technol. 2019, 291, 121892. [Google Scholar] [CrossRef]
- Varma, A.K.; Lal, N.; Rathore, A.K.; Katiyar, R.; Thakur, L.S.; Shankar, R.; Mondal, P. Thermal, kinetic and thermodynamic study for co-pyrolysis of pine needles and styrofoam using thermogravimetric analysis. Energy 2020, 218, 119404. [Google Scholar] [CrossRef]
- El-Sayed, S.A.; Mostafa, M.E. Kinetics, thermodynamics, and combustion characteristics of Poinciana pods using TG/DTG/DTA techniques. Biomass Conv. Bioref. 2021, 11, 1–25. [Google Scholar] [CrossRef]
- García, R.; Pizarro, C.; Lavín, A.G.; Bueno, J.L. Characterization of Spanish biomass wastes for energy use. Bioresour. Technol. 2012, 103, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Emiola-Sadiq, T.; Zhang, L.; Dalai, A.K. Thermal and Kinetic Studies on Biomass Degradation via Thermogravimetric Anal-ysis: A Combination of Model-Fitting and Model-Free Approach. ACS Omega 2021, 6, 22233–22247. [Google Scholar] [CrossRef] [PubMed]
- Güleç, F.; Pekaslan, D.; Williams, O.; Lester, E. Predictability of higher heating value of biomass feedstocks via proximate and ultimate analyses—A comprehensive study of artificial neural network applications. Fuel 2022, 320, 123944. [Google Scholar] [CrossRef]
- Imam, T.; Capareda, S. Characterization of bio-oil, syn-gas and bio-char from switchgrass pyrolysis at various temperatures. J. Anal. Appl. Pyrolysis 2012, 93, 170–177. [Google Scholar] [CrossRef]
- Aboulkas, A.; Hammani, H.; El Achaby, M.; Bilal, E.; Barakat, A.; El harfi, K. Valorization of algal waste via pyrolysis in a fixed-bed reactor: Production and characterization of bio-oil and bio-char. Bioresour. Technol. 2017, 243, 400–408. [Google Scholar] [CrossRef]
- Pandey, D.S.; Katsaros, G.; Lindfors, C.; Leahy, J.J.; Tassou, S.A. Fast Pyrolysis of Poultry Litter in a Bubbling Fluidised Bed Reactor: Energy and Nutrient Recovery. Sustainability 2019, 11, 2533. [Google Scholar] [CrossRef]
- Panchasara, H.; Ashwath, N. Effects of Pyrolysis Bio-Oils on Fuel Atomisation—A Review. Energies 2021, 14, 794. [Google Scholar] [CrossRef]
- Alvarez, J.; Lopez, G.; Amutio, M.; Artetxe, M.; Barbarias, I.; Arregi, A.; Bilbao, J.; Olazar, M. Characterization of the bio-oil obtained by fast pyrolysis of sewage sludge in a conical spouted bed reactor. Fuel Process. Technol. 2016, 149, 169–175. [Google Scholar] [CrossRef]
- Nasirpour-Tabrizi, P.; Azadmard-Damirchi, S.; Hesari, J.; Piravi-Vanak, Z. Amaranth Seed Oil Composition; Waisundara, V.Y., Ed.; IntechOpen: London, UK, 2020. [Google Scholar]
- Griessacher, T.; Antrekowitsch, J.; Steinlechner, S. Charcoal from agricultural residues as alternative reducing agent in metal recycling. Biomass Bioenergy 2012, 39, 139–146. [Google Scholar] [CrossRef]
- Peer, V.; Frantík, J.; Kielar, J.; Mašek, D. Substrates for slow pyrolysis. In Proceedings of the XXI International Scientific Conference—The Application of Experimental and Numerical Methods in Fluid Mechanics and Energy, Teplice, Slovakia, 25–27 April 2018. [Google Scholar]
- Das, S.K.; Ghosh, G.K.; Avasthe, R.K.; Sinha, K. Compositional heterogeneity of different biochar: Effect of pyrolysis temper-ature and feedstocks. J. Environ. Manag. 2021, 278, 2. [Google Scholar] [CrossRef] [PubMed]
- Tangmankongworakoon, N. An approach to produce biochar from coffee residue for fuel and soil amendment purpose. Int. J. Recycl. Org. Waste Agric. 2019, 8 (Suppl. S1), 37–44. [Google Scholar] [CrossRef]
- Baidoo, I.K.; Sarpong, D.B.; Bolwig, S.; Ninson, D. Biochar amended soils and crop productivity: A critical and meta-analysis of literature. Int. J. Dev. Sustain. 2016, 5, 414–432. [Google Scholar]
- Yuan, J.-H.; Xu, R.-K.; Qian, W.; Wang, R.-H. Comparison of the ameliorating effects on an acidic ultisol between four crop straws and their biochars. J. Soils Sediments 2011, 11, 741–750. [Google Scholar] [CrossRef]
- Anca-Couce, A. Reaction mechanisms and multi-scale modelling of lignocellulosic biomass pyrolysis. Prog. Energy Combust. Sci. 2016, 53, 41–79. [Google Scholar] [CrossRef]
- Pielsticker, S.; Gövert, B.; Umeki, K.; Kneer, R. Flash Pyrolysis Kinetics of Extracted Lignocellulosic Biomass Components. Front. Energy Res. 2021, 9, 497. [Google Scholar] [CrossRef]
- Senneca, O.; Cerciello, F.; Russo, C.; Wütscher, A.; Muhler, M.; Apicella, B. Thermal treatment of lignin, cellulose and hemi-cellulose in nitrogen and carbon dioxide. Fuel 2020, 271, 117656. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction, 3rd ed.; Academic Press: Cambridge, MA, USA, 2018; p. 564. [Google Scholar]
- Shen, D.K.; Gu, S.; Bridgwater, A.V. The thermal performance of the polysaccharides extracted from hardwood: Cellulose and hemicellulose. Carbohydr. Polym. 2010, 82, 39–45. [Google Scholar] [CrossRef]
- Usino, D.O.; Ylitervo, P.; Moreno, A.; Sipponen, M.H.; Richards, T. Primary interactions of biomass components during fast pyrolysis. J. Anal. Appl. Pyrolysis 2021, 159, 105297. [Google Scholar] [CrossRef]
- Yu, J.; Paterson, N.; Blamey, J.; Millan, M. Cellulose, xylan and lignin interactions during pyrolysis of lignocellulosic biomass. Fuel 2017, 191, 140–149. [Google Scholar] [CrossRef]
- Shen, D.; Xiao, R.; Gu, S.; Luo, K. The pyrolytic behavior of cellulose in lignocellulosic biomass: A review. RSC Adv. 2011, 1, 1641–1660. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, B.; Ji, Y.; Xu, F.; Zong, P.; Zhang, J.; Tian, Y. Thermal decomposition of castor oil, corn starch, soy protein, lignin, xylan, and cellulose during fast pyrolysis. Bioresour. Technol. 2019, 278, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lin, H.; Ru, B.; Sun, W.; Wang, Y.; Luo, Z. Comparison of the pyrolysis behavior of pyrolytic lignin and milled wood lignin by using TG–FTIR analysis. J. Anal. Appl. Pyrolysis 2014, 108, 78–85. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, G.; Yue, J.; Xu, G. Pyrolysis of black liquor for phenols and impact of its inherent alkali. Fuel Process. Technol. 2014, 127, 149–156. [Google Scholar] [CrossRef]
- Lin, F.; Waters, C.L.; Mallinson, R.G.; Lobban, L.L.; Bartley, L.E. Relationships between Biomass Composition and Liquid Products Formed via Pyrolysis. Front. Energy Res. 2015, 3, 45. [Google Scholar] [CrossRef]
- Laougé, Z.B.; Merdun, H. Pyrolysis and combustion kinetics of Sida cordifolia L. using thermogravimetric analysis. Bioresour. Technol. 2020, 299, 122602. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Antunes, E.; Sanchez, P.B.; Duan, H.; Zhao, M. Influence of microalgae on synergism during co-pyrolysis with organic waste biomass: A thermogravimetric and kinetic analysis. Renew. Energy 2020, 167, 42–55. [Google Scholar] [CrossRef]
- Mallick, D.; Bora, B.J.; Baruah, D.; Barbhuiya, S.A.; Banik, R.; Garg, J.; Sarma, R. Mechanistic investigation and thermal deg-radation of Eichhornia crassipes using Thermogravimetric analysis. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Baruah, D.; Mallick, D.; Kalita, P.; Moholkar, V.S. A Detailed Study of Pyrolysis Kinetics of Elephant Grass Using Thermogravimetric Analysis. In Proceedings of the 2nd International Conference on Energy Power and Environment, Shillong, India, 1–2 June 2018. [Google Scholar]
- Patidar, K.; Singathia, A.; Vashishtha, M.; Sangal, V.K.; Upadhyaya, S. Investigation of kinetic and thermodynamic parameters approaches to non-isothermal pyrolysis of mustard stalk using model-free and master plots methods. Mater. Sci. Energy Technol. 2021, 5, 6–14. [Google Scholar] [CrossRef]
- Fernandez, A.; Ortiz, L.R.; Asensio, D.; Rodriguez, R.; Mazza, G. Kinetic analysis and thermodynamics properties of air/steam gasification of agricultural waste. J. Environ. Chem. Eng. 2020, 8, 103829. [Google Scholar] [CrossRef]
- Wang, S.; Wen, Y.; Shi, Z.; Niedzwiecki, L.; Baranowski, M.; Czerep, M.; Mu, W.; Kruczek, H.P.; Jönsson, P.G.; Yang, W. Effect of hydrothermal carbonization pretreatment on the pyrolysis behavior of the digestate of agricultural waste: A view on kinetics and thermodynamics. Chem. Eng. J. 2022, 431, 133881. [Google Scholar] [CrossRef]
- Ozawa, T. Estimation of activation energy by isoconversion methods. Thermochim. Acta 1992, 203, 159–165. [Google Scholar] [CrossRef]
- Mandapati, R.N.; Ghodke, P.K. Kinetics of pyrolysis of cotton stalk using model-fitting and model-free methods. Fuel 2021, 303, 121285. [Google Scholar] [CrossRef]
- Hu, S.; Jess, A.; Xu, M. Kinetic study of Chinese biomass slow pyrolysis: Comparison of different kinetic models. Fuel 2007, 86, 2778–2788. [Google Scholar] [CrossRef]
- Hilten, R.; Vandenbrink, J.; Paterson, A.; Feltus, F.; Das, K. Linking isoconversional pyrolysis kinetics to compositional characteristics for multiple Sorghum bicolor genotypes. Thermochim. Acta 2013, 577, 46–52. [Google Scholar] [CrossRef]
- Müsellim, E.; Tahir, M.H.; Ahmad, M.S.; Ceylan, S. Thermokinetic and TG/DSC-FTIR study of pea waste biomass pyrolysis. Appl. Therm. Eng. 2018, 137, 54–61. [Google Scholar] [CrossRef]
- Sharma, A.; Mohantya, B. Thermal degradation of mango (Mangifera indica) wood sawdust in a nitrogen environment: Characterization, kinetics, reaction mechanism, and thermodynamic analysis. RSC Adv. 2021, 11, 13396–13408. [Google Scholar] [CrossRef]
- Phuakpunk, K.; Chalermsinsuwan, B.; Assabumrungrat, S. Pyrolysis kinetic parameters investigation of single and tri-component biomass: Models fitting via comparative model-free methods. Renew. Energy 2022, 182, 494–507. [Google Scholar] [CrossRef]
- Colpani, D.; Santos, V.O.; Araujo, R.O.; Lima, V.M.R.; Tenório, J.A.S.; Coleti, J.; Chaar, J.S.; de Souza, L.K.C. Bioenergy po-tential analysis of Brazil nut biomass residues through pyrolysis: Gas emission, kinetics, and thermodynamic parameters. Clean. Chem. Eng. 2022, 1, 100002. [Google Scholar]
- Poletto, M.; Zattera, A.J.; Santana, R.M. Thermal decomposition of wood: Kinetics and degradation mechanisms. Bioresour. Technol. 2012, 126, 7–12. [Google Scholar] [CrossRef]
- Mallick, D.; Poddar, M.K.; Mahanta, P.; Moholkar, V.S. Discernment of synergism in pyrolysis of biomass blends using ther-mogravimetric analysis. Bioresour. Technol. 2018, 261, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Vikraman, V.K.; Boopathi, G.; Kumar, D.P.; Mythili, R.; Subramanian, P. Non-isothermal pyrolytic kinetics of milk dust powder using thermogravimetric analysis. Renew. Energy 2021, 180, 838–849. [Google Scholar] [CrossRef]
- Kumar, M.; Mishra, P.K.; Upadhyay, S.N. Thermal degradation of rice husk: Effect of pre-treatment on kinetic and thermo-dynamic parameters. Fuel 2020, 268, 117164. [Google Scholar] [CrossRef]
- Postawa, K.; Fałtynowicz, H.; Szczygieł, J.; Beran, E.; Kułażyński, M. Analyzing the kinetics of waste plant biomass pyrolysis via thermogravimetry modeling and semi-statistical methods. Bioresour. Technol. 2022, 344, 126181. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Yang, W.; Cong, X.; Dong, K.; Xu, J.; Wang, D.; Yang, X. Thermogravimetric analysis and reaction kinetics of ligno-cellulosic biomass pyrolysis. Energy 2020, 201, 117537. [Google Scholar] [CrossRef]
- Maia, A.A.D.; De Morais, L.C. Kinetic parameters of red pepper waste as biomass to solid biofuel. Bioresour. Technol. 2016, 204, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.; Kumar, S.; Mohanty, K. Kinetic and thermodynamic analysis of Putranjiva roxburghii (putranjiva) and Cassia fistula (amaltas) non-edible oilseeds using thermogravimetric analyzer. Renew. Energy 2021, 165, 261–277. [Google Scholar] [CrossRef]
- Hu, L.; Wei, X.-Y.; Guo, X.-H.; Lv, H.-P.; Wang, G.-H. Investigation on the kinetic behavior, thermodynamic and volatile products analysis of chili straw waste pyrolysis. J. Environ. Chem. Eng. 2021, 9, 105859. [Google Scholar] [CrossRef]
- Mishra, A.; Kumari, U.; Turlapati, V.Y.; Siddiqi, H.; Meikap, B. Extensive thermogravimetric and thermo-kinetic study of waste motor oil based on iso-conversional methods. Energy Convers. Manag. 2020, 221, 113194. [Google Scholar] [CrossRef]
- Simões, L.M.S.; Setter, C.; Sousa, N.G.; Cardoso, C.R.; de Oliveira, T.J.P. Biomass to biofuel densification of coconut fibers: Kinetic triplet and thermodynamic evaluation. Biomass Conv. Bioref. 2022, 12. [Google Scholar] [CrossRef]
- Singh, S.; Chakraborty, J.P.; Mondal, M.K. Intrinsic kinetics, thermodynamic parameters and reaction mechanism of non-isothermal degradation of torrefied Acacia nilotica using isoconversional methods. Fuel 2020, 259, 116263. [Google Scholar] [CrossRef]
- Muigai, H.H.; Choudhury, B.J.; Kalita, P.; Moholkar, V.S. Co–pyrolysis of biomass blends: Characterization, kinetic and thermodynamic analysis. Biomass Bioenergy 2020, 143, 105839. [Google Scholar] [CrossRef]




| Analysis | Values |
|---|---|
| Proximate (wt.%)—based on air-dried basis: | |
| Moisture | 7.42 ± 0.02 |
| Volatile matter | 74.65 ± 0.30 |
| Ash | 8.76 ± 0.01 |
| Fixed carbon | 9.17 ± 0.06 |
| HHV, MJ/kg | 17.87 |
| Ultimate (wt.%)—based on dry basis: | |
| Carbon | 41.83 ± 0.26 |
| Hydrogen | 6.81 ± 0.08 |
| Nitrogen | 4.71 ± 0.13 |
| Oxygen | 37.89 ± 0.17 |
| N° | Area, % | Name | Formula | Mw, g/mol |
|---|---|---|---|---|
| 1 | 12.36 | Tetratetracontane | C44H90 | 619.8 |
| 2 | 9.70 | Tetracontane | C40H82 | 563.1 |
| 3 | 8.20 | 1-Octacosanol | C28H58O | 410.8 |
| 4 | 5.44 | 2,6,10,14,18,22-Tetracosahexaene, 2,6,10,15,19,23-hexamethyl-, (all-E)- | C30H50 | 410.7 |
| 5 | 2.19 | Octacosanoic acid, methyl ester | C29H58O2 | 438.8 |
| 6 | 2.00 | Phenol | C6H5OH | 94.11 |
| 7 | 1.80 | Pentadecane | C15H32 | 212.41 |
| 8 | 1.50 | Triacontanoic acid, methyl ester | C31H62O2 | 466.82 |
| 9 | 1.43 | Tetracosane | C24H50 | 338.7 |
| 10 | 1.40 | Phenol, 2-methoxy- | C7H8O2 | 124.12 |
| 11 | 1.24 | Pyridine, 3-methyl- | C6H7N | 93.13 |
| 12 | 1.11 | Octadecane | C18H38 | 254.49 |
| Component | CO2 | CO | CH4 | C2H6 | CxHy | C2H4 | H2 |
|---|---|---|---|---|---|---|---|
| Concentration, % | 47.30 | 47.14 | 3.51 | 1.17 | 0.75 | 0.12 | 0.01 |
| Analysis | Biomass | ||||
|---|---|---|---|---|---|
| AIW | Maize Stalk [58] | Lantana Camara [58] | Pine Needles [58] | Black Gram [58] | |
| Proximate (wt.%) | |||||
| Volatile matter | 21.34 ± 0.03 | 20.67 | 22.56 | 27.62 | 23.56 |
| Ash | 20.49 ± 0.01 | 19.7 | 15.7 | 13.5 | 23.3 |
| Moisture | 4.4 ± 0.19 | 11.5 | 6.13 | 8.05 | 12.41 |
| Fixed carbon | 53.77 ± 0.0,9 | 48.13 | 55.61 | 50.83 | 40.73 |
| HHV *, MJ/kg | 20.92 | 23.7 | 25.87 | 22.33 | 21.06 |
| Ultimate (wt.%) | |||||
| carbon | 56.56 ± 0.17 | 61.9 | 70.5 | 65.8 | 56.7 |
| hydrogen | 3.09 ± 0.05 | 3.56 | 2.69 | 2.13 | 3.14 |
| nitrogen | 4.12 ± 0.01 | 1.17 | 0.86 | 0.78 | 1.24 |
| oxygen | 15.75 ± 0.14 | 13.67 | 10.25 | 17.79 | 15.62 |
| N° | Pyrolysis Stage | Heating Rate (°C/min) | Starting Temperature (°C) | Ending Temperature (°C) | Temperature Peak (°C) |
|---|---|---|---|---|---|
| I | Moisture evaporation | 10 15 20 | 40 40 40 | 191.26 190.76 191.77 | 103.1 115.4 126.7 |
| II | Devolatilization | 10 15 20 | 191.26 190.76 191.77 | 529.5 544.48 558.95 | 317.7 322.6 328.5 |
| III | Degradation of char and minerals | 10 15 20 | 529.5 544.48 558.95 | 1000 1000 1000 | 668.2 685.6 690.6 |
| Heating Rate (°C/min) | Mass Loss, wt.% | Residual Mass, wt.% | ||
|---|---|---|---|---|
| Moisture Evaporation | Devolatilization | Degradation of Char and Minerals | ||
| 10 | 9.14 | 58.65 | 5.79 | 26.24 |
| 15 | 9.33 | 59.17 | 5.89 | 25.61 |
| 20 | 9.28 | 61.08 | 4.88 | 24.76 |
| Average, % | 9.25 | 59.63 | 5.52 | 25.54 |
| α | Friedman | KAS | OFW | |||
|---|---|---|---|---|---|---|
| Eα (kJ/mol) | Log A (1/s) | Eα (kJ/mol) | Log A (1/s) | Eα (kJ/mol) | Log A (1/s) | |
| 0.1 | 164.48 | 13.22 | 185.27 | 15.72 | 185.42 | 15.68 |
| 0.2 | 152.52 | 11.44 | 156.78 | 12.26 | 157.00 | 12.23 |
| 0.3 | 184.77 | 14.16 | 167.31 | 12.93 | 167.50 | 12.90 |
| 0.4 | 212.88 | 16.47 | 189.57 | 14.76 | 189.74 | 14.72 |
| 0.5 | 231.03 | 17.80 | 209.08 | 16.31 | 209.23 | 16.26 |
| 0.6 | 250.60 | 19.10 | 225.76 | 17.52 | 225.91 | 17.48 |
| 0.7 | 291.94 | 21.85 | 253.73 | 19.48 | 253.88 | 19.44 |
| 0.8 | 244.29 | 16.61 | 265.25 | 19.30 | 265.43 | 19.26 |
| 0.9 | 213.02 | 13.17 | 223.21 | 14.75 | 223.43 | 14.70 |
| Average | 216.17 | 15.98 | 208.44 | 15.89 | 208.61 | 15.85 |
| Fuel | Heating Rate (K/min) | Used Methods | Activation Energy (kJ/mol) | Reference |
|---|---|---|---|---|
| AIW | 10, 15, and 20 | Friedman, KAS, OFW | 216.17 208.44 208.61 | Present Study |
| Cotton stalk | 10–40 | KAS, OFW | 223–230 213–240 | [82] |
| Sugarcane leaves | 5–40 | Friedman, KAS, OFW | 239.58 226.75 226.97 | [21] |
| Prosopis juliflora fuelwood | 2–25 | Friedman, KAS, OFW | 219.3 204.0 203.2 | [22] |
| Phyllanthus emblica seeds | 10–50 | Friedman, KAS, OFW | 189.95 184.77 195.10 | [23] |
| Camphor branch | 2.5, 5, and 10 | Ozawa | 190 | [83] |
| Microalgae Chlorella vulgaris | 10–40 | Kissinger, Friedman, OFW, KAS, Vyazovkin, DAEM | 135.6–337.1 | [24] |
| Digested biomass wastes | 10, 15, and 20 | Friedman, KAS | 202.55 202.21 | [75] |
| Sorghum bicolor | 2, 5, and 8 | Friedman and KAS | 226.6 | [84] |
| Pea waste | 10–40 | KAS, OFW | 212.71 211.55 | [85] |
| Basswood waste | 20–40 | KAS, OFW | 197.2 207.9 | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaeva, J.; Timofeeva, S.; Islamova, S.; Bulygina, K.; Aliev, F.; Panchenko, V.; Bolshev, V. Pyrolysis of Amaranth Inflorescence Wastes: Bioenergy Potential, Biochar and Hydrocarbon Rich Bio-Oil Production. Agriculture 2023, 13, 260. https://doi.org/10.3390/agriculture13020260
Karaeva J, Timofeeva S, Islamova S, Bulygina K, Aliev F, Panchenko V, Bolshev V. Pyrolysis of Amaranth Inflorescence Wastes: Bioenergy Potential, Biochar and Hydrocarbon Rich Bio-Oil Production. Agriculture. 2023; 13(2):260. https://doi.org/10.3390/agriculture13020260
Chicago/Turabian StyleKaraeva, Julia, Svetlana Timofeeva, Svetlana Islamova, Kseny Bulygina, Firdavs Aliev, Vladimir Panchenko, and Vadim Bolshev. 2023. "Pyrolysis of Amaranth Inflorescence Wastes: Bioenergy Potential, Biochar and Hydrocarbon Rich Bio-Oil Production" Agriculture 13, no. 2: 260. https://doi.org/10.3390/agriculture13020260
APA StyleKaraeva, J., Timofeeva, S., Islamova, S., Bulygina, K., Aliev, F., Panchenko, V., & Bolshev, V. (2023). Pyrolysis of Amaranth Inflorescence Wastes: Bioenergy Potential, Biochar and Hydrocarbon Rich Bio-Oil Production. Agriculture, 13(2), 260. https://doi.org/10.3390/agriculture13020260









