Changes in Soil Organic Matter and Biological Parameters as a Result of Long-Term Strip-Till Cultivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Design
- –
- conventional tillage, mouldboard plough (CT);
- –
- reduced tillage, ploughless cultivation (RT);
- –
- strip cultivation, strip-till one-pass (ST-OP).
2.2. Methods
2.2.1. Content and Fractional Composition of Organic Matter
2.2.2. Microbial Analyses
2.2.3. Activity of Enzymes in the Soil
- –
- The activity of dehydrogenases (DEHs) (EC 1.1.1) was determined by the method of Thalmann [50];
- –
- Catalase activity (CAT) (EC 1.11.1.6) was determined by the method of Johnson and Temple [51];
- –
- Alkaline (AlP) (EC 3.1.3.1) and acid (AcP) (3.1.3.2) activity was determined by the method of Tabatabai and Bremner [52].
- –
- enzymatic pH indicator [53]:
- –
- geometric mean GMea [54]:
- –
- biological index of fertility BIF [55]:where: k is a factor proportionality equal to 0.01.
- –
- to assess the total level of enzyme activity (TEI), the following was calculated [56]:where Xi is the activity of soil enzyme i and is the mean activity of enzyme i in all samples.
- –
- biochemical soil activity (BA12) [57] was proposed based on the activities of soil enzymes and the content of the total organic carbon:
2.3. Statistical Analyses
3. Results and Discussion
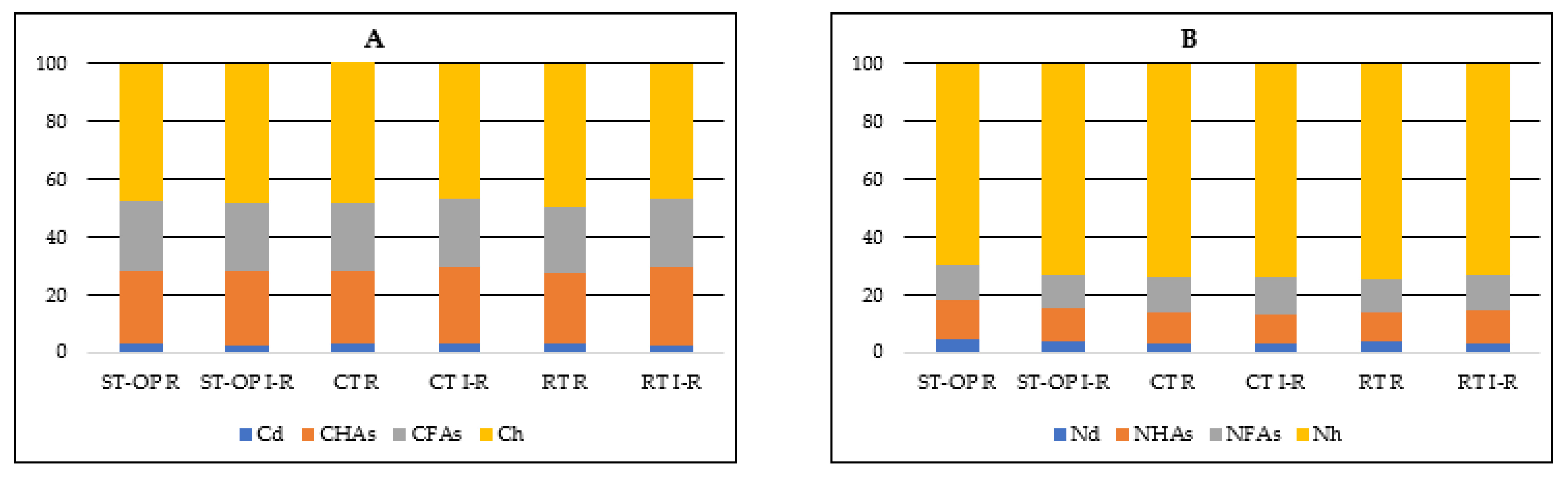
3.1. Content and Fractional Composition of Organic Matter
3.2. Occurrence of Soil Microorganisms
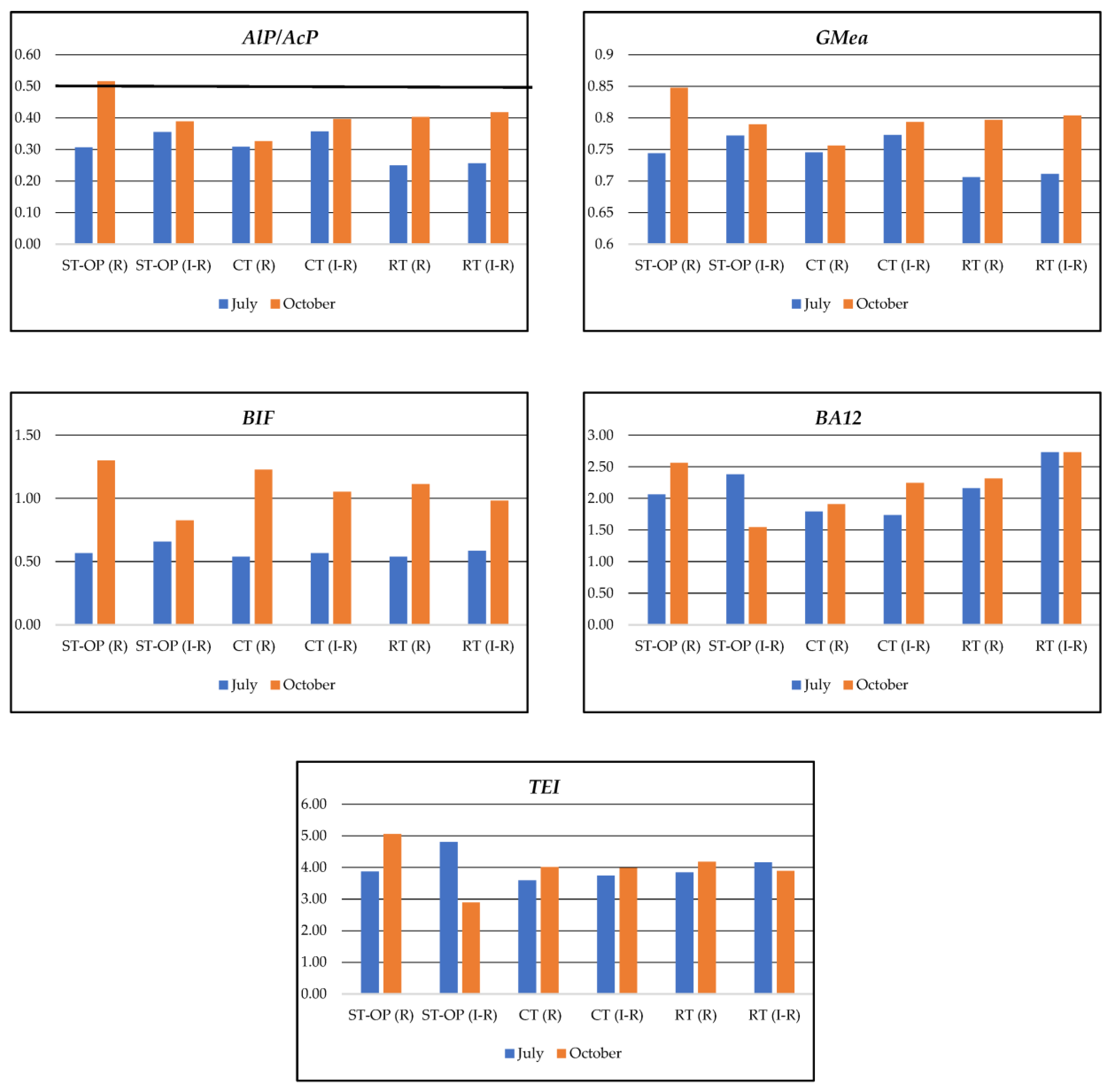
3.3. Activity of Enzymes in the Soil
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassan, W.; Li, Y.; Saba, T.; Jabbi, F.; Wang, B.; Cai, A.; Wu, J. Improved and sustainable agroecosystem, food security and environmental resilience through zero tillage with emphasis on soils of temperate and subtropical climate regions: A review. Int. Soil Water Conserv. Res. 2022, 10, 530–545. [Google Scholar] [CrossRef]
- Swędzyńska, D.; Małecka, I.; Blecharczyk, A.; Swędrzyńskki, A.; Starzyk, J. Effects of various long-term tillage systems on some chemical and biological properties of soil. Pol. J. Environ. Stud. 2013, 22, 1835–1844. [Google Scholar]
- Salinas-Garcia, J.R.; Velázquez-García, J.d.J.; Gallardo-Valdez, M.; Díaz-Mederos, P.; Caballero-Hernández, F.; Tapia-Vargas, L.M.; Rosales-Robles, E. Tillage effects on microbial biomass and nutrient distribution in soils under rain-fed corn production in central-western Mexico. Soil Tillage Res. 2002, 66, 143–152. [Google Scholar] [CrossRef]
- Reicosky, D.C. Conservation tillage is not conservation agriculture. J. Soil Water Conserv. 2015, 70, 103A–108A. [Google Scholar] [CrossRef]
- Kassam, A.; Friedrich, T.; Derpsch, R. Global spread of conservation agriculture. Int. J. Environ. Stud. 2019, 76, 29–51. [Google Scholar] [CrossRef]
- Wittwer, R.A.; Bender, S.F.; Hartman, K.; Hydbom, S.; Lima, R.A.A.; Loaiza, V.; Nemecek, T.; Oehl, F.; Olsson, P.A.; Petchey, O.; et al. Organic and conservation agriculture promote ecosystem multifunctionality. Sci. Adv. 2021, 7, eabg6995. [Google Scholar] [CrossRef]
- Morris, N.L.; Miller, P.C.H.; Orson, J.H.; Froud-Williams, R.J. The adoption of noninversion tillage systems in the United Kingdom and the agronomic impact on soil, crops and the environment—A review. Soil Tillage Res. 2010, 108, 1–15. [Google Scholar] [CrossRef]
- Jaskulska, I.; Jaskulski, D.; Różniak, M.; Radziemska, M.; Gałęzewski, L. Zonal tillage as innovative element of the technology of growing winter wheat: A field experiment under low rainfall conditions. Agriculture 2020, 10, 105. [Google Scholar] [CrossRef]
- Li, K.; Hao, Z.; Chen, L.; Sha, Y.; Wang, E.; Sui, X.; Mi, G. Conservation strip-till modifies rhizosphere ammonia-oxidizing archaea and bacteria, increases nitrate accumulation and promotes maize growth at grain filling stage. Soil Tillage Res. 2023, 234, 105821. [Google Scholar] [CrossRef]
- Morrison, J.E. Strip tillage for “no-till” row crop production. Appl. Eng. Agric. 2002, 18, 277–284. [Google Scholar] [CrossRef]
- Townsend, T.J.; Ramsden, S.J.; Wilson, P. How do we cultivate in England? Tillage practices in crop production systems. Soil Use Manag. 2016, 32, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Jaskulska, I.; Jaskulski, D. Strip-Till One-Pass Technology in Central and Eastern Europe: A MZURI Pro-Til Hybrid Machine Case Study. Agronomy 2020, 10, 925. [Google Scholar] [CrossRef]
- Różewicz, M. Review of current knowledge on strip-till cultivation and possibilities of its popularization in Poland. Pol. J. Agron. 2022, 49, 20–30. [Google Scholar] [CrossRef]
- Tabatabaeekoloor, R. Soil characteristics at the in-row and inter-row zones after strip-tillage. Afr. J. Agric. Res. 2011, 6, 6598–6603. [Google Scholar] [CrossRef]
- Williams, A.; Davis, A.S.; Ewing, P.M.; Grandy, A.S.; Kane, D.A.; Koide, R.T.; Mortensen, D.A.; Smith, R.G.; Snapp, S.S.; Spokas, K.A.; et al. A comparison of soil hydrothermal properties in zonal and uniform tillage systems across the US Corn Belt. Geoderma 2016, 273, 12–19. [Google Scholar] [CrossRef]
- Campbell, C.A.; McConkey, B.G.; Zentner, R.P.; Selles, F.; Curtin, D. Long-term effects of tillage and crop rotations on soil organic C and total N in a clay soil in southwestern Saskatchewan. Can. J. Soil Sci. 1996, 76, 395–401. [Google Scholar] [CrossRef]
- Islam, A.K.M.S.; Saleque, M.A.; Hossain, M.M.; Islam, A.K.M.A. Effect of conservation tillage on soil chemical properties in rice-maize cropping system. Agriculturists 2015, 13, 62–73. [Google Scholar] [CrossRef]
- Strickland, T.C.; Scully, B.T.; Hubbard, R.K.; Sullivan, D.G.; Abdo, Z.; Savabi, M.R.; Lee, R.D.; Olson, D.M.; Hawkins, G.L. Effect of conservation practices on soil carbon and nitrogen accretion and crop yield in a corn production system in the southeastern coastal plain, United States. J. Soil Water Conserv. 2015, 70, 170–181. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Hedlund, K.; Jackson, L.E.; Kätterer, T.; Lugato, E.; Thomsen, I.K.; Jørgensen, H.B.; Isberg, P.-E. How does tillage intensity affect soil organic carbon? A systematic review protocol. Environ. Evid. 2017, 6, 30. [Google Scholar] [CrossRef]
- Si, P.; Liu, E.; He, W.; Sun, Z.; Dong, W.; Yan, C.; Zhang, Y. Effect of no-tillage with straw mulch and conventional tillage on soil organic carbon pools in Northern China. Arch. Agron. Soil Sci. 2018, 64, 398–408. [Google Scholar] [CrossRef]
- Bhardwaj, A.K.; Jasrotia, P.; Hamilton, S.K.; Robertson, G.P. Ecological management of intensively cropped agro-ecosystems improves soil quality with sustained productivity. Agric. Ecosyst. Environ. 2011, 140, 419–429. [Google Scholar] [CrossRef]
- Scharlemann, J.P.W.; Tanner, E.V.J.; Hiederer, R.; Kapos, V. Global soil carbon: Understanding and managing the largest terrestrial carbon pool. Carbon Manag. 2014, 5, 81–91. [Google Scholar] [CrossRef]
- Mayer, M.; Prescott, C.E.; Abaker, W.E.A.; Augusto, L.; Cécillon, L.; Ferreira, G.W.D.; James, J.; Jandl, R.; Katzensteiner, K.; Laclau, J.-P.; et al. Tamm Review: Influence of forest management activities on soil organic carbon stocks: A knowledge synthesis. For. Ecol. Manag. 2020, 466, 118127. [Google Scholar] [CrossRef]
- Stockmann, U.; Adams, M.A.; Crawford, J.W.; Field, D.J.; Henakaarchchi, N.; Jenkins, M.; Minasny, B.; McBratney, A.B.; Courcelles, V.R.; Singh, K.; et al. The knowns, known unknowns and unknowns of sequestration of soil organic carbon. Agr. Ecosyst. Environ. 2013, 164, 80–99. [Google Scholar] [CrossRef]
- Yang, Z.H.; Singh, B.R.; Sitaula, B.K. Soil organic carbon fractions under different land uses in Mardi Watershed of Nepal. Commun. Soil Sci. Plant Anal. 2006, 35, 615–629. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, Y.; Li, J.; Zhang, J.; He, N. Soil organic carbon contents, aggregate stability, and humic acid composition in different alpine grasslands in Qinghai-Tibet Plateau. J. Mt. Sci. 2016, 13, 2015–2027. [Google Scholar] [CrossRef]
- Chantigny, M.H. Dissolved and water-extractable organic matter in soils: A review on the influence of land use and management practice. Geoderma 2003, 113, 357–380. [Google Scholar] [CrossRef]
- Kalbitz, K.; Geyer, S. Different effects of peat degradation on dissolved organic carbon and nitrogen. Org. Geochem. 2002, 33, 319–326. [Google Scholar] [CrossRef]
- Debska, B.; Drag, M.; Tobiasova, E. Effect of post-harvest residue of maize, rapeseed, and unflower on humic acids properties in various soils. Pol. J. Environ. Stud. 2012, 21, 603–613. [Google Scholar]
- Debska, B.; Dlugosz, J.; Piotrowska-Dlugosz, A.; Banach-Szott, M. The impact of a bio- fertilizer on the soil organic matter status and carbon sequestration—Results from a field-scale study. J. Soils Sediments 2016, 16, 2335–2343. [Google Scholar] [CrossRef]
- Debska, B.; Jaskulska, I.; Jaskulski, D. Method of tillage with the factor determining the quality of organic matter. Agronomy 2020, 10, 1250. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Zhang, Q.; Zhu, H.; Reich, P.B.; Banerjee, S.; van der Heijden, M.G.; Sadowsky, M.J.; Ishii, S.; Jia, X.; Shao, M.; et al. Erosion reduces soil microbial diversity, network complexity and multifunctionality. ISME J. 2021, 15, 2474–2489. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Walder, F.; Büchi, L.; Meyer, M.; Held, A.Y.; Gattinger, A.; Keller, T.; Charles, R.; van der Heijden, M.G.A. Agricultural intensification reduces microbial network complexity and the abundance of keystone taxa in roots. ISME J. 2019, 13, 1722–1736. [Google Scholar] [CrossRef] [PubMed]
- Laufer, D.; Loibl, B.; Märländer, B.; Koch, H.J. Soil erosion and surface runoff under strip tillage for sugar beet (Beta vulgaris L.) in Central Europe. Soil Tillage Res. 2016, 162, 1–7. [Google Scholar] [CrossRef]
- Schmidt, R.; Gravuer, K.; Bossange, A.V.; Mitchell, J.; Scow, K. Long-term use of cover crops and no-till shift soil microbial community life strategies in agricultural soil. PLoS ONE 2018, 13, e0192953. [Google Scholar] [CrossRef]
- Martínez-García, L.B.; Korthals, G.; Brussaard, L.; Jørgensen, H.B.; De Deyn, G.B. Organic management and cover crop species steer soil microbial community structure and functionality along with soil organic matter properties. Agric. Ecosyst. Environ. 2018, 263, 7–17. [Google Scholar] [CrossRef]
- Lemanowicz, J.; Bartkowiak, A.; Lamparski, R.; Wojewódzki, P.; Pobereżny, J.; Wszelaczyńska, E.; Szczepanek, M. Physicochemical and enzymatic soil properties influenced by cropping of primary wheat under organic and conventional farming systems. Agronomy 2020, 10, 1652. [Google Scholar] [CrossRef]
- Lemanowicz, J.; Bartkowiak, A.; Zielińska, A.; Jaskulska, I.; Rydlewska, M.; Klunek, K.; Polkowska, M. The Effect of Enzyme activity on carbon sequestration and the cycle of available macro- (P, K, Mg) and microelements (Zn, Cu) in Phaeozems. Agriculture 2023, 13, 172. [Google Scholar] [CrossRef]
- Niewiadomska, A.; Gaj, R.; Przybył, J.; Budka, A.; Mioduszewska, N.; Wolna-Maruwka, A. Analysis of microbial parameters of soil in different tillage systems under sugar beets (Beta vulgaris L.). Pol. J. Environ. Stud. 2016, 25, 1803–1812. [Google Scholar] [CrossRef] [PubMed]
- Niewiadomska, A.; Majchrzak, L.; Borowiak, K.; Wolna-Maruwka, A.; Waraczewska, Z.; Budka, A.; Gaj, R. The influence of tillage and cover cropping on soil microbial parameters and spring wheat physiology. Agronomy 2020, 10, 200. [Google Scholar] [CrossRef]
- Bielińska, J.; Mocek-Płóciniak, A. Impact of the tillage system on the soil enzymatic activity. Arch. Environ. Prot. 2012, 38, 75–82. [Google Scholar] [CrossRef]
- Pérez-Brandán, C.; Huidobro, J.; Galván, M.; Vargas-Gil, S.; Meriles, J.M. Relationship between microbial functions and community structure following agricultural intensification in South American Chaco. Plant Soil Environ. 2016, 62, 321–328. [Google Scholar] [CrossRef]
- Sharma, K.L.; Mandal, U.K.; Srinivas, K.; Vittal, K.P.R.; Mandal, B.; Grace, J.K.; Ramesh, V. Long-term soil management effects on crop yields and soil quality in a dryland Alfisol. Soil Tillage Res. 2005, 83, 246–259. [Google Scholar] [CrossRef]
- Jaskulska, I.; Romaneckas, K.; Jaskulski, D.; Gałęzewski, L.; Breza-Boruta, B.; Dębska, B.; Lemanowicz, J. Soil Properties after Eight Years of the Use of Strip-Till One-Pass Technology. Agronomy 2020, 10, 1596. [Google Scholar] [CrossRef]
- Atlas, R.M. Handbook of Microbiological Media; CRC Press: Boca Raton, FL, USA, 2010; ISBN 978-0-429-13049-6. [Google Scholar]
- Crawford, D.L.; Lynch, J.M.; Whipps, J.M.; Ousley, M.A. Isolation and Characterization of Actinomycete Antagonists of a Fungal Root Pathogen. Appl. Environ. Microbiol. 1993, 59, 3899–3905. [Google Scholar] [CrossRef]
- Gupta, P.; Samant, K.; Sahu, A. Isolation of cellulose-degrading bacteria and determination of their cellulolytic potential. Int. J. Microbiol. 2012, 2012, 578925. [Google Scholar] [CrossRef]
- Thalmann, A. Zur Methodik der Bestimung der Dehydrogenaseaktivität im Boden mittels Triphenyltetrazoliumchlorid (TTC). Landwirtsch. Forsch. 1968, 21, 249–258. [Google Scholar]
- Johnson, J.I.; Temple, K.I. Some variables affecting the measurements of catalase activity in soil. Soil Sci. Soc. Am. 1964, 28, 207–209. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p–nitrophenol phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Dick, W.A.; Cheng, L.; Wang, P. Soil acid alkaline phosphatase activity as pH adjustment indicators. Soil Biol. Biochem. 2000, 32, 1915–1919. [Google Scholar] [CrossRef]
- Hinojosa, M.B.; Garcia-Ruiz, R.; Viñegla, B.; Carreira, J.A. Microbiological rates and enzyme activities as indicators of functionality in soils affected by the Aznalcóllar toxic spill. Soil Biol. Biochem. 2004, 36, 1637–1644. [Google Scholar] [CrossRef]
- Stefanic, F.; Ellade, G.; Chirnageanu, J. Researches concerning a biological index of soil fertility. In Proceeding of the Fifth Symposium of Soil Biology; Nemes, M.P., Kiss, S., Papacostea, P., Stefanic, C., Rusan, M., Eds.; Romanian National Society of Soil Science: Bucharest, Romania, 1984; pp. 35–45. [Google Scholar]
- Tan, X.; Xie, B.; Wang, J.; He, W.; Wang, X.; Wei, G. County-scale spatial distribution of soil enzyme activities and enzyme activity indices in agricultural land: Implications for soil quality assessment. Sci. World J. 2014, 2014, 535768. [Google Scholar] [CrossRef] [PubMed]
- Wyszkowska, J.; Borowik, A.; Kucharski, M.; Kucharski, J. Applicability of biochemical indices to quality assessment of soil polluted with heavy metals. J. Elemen. 2013, 18, 733–756. [Google Scholar] [CrossRef]
- Ward, J.H. Hierarchical grouping to optimize an objective function. J. Am. Stat. Ass. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Geisseler, D.; Horwath, W.R. Relationship between carbon and nitrogen availability and extracellular enzyme activities in soil. Pedobiologia 2009, 53, 87–98. [Google Scholar] [CrossRef]
- Debska, B.; Kotwica, K.; Banach-Szott, M.; Spychaj-Fabisiak, E.; Tobiašová, E. Soil fertility improvement and carbon sequestration through exogenous organic matter and biostimulant application. Agriculture 2022, 12, 1478. [Google Scholar] [CrossRef]
- Małecka, I.; Blecharczyk, A.; Dobrzeniecki, T. The productivity and environmental consequences of long-term ploughless tillage systems in field pea. Fragm. Agron. 2009, 26, 118–127. [Google Scholar]
- Jaskulska, I.; Romaneckas, K.; Jaskulski, D.; Wojewódzki, P. A Strip-till one-pass system as a component of conservation agriculture. Agronomy 2020, 10, 2015. [Google Scholar] [CrossRef]
- Rożewicz, M.; Grabiński, J.; Wyzińska, M. Effect of the strip-till method on the yield and quality of winter wheat grain depending on the cultivar and scope of post-harvest cultivation. Part I. Yield. Agron. Sci. 2023, 78, 21–28. [Google Scholar] [CrossRef]
- Breza Boruta, B.; Paluszak, Z. The antagonistic activity of actinomycetes of Streptomyces genus in relation to Trichoderma koningii. J. Ecol. Eng. 2016, 17, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Pengproh, R.; Thanyasiriwat, T.; Sangdee, K.; Kawicha, P.; Sangdee, A. Antagonistic ability and genome mining of soil Streptomyces spp. against Fusarium oxysporum f. sp. lycopersici. Eur. J. Plant Pathol. 2023, 167, 251–270. [Google Scholar] [CrossRef]
- Ghosh, P.K.; Das, A.; Saha, R.; Kharkrang, E.; Tripathy, A.K.; Mundu, G.C.; Ngachan, S.V. Conservation agriculture towards achieving food security in noth east India. Curr. Sci. 2010, 99, 915–921. [Google Scholar]
- Godsey, C.; Kochenower, R.; Taylor, R. Strip-till considerations in Oklahoma. Okla. Coop. Ext. Serv. 2017, 2, PSS-2134. [Google Scholar]
- Lv, L.; Gao, Z.; Liao, K.; Zhu, Q.; Zhu, J. Impact of conservation tillage on the distribution of soil nutrients with depth. Soil Tillage Res. 2023, 225, 105527. [Google Scholar] [CrossRef]
- Wen, L.; Peng, Y.; Zhou, Y.; Cai, G.; Lin, Y.; Li, B. Effects of conservation tillage on soil enzyme activities of global cultivated land: A meta-analysis. J. Environ. Manag. 2023, 345, 118904. [Google Scholar] [CrossRef] [PubMed]
- Holatko, J.; Hammerschmiedt, T.; Kintl, A.; Kucerik, J.; Malicek, O.; Latal, O.; Baltazar, T.; Brtnicky, M. Effects of strip-till and simultaneous fertilization at three soil depths on soil biochemical and biological properties. Agronomy 2022, 12, 2597. [Google Scholar] [CrossRef]
- Meena, A.; Rao, K.S. Assessment of soil microbial and enzyme activity in the rhizosphere zone under different land use/cover of a semiarid region, India. Ecol. Process. 2021, 10, 16. [Google Scholar] [CrossRef]
- He, L.; Lu, S.; Wang, C.; Mu, J.; Zhang, Y.; Wang, X. Changes in soil organic carbon fractions and enzyme activities in response to tillage practices in the Loess Plateau of China. Soil Tillage Res. 2021, 209, 104940. [Google Scholar] [CrossRef]
- Zuber, S.M.; Villamil, M.B. Meta-analysis approach to assess effect of tillage on microbial biomass and enzyme activities. Soil Biol. Biochem. 2016, 97, 176–187. [Google Scholar] [CrossRef]
- Piazza, G.; Ercoli, L.; Nuti, M.; Pellegrino, E. Interaction between conservation tillage and nitrogen fertilization shapes prokaryotic and fungal diversity at different soil depths: Evidence from a 23-year field experiment in the mediterranean area. Front. Microbiol. 2019, 10, 2047. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, K.; Heidari, G.; Nezhad, M.T.K.; Ghamari, S.; Sohrabi, Y. Contrasting soil microbial responses to fertilization and tillage systems in canola rhizosphere. Saudi J. Biol. Sci. 2012, 19, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Davis, A.S.; Jilling, A.; Grandy, A.S.; Koide, R.T.; Mortensen, D.A.; Smith, R.G.; Snapp, S.S.; Spokas, K.A.; Yannarell, A.C.; et al. Reconciling opposing soil processes in row-crop agroecosystems via soil functional zone management. Agric. Ecosyst. Environ. 2017, 236, 99–107. [Google Scholar] [CrossRef]
- Si, P.; Qiao, X.S. Effect of clean and sod cultivation on soil enzyme activity in sandy vineyard. J. Fruit Sci. 2014, 31, 238–244. [Google Scholar]
- Williams, A.; Kane, D.A.; Ewing, P.M.; Atwood, L.W.; Jilling, A.; Li, M.; Lou, Y.; Davis, A.S.; Grandy, A.S.; Huerd, S.C.; et al. Soil functional zone management: A vehicle for enhancing production and soil ecosystem services in row-crop agroecosystems. Front. Plant Sci. 2016, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, J.L.; Allmaras, R.R.; Rehm, G.W.; Lowery, B. Ridge tillage for corn and soybean production: Environmental quality impacts. Soil Tillage Res. 1998, 48, 145–154. [Google Scholar] [CrossRef]
- Datta, A.; Gujre, N.; Gupta, D.; Agnihotri, R.; Mitra, S. Application of enzymes as a diagnostic tool for soils as affected by municipal solid wastes. J. Environ. Managem. 2021, 286, 112169. [Google Scholar] [CrossRef]
- Dantas, G.; Sommer, M.O.A.; Oluwasegun, R.D.; Church, G.M. Bacterial subsisting on antibiotics. Science 2008, 320, 100–103. [Google Scholar] [CrossRef]
- Criquet, S.; Ferre, E.; Farnet, A.M.; Petit, J.L. Annual dynamics of phosphatase activities in an evergreen oak litter: Influence of biotic and abiotic factors. Soil Biol. Biochem. 2004, 36, 1111–1118. [Google Scholar] [CrossRef]
- Stone, M.M.; Weiss, M.S.; Goodale, C.L.; Adams, M.B.; Fernandez, I.J.; German, D.P.; Allison, S.D. Temperature sensitivity of soil enzyme kinetics under N-fertilization in two temperate forests. Glob. Chang. Biol. 2012, 18, 1173–1184. [Google Scholar] [CrossRef]
- Jing, X.; Wang, Y.; Chung, H.; Mi, Z.; Wang, S.; Zeng, H.; He, J.-S. No temperature acclimation of soil extracellular enzymes to experimental warming in an alpine grassland ecosystem on the Tibetan Plateau. Biogeochemistry 2014, 117, 39–54. [Google Scholar] [CrossRef]
- Qi, R.; Li, J.; Lin, Z.; Li, Z.; Li, Y.; Yang, X.; Zhang, J.; Zhao, B. Temperature effects on soil organic carbon, soil labile organic carbon fractions, and soil enzyme activities under long-term fertilization regimes. Appl. Soil Ecol. 2016, 102, 36–45. [Google Scholar] [CrossRef]
- Brzostek, E.R.; Adrien, C.F. Seasonal variation in the temperature sensitivity of proteolytic enzyme activity in temperate forest soils. J. Geophys. Res. Biogeosci. 2012, 117. [Google Scholar] [CrossRef]
- German, D.P.; Marcelo, K.R.B.; Stone, M.M.; Allison, S.D. The Michaelis–Menten kinetics of soil extracellular enzymes in response to temperature: A cross-latitudinal study. Glob. Chang. Biol. 2012, 18, 1468–1479. [Google Scholar] [CrossRef]
- Gil-Sotres, F.; Trasar-Cepeda, C.; Leiros, M.C.; Seoane, S. Different approaches to evaluating soil quality using biochemical properties. Soil Biol. Biochem. 2005, 37, 877–887. [Google Scholar] [CrossRef]
- Lemanowicz, J. Activity of selected enzymes as markers of ecotoxicity in technogenic salinization soils. Environ. Sci. Pollut. Res. 2019, 26, 13014–13024. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Gascó, G.; Gutierrez, B.; Mendez, A. Soil biochemical activities and geometric mean of enzyme activities after application of sewage sludge and sewage sludge biochar to soil. Biol. Fertil. Soils 2012, 48, 511–517. [Google Scholar] [CrossRef]
- Górski, R.; Rosa, R.; Niewiadomska, A.; Wolna-Maruwka, A.; Płaza, A. Innovative spring barley cultivation technology based on the use of microbial products together with living mulch in organic farming. Agronomy 2023, 13, 1914. [Google Scholar] [CrossRef]
- Mierzwa-Hersztek, M.; Gondek, K.; Klimkowicz-Pawlas, A.; Chmiel, M.J.; Dziedzic, K.; Taras, H. Assessment of soil quality after biochar application based on enzymatic activity and microbial composition. Int. Agrophys. 2019, 33, 331–336. [Google Scholar] [CrossRef]
- Picariello, E.; Baldantoni, D.; Muniategui-Lorenzo, S.; Concha-Granã, E.; De Nicola, F. A synthetic quality index to evaluate the functional stability of soil microbial communities after perturbations. Ecol. Indic. 2021, 128, 107844. [Google Scholar] [CrossRef]
- Wojewódzki, P.; Lemanowicz, J.; Debska, B.; Haddad, S.A.; Tobiasova, E. The application of biochar from waste biomass to improve soil fertility and soil enzyme activity and increase aarbon sequestration. Energies 2023, 16, 380. [Google Scholar] [CrossRef]
- Nurzhan, A.; Tian, H.; Nuralykyzy, B.; He, W. Soil enzyme activities and enzyme activity indices in long-term arsenic-contaminated soils. Eurasian Soil Sci. 2022, 55, 1425–1435. [Google Scholar] [CrossRef]
- García-Ruiz, R.; Ochoa, V.; Hinojosa, M.B.; Carreira, J.A. Suitability of enzyme activities for the monitoring of soil quality improvement in organic agricultural systems. Soil Biol. Biochem. 2008, 40, 2137–2145. [Google Scholar] [CrossRef]
- Bach, C.E.; Warnock, D.D.; Van Horn, D.J.; Weintraub, M.N.; Sinsabaugh, R.L.; Allison, S.D.; German, D.P. Measuring phenol oxidase and peroxidase activities with pyrogallol, l-DOPA, and ABTS: Effect of assay conditions and soil type. Soil Biol. Biochem. 2013, 67, 183–191. [Google Scholar] [CrossRef]
- Liu, C.W.; Lin, K.H.; Kuo, Y.M. Application of factor analysis in the assessment of groundwater quality in a blackfoot disease area in Taiwan. Sci. Total Environ. 2003, 313, 77–89. [Google Scholar] [CrossRef]


| Month | Year | 1981–2010 | Year | 1981–2010 | ||||
|---|---|---|---|---|---|---|---|---|
| 2018 | 2019 | 2020 | 2018 | 2019 | 2020 | |||
| Air Temperature (°C) | Precipitation (mm) | |||||||
| January | 0.8 | −0.7 | 2.6 | −1.8 | 46.3 | 32.6 | 37.7 | 26.8 |
| February | −3.2 | 2.6 | 3.6 | −0.9 | 5.8 | 18.1 | 36.0 | 20.7 |
| March | −0.2 | 5.4 | 3.9 | 2.5 | 16.4 | 28.8 | 26.1 | 31.9 |
| April | 12.0 | 9.3 | 8.2 | 7.9 | 40.4 | 1.5 | 0.7 | 27.0 |
| May | 16.9 | 12.1 | 11.2 | 13.3 | 14.2 | 89.2 | 34.2 | 49.3 |
| June | 18.4 | 21.9 | 17.9 | 16.1 | 26.4 | 17.7 | 142.0 | 52.8 |
| July | 20.5 | 18.6 | 18.3 | 18.6 | 86.0 | 22.4 | 67.2 | 69.8 |
| August | 19.9 | 19.7 | 19.9 | 17.9 | 23.7 | 37.7 | 114.4 | 62.6 |
| September | 15.6 | 13.5 | 15.1 | 13.1 | 17.0 | 98.5 | 66.7 | 46.0 |
| October | 9.8 | 9.8 | 10.5 | 8.2 | 34.1 | 35.9 | 72.9 | 31.5 |
| November | 4.5 | 5.5 | 6.0 | 2.9 | 7.2 | 69.6 | 12.4 | 32.4 |
| December | 2.0 | 2.7 | 1.8 | −0.6 | 50.3 | 21.1 | 33.8 | 34.0 |
| Average | 9.8 | 10.0 | 17.9 | 8.1 | 367.8 | 473.1 | 142.0 | 484.8 |
| Tillage Systems I Factor * | TOC (g kg−1) | TN (g kg−1) | TOC/TN | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Zone II Factor | |||||||||
| R | IR | Mean | R | IR | Mean | R | IR | Mean | |
| CT | 9.62 | 9.11 | 9.36 | 1.15 | 1.08 | 1.11 | 8.40 | 8.48 | 8.44 |
| RT | 12.44 | 12.78 | 12.61 | 1.43 | 1.42 | 1.42 | 8.70 | 9.03 | 8.87 |
| ST-OP | 12.47 | 13.70 | 13.08 | 1.45 | 1.56 | 1.50 | 8.63 | 8.28 | 8.45 |
| Mean | 11.51 | 11.86 | 11.67 | 1.34 | 1.35 | 1.34 | 8.58 | 8.60 | 8.59 |
| LSD0.05 | I 0.12; II n.s.; I/II 0.69; II/I 0.78 | I 0.040; II n.s.; I/II 0.048; II/I 0.059 | I 0.22; II n.s.; I/II 0.38; II/I 0.35 | ||||||
| Tillage Systems I Factor * | Cd (mg kg−1) | CHAs (mg kg−1) | CFAs (mg kg−1) | CHAs/CFAs | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zone II Factor | ||||||||||||
| R | IR | Mean | R | IR | Mean | R | IR | Mean | R | IR | Mean | |
| CT | 271 | 305 | 288 | 2447 | 2470 | 2458 | 2240 | 2200 | 2220 | 1.1 | 1.1 | 1.1 |
| RT | 355 | 310 | 333 | 3203 | 3465 | 3334 | 2767 | 2938 | 2852 | 1.2 | 1.2 | 1.2 |
| ST-OP | 375 | 298 | 336 | 3164 | 3539 | 3352 | 3001 | 3198 | 3099 | 1.0 | 1.1 | 1.1 |
| Mean | 333 | 304 | 319 | 2938 | 3158 | 3048 | 2669 | 2778 | 2724 | 1.1 | 1.1 | 1.1 |
| LSD0.05 | I 38.6; II n.s.; I/II n.s.; II/I n.s. | I 363.7; II 163.8; I/II n.s.; II/I n.s. | I 143.6; II n.s.; I/II n.s.; II/I n.s. | I n.s.; II n.s.; I/II n.s.; II/I n.s. | ||||||||
| Tillage Systems I Factor * | Nd | NHAs | NFAs | NHAs/NFAs | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zone II Factor | ||||||||||||
| R | IR | Mean | R | IR | Mean | R | IR | Mean | R | IR | Mean | |
| CT | 45 | 48 | 47 | 112 | 114 | 113 | 140 | 145 | 143 | 0.8 | 0.8 | 0.8 |
| RT | 49 | 46 | 47 | 177 | 175 | 176 | 161 | 161 | 161 | 1.1 | 1.1 | 1.1 |
| ST-OP | 63 | 59 | 62 | 199 | 180 | 189 | 178 | 172 | 175 | 1.1 | 1.0 | 1.1 |
| Mean | 52 | 51 | 52 | 162 | 156 | 159 | 160 | 159 | 160 | 1.0 | 1.0 | 1.0 |
| LSD0.05 | I 1.5; II 2.4; I/II n.s.; II/I n.s. | I 52.1; II n.s.; I/II n.s.; II/I n.s. | I 15.0; II n.s.; I/II n.s.; II/I n.s. | I n.s.; II n.s.; I/II n.s.; II/I n.s. | ||||||||
| Tillage Systems I Factor * | B (106 cfu g−1 d.m. of Soil) | F (104 cfu g−1 d.m. of Soil) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| July | October | July | October | |||||||||
| Zone II Factor | Zone II Factor | |||||||||||
| R | IR | Mean | R | IR | Mean | R | IR | Mean | R | IR | Mean | |
| CT | 27.8 | 28.5 | 28.13 | 27.0 | 33.6 | 30.31 | 28.9 | 27.8 | 28.31 | 36.3 | 44.3 | 40.25 |
| RT | 49.8 | 37.1 | 43.44 | 42.5 | 50.5 | 46.50 | 42.3 | 51.3 | 46.75 | 81.9 | 69.4 | 75.63 |
| ST-OP | 25.3 | 37.9 | 31.60 | 66.7 | 34.5 | 50.60 | 46.1 | 46.8 | 46.47 | 108.8 | 68.4 | 88.57 |
| Mean | 34.28 | 34.51 | 34.39 | 45.40 | 39.54 | 42.47 | 39.08 | 41.94 | 40.51 | 75.63 | 60.67 | 68.15 |
| LSD0.05 | I 13.41; II n.s. I/II n.s.; II/I n.s. | I 19.98; II n.s.; I/II 28.26; II/I 23.27 | I 17.81; II n.s I/II n.s.; II/I n.s. | I 27.50; II n.s. I/II n.s.; II/I n.s. | ||||||||
| Tillage Systems I Factor * | Ac (105 cfu g−1 d.m. of Soil) | Ce (106 cfu g−1 d.m. of Soil) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| July | October | July | October | |||||||||
| Zone II Factor | Zone II Factor | |||||||||||
| R | IR | Mean | R | IR | Mean | R | IR | Mean | R | IR | Mean | |
| CT | 59.6 | 58.6 | 59.13 | 38.9 | 43.8 | 41.31 | 13.8 | 13.3 | 13.50 | 18.5 | 14.0 | 16.25 |
| RT | 116.8 | 95.8 | 106.3 | 95.4 | 91.6 | 93.50 | 17.3 | 16.3 | 16.78 | 28.5 | 26.1 | 27.31 |
| ST-OP | 47.7 | 72.8 | 60.25 | 104.5 | 72.3 | 88.38 | 12.5 | 20.6 | 16.55 | 32.1 | 20.0 | 26.04 |
| Mean | 74.69 | 75.73 | 75.21 | 79.58 | 69.21 | 74.40 | 14.53 | 16.69 | 15.61 | 26.37 | 20.03 | 23.20 |
| LSD0.05 | I 12.29; II n.s I/II 17.39; II/I 14.31 | I 24.31; II n.s. I/II n.s.; II/I n.s. | I n.s.; II n.s. I/II n.s.; II/I n.s. | I 3.65; II 2.46; I/II 5.16.; II/I 4.25 | ||||||||
| Tillage Systems I Factor * | DEH (mg TPF kg−1 24 h−1) | CAT (mg H2O2 kg−1 h−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| July | October | July | October | |||||||||
| Zone II Factor | Zone II Factor | |||||||||||
| R | IR | Mean | R | IR | Mean | R | IR | Mean | R | IR | Mean | |
| CT | 0.509 | 0.528 | 0.518 | 1.121 | 1.155 | 1.138 | 0.311 | 0.340 | 0.326 | 0.632 | 0.720 | 0.678 |
| RT | 0.510 | 0.553 | 0.532 | 1.010 | 1.066 | 1.038 | 0.311 | 0.342 | 0.326 | 0.588 | 0.626 | 0.607 |
| ST-OP | 0.532 | 0.616 | 0.574 | 0.967 | 1.273 | 1.120 | 0.334 | 0.392 | 0.363 | 0.586 | 0.689 | 0.638 |
| Mean | 0.517 | 0.566 | 0.541 | 1.033 | 1.165 | 1.099 | 0.319 | 0.358 | 0.338 | 0.603 | 0.678 | 0.641 |
| LSD0.05 | I 0.09.; II 0.10; I/II n.s.; II/I n.s. | I 0.08.; II 0.11; I/II n.s.; II/I n.s. | I 0.011; II 0.019; I/II n.s.; II/I n.s. | I 0.015; II 0.028; I/II n.s.; II/I n.s. | ||||||||
| Tillage Systems I Factor * | AlP | AcP | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| July | October | July | October | |||||||||
| Zone II Factor | Zone II Factor | |||||||||||
| R | IR | Mean | R | IR | Mean | R | IR | Mean | R | IR | Mean | |
| CT | 0.658 | 0.729 | 0.692 | 0.651 | 0.686 | 0.669 | 2.146 | 2.024 | 2.085 | 1.996 | 2.103 | 2.050 |
| RT | 0.675 | 0.739 | 0.707 | 0.906 | 0.947 | 0.927 | 2.711 | 2.885 | 2.798 | 2.288 | 2.378 | 2.333 |
| ST-OP | 0.724 | 1.026 | 0.875 | 0.951 | 1.353 | 1.152 | 2.364 | 2.889 | 2.626 | 2.298 | 2.626 | 2.462 |
| Mean | 0.686 | 0.831 | 0.758 | 0.836 | 0.996 | 0.916 | 2.407 | 2.599 | 2.503 | 2.194 | 2.369 | 2.281 |
| LSD0.05 | I 0.08; II 0.10; I/II n.s.; II/I n.s. | I 0.386; II 0.135; I/II n.s.; II/I n.s. | I 0.699; II 0.186; I/II 0.754; II/I 0.323 | I 0.267; II 0.156; I/II n.s.; II/I n.s. | ||||||||
| AlP/AcP | GMea | BIF | BA12 | TEI | |
|---|---|---|---|---|---|
| TOC | 0.642 | 0.652 | n.s. | 0.871 | 0.773 |
| TN | 0.651 | 0.661 | n.s. | 0.904 | 0.780 |
| CHAs | 0.638 | 0.648 | n.s. | 0.917 | 0.774 |
| CFAs | 0.632 | 0.644 | n.s. | 0.954 | 0.774 |
| CHA/CFA | n.s. | n.s. | 0.759 | 0.766 | 0.836 |
| CHAs% | n.s. | n.s. | 0.672 | 0.835 | 0.758 |
| CFAs% | n.s. | n.s. | 0.612 | 0.816 | 0.677 |
| CH% | n.s. | n.s. | 0.767 | 0.573 | 0.792 |
| Nd | 0.701 | 0.723 | n.s. | 0.872 | n.s. |
| NHAs | 0.575 | 0.592 | n.s. | 0.978 | 0.573 |
| NFAs | 0.508 | 0.520 | n.s. | 0.962 | 0.702 |
| NHA/NFA | n.s. | n.s. | n.s. | 0.982 | 0.668 |
| Nd% | 0.507 | 0.526 | n.s. | 0.846 | n.s. |
| NFAs% | n.s. | n.s. | 0.560 | 0.699 | 0.562 |
| B | n.s. | n.s. | n.s. | 0.707 | n.s. |
| F | n.s. | n.s. | n.s. | 0.817 | n.s. |
| Ac | n.s. | n.s. | n.s. | 0.766 | n.s. |
| Ce | n.s. | n.s. | n.s. | 0.639 | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaskulska, I.; Lemanowicz, J.; Dębska, B.; Jaskulski, D.; Breza-Boruta, B. Changes in Soil Organic Matter and Biological Parameters as a Result of Long-Term Strip-Till Cultivation. Agriculture 2023, 13, 2188. https://doi.org/10.3390/agriculture13122188
Jaskulska I, Lemanowicz J, Dębska B, Jaskulski D, Breza-Boruta B. Changes in Soil Organic Matter and Biological Parameters as a Result of Long-Term Strip-Till Cultivation. Agriculture. 2023; 13(12):2188. https://doi.org/10.3390/agriculture13122188
Chicago/Turabian StyleJaskulska, Iwona, Joanna Lemanowicz, Bożena Dębska, Dariusz Jaskulski, and Barbara Breza-Boruta. 2023. "Changes in Soil Organic Matter and Biological Parameters as a Result of Long-Term Strip-Till Cultivation" Agriculture 13, no. 12: 2188. https://doi.org/10.3390/agriculture13122188
APA StyleJaskulska, I., Lemanowicz, J., Dębska, B., Jaskulski, D., & Breza-Boruta, B. (2023). Changes in Soil Organic Matter and Biological Parameters as a Result of Long-Term Strip-Till Cultivation. Agriculture, 13(12), 2188. https://doi.org/10.3390/agriculture13122188






