3.1. Chemical Composition
The chemical composition of the dehydrated macroalgae varied widely (
Table 2). The coefficients of variation ranged from 21% for TDF to 66% for RES (100-ash-CP-TDF). The chemical composition of dehydrated
H.
elongata was found to be rather similar to that of samples previously collected in Galicia, but dehydrated
S. latissima and
U. pinnatifida showed different composition (
Table 3). As already pointed out, the chemical composition of macroalgae varies depending on geographical location, season, environmental factors, and stressors [
21,
25,
26,
58,
59]. The chemical composition of both types of extracts was also very variable, with coefficients of variation of 8 for NDF and 106% for SF.
The ash contents of the dehydrated macroalgae were very high (20.8–50.1% DM), which is consistent with the results reported by other authors for these macroalgae species (
Table 3), indicating a much higher mineral content than in terrestrial plants [
25]. In this respect, it is important to take into account the mineral content of each macroalgae for incorporation in diets, mainly due to the iodine and heavy metals contents [
58]. The CP of the dehydrated macroalgae ranged between 7.10 and 16.4% DM, which is consistent with previous reports (5–15% for brown and 10–25% for green algae [
23], and with the values previously obtained for these macroalgae in Galicia (
Table 3).
Ulva spp. (16.4%) and
U.
pinnatifida (14.8%) contained almost twice as much protein as SBP (7.99%), and it has been reported that their CP contains relevant proportions of leucine and valine [
60,
61]. However, CP content in all macroalgae tested was lower than other protein feeds commonly used in livestock feeding, as sunflower meal or soybean meal.
In the present study, the TDF content varied depending on the species of macroalgae and the type of extract. Dehydrated macroalgae contained large amounts of TDF (28.6–52.7%), although not as high as cereal straw (78.1%) or SBP (71.7%). However, in contrast to straw, macroalgae TDF contained approximately equal parts of NDF and SF (NDF: from 17.1 to 31.3% for macroalgae and 74.3% for straw; SF: from 11.6 to 22.5% for macroalgae and 3.79% for straw), being the values of the macroalgae closer to those of SBP (36.5% NDF and 35.2% of SF). Unlike SBP, some dehydrated macroalgae contained remarkable amounts of ADL (3.63–14.2% for macroalgae; 1.91% for SBP), which may be associated with polyphenolic compounds other than lignin (such as phlorotannins), as lignin is only found in red macroalgae [
62]. The results for TDF, although very variable, are broadly consistent with previous reports for samples collected in Galicia (
Table 3). The results obtained for SF and other components of TDF are not easy to compare with previously published data because of the variable methods used for determination of macroalgae fibre components in different studies [
23,
25,
26].
Table 2.
Chemical composition (g/100 g; dry matter basis) of macroalgae products and reference ingredients (untreated cereal straw and sugar beet pulp) 1.
Table 2.
Chemical composition (g/100 g; dry matter basis) of macroalgae products and reference ingredients (untreated cereal straw and sugar beet pulp) 1.
| | Ash | CP | TDF | NDF | SF | ADF | ADL | RES | RES + SF |
|---|
| Dehydrated macroalgae | | | | | | | | | |
|
Fucus vesiculosus | 23.1 | 10.6 | 52.7 | 31.3 | 21.4 | 30.7 | 14.2 | 13.6 | 35.0 |
|
Himanthalia elongata | 41.4 | 9.51 | 41.7 | 19.4 | 22.3 | 17.8 | 13.3 | 7.39 | 29.7 |
|
Saccharina latissima | 20.8 | 7.10 | 43.1 | 22.6 | 20.5 | 10.1 | 3.63 | 29.0 | 49.5 |
| Ulva spp. | 27.7 | 16.4 | 44.1 | 21.6 | 22.5 | 14.7 | 7.87 | 11.8 | 34.3 |
|
Undaria pinnatifida | 50.1 | 14.8 | 28.6 | 17.1 | 11.5 | 11.6 | 7.10 | 6.5 | 18.0 |
| Hydrolyzed macroalgae | | | | | | | | | |
|
Fucus vesiculosus | 28.9 | 10.6 | 53.2 | 30.2 | 23.0 | 21.3 | 15.9 | 7.3 | 30.3 |
|
Himanthalia elongata | 45.5 | 8.92 | 40.9 | 15.3 | 25.6 | 16.4 | 12.5 | 4.7 | 30.3 |
|
Laminaria ochroleuca | 43.4 | 8.36 | 33.0 | 11.2 | 21.8 | 11.4 | 3.85 | 15.2 | 37.0 |
| Ulva spp. | 39.1 | 13.0 | 34.1 | 5.63 | 28.5 | 6.07 | 4.19 | 13.8 | 42.3 |
|
Undaria pinnatifida | 58.2 | 14.2 | 21.3 | 5.80 | 15.5 | 5.68 | 4.66 | 6.3 | 21.8 |
| Aqueous extract | | | | | | | | | |
|
Fucus vesiculosus | 28.7 | 8.23 | 10.4 | 4.83 | 5.60 | 4.72 | 4.32 | 52.7 | 58.3 |
|
Himanthalia elongata | 55.1 | 3.74 | 29.9 | 0.00 | 29.9 | 0.14 | 0.17 | 11.3 | 41.2 |
|
Saccharina latissima | 13.6 | 3.49 | 64.7 | 0.00 | 64.7 | 0.00 | 0.00 | 18.2 | 82.9 |
|
Undaria pinnatifida | 68.7 | 8.91 | 18.6 | 0.26 | 18.3 | 0.17 | 0.16 | 3.8 | 22.1 |
| Hydrolyzed extract | | | | | | | | | |
|
Fucus vesiculosus | 32.0 | 9.22 | 29.5 | 1.61 | 27.9 | 1.51 | 1.29 | 29.3 | 57.2 |
|
Laminaria ochroleuca | 44.4 | 2.37 | 2.70 | 0.54 | 2.16 | 0.82 | 0.77 | 50.5 | 53.1 |
|
Saccharina latissima | 19.3 | 4.09 | 2.94 | 0.00 | 2.94 | 0.24 | 0.18 | 73.7 | 76.6 |
| Ulva spp. | 34.1 | 4.88 | 30.2 | 0.00 | 30.2 | 0.09 | 0.06 | 30.8 | 61.0 |
|
Mastocarpus stellatus | 31.8 | 10.4 | 43.4 | 2.09 | 41.3 | 0.14 | 0.14 | 14.4 | 55.7 |
| Reference ingredients | | | | | | | | | |
| Untreated cereal straw | 8.80 | 2.90 | 78.1 | 74.3 | 3.79 | 43.8 | 4.20 | 10.2 | 13.9 |
| Sugar beet pulp | 4.70 | 7.99 | 71.7 | 36.5 | 35.2 | 23.6 | 1.91 | 15.6 | 50.8 |
Table 3.
Review of published data for chemical composition (g/100 g; dry matter basis) of the macroalgae tested in this study.
Table 3.
Review of published data for chemical composition (g/100 g; dry matter basis) of the macroalgae tested in this study.
| Location and Reference | Macroalgae | Ash | Crude Protein | Total Dietary Fibre |
|---|
| Galicia, Spain [25] | Himanthalia elongata | 36.4 | 14.1 | 37.1 |
| Saccharina latissima | 34.8 | 25.7 | 30.2 |
| Mastocarpus stellatus | 25.0 | 21.3 | 31.7 |
| Galicia, Spain [63] | Himanthalia elongata | 31.0 | 6.80 | 39.0 |
| Laminaria ochroleuca | 33.0 | 8.5 | 45.0 |
| Undaria pinnatifida | 35.0 | 20.5 | 39.0 |
| Galicia, Spain [42] | Himanthalia elongata | 33.2 | 7.50 | 36.0 |
The enzymatic treatment of the macroalgae (hydrolyzed macroalgae products) did not substantially change their chemical composition, especially in F. vesiculosus and H. elongata. Both the ash (28.9–58.2%) and CP content (8.36–14.2%) were similar to those of the dehydrated samples. In some cases, there was a slight reduction in TDF content due to a lower NDF (5.63–30.2%) but a higher SF level (15.5–28.5%), as it was observed for Ulva spp. and U. pinnatifida.
As expected, both types of extractions influenced the composition of the extract obtained, although in a slightly different way for each macroalgae. No information is available in the literature about the composition of the same extracts for accurate comparisons. The ash content of the extracts ranged from 13.6 to 68.7% DM, and it was numerically higher than that in the corresponding dehydrated macroalgae in most samples. In contrast, the CP content of most extracts was much lower than in the dehydrated macroalgae. For
H.
elongata and
S. latissima, it was around half in the aqueous extract than in the dehydrated macroalgae (decreased from 9.51% to 3.74%, and from 7.10% to 3.49%, respectively); and the CP reduction was much marked for
Ulva spp., decreasing from 16.4% in the dehydrated samples to 4.88% in the hydrolyzed extract. In contrast, both aqueous and hydrolyzed extracts of
F.
vesiculosus contained only slightly lower amounts of CP (8.23 and 9.22%, respectively) than the dehydrated macroalgae (10.6%), and CP reductions in the aqueous extract of
U.
pinnatifida were intermediate (8.91 and 14.8% for the extract and the dehydrated macroalgae, respectively). These results suggest a high variability between macroalgae in the CP extraction efficiency, even using the same extraction procedure for all samples. In order to analyze the dietary fibre in the different extracts, it is important to take into account the RES fraction (calculated as RES = 100 − (ash + CP + TDF)), which was very high in some extracts. Considering the low fat and sugar contents usually reported for macroalgae [
59,
64,
65,
66], the RES fraction probably contained mainly soluble carbohydrates, which could not be identified, although in some cases they accounted for a high proportion of the macroalgae. The RES fraction would include the SF that is not precipitated by ethanol or other soluble compounds [
67]. In brown macroalgae, such as
S.
latissima, this fraction may correspond to laminarin (1,3-β-D-glucans), which is soluble in water, and alginate (1,4-D-mannuronic acid combined with 1,4-α-L-glucuronic acid), which is soluble at pH between 6 and 9, and/or mannitol [
25,
68]. These results showed that the standard techniques used to characterize fibre fractions in terrestrial plants do not enable precise quantification of the composition of macroalgae products [
25,
58,
59]. The RES was remarkable in
S.
latissima (29.0% for dehydrated macroalgae and 73.7% for hydrolyzed extract),
F.
vesiculosus (52.7 and 29.3% for aqueous and hydrolyzed extracts, respectively), and the hydrolyzed extracts of
Ulva spp. (30.8%) and
L.
ochroleuca (50.5%). Therefore, if the RES is considered part of the TDF, and the sum of SF and RES fractions is calculated (
Table 2), both the aqueous and hydrolyzed extracts were characterized by high soluble polysaccharides (22.1–82.9%) and very low NDF contents (0–4.83%). These results were expected, as the aim of these extraction processes was to recover the polysaccharides of interest due to their properties and prebiotic potential. Except for
U.
pinnatifida, the sum (RES + SF) was similar or even greater than that of SBP, which is promising due to the fermentation potential of these macroalgae [
30,
32,
69]. Of all the macroalgae considered,
S.
latissima deserves special attention because of the high values of the RES + SF fraction observed in both the aqueous (82.9%) and the hydrolyzed (76.6%) extract, probably due to its high content of laminarin in agreement with the high glucose content in the aqueous extract (80.5%: unpublished data).
3.2. In Vitro Digestibility and Estimated Energy Content
The values of ivIDMd of dehydrated macroalgae ranged from 38.4 to 73.2%, being all higher than that for SBP (43.3%, except in
F.
vesiculosus. Table 4). When the macroalgae were hydrolyzed the ivIDMd increased by 25% on average. In most dehydrated macroalgae, the values of ivFDMd were similar (42.2–75.0%) than those of SBP (76.8%). The exceptions were
F.
vesiculosus and
H. elongata, both of which had relatively high sulphate contents (4–7%: unpublished data), and the latter also had a high fucose content (31.5%: unpublished data) probably associated with fucoidans. However, the ivFDMd values were higher in all macroalgae than in the cereal straw (19.8%). Conversely, CP digestibility (ivFCPd) was lower in all macroalgae than in SBP (22.6–59.0% for dehydrated macroalgae and 68.1% for SBP), with some macroalgae (i.e.,
F.
vesiculosus and
H. elongata) having values close to that of cereal straw (17.7%). Both DM and CP digestibility increased when the macroalgae were hydrolyzed (by 20% ivFDMd, and 80% ivFCPd, on average, except in
F.
vesiculosus). The low
in vitro CP digestibility is consistent with the low
in vivo CP digestibility reported for
S.
latissima and
Ulva spp. in rats [
70], despite these samples showing the numerically highest values
in vitro in our study. The ivFDMd and ivFCPd values of the dehydrated macroalgae were negatively correlated with ADF content (r = −0.97;
p = 0.005;
n = 5) and the protein associated with TDF (r = −0.96;
p = 0.011;
n = 5), respectively, but they were not correlated with either SF or the sum of RES + SF fractions (
p ≥ 0.35). The negative correlation between ivFDMd and ADF was previously observed for compound feeds for rabbits based on terrestrial plant ingredients [
47].
As expected, the different extracts obtained from the macroalgae were almost completely digested
in vitro (78.6–99.8% ivIDMd; 97.9–99.7% ivFDMd; 100.0% ivFCPd) due to their soluble nature. However, the polysaccharides quantified in both the TDF and the RES fractions (possibly laminarin, alginate, fucoidans, ulvans) cannot be hydrolyzed by the endogenous enzymes and can only be fermented by the intestinal microbiota. The
in vivo protein digestibility of these extracts might be higher than in the dehydrated macroalgae although it would not be expected to be high. Specific protein extraction in
Ulva spp. has been shown to increase
in vitro proteolysis [
71], which was in agreement with the increased ivFCPd observed in our study for the hydrolyzed
Ulva spp. and
U.
pinnatifida compared with the dehydrated macroalgae, but these results should be confirmed
in vivo. None of the
in vitro digestibility values measured in the extracts were correlated with any fibrous fractions analyzed (
p ≥ 0.30;
n = 9). When considering all of the 19 macroalgae products together, the ivFDMd and ivFCPd values were negatively correlated with NDF (r = −0.96;
p < 0.001;
n = 19), and with the protein linked to NDF (r = −0.82;
p < 0.001;
n = 19) content, respectively.
The dehydrated macroalgae showed similar values of digestible energy (7.96–12.9 MJ/kg DM) than SBP (13.1 MJ/kg DM), with the exception of
F.
vesiculosus, but higher values than straw (4.60 MJ/kg DM) (
Table 4). Hydrolyzation of the macroalgae slightly improved their energy content, except for
F.
vesiculosus. In both types of extracts, DE values were higher (16.5 MJ/kg DM, on average) than that of SBP, which is in good accordance with the high DM digestibility of the extracts, although the real value will depend on their fermentability.
3.3. In Vitro Gas and VFA Production
Values for the macroalgae products and reference materials are shown in
Table 5, whereas those for the insoluble residue of the ivIDMd are shown in
Table 6.
The different kinetics of gas production observed in the samples precluded the data being fitted with a single mathematical model, and comparison between samples was thus impossible. Accordingly, only data for 12, 24, and 48 h of incubation are shown for comparison of the samples, as they were more closely correlated with the real in vivo fermentation and fibre digestibility in the rabbit (unpublished results). At 48 h, 14 out of 33 samples (macroalgae products + reference ingredients + insoluble residues of 2-step in vitro digestibility) have reached the whole potential gas production. When data were analyzed as repeated measures, in vitro gas production was influenced by the incubated sample, measurement time and their interaction (p < 0.001).
Surprisingly, the two samples that showed the closest (
p > 0.05) gas production than SBP after 12 h were the dehydrated
Ulva spp. and the hydrolyzed
L.
ochroleuca samples (
Table 5), whereas for the hydrolyzed
Ulva spp., both extracts of
S.
latissima and the hydrolyzed extract of
F. vesiculosus showed intermediate values between SPB and straw. At 24 h, gas production in dehydrated
S.
latissima, the aqueous extract of
S. latissima and the hydrolyzed extract of
L. ochroleuca was similar to that in SBP, while gas production in the hydrolyzed extract of
S. latissima was higher than in SBP (
p < 0.05). Similar results were obtained at 48 h. The results indicated a high fermentability of the different
S.
latissima products, which is probably related to the high content in laminarin and alginate previously reported [
72], especially when these polysaccharides are concentrated, as it may occur in the extracts. Laminarin is a non-sulphated water-soluble storage polysaccharide composed of 1,3-β-D-glucans with β1,6 ramifications, and alginate is composed of 1,4-β-D-manuronic acid and 1,4-β-L-guluronic acid and is water soluble at pH 6–9; however, both cannot be hydrolyzed by endogenous enzymes and can be only used by intestinal microbiota [
73].
S.
latissima was also previously found to yield a relatively high level of ruminal gas production
in vitro, lower than SBP but higher than alfalfa meal [
59]. By contrast, gas production was lower in the macroalgae containing relatively high levels of sulphated polysaccharides (ulvans and fucoidans), such as
F.
vesiculosus and
Ulva spp., which had been also reported to be less fermentable in other studies [
74,
75].
The other macroalgae products yielded similar gas production to that produced by straw, suggesting a low fermentability, with the extracts of H. elongata (aqueous), F. vesiculosus (hydrolyzed), and Ulva spp. (hydrolyzed) yielding intermediate values between SBP and straw at 48 h.
The different fermentability of samples at 12 h suggests the need of adaptation for the microbiota, especially to the most purified extracts, in which the chemical composition is more homogeneous and different from that of substrates usually fermented by the rabbit intestinal microbiota. The caecal inocula were obtained from rabbits that were not fed macroalgae, and the adaptation required would have delayed the beginning of the fermentation process. The need for substrate adaptation by the rabbit intestinal microbiota has been already observed when using a very specific substrate (cellobiose) [
50].
Most of the insoluble residues of ivIDMd yielded similar gas production to the cereal straw (
Table 6), independently of the fermentation time, which is consistent with the higher fermentability of soluble compared with insoluble fibre in rabbits [
76]. Only the insoluble residues of
S.
latissima and SBP produced more gas than the cereal straw not subjected to
in vitro digestion but less than SBP.
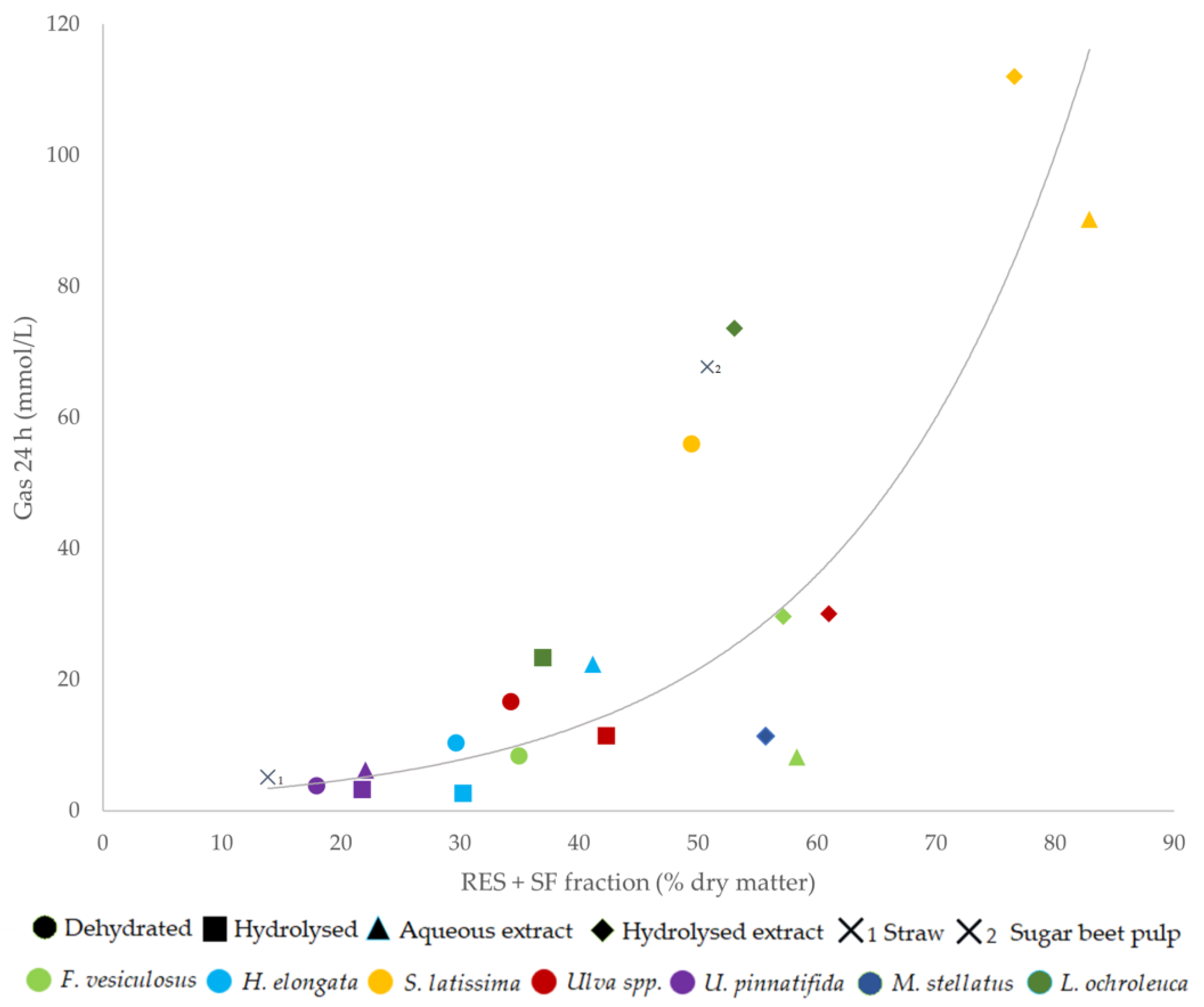
Although a positive correlation between the gas production at 24 h and the RES fraction was observed for the dehydrated macroalgae (r = 0.96;
p = 0.011;
n = 5), no correlation was found with SF or the ivIDMd, ivFDMd, and ivFCPd values (
p ≥ 0.54). The gas production at 24 h of the 9 extracts (aqueous and hydrolyzed) was positively correlated with the sum of RES + SF (r =0.72;
p = 0.028;
n = 9), but was not correlated with SF or ivIDMd, ivFDMd, and ivFCPd (
p ≥ 0.34). When the relationships between the gas production at 24 h of the 19 macroalgae products and their chemical composition were analyzed, positive correlations were again observed with the RES fraction (r = 0.71;
p < 0.001;
n = 19) and with the sum (RES + SF), both linearly (R
2 = 0.62;
p < 0.001;
n = 19) and quadratically (R
2 = 0.68;
p = 0.095.
Figure 1).
Gas production at 24 h was also negatively correlated with CP (r = −0.69;
p = 0.001;
n = 19) and ADL content (r = −0.51;
p = 0.025;
n = 19), but less significant correlations were observed with ivIDMd, ivFDMd, and ivFCPd (r = 0.37–0.40;
p = 0.086–0.12;
n = 19), and no correlations were observed with SF fraction (
p = 0.85). These results indicate the complexity of characterizing macroalgae products with the standard techniques used for terrestrial plant ingredients. In addition, solubility does not equate to fermentability in these samples, which could be due to the presence of either substances that inhibit enzyme action [
66,
77], or highly fermentable compounds not collected in the TDF fraction. The negative influence of ADL on
in vitro gas production is similar to that observed by Bikker et al. [
59] for macroalgae and to that observed in terrestrial plant ingredients [
78], although the chemical meaning of ADL in macroalgae may not be the same than in terrestrial plants.
The
in vitro VFA production was determined only in the most fermentable macroalgae products and the two reference materials (
Table 7). As expected, gas and total VFA production at 24 h were positively correlated (r = 0.84;
p = 0.018;
n = 9). All products derived from
S.
latissima showed total VFA production similar to that for SBP. Both extracts of
S.
latissima had increased VFA production compared with the dehydrated macroalgae, probably due to the higher concentration of fermentable polysaccharides such as laminarin, as previously observed
in vitro in ruminants [
79,
80]. The other analyzed macroalgae products had lower (
p < 0.05) total VFA production than
S. latissima products.
Saccharina latissima products also produced high proportions of propionate (25.0, 20.7, and 21.2% for the dehydrated sample, aqueous extract, and hydrolyzed extract, respectively), but they did not differ with that produced by SBP (14.1%) or straw (10.2%). The fermentation of the aqueous extract of
H.
elongata and the hydrolyzed extract of
L. ochroleuca resulted in higher (
p < 0.05) proportions of butyrate than the reference ingredients, which may be associated with the fucoidan content [
81]. A high proportion of butyrate is of interest due to its benefits on gut health, as it is known to be the main energy source for colonocytes [
82]. In rabbits, the ileal concentration of butyrate has been associated with better growth traits when cellobiose was supplied in the diet [
83]. The hydrolyzed extract of
Ulva spp. produced a remarkable amount of isobutyrate (10.7%), much higher than SBP (0.17%) or any other macroalgae product.
Macroalgae polysaccharides, particularly laminarin and fucoidan, have been shown to have a prebiotic effect in several
in vitro and
in vivo studies (for a review, [
32]), but their effects on VFA proportions is variable. In most studies, laminarin (derived from macroalgae such as
S.
latissima and
L. ochroleuca) has been found to yield high levels of gas production and increased levels of acetate [
84,
85], propionate [
84,
85,
86], and butyrate [
86,
87]. Fermentation of fucoidan, present in macroalgae such as
F.
vesiculosus and
H. elongata, among others, also showed an increase in the proportions of acetate and butyrate [
88], but a decrease in that of propionate [
86,
88]. It is important to consider that the
in vitro VFA proportions are influenced by the incubated macroalgae, but probably also by the source of the inoculum used [
49].
3.4. Minimal Inhibitory Concentrations
Most of the macroalgae products tested did not inhibit the bacterial growth at the maximal concentration tested (8.2 mg/mL.
Table 8).
Two of the dehydrated macroalgae (
H. elongata and
U. pinnatifida) inhibited bacterial growth at the maximal concentration (8.2 mg/mL, except 2.05 mg/mL for
U. pinnatifida against LM), but their products did not have the same effect. Previous findings indicate that these macroalgae could have some antimicrobial properties [
89,
90]. Although it is not known what causes the antimicrobial effect of some macroalgae, it has been mainly attributed to the phenolic compounds [
90,
91]. In the present study, the extracts were obtained with the objective of increasing digestibility and/or fermentability and concentrating the SF of the macroalgae, so any antimicrobial effect may have disappeared at least partly in these extracts because of the absence of these compounds.
Within the macroalgae products, hydrolyzed
L. ochroleuca and the hydrolyzed extract of
S. latissima also showed inhibitory responses when tested at the maximal concentrations. Unfortunately, a sample of dehydrated
L. ochroleuca was not available for testing, and it is possible that it could also show an inhibitory effect. By contrast, dehydrated
S. latissima did not show any inhibitory effect, suggesting that the positive effect observed in the hydrolyzed extract may be associated with other compounds concentrated in this macroalgae product [
90]. Further studies with different solvents and extracts are required to enable solid conclusions to be reached regarding the potential inhibitory effects of these macroalgae. Nonetheless, the positive results appeared only at the maximal concentration, which would correspond in vivo to an inclusion of the macroalgae at a proportion of 2.5% in the diet. Moreover, it must be taking into account that
H. elongata,
U. pinnatifida,
L. ochroleuca, and
S. latissima are brown macroalgae with a high iodine content, which limits the amount that could be included in rabbit feed (Regulation EC 1334/2003).