1. Introduction
The practice of controlled or indoor environment agriculture for food production is gaining significant popularity on a global scale. The efficacy of controlled environment agriculture lies in its capacity to shield plants from external weather conditions and regulate the internal climate of the cultivation area. This enables the manipulation, control, and monitoring of environmental factors to achieve enhanced and consistent yields and quality throughout the entire year of production [
1,
2]. Furthermore, crop yields and quality of conventional cultivation vary substantially due to seasonality, weather extremes, pests, and diseases, particularly soil-borne diseases, and cultivating most crops throughout the year is only possible in certain areas. In addition, controlled environment systems can overcome the problem of decreasing agricultural land and optimize an area’s utilization. This is because environmentally controlled structures can be installed in a wide range of facilities and sizes, including high tunnels, greenhouses, growth chambers, warehouses, abandoned buildings, etc. [
3,
4,
5]. The controlled environment crop production system mainly utilizes soilless cultivation techniques, saving up to 70–95% of the water used in conventional farming [
5,
6,
7]. In addition, it can also optimize the use of nutrients. Therefore, the controlled environment agricultural practices that integrate science- and engineering-based approaches can increase productivity and optimize resource use [
8]. The soilless systems are also suitable for vertical farming, particularly in urban areas. This is due to their long-term resource efficiency and low weight [
9]. According to Gruda [
10] and Hong et al. [
11], soilless systems are used to develop new crops and enhance biochemicals in herbs and aromatic plants. As a result, the demand for soilless agriculture systems is growing globally, and innovative research is required to overcome constraints and keep providing chances for this industry [
12].
Soilless culture is a growing medium that replaces soil where the nutrients needed by plants are supplied through irrigation water [
13]. Soilless culture is ideal for this system as water culture or hydroponics such as floating hydroponics, aeroponics, deep-water culture, and nutrient film technique. Other than hydroponics, the soilless culture widely uses organic (sawdust, peat, coconut coir, bark, straw, rice hull, etc.) and inorganic substrate (pebbles, sand, perlite, gravel, rock wool, vermiculite, etc.) [
13,
14,
15]. The soilless substrate is widely used in soilless cultivation because, in addition to saving water, substrates also retain nutrient solution reserves, buffering water and nutrient supply disruptions and protecting roots from temperature fluctuations [
13]. In addition, good qualities of the soilless substrate are good aeration and drainage, good water holding capacity and being easily wetted, lasting long in the pot and resisting decomposition, and good cation exchange capacity (CEC) and pH [
16]. Clay mineral aggregate, also known as expanded clay aggregate, is widely used in soilless agriculture because it meets the characteristics of a good quality substrate with a neutral pH of 7.0 and has a long lifespan due to its reusability [
13,
16]. Therefore, it not only meets the ideal characteristics of the substrate but is also more practical and cost-effective.
Various crops, such as herbs, leafy vegetables, flowers, strawberries (
Fragaria x ananassa), tomatoes (
Solanum lycopersicum), cucumbers (
Cucumis sativus), and peppers (
Capsicum annuum) can be grown in a controlled condition utilizing a soilless crop system [
17]. However, various other crops are suitable and have the potential to be grown in a controlled condition utilizing a soilless culture, including soybean. Soybean is an essential crop because it is a high protein crop whose chemical composition consists of protein (38%), carbohydrate (27%), oil (19%), water (12%), and ashes (4%) [
18]. Soybeans are an essential source of food for humans as well as animals [
19,
20,
21]. Therefore, soybean is widely used in food products such as tofu, soy milk, soy source, fermented bean paste, tempeh, and animal feed. Soybean cultivation in a soilless culture system is being investigated because soybeans not only can use nitrogen from biological nitrogen fixation and reduce the requirement for mineral fertilizer, but can also improve the production of necessary amino acids and functional ingredients, including minerals as well as vitamins when planted in a controlled environment [
22]. Soybean is also one of the candidate crops for cultivation in bioregenerative life-support systems (BLSS) for space missions [
23]. Therefore, the study of soybeans in a controlled environment using soilless culture is essential to be carried out, especially for specific varieties, so that the findings obtained can be used as a guide and as a source of reference.
An essential factor that needs to be emphasized in the planting system in a controlled environment besides light, temperature, CO
2, and water is the need for nutrients. In a soilless cultivation system, all essential plant nutrients should be supplied via the nutrient solution, where inorganic fertilizers are used as nutrient sources [
9,
24]. According to Savvas [
25], due to the substrate’s limited capacity as a buffer and a limited supply of nutrients, it is necessary to continuously supply the essential elements, including micronutrients. The nutrient solution can be formulated with a mixture of several fertilizer sources or using fertilizers that were formulated and are commercially available in the market, such as ‘Advance Hydroponics of Holland (Dutch Formula)’. This hydroponics fertilizer was developed in 1993 in the Netherlands and consisted of three-part mineral fertilizers (Grow, Bloom, and Micro) [
26].
The precise amount of the nutrient solution differs depending on the crop, variety, stage of plant growth, conditions of the environment, and irrigation system. The fertilizer used in the soilless cultivation system should have balanced nutrients with no precipitates [
27]. The guide for fertilizers developed for field cultivation can be used for planting soybeans in soilless culture. Total nutrients absorbed by the soybean plant for field planting per metric tonne of production of grains is 146 kg nitrogen (N), 25 kg phosphorus pentoxide (P
2O
5), 53 kg potassium oxide (K
2O), 22 kg magnesium oxide (MgO), 28 kg calcium oxide (CaO), 5 kg sulfur (S), 476 kg iron (Fe), 104 kg zinc (Zn), 123 kg manganese (Mn), 41 kg copper (Cu), 55 kg boron (B), and 13 kg molybdenum (Mo) [
28]. For soilless crop production, Wheeler et al., 2008 [
29] used a nutrient solution containing substances such as 7.5 mM N, 0.5 mM phosphorus (P), 3.0 mM potassium (K), 2.5 mM calcium (Ca), 1.0 mM S, 1.0 mM magnesium (Mg), 60 μM Fe, 7.4 μM Mn, 1.04 μM Cu, 0.96 μM Zn, 7.13 μM B, and 0.01 μM Mo in his study on the performance of wheat, potato, tomato, lettuce, and soybean (var. McCall and Hoyt) that were carried out in a biomass production chamber using one of the soilless system methods namely the nutrient film technique. The pH was controlled to 5.8, adding 0.4 mM nitric acid and EC to 1.2 dS m
−1. Another study on soybean varieties McCall and Hoyt in a controlled environment using soilless substrate peat: perlite at a ratio of 1:1 and nutrient solution containing substances such as 1.0 mM calcium nitrate [Ca(NO
3)
2], 5 mM potassium nitate (KNO
3), 0.75 mM magnesium sulfate (MgSO
4), 1.5 μM ferric nitrate [Fe(NO
3)
3], 5 μM ferric-hydroxyethylenediaminetriacetic acid (Fe-HEDTA), 9 μM manganese dichloride (MnCl
2), 2 μM zinc sulfate (ZnSO
4), 40 μM boric acid (H
3BO
3), 0.6 μM copper sulfate (CuSO
4), and 0.06 μM sodium molybdate (Na
2MoO
4) [
30]. According to Basal et al., 2020 [
31], who studied the effect of water stress using polyethylene glycol (PEG) to control the water level on the growth of soybean variety ES Mentor and Pedro, used a nutrient solution containing substances such as 0.7 mM potassium sulfate (K
2SO
4), 0.1 mM KCl, 2.0 mM Ca(NO
3)
2, 0.5 mM MgSO
4, 0.1 mM potassium dihydrogen phosphate (KH
2PO
4), 10 μM H
3BO
3, 0.5 μM manganese sulfate (MnSO
4), 0.5 μM ZnSO
4, 0.2 μM CuSO
4, and 10
−4 M Fe-EDTA. These three studies use nutrient solutions with varying concentrations because the soilless culture type and variety are different. Thus, the study of nutrient concentration for specific varieties, such as the Martina and Johanna varieties, is essential so that the nutrients supplied are according to the needs of the variety. There is still no research on the requirements and effects of nutrients for the two varieties using soilless culture systems in a controlled environment.
Some essential things that must be emphasized in managing nutrient solutions for soilless cultivation systems are pH and electrical conductivity (EC). The proper pH value of the nutrient solution for plant growth is between 5.5 and 6.5 [
32]. Meanwhile, EC is a salt level index that measures the quantity of salts in a solution and can be utilized for estimating nutrient solution osmotic pressure. As a result, the EC of the nutrient solution can be used as an indicator to determine the quantity of available ions in the root area of the plant [
33]. The optimum EC varies according to crop and environmental conditions. However, the EC standards for soilless culture or hydroponics systems range between 1.5 and 2.5 ds/m [
34]. Higher EC reduces the absorption of nutrients by increasing the pressure of osmosis, while lower EC could be detrimental to the health and productivity of the plant [
35]. Therefore, optimal pH and EC values are essential in ensuring that the nutrient solution is in a proper condition to be supplied to the plant.
Thus, the study of a nutrient solution, including nutrient concentration for soybean plants with certain varieties, is essential to implement. Therefore, an investigation was conducted to study the influence of different nutrient concentrations on shoot and root growth, yield components, yield, and the chemical composition of two soybean varieties grown in a controlled environment using a soilless substrate. The influence of nutrient concentration on the soybean varieties varied. However, the study hypothesis was accepted, which predicts that the growth, yield, and protein composition of soybean varieties will increase with increasing nutrient concentrations up to a certain level. The findings from this research can be used as a guideline for developing high-yielding, high-quality, and pesticide-free soybean production technologies.
2. Materials and Methods
2.1. Experimental Setup
The research was conducted in a 7.2 m
2 controlled environment growth chamber at the research field, Institute of Agronomy, the Hungarian University of Agriculture and Life Sciences (MATE), Gödöllő, Hungary, which is located at 47°46′ N, 19°21′ E, and 242 m above sea level. The chamber is equipped with planting tools, including pots with a capacity of 10 L and dimension of 19 cm in diameter by 22 cm in height, 117 cm × 60 cm fertilizer solution tanks, 1000 L/h water pumps (Newa Maxi IP68, Loreggia, PD, Italy; 220–240 V, 13 W), drip irrigation system, and timer. A total of 24 pots were placed on top of 4 nutrient solution tanks, where 6 pots were placed for each tank. Each tank was installed with a water pump that pumps the fertilizer solution in the tank through a drip irrigation system to each pot controlled by a timer. The arrangement of some equipment in this planting system is shown in
Figure 1.
In order to optimize the growing conditions for soybean growth, the chamber was installed with air conditioning to control the temperature, fluorescent lamps to supply enough light, fans for a sound ventilation system, and an exhaust fan for airflow. The growth chamber’s temperature was 22 °C during the day and 16 °C at night. Each nutrient concentration treatment (main plot) had two fluorescent lamps installed, each 58 watts, with a combination of red and blue lights. The lamp was turned on automatically for 16 h at 950 Lux and turned off automatically for 8 h at night. The humidity percentage in the chamber was between 40 and 60%. Before planting, pots were filled with substrate culture as a growing medium, which used expanded clay aggregate. The research was carried out from January 2022 to November 2022.
2.2. Experimental Treatments and Design
This study examined four different nutrient concentrations and two different soybean varieties. There were four different nutrient concentration treatments: 0, 50, 100, and 150%. The control treatment was 0%, which means no nutrients were supplied to the soybean plants under this treatment. Meanwhile, Martina and Johanna were the two tested varieties. Martina and Johanna are early maturing varieties from Hungary, with standard maturation times of 138 days for Martina and 140 days for Johanna. The Martina variety has a standard plant height of 106 cm, and the Johanna variety has a standard plant height of 91 cm. Both varieties have mature-brown pods and yellow seeds. Martina has a standard thousand-grain weight of 187.1 g, and Johanna weights 193.4 g. The fertilizer used was ‘Advance Hydroponics of Holland (Dutch Formula)’ in liquid form, a commercial fertilizer. This fertilizer is suitable for hydroponics cultivation and consists of three different formulas, namely formula 1 (Grow), 2 (Bloom), and 3 (Micro).
Table 1 indicates the nutrient content of each of the three formulas.
The use of fertilizer was in combination, and the rate was according to the growth stage of the soybean plant. Formula 1 was applied during the first vegetative stage (V1), the second vegetative stage (V2), the third vegetative stage (V3), the fourth vegetative stage (V4), the fifth vegetative stage (V5), and the flowering stage. The fertilizer formulations 2 and 3 were given at all stages of plant growth, including the end of the flowering stage. Identification of each of these stages is crucial, especially when deciding when to apply nutrients. According to Purcell et al. [
36], the vegetative stages of soybean plants can be determined based on their leaves and nodes. When the unifoliated leaves are fully developed, the plant is at its V1 stage.
Meanwhile, when the plant has unifoliate leaves and two trifoliate leaves, the edges of the early developing trifoliate are not touching, indicating that the plant is in V2. The nodes above the unifoliate leaves and vegetative growth are identified from V2 to the highest node of the plant (Vn). If a minimum of 50% of the examined plants reached that stage, the whole field can be considered in that stage [
37]. Each fertilizer formulation needs to be diluted with water, and in this study, as much as 25 L of water was used in each fertilizer tank. Therefore, the amount of fertilizer used based on 25 L of water and according to the plant growth stage and treatment is shown below (
Table 2). The nutrient solutions started to be supplied to the plants for all treatments after 10 days of germination.
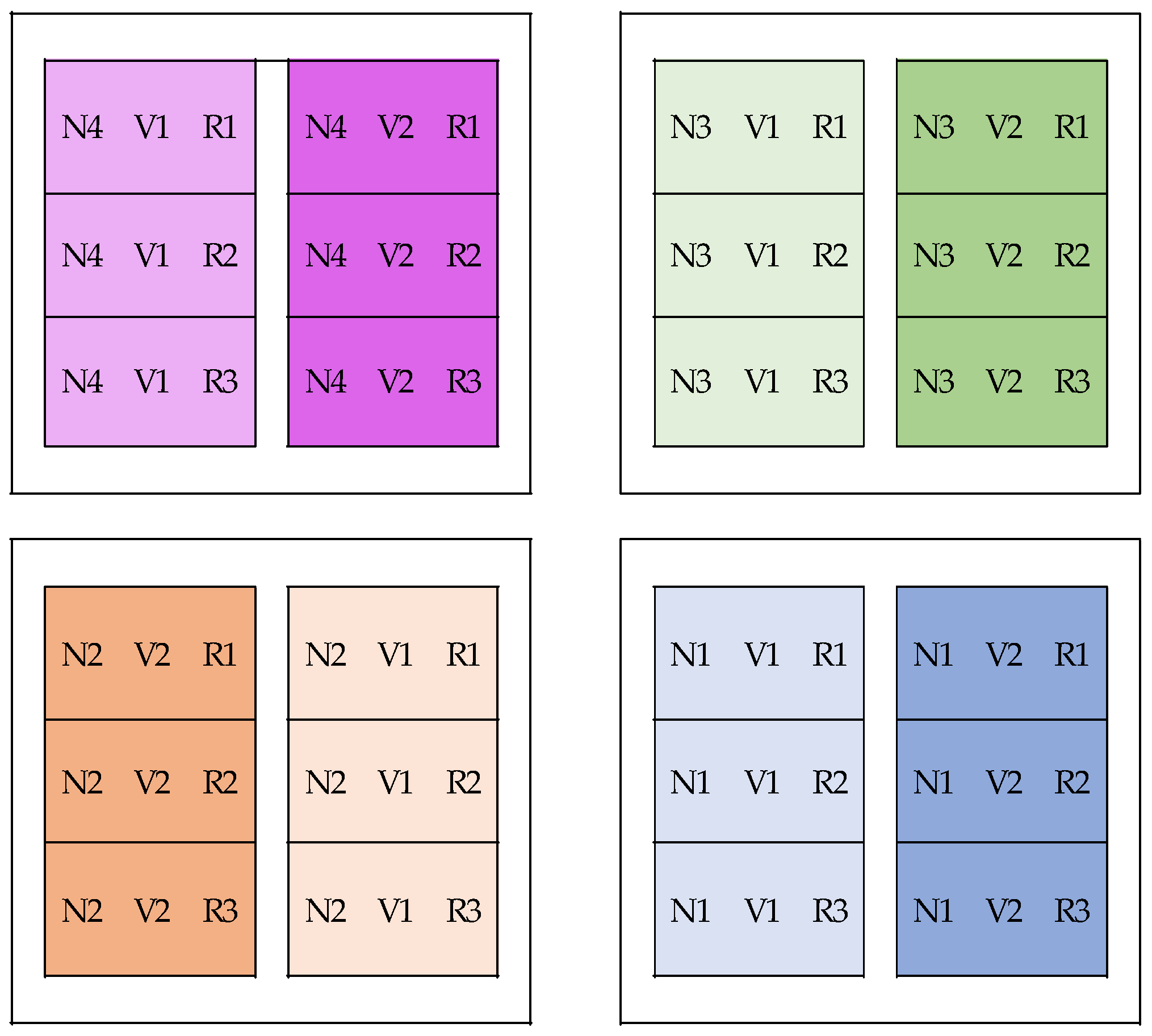
The experimental design for this study was a split plot with three replications. The nutrient concentration factor was a central plot, while the variety factor was a subplot.
Figure 2 below shows the experimental layout for this study.
2.3. Planting and Crop Management
Soybean seeds of both varieties that have a growth rate of more than 90% were used in this study. A total of 8 seeds were sown directly into the pot by planting the seeds 3 cm deep into the growing medium. Six seedlings that were healthy and growing uniformly were retained in the pots, while the other 2 were uprooted and discarded after 10 days of planting.
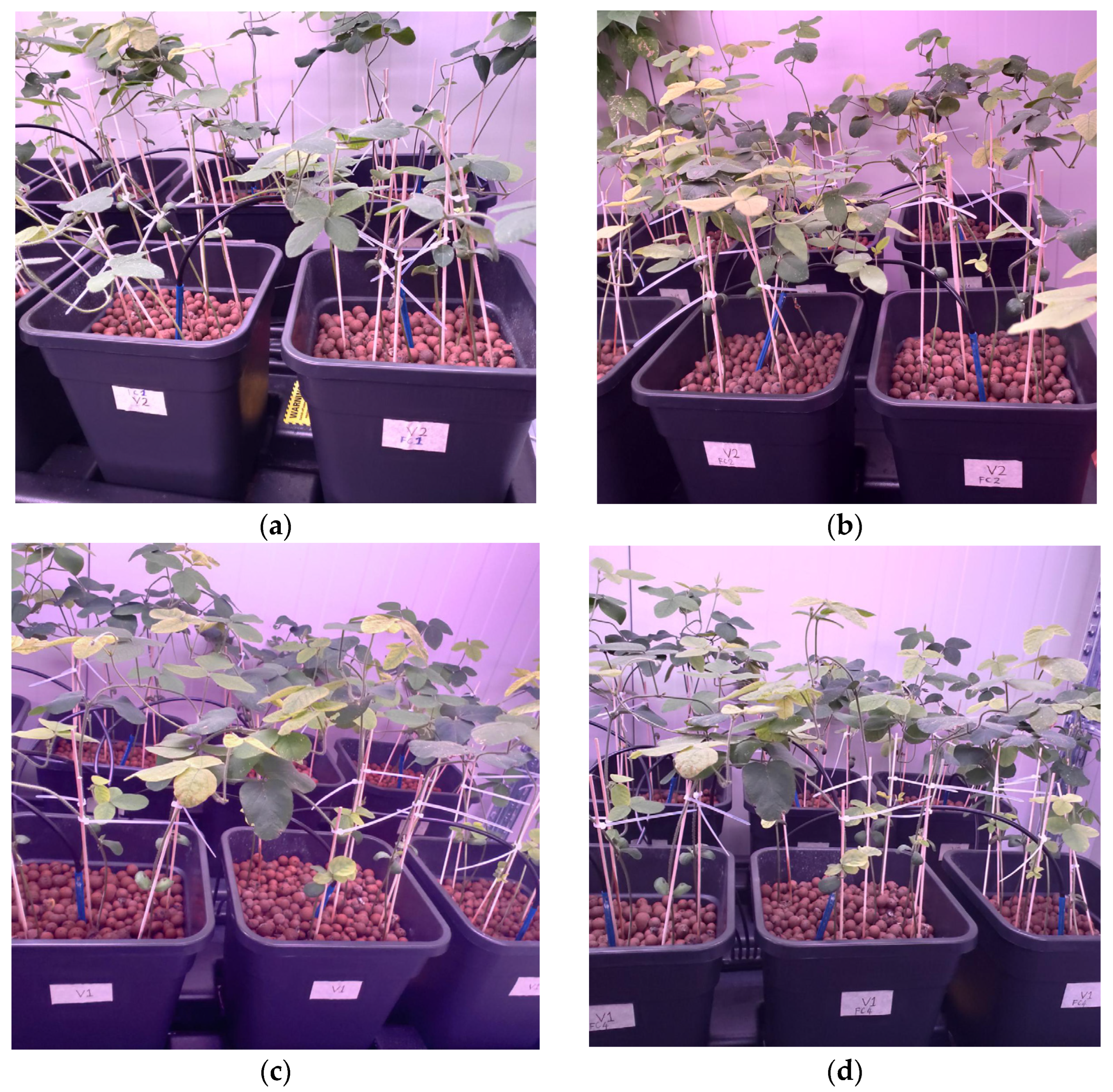
For the first 10 days of planting, during the germination period, each pot was irrigated without nutrients thrice daily for 30 min each irrigation. Irrigation with fertilizer solution was then given according to the treatment and growth stage of the plant from the 11th day after planting. The fertilizer solution was automatically given thrice daily for 30 min per irrigation. The solution was manually replaced weekly by pumping it out of the tank and refilling it with a new fertilizer solution. The aim was to maintain the proper range for the EC and pH readings. The plants treated with different nutrient concentration treatments 60 days after planting are shown in
Figure 3. Fallen leaves in and around the pot were collected and disposed of to prevent the growth of fungi that would damage the plant.
2.4. Harvesting and Sampling
Soybean plants are mature when the seeds, pods, and stem turn yellow. However, harvesting should be conducted when the soybean pods are completely dry and turn brown. In this study, plants were harvested at the age of 162 days after planting. After the pods were harvested, they were oven-dried for two days at 50 °C to reduce the moisture in the seeds and reach the appropriate moisture of less than 13%. This purpose was to prevent seed damage, mould, and insect attacks. After the pods were completely dried, the seeds were then removed from the pods and stored in a covered and dry area.
After all the pods were harvested, six pot plants were uprooted and separated into two parts: above ground (leaves and stem) and below ground (root). Both parts were then oven-dried at 50 °C for dried weight measurement for two days.
2.5. Data Collections
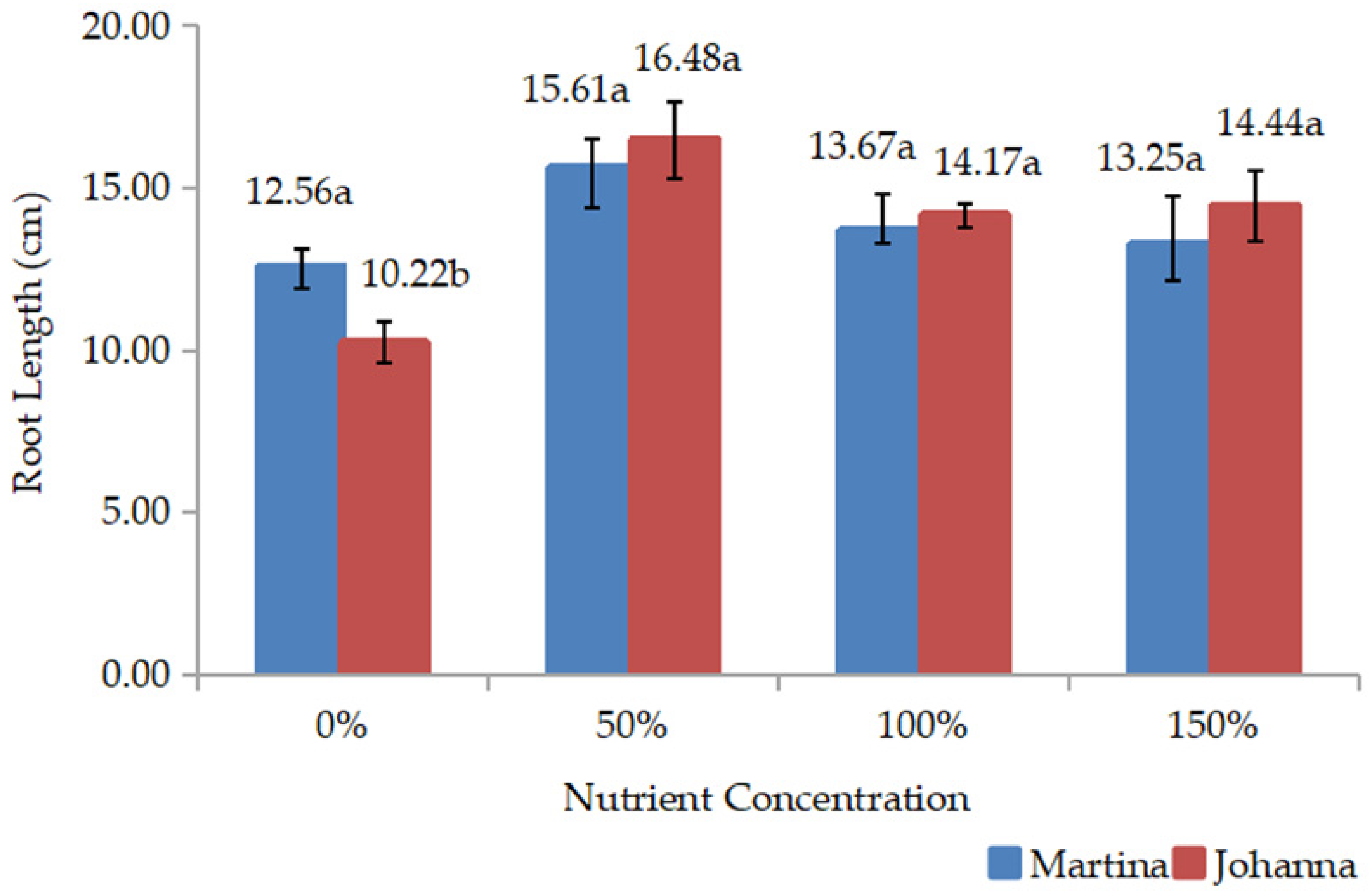
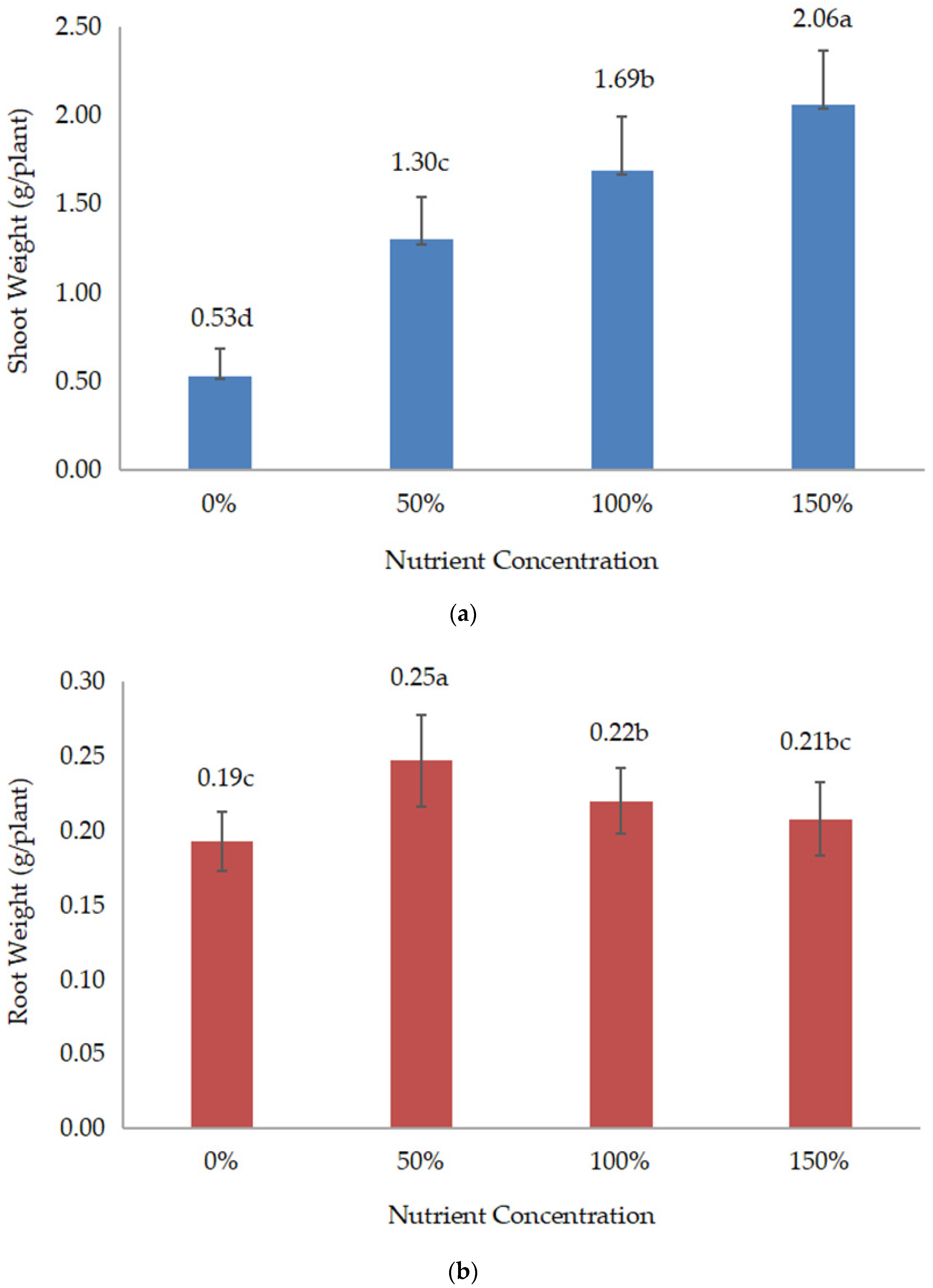
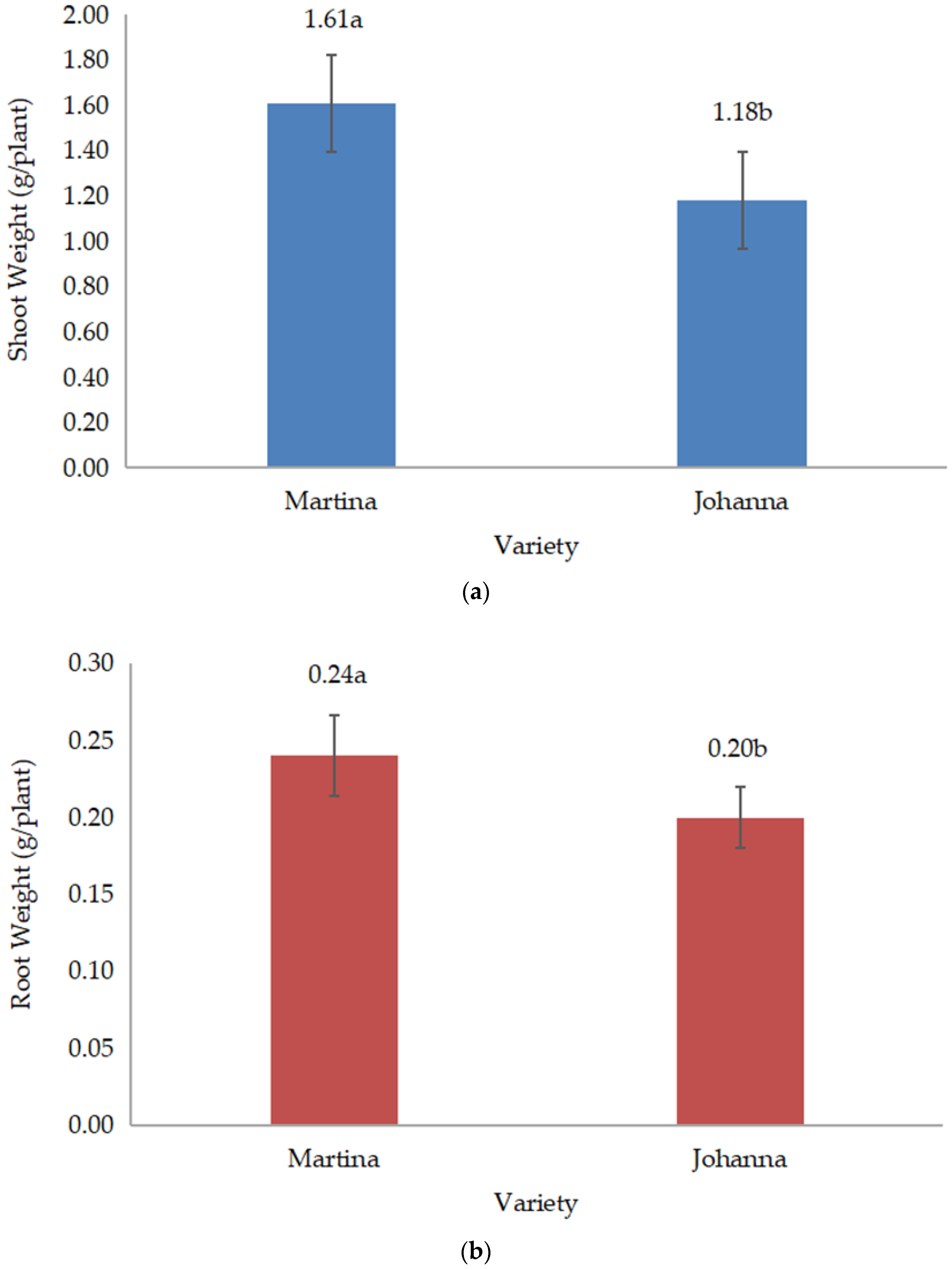
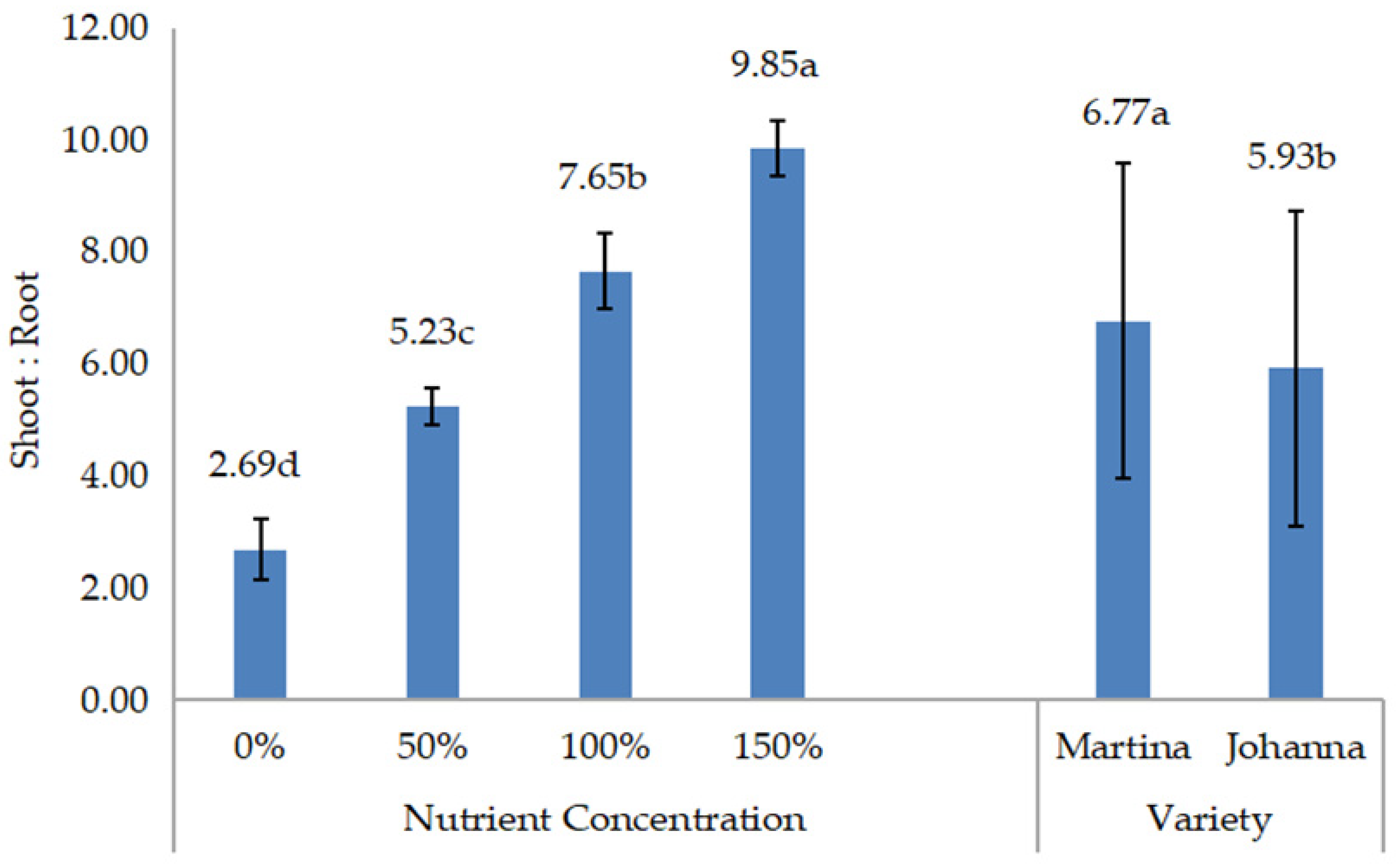
All data were collected during and after harvest. The collected data include root length, root weight, shoot weight, shoot:root, yield components, yield, and chemical composition. Root length or deep root was measured vertically, which was conducted during harvest. Meanwhile, shoot (above the ground) and root (below the ground) weights were measured by weighing the weight of both parts of the plant after drying. When the root and shoot weights were measured, the shoot:root ratio was calculated and recorded. Data on yield components, such as the number of pods/plants and the number of grains/pods, were recorded during harvesting.
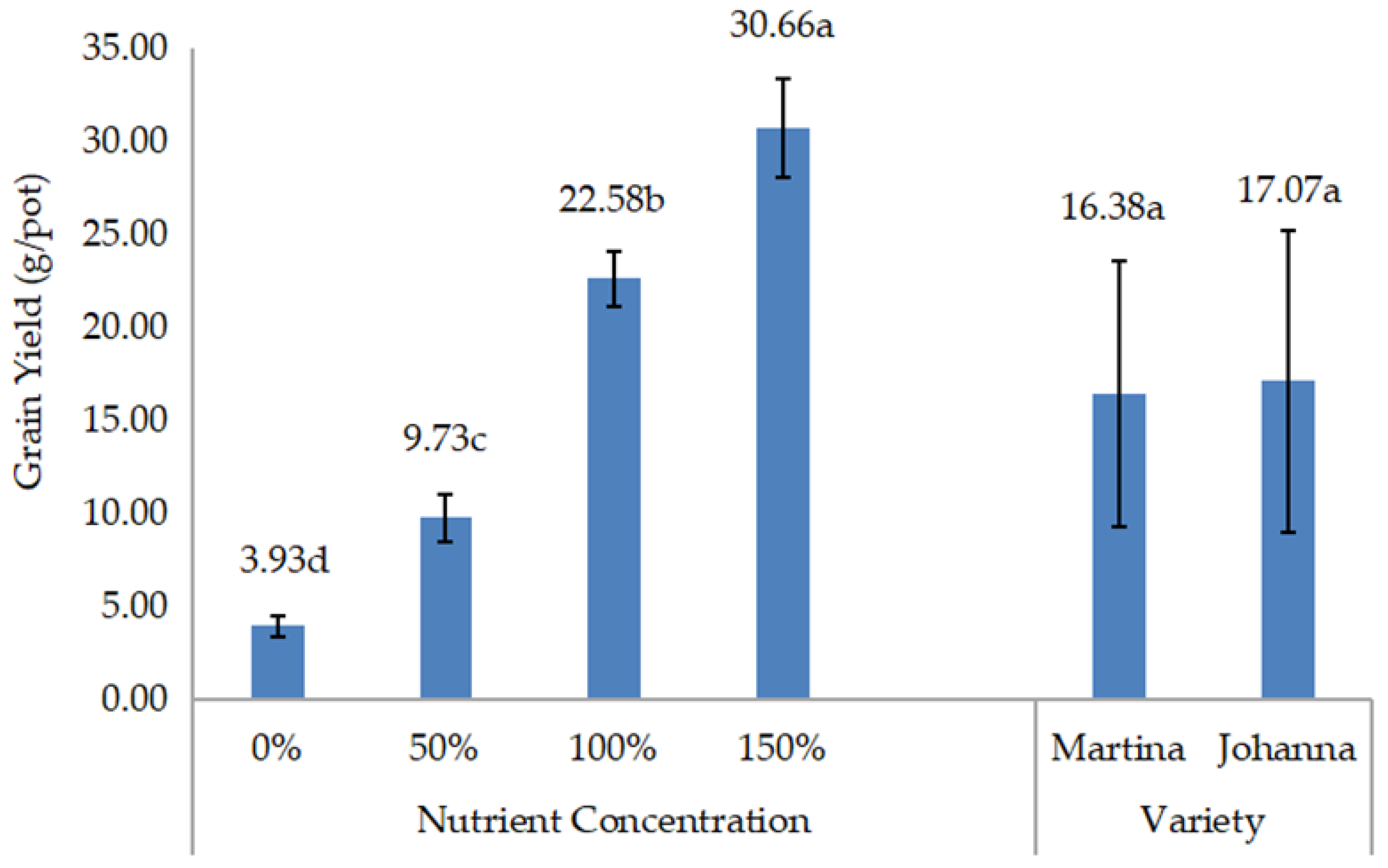
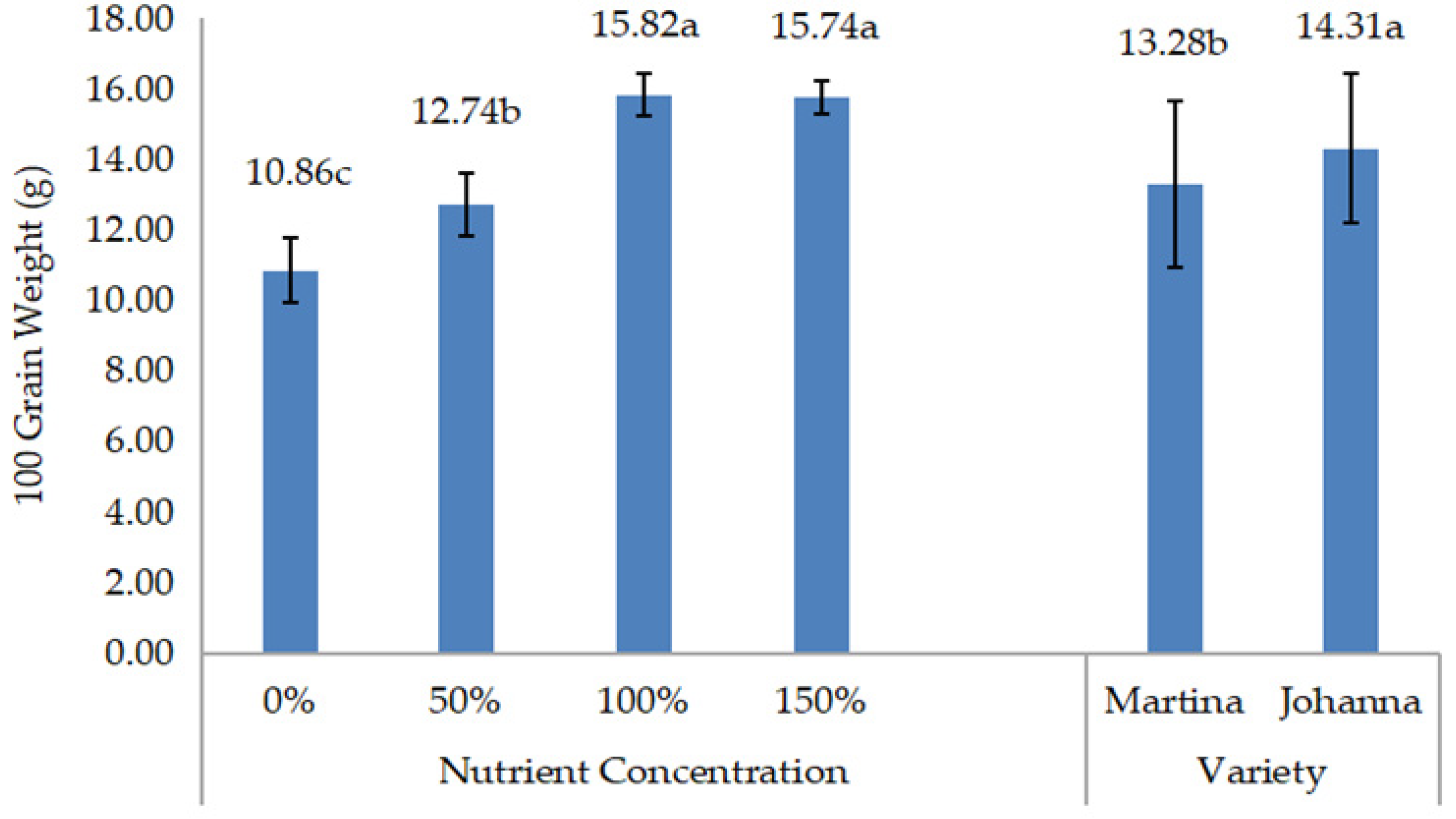
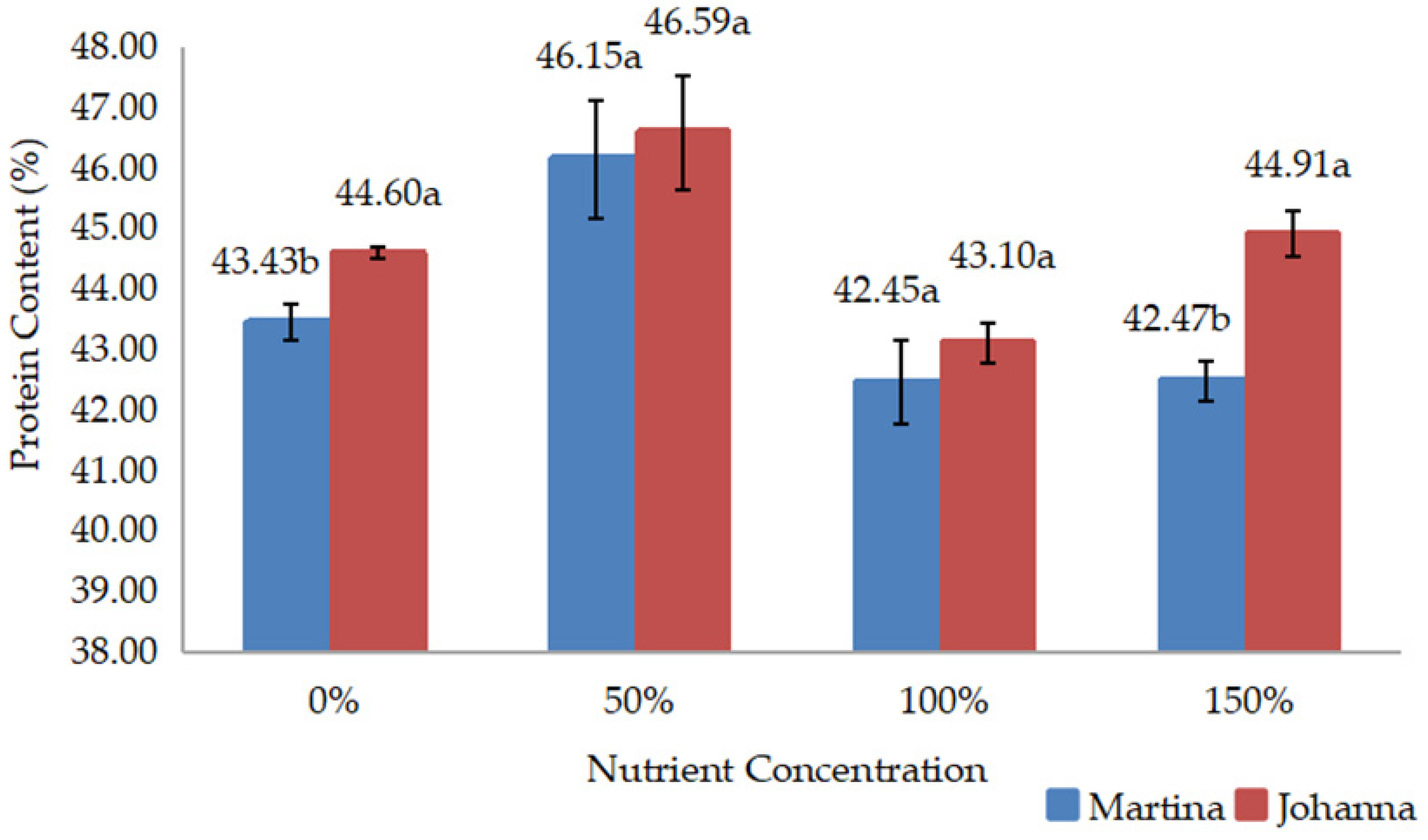
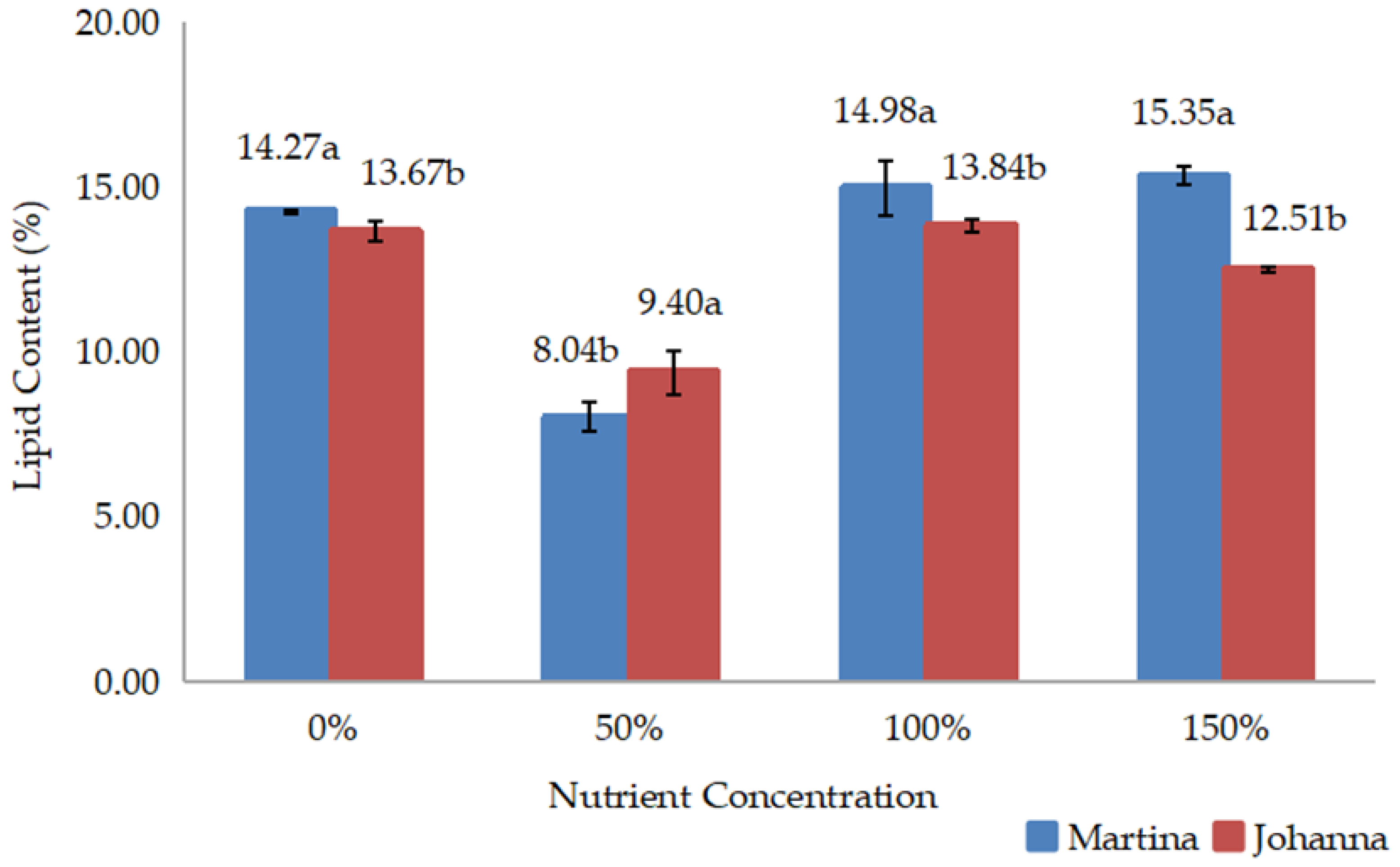
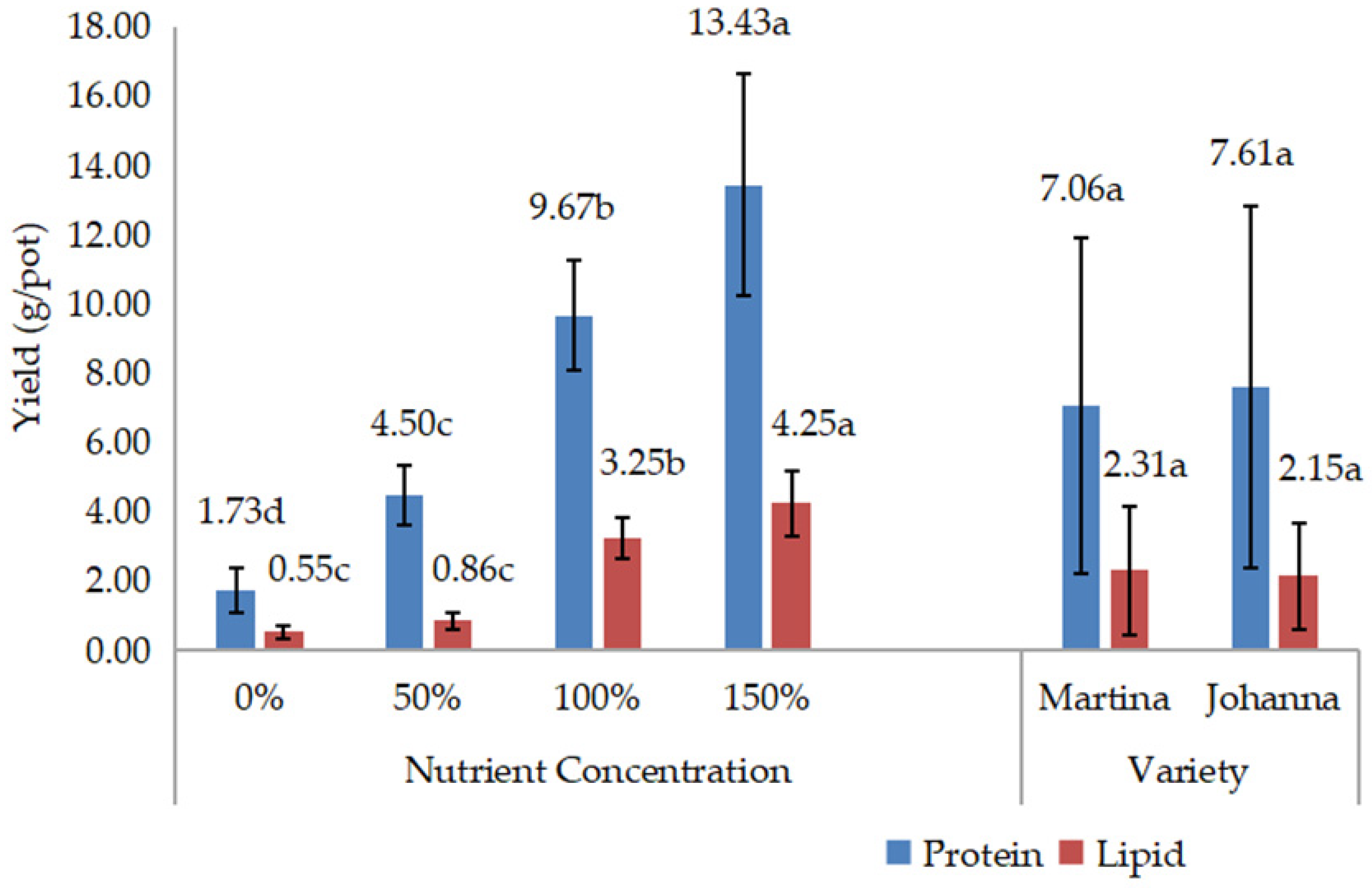
Meanwhile, yield data such as grain weight/pod, grain yield/pot, and 100-grain weight were recorded three days after harvest, when the pods were dried. The grains from each treatment were then grounded to measure chemical composition, including protein and lipid content. Protein and lipid content expressed as percentages were measured in the laboratory using a NIR Product Analyzer (INSTALAB 600, Auburn, IL, USA). The yields of protein and lipids were then calculated by multiplying their contents by grain yield.
2.6. Statistical Analysis
The reported results and data are presented as averages for the main effect of nutrient concentration, variety, and the interaction of the main effects. At the significance level of p = 0.05, a two-way analysis of variance (ANOVA) was carried out to compare the effects of nutrient concentration and variety on all recorded parameters. Post hoc comparisons were then performed using the least significant difference (LSD) test at p < 0.05. All the statistical analyses in this study used a 5% significance level. Meanwhile, the software of IBM SPSS V.23 (SPSS Inc., Chicago, IL, USA) was used to analyze the data.