Abstract
Arbuscular mycorrhizal fungi are biotrophic fungi that form an association with plant roots and render benefits in nutrient uptake, disease control and plant tolerance to stress conditions. Plant–mycorrhizal fungi interaction has been proposed as a suitable tool for contributing to sustainable agriculture and reducing the dependence on agrochemicals. Interactions between plants and arbuscular mycorrhizal fungi are regulated by several factors ranging from host traits to environmental conditions that affect the species richness, diversity and functions. In this review, we highlight recent advances on how host traits and environmental conditions in farming systems and/or in natural ecosystems affect the richness, physiology and ecological functions of arbuscular mycorrhizal fungi while specifying the gaps that need to be filled through research.
1. Introduction
During the mutualistic association, mycorrhizal fungi mediate the interactions between their host plants and soil microbiomes (pathogens, beneficial microbes and mycorrhizosphere mutualists) that contribute to belowground plant traits and to plant–plant interactions [1,2]. The mycorrhizal fungal influence on plants and the soil microbiome could have corresponding effects on ecosystem processes like the biogeochemical cycling of nutrients. Mycorrhizal fungi, based on their morphologies, type of association with plants, ecology, arrangement of hyphae within plant root tissues and the plant species associated with them, can be grouped as ectomycorrhizae (ectomycorrhizas category), arbuscular mycorrhizae, orchid mycorrhizae and ericoid mycorrhizae [3]. Each of these ubiquitous mycorrhizal fungi forms a common mycelial network, which penetrates into the soil to extract and transports nutrients and water to its hosts. The mycelia equally return plants’ photosynthates to the soil to support the fungi-associated microbiome and connect one plant to another for ease of redistribution of resources (organic carbon, signal molecules, water and nutrients) among plants of the same or different species [2]. In this review, we highlight recent advances on how host traits and environmental conditions in farming systems and/or in natural ecosystems affect the richness, physiology and ecological functions of arbuscular mycorrhizal fungi while specifying the gaps that need to be filled through research.
Arbuscular mycorrhizal fungi (AMF) are a group of mutualistic fungi with a lifestyle of biotrophism during symbiosis. They depend on their host plants for carbon, which is transferred from the plants to the fungi during their symbiotic relationship. Most terrestrial plants (about 80 percent) are colonized by arbuscular mycorrhizal fungi, making their biotrophic nature a prerequisite for their association with plants as well as for aiding plants’ colonization, invasiveness, nutrient and water uptake and adaptation to new and harsh environments [4,5].
Besides nutrient uptake and exchange of nutrients among plants, arbuscular mycorrhizal fungi also communicate warning signals from plants under attack by herbivores and phytopathogens to healthy plants [6,7]. This signal communication aids in plants’ activation of pathogenesis defense genes and resistance phenotypes. In some cases, direct colonization of host plants by AMF could result in plant resistance to pathogens. For instance, through the activation of salicylic acid and jasmonic acid defense-related genes in tomato plants by the arbuscular mycorrhizal fungus, Gigaspora margarita, tomato plants developed immunity against Botrytis cinerea and Pseudomonas syringae pv. tomato DC3000 pathogens [8]. In another study, a tomato plant was observed to upregulate defense genes, phenylpropanoids, chlorogenic acid and flavonoids, resulting in the control of Tomato Mosaic Virus accumulation level and its associated Tomato Mosaic disease in infected plants under the influence of mycorrhizal fungi [9]. A recent review by Dowarah et al. [10] has shed light on the various mechanisms employed by arbuscular mycorrhizal fungi in enhancing plants’ tolerance and resistance to phytopathogens in the soil.
In addition to pathogen control, AMF could facilitate plant recognition of their kin through chemical signatures in their exudates [11] and increase resource exchange among plants of the same species. This might be the rationale behind the observed increase in nutrient uptake, recovery and use efficiency of densely populated chili peppers growing together at short planting distances on farmland, as reported by Mali et al. [12]. Taken together, AMF are highly essential for plants’ health, survival and productivity. Here, we integrate experimental results from different studies and seek to understand the effects of host traits and environmental parameters on mycorrhizal (arbuscular mycorrhiza) fungal richness and ecological functions. We will discuss the mechanisms of AMF recognition and colonization of plants; the relation between AMF richness, plant biodiversity and ecological functions; and factors affecting AMF richness and functions in the soil.
2. Arbuscular Mycorrhizal Fungi Recognition and Colonization of Plants
Recall that arbuscular mycorrhizal fungi are ubiquitous soil-borne fungi that are components of plant holobionts. Their biotrophic nature and significant role in plant growth and development have made their association important in sustainable plant production. The genetic composition of AMF and plants promotes the ease of establishment and sustenance of beneficial symbiotic associations among them. The phases of this association begin with pre-colonization, penetration/colonization and establishment. The process begins with the growth of plants on soil under a light source. The light absorption by plants activates shoot phyB (phytochrome B), which triggers the accumulation of shoot-produced mobile HY5 proteins (ELONGATED HYPOCOTYL 5) in the plant roots. These accumulated HY5 proteins further activate and/or positively regulate the transcription of SL (strigolactones) synthetic genes and the subsequent production of strigolactones in the roots [13]. These strigolactones signaling molecules (phytohormones) produced in the roots are discharged into the soil through the aid of HPC (suberin-free hypodermal passage cells) present in the exodermis of the roots and in conjunction with PDR1 (Pleiotropic Drug Resistance 1) exporter protein channels. This suberin-free HPC is the channel for fungi penetration and colonization of the root cortex and influences the fungi penetration level in the colonized roots. It controls the quantity of strigolactones excreted by the plants and its concentration gradients in the environment [14,15,16]. Besides the influence of light on strigolactones production, low P availability is another trigger of strigolactones production. Plants growing under low soil phosphorus levels increase their production of strigolactones for efficient adaptation to the conditions. Phosphorus deprivation is indeed a good measure for improving plant strigolactones production and enhancing AMF hyphal branching. The elevated strigolactones that are produced increase root hair density, lower shoot branching and stimulate lateral root formation [17,18,19,20]. The presence of strigolactones in the soil triggers the germination, elongation and branching of fungal hyphae as well as the production and release of mycorrhizal factors (a group of short-chain chitooligosaccharide molecules) that aid plants’ recognition of the fungi (Figure 1).
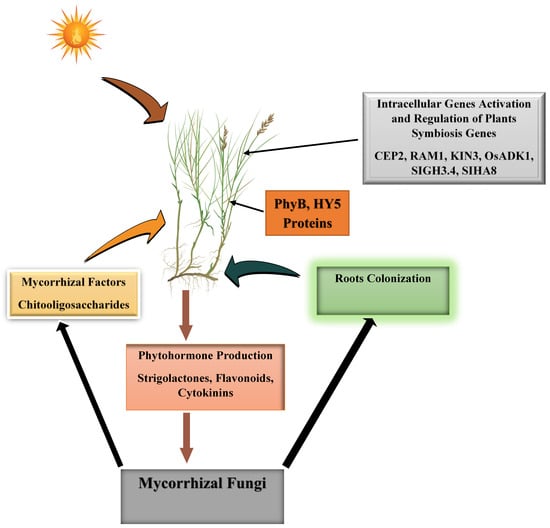
Figure 1.
Environmental and biological processes involved in arbuscular mycorrhizal fungi recognition and colonization of plant roots and symbiosis. phyB (phytochrome B), HY5 proteins (ELONGATED HYPOCOTYL 5), CEP2 (C-terminally encoded peptide), RAM 1 (Require for Arbuscular Mycorrhization 1), OsADK1 (arbuscule development kinase 1), SlHA8 (a periarbuscular membrane-bound H+-ATPase), Kinase 3 (KIN3), SlGH3.4 (encoding IAA-amido synthetase).
To sense these mycorrhizal factors, the plant uses its receptor (Myc Factor) and a receptor-like kinase (SYMPK) that transmit the signal from the cytoplasm to the nucleus through a cascade of phosphorylation (of CYCLOPS—a SYM gene product and transcription factor) as well as calcium oscillation-dependent processes. This leads to the regulation of some important immune defense genes and other genes that eventually permit the penetration of the fungi into the plant tissues [21]. During this process, the gene for CEP2 (C-terminally encoded peptide) expression, which is responsible for controlling lateral root growth and development through auxin-related pathways, was downregulated to permit lateral root formation and symbiosis [22]. In response to the presence of mycorrhizal fungal symbionts, the plant activates the KIN3 gene, which, through the action of RAM 1 (Require for Arbuscular Mycorrhization 1—a transcriptional factor), acts upstream of the KIN3 gene and facilitates the suppression of plant immune defenses. Plants defective in KIN3 genes exhibit decreased root colonization [23]. Another essential gene for promoting root colonization by mycorrhizal fungi is arbuscule development kinase 1 (OsADK1). This gene is required for the development of arbuscules by the fungi and arbuscules branching within the infected root tissues. It is induced in the arbusculated root cortex. A study has shown that mutation of this kinase gene in rice plants could result in a significant decrease in Rhizophagus irregularis colonization of rice roots [24], indicating its importance during plant–mycorrhizal fungi symbiosis. Additionally, maintenance of auxin homeostasis within the roots by IAA-amido synthetase (a gene product of GH3 gene—SlGH3.4) is implicated in the mycorrhization regulation. Auxin (indole-3-acetic acid) accumulation in plants facilitates mycorrhizal fungi symbiosis; however, high cellular concentrations of auxin repress mycorrhization processes. Perhaps the presence of moderate free indole acetic acid in the root cells due to the loss of SlGH3.4 gene function will lead to an increased incidence of arbuscules in the root.
Therefore, the colonization of roots by arbuscular mycorrhizal fungi will downregulate the indole acetic acid biosynthesis gene while promoting auxin response and indole acetic acid accumulation [25]. Another important hormone similar to auxin is cytokinin. Cytokinin has an efficient stimulatory effect on mycorrhizal fungi colonization, and its suppression or absence reduces the mycorrhization activity of the fungi. A study has shown that wild-type pea plants treated with synthetic chemical compounds known to alter the cytokinin status of the plants in the presence of arbuscular mycorrhizal fungi resulted in a lowering of plant nucleotides (precursor for cytokinin production) and more colonization than the mutant pea plants under the same treatment [26]. Cytokinin is essential for the establishment of mycorrhizal fungi–plant symbiosis.
Suffice it to say that yet another important gene that promotes efficient root colonization, arbuscule development and exchange of nutrients between fungi and plants is SlHA8 (a periarbuscular membrane-bound H+-ATPase). This hydrogen ion gradient generation system facilitates phosphate ion transport from the interfacial apoplast into the plant cells upon reception of phosphate ions from the fungi. Deletion of this gene (SlHA8) has been implicated in the truncation of arbuscules’ morphology, decreased accumulation of phosphorus and nitrogen in plant shoots, and acidification of the apoplastic spaces in the arbusculated root cortical cells. But, the reverse is the case with the overexpression of this ATPase gene, with no observed effect on arbuscule morphology [27]. Therefore, the SlHA8 gene is needed in plant–fungi symbiosis. Since these genes are paramount for mycorrhizal fungi symbiosis, studies should focus on the biotechnological potentials of these genes and how they can be exploited in the genetic engineering of plants to increase biofortification of mineral nutrients in crops and aid them in tolerance to climate-change-associated drought stress. Within the plants, there are also differentially expressed genes that are activated during mycorrhizal symbiosis and/or arbuscular mycorrhizal fungal infection of the plant roots. These genes are essential for many physiological and morphological changes in plants and are upregulated during colonization. The products of these genes participate in cell wall structural adjustments, membrane transport, transcriptional factors and plant accommodation of the fungi [28].
Other chemical substances that influence fungal hyphal growth toward the plant roots are flavonoids and 2-hydroxy fatty acids. They induce hyphal elongation and branching and consequently promote plant–mycorrhizal fungi interactions [29]. Flavonoid excretion, though a constituent of root exudates, requires further study to understand the protein channels responsible for its transport from the roots into the soil. This will now lead us to comprehend how the establishment of this association depends on host traits and environmental factors and its corresponding effects on arbuscular mycorrhizal fungal richness.
3. Arbuscular Mycorrhizal Fungal Richness, Plant Biodiversity and Ecological Functions
Plant diversity and species composition affect terrestrial ecosystem stability and functions [30,31]. The diversity of plants, on the other hand, is influenced by a number of factors such as spatiotemporal partitioning of resources, competition among plants, plant–herbivore–pathogens interactions, etcetera [32,33,34,35]. Of all these factors, one major determinant of plant biodiversity and ecological function is mycorrhizal fungal richness (Figure 2). Arbuscular mycorrhizal fungi, as previously stated, form symbiotic associations with terrestrial plants’ roots and facilitate plants’ nutrient uptake (phosphorus) as well as increase plant biodiversity [36,37]. An increase in the population of mycorrhizal-dependent plants over the low and independent AMF plants has been linked to AMF-mediated resource allocation to the associated plants. The supply of nutrients to plants by AMF aids the establishment and coexistence of mycorrhizal-dependent plants with other plant species in a given ecosystem [38]. This mycorrhizal fungal influence on plant diversity and structure is a function of the fungal species composition within the mycorrhizal fungal community. To illustrate, different plants respond differently to arbuscular mycorrhizal fungi association. While observing the effect of single and mixed species of arbuscular mycorrhizal fungi on plant species (Hieracium pilosella, Festuca ovina and Bromus erectus), Van der Heijden et al. [39] observed that the response of the plant species differed in their level of dependency on the arbuscular mycorrhizal fungi. Plant growth variables and variation in growth response were also different among the plant species. The growth response of the Hieracium pilosella plant, a mycorrhizal-dependent plant, differed with different arbuscular mycorrhizal fungi species. While those of the less dependent mycorrhizal plants, Bromus erectus, showed no variation in their response to different arbuscular mycorrhizal fungi species present. Thus, the types or species of arbuscular mycorrhizal fungi colonizing Bromus erectus do not affect its physiology like the former. For instance, the authors also observed that on the basis of plant biomass (dry mass) and in the presence of Glomus geosporum, the coexistence of Hieracium pilosella and Bromus erectus will result in the growth reduction of the former due to the competitive exclusion effect of the Bromus erectus on the Hieracium pilosella which has low biomass. However, in the presence of mixed species of arbuscular mycorrhizal fungi, the growth variables (biomass) of Hieracium pilosella are significantly enhanced, and the two plant species can coexist efficiently.
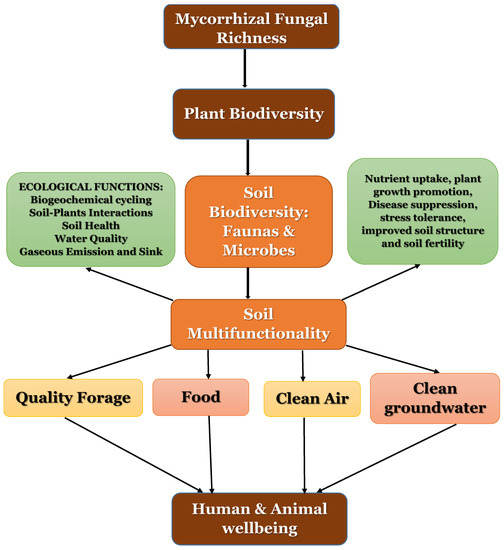
Figure 2.
Schematic overview on the influence of arbuscular mycorrhizal fungal richness on plant biodiversity and ecosystem functions. Here, arbuscular mycorrhizal fungal diversity influences the selection, establishment and maintenance of plant biodiversity with corresponding effects on soil biodiversity and soil multifunctionality.
In another study, Van Der Heijden et al. [37] posited that the richness of arbuscular mycorrhizal fungi species is a remarkable contributor to plant species diversity, composition and productivity. They found that several plant species were dependent on arbuscular mycorrhizal fungal species for establishment and successful coexistence. These plants are mycorrhizal-dependent plants. In contrast, regardless of the presence or absence of arbuscular mycorrhizal fungi, Carex flacca plants recorded an increased tissue biomass. Similarly, another plant that showed no response to the alteration of arbuscular mycorrhizal species richness was Bromus erectus. These plants, as described above, do not depend on mycorrhizal fungi to meet their nutritional needs and can exit with or without AMF association. Taken together, arbuscular mycorrhizal fungal association is required for plant biodiversity maintenance. At low mycorrhizal fungi diversity or species richness, large fluctuations in the structure, diversity and composition of plants will occur simultaneously if the composition and richness of the arbuscular mycorrhizal species present is altered. Therefore, high mycorrhizal fungal species richness will result in a corresponding increase in plant diversity. And low plant diversity and productivity will positively correlate with the absence or low richness of mycorrhizal fungi species.
On the basis of this evidence, arbuscular mycorrhizal fungal richness has been shown to significantly affect plant biodiversity and ecological functions in the soil. As microbes contribute to the performance, health and productivity of plants, so do plants, in return, enhance the activities, richness and productivity of microbes in the soil. High plant species richness has been shown to increase the catabolic diversity of soil-borne culturable bacteria, as observed by Bartelt-Ryser et al. [40]. In another study, very importantly, it was uncovered that vegetation types comprising different plant species support very high microbial activities and functions in the soil [41]. Nevertheless, the opposite is also true, as shown by the plant diversity hotspots in the Mediterranean regions of South Africa and Australia, which have extremely poor soils with very low microbial activities and communities. In these regions, the effects of plant diversity on soil microbes are impaired by the prevailing water and nutrient stress conditions orchestrated by extreme climate conditions [42]. It has been noticed that soil water and nutrient availability promote plant productivity and the nutritional quality of its litter, which stimulate enhanced microbial activities in the soil [43,44]. Although plant communities in the Mediterranean regions form associations with mycorrhizal fungi, environmental stress (drought, temperature and poor soil nutrients) reduces the population density of the fungi but not their richness since these organisms are evolving adaptive strategies to cope with the stress [45,46]. Through the metabolic actions of roots in the release of exudates, plant species tend to recruit and support soil-dwelling microbes. Plants also provide suitable habitats for the establishment of diverse microbial communities through the release of litter with a high nutritional composition, root exudation and enhancing soil organic matter composition, which together influence the microbial biomass, catabolic capacity and their community structure [41,47]. Therefore, an increase in mycorrhizal fungal richness correlates positively with an increase in plant species richness. The composition of plant species, as well as their functional groups, determines the composition of specific fungal and bacterial communities present in the soil. This effect is a result of plants’ rhizosphere effects in recruiting diverse species of microbes that could be associated with the diverse species of plants present in the habitat [48,49]. In return, soil microbes play significant roles in nutrient cycling, decomposition of organic molecules or polymers, plant growth promotion, homeostasis, phytohormone production, acting as a sink for carbon, maintenance of soil health, disease suppression and plant immunity [50,51,52,53,54]. These ecological functions performed by microbes in the soil have resultant effects on the environment, humans and animals. On the premise of the above-presented evidence, arbuscular mycorrhizal fungal richness is a major driver of plant and soil biodiversity as well. In the next section, we will be discussing the various factors affecting mycorrhizal fungal diversity.
4. Factors Affecting Mycorrhizal Fungal Richness and Function in the Soil
The degree of richness, survival, development, distribution and ecological functions of arbuscular mycorrhizal fungi are under the influence of environmental and plant variables. Among these variables are host traits and succession, soil fertility and soil fertilization, altitude, soil pedogenesis, tillage, land use changes or practices, seasonal variation, temperature, and soil moisture (Figure 3). Within these variables lies the ecological functional efficiency of arbuscular mycorrhizal fungi, their reproduction and their abundance in the soil. Here, we will explain the various factors that influence the richness and functions of mycorrhizal fungi.
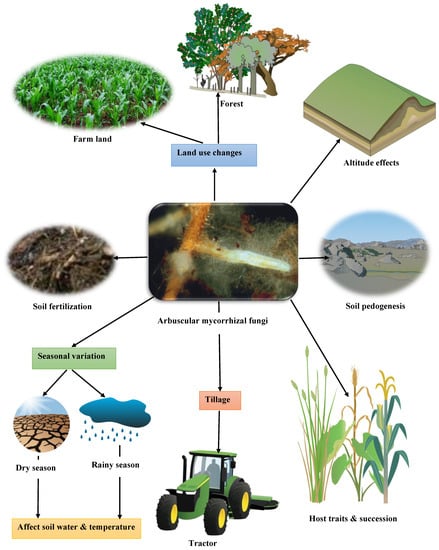
Figure 3.
Comprehensive representation of the biotic and abiotic factors that affect arbuscular mycorrhizal fungi richness and functions in the soil. The picture of the mycorrhizal fungi in the middle was adapted from Egli and Brunner [55] published in 2011 by Swiss Federal Research Institute WSL, Birmensdorf, with permission from the publisher. Some of the other pictures in the diagram were created using IAN Image Library, accessed on 10 August 2023 (http://ian.umces.edu).
4.1. Host Traits and Succession
As we are aware, different plants bear different genes with varying distributions and abundance of symbiosis-activated genes and phenotypes. Plant–fungi interactions vary with arbuscular mycorrhizal fungal species that are under the influence of host genotypic and phenotypic characteristics. For instance, it was recorded that sorghum plants exposed to two different species of arbuscular mycorrhizal fungi (Rhizophagus irregularis and Gigaspora gigantea) exhibited different levels and types of association. Rhizophagus irregularis increased plants’ phosphorus uptake and exhibited mutualistic and plant growth promotion effects on the host plants. Meanwhile, Gigaspora gigantea showed no evidence of benefits on nutrient acquisition or growth in the plants. It equally retarded the growth of the plants and activated the production of antifungal substances (p-hydroxyphenylacetaldoxime) in the host plants. Differences in the root metabolome of these plants exposed to different species of arbuscular mycorrhizal fungi have clearly shown that plant genotypes greatly determine the outcome of the association [56]. In the same study, it was also observed that co-inoculation of the plants with both species of arbuscular mycorrhizal fungi transformed the outcome of the association into a mutualistic symbiotic association between the plants and the fungi. The question that was not answered by the study was to what extent Rhizophagus irregularis was different from Gigaspora gigantea in the suppression of host immunity to aid the establishment of symbiosis. Every symbiotic association is first challenged with plant immune defenses, but the successful suppression of the plant immune system will aid the colonization of plants by the fungi [57].
Therefore, different plants respond differently to different species of mycorrhizal fungi. This specificity in plant response is a prerequisite for arbuscular mycorrhizal fungi to exert an effect on the plant community. Nevertheless, most plants in nature are colonized simultaneously by several species of arbuscular mycorrhizal fungi, which leads to the enhancement of resource trading among the plants, with a corresponding influence on the population dynamics, diversity and coexistence among the plant species [58]. The life history of the plants under the influence of evolution could lead to changes in the phenotypic and genotypic properties of the plants. These intrinsic changes in plant physiology, based on their successional stage and growth rate, determine their responsiveness to arbuscular mycorrhizal fungal association [59]. Koziol and Bever [60] reported that tallgrass prairie at different successional stages have different degrees of responsiveness to the arbuscular mycorrhizal fungal association. Late successional plants, according to the authors, were more responsive, with greater specificity to the mycorrhizal fungi species involved than early successional plants. This difference could be related to coevolutionary pressure and adaptation of the fungi to the plants. Plants are constantly evolving to either accommodate or resist mycorrhizal fungal infection, colonization and association. Hence, plant species vary in their responses to different arbuscular mycorrhizal fungi [61,62], with resultant effects on the fungi richness. The quality of plants as hosts for arbuscular mycorrhizal fungi determines the mycorrhizal fungal growth, reproduction and abundance in the soil. However, plant community assembly, productivity and nutritional status are equally influenced by their tolerance, accommodation and establishment of symbiotic associations with arbuscular mycorrhizal fungi [58]. The ecological restoration of plants in an environment depends entirely on their association with mycorrhizal fungi [58]. Ancient and restored grasslands have different arbuscular mycorrhizal fungi taxa peculiar to the site. And upon complete restoration of the plant community to the site, it will significantly increase the diversity, richness and abundance of the mycorrhizal fungi. The mycorrhizal fungi community (arbuscular mycorrhizal fungi) composition of a restored grassland is the same as that of an ancient grassland but differs from that of an afforested grassland or grassland at the early stages of restoration [63]. This could be attributed to the physiology of plant species present, their developmental stages, plant–soil feedback and soil legacies (soil microbial legacies) prevalent at those sites [64,65]. Plant species exert selective pressure on the soil arbuscular mycorrhizal fungal communities. Conversely, the progression of aboveground communities influences and is influenced by that of AMF.
It is noteworthy to mention that despite plant traits influencing the community compositions of belowground associated microbes, they also determine the functional diversity and the phylogenetic structure of arbuscular mycorrhizal fungi communities associated with plants of different life cycles (annual, biannual or perennial plants), growth and life forms. The genetic makeup of plants determines the environment in which they will grow and thrive. For instance, a ruderal plant (with a characteristic nature of growing in waste ground or roadsides) is found to be associated with phylogenetically clustered arbuscular mycorrhizal fungal communities, while nutrient-conservative plants are associated with widely dispersed communities of arbuscular mycorrhizal fungi with different functional diversity [66]. Still, on plant traits, legumes and non-leguminous plants harbour different compositions of mycorrhizal fungi. Legumes are characterized by low taxonomic diversity of arbuscular mycorrhizal fungi. They have a high degree of specificity in their selection and association with specific groups of arbuscular mycorrhizal fungi, unlike non-legumes characterized by diverse communities of associated mycorrhizal fungi [67]. This could probably be a function of the differences in the genetic and phenotypic traits of the plants.
Nevertheless, the variation in the selection and responsiveness of plants to arbuscular mycorrhizal fungi in relation to the functional roles of these fungi to the plants could best be described by the work of Cobb et al. [68]. In their study, to ascertain the responsiveness of open-pollinated and commercial or hybrid sorghum species, it was observed that open-pollinated sorghum cultivars associated more with arbuscular mycorrhizal fungi and, as a result, recorded an increase of 320 percent in protein contents, 285 percent in grain yield per plant and 206 percent in vegetative biomass more than the commercial hybrid species. This study showed a strong correlation between arbuscular mycorrhizal fungi and plant species responsiveness. Therefore, an increase in the quality and quantity of grains and biomass of sorghum crops is directly related to a 149 percent increase in the arbuscular mycorrhizal fungi colonization of the open-pollinated plants. Plants with different genetic traits, as mentioned earlier, respond differently to the same or different species of arbuscular mycorrhizal fungi. However, the sorghum plants used in the above study were hybrid and non-hybrid species. Crop breeding and genetic modification of plants to increase their overall yield, nutrient quality, adaptation and biomass could become counterproductive in interfering with the successful interaction between plants and their associated mycorrhizal fungi. One of the critical challenges with the genetic engineering of crops could be alteration in the functions of non-targeted genes or disruption of plant–soil feedback loops. During gene modification, some specific non-targeted genes responsible for the production of strigolactones, mycorrhizal factor receptors, or transport proteins may be deleted, or their functions may be interfered with by the expression of the inserted foreign genes or their gene products. This defect could incidentally lead to a decrease in plant–fungal interactions or symbiosis [69,70]. Therefore, an adequate understanding of plants–mycorrhizal fungi interactions and the genes facilitating the process should be adhered to. This will allow plant biotechnologists or breeders to achieve both an increase in plant productivity and sustenance of plant–arbuscular mycorrhizal fungi symbiotic interactions. Similarly, future studies should focus on characterizing the various transport proteins that regulate the concentration gradients of strigolactones released by plants, the genes responsible for strigolactones production and the mycorrhizal factor-producing genes. The knowledge of these facilitator symbiosis genes will aid breeders in engineering crops with maximum potential to form symbiotic relationships with arbuscular mycorrhizal fungi while increasing the yield, tolerance and productivity of the plants.
Surprisingly, plants with varying growth rates, tolerance to environmental conditions and plants belonging to either C4 (i.e., plants that transform carbon dioxide during photosynthesis into sugars with four carbon atoms) or C3 (i.e., plants that form sugars with three carbon atoms) have remarkable differences in their degree of mycorrhization. C4 plants grow and adapt well to warmer seasons/climates and have more root colonization than C3 plants, which grow and adapt to cold climates. More root colonization implies greater nutrient uptake, biomass quality and yield [71,72]. In fact, the association of arbuscular mycorrhizal fungi with plant roots is entirely influenced by plant traits and not just by random assemblages. Similarly, different species of plants select and associate based on their traits with different mycorrhizal fungi. This difference in mycorrhizal fungi recruitment by plants is equally influenced by local environmental conditions. These environmental conditions will form the basis of our discussion in the following sections. Before we discuss that, let us briefly examine the effects of AMF on the yield and quality of cultivated plants (Table 1).

Table 1.
Effect of AMF on the yield and quality of cultivated plants.
From the table above, an association of different crops with AMF resulted in an overall increase in the yield and nutritional quality of the crops. These crops, with their diverse inherent traits, were able to benefit positively from their symbiotic association with arbuscular mycorrhizal fungi. The fungi enhanced nutrient acquisition from the soil and promoted plant health. To illustrate this pictorially, we can see in Figure 4 that the quantities of phosphorus in different parts of cotton plants are absorbed through the aid of the AMF nutrient uptake pathway. In this figure, higher concentrations of phosphorus in the plant parts (roots, stems and leaves) were found in plants inoculated with Rhizophagus irregularis CD1 compared to the uninoculated control.
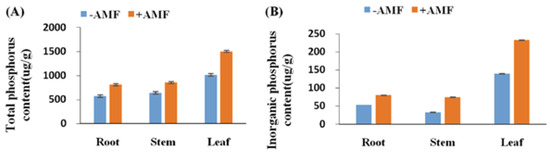
Figure 4.
A case study on the absorbed phosphorus concentrations on different parts of plants (cotton) in the presence of −AMF and +AMF plots. (A) The total phosphorus concentrations in −AMF and +AMF plots; (B) the inorganic phosphorus concentrations in −AMF and +AMF plots. The means ± standard error values are shown in the figure. Bars labeled with a different letter at the top of each parameter show a significant difference at p < 0.05 by statistical analysis of a single-factor experiment. Each parameter was analyzed independently. Adapted from Gao et al. [82] published by Springer Nature (Scientific report) in 2020 with CC BY license permission.
4.2. Soil Pedogenesis
Pedogenesis can be described as various means or processes involved in soil formation. Different soils originate from different parent materials such as rocks, minerals, organic matter or a combination of them. Each of these parent materials is made of chemical constituents ranging from metals to non-metallic elements. During the weathering process, either through biological (microbial, plant or animal mediated) or non-biological means like rock weathering, or the action of agents of denudation will result in the formation of soil. As soil forms, the principle of conservation of matter (which states that matter can neither be created nor destroyed but transformed from one form to another) prevails. The quality of the parent material determines the nutrient quality of the soil formed. This inevitably determines the microbial ecology in the soil, and arbuscular mycorrhizal fungi are no exception. The structure of mycorrhizal fungi is perhaps dependent on the ecosystem age [88,89]. It was reported that ecosystem age could significantly influence the structural differentiation in niche features of mycorrhizal fungi with a decrease in fungal alpha diversity and/or an increase in beta diversity [90]. One may wonder why ecosystem age based on soil formation will determine the structure of soil mycorrhizal fungi diversity. The answer lies in the nutrient concentration or quality of the soil. For instance, it was recorded that the diversity and richness of ectomycorrhizal fungi was reduced in younger terraces about 100,000 years old (a newly formed soil with high fertility) compared to older terraces of about 300,000 years old (with low soil fertility), which increased both the diversity and richness of the fungi [91]. Soil nutrients are among the vital factors that influence root colonization by mycorrhizal fungi. The presence of nutrients, such as potassium, magnesium, sulfate, chlorides, phosphorus, etcetera, are essential nutrients required for both plants and microbial growth. These nutrients affect soil pH and electrical conductivity, which also play a role in the distribution and diversity of mycorrhizal fungi [92,93]. Studies have shown that the addition of phosphorus and nitrogen decreased the abundance of arbuscular mycorrhizal fungi belonging to Gigaspora sp., Paraglomus sp. and Scutellospora sp. and increased the richness of Rhizophagus intraradices [94,95,96]. Therefore, high phosphorus content exerts negative effects on the diversity, richness and functions of mycorrhizal fungi. Some soil elements, especially P elements, can be appropriately supplemented to regulate AMF.
The older the soil, the lower the available nutrients if there is no anthropogenic influence like soil fertilization. A soil that is not under any fertilization regime will continue to experience nutrient extraction or loss through successive plant growth, nutrient absorption, leaching and consumption by animals. The more nutrients are being depleted, the more the establishment of arbuscular mycorrhizal fungi symbiosis with plants to increase their chances of adaptation and survival. In contrast, young soils are often rich in nutrients that are readily available for plant absorption. The more the available nutrients, the lesser the dependence of plants on mycorrhizal association and the lower the plants’ allocation of nutrients to the associated fungi. Soil pedogenesis affects soil conditions, which, in turn, could determine the distribution of mycorrhizal fungi in the soil [97]. Soil pedogenesis and parent material composition determine soil nutrient composition and its physicochemical composition. Although the parent material and the age of the soil have been described to have effects on mycorrhizal fungi richness, function and distribution, there is still a knowledge gap on the effects of soil pedogenesis on the co-evolution of plants and mycorrhizal fungi symbiosis. Understanding the soil pedogenesis effects on arbuscular mycorrhizal fungal biology, physiology, hyphal exploration type and ecology will guide ecologists and agriculturists in the proper application and exploitation of these fungi in maximizing their ecological benefits to the plants. Finally, studies should equally address the paucity of knowledge on how plants’ nutritional requirements, pedogenesis and environmental conditions influence the actual functions of arbuscular mycorrhizal fungi recruited by plants and the interactions between ecosystem evolution (age), plants, mycorrhizal fungi and mycorrhizosphere microbiomes.
4.3. Altitude Effects
An increase in the height of a place above sea level carries with it a corresponding variation in environmental conditions, such as a decrease in temperature, increased air flow rate and high drainage/leaching of nutrients. Note that in the previous section, soil conditions and environmental factors are among the drivers of arbuscular mycorrhizal fungal diversity, richness and functions, as well as plant traits. Here, we argue that high-rise soils (hills and mountains) have fragile environmental conditions. On these high-raised soils, plants grow on sloped surfaces and, depending on the density of the growing plants, could determine the quantity of sunlight penetration. Some slopes could be sunny (receiving a high amount of sunlight) or shady (the majority of the plants have little access to sunlight). Studies have shown that sunny slopes increase the richness, diversity, root colonization and spore density of arbuscular mycorrhizal fungi better than the shady sloped areas [98]. The intensity of the sunlight received by plants will determine their photosynthetic potential and the amount of carbon allocated to the root-associated fungi, which correspondingly affects the spore density. Also, vigorously growing plants under good sunlight penetration will absorb significant amounts of essential nutrients like phosphorus. The greater the uptake of phosphate from the soil, the less the available soil phosphorus and the more the need for plant–arbuscular mycorrhizal fungal symbiosis, hence the observed increase in root colonization and fungi richness. What was not considered in the above study was the effect of soil mineral layers and horizons on the distribution, abundance and diversity of the fungi.
The slope of a landform has marked effects on soil nutrients and plant diversity [99,100]. Still, on soil elevation, a decrease in the abundance of mycorrhizal fungal species was recorded by Vašutová et al. [101]. The increase in elevation correlates with stress like drought and a decrease in soil nutrients. These conditions affect the abundance, vitality and carbon allocation capacity of plants to associated mycorrhizal fungi. It was observed in another study that an increase in altitude promoted an increase in mycorrhizal fungal richness and diversity [102]. This observation was a result of plant species present in that area and not the soil type since their parent material originated from volcanic soils. Among mycorrhizal fungi, arbuscular mycorrhizal fungi are susceptible to elevation-induced stress and decline in richness with an increase in elevation [103]. Within the arbuscular mycorrhizal fungi, Gigasporaceae are the most severely affected, whereas Acaulosporaceae species are the least altitude-affected arbuscular mycorrhizal fungi. These fungal species have different features, with the former producing large amounts of mycelial biomass and the latter producing a small amount of biomass. Based on the principle of phylogenetic trait conservatism, mycorrhizal fungi that are able to produce small biomass will be more resistant to environmental stress than those producing large mycelial biomass. The carbon needs of the mycorrhizal fungi with few propagules could easily be met by plants undergoing environmental stress better than mycorrhizal fungi with large propagules biomass. Thus, the distribution of biomass between mycelium and spores is influenced by carbon quality, availability and quantity [104,105,106,107]. The lower the elevation, the more environmental conditions will favour plant productivity, abundance and association with arbuscular mycorrhizal fungi and the resultant increase in the richness of large biomass-producing mycorrhizal fungi. Apart from environmental conditions prevailing with an increase in altitude, further studies should focus on understanding other determinants such as pressure, air flow rate, aeration, mineral constituents of the soil that could affect the assemblages, phylogenetic composition, richness and functions of arbuscular mycorrhizal fungi.
4.4. Tillage Practice
Tillage, an agricultural practice of softening the soil for ease of plant root penetration, break-up of residue, incorporation of manure and control of weeds through mechanical means, is constantly applied in both commercial and subsistence agriculture. Although this agricultural practice is beneficial, its drawback is obvious in the reduction of soil organic matter, soil compaction, soil aggregate break-up, water loss, erosion and the breaking or distortion of fungi mycelia or hyphae. With these prevailing negative effects, our focus will be on how they affect arbuscular mycorrhizal fungi. Tillage results in hyphal breakage of mycorrhizal fungi, and constant breakage and healing of the broken hyphal strand negatively affects the richness, diversity and ecological functions of the fungi. These damaging effects are not observed in no-till or reduced-tilled soils [108,109]. No-tillage has been reported to increase soil organic carbon, soil enzymes, microbial biomass and microbial functions better than tilled soil [110]. These improvements in soil characteristics are good indicators of soil health.
There are three types of tillage systems: conventional tillage, reduced tillage and no-tillage systems. In conventional tillage, the soil’s outer surface is inverted using mechanical means of ploughing to a depth that exceeds 20 cm with small amounts of crop residue retained on the soil. In reduced tillage, a shallow soil depth is tilled with no inversion of soil. Crop residues are retained in large quantities in the field, while in the no-tillage system, the soil is undisturbed, and stubbles are retained year-round [111,112]. Reduced tillage and no-tillage are now widely accepted and practiced by farmers to prevent nutrient loss and erosion and increase soil water and organic matter contents [113]. Evaluating the effects of tillage practices, de Pontes et al. [114] reported that conventional tillage reduced the spore density and species richness of arbuscular mycorrhizal fungi by a magnitude of 3 to 4 spores per gram and 12 to 17 species of the fungi. No-tillage increased the spore density by 4 to 6 spores per gram and species richness by 15 to 18 species. Natural Savannah forest soil has a spore density between 9 and 11 spores per gram of soil and a richness of 16 to 22 fungal species in the sampled soil. Each of these soils is characterized by different dominant species of arbuscular mycorrhizal fungi. The tilled soil was dominated by Racocetra coralloidea, Gigaspora margarita and Racocetra fulgida. Natural forest soil contains Sclerocystis sinuosa and Glomus macrocarpum, whereas untilled soil contains an abundance of Sclerocystis coremioides. The evidence presented herein could possibly explain the effects of tillage practice on soil microbiome richness and diversity. The fungal species richness and spore densities are higher in no-tilled soil than in the tilled one but are highest in the natural forest soil (Figure 5). Although studies are examining the effects of tillage on species richness and diversity of arbuscular mycorrhizal fungi, no study, to the best of our knowledge, to date, has examined how soil tillage affects the mycorrhizal fungi microbiome, the evolution and ecological functions of bacterial symbionts of mycorrhizal fungi, resilience, and functions in the soil. Does tillage improve the quality and quantity of arbuscular mycorrhizal fungal–bacterial symbiont interactions? Looking beyond soil physiochemical conditions’ effects on fungal richness and diversity as they are affected by soil tillage on the bacterial symbionts of mycorrhizal fungi under the influence of tillage systems will expose the mysteries behind the observations recorded in contemporary studies published to date.
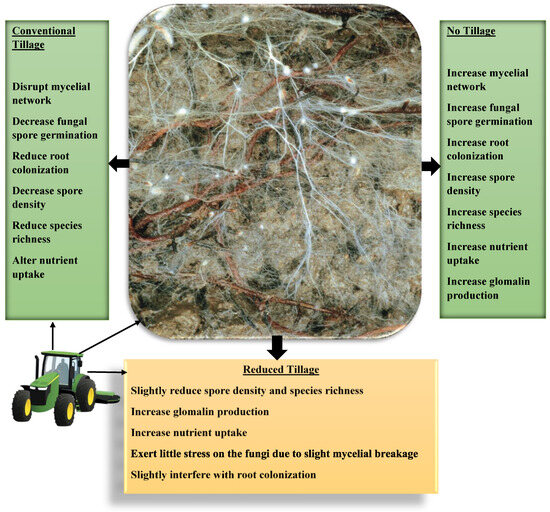
Figure 5.
Pictorial representation of the effects of different forms of tillage on the arbuscular mycorrhizal fungi species richness, spore density and its ecological functions in the soil. The picture of the soil mycorrhizal fungal network in the center was adapted from Egli and Brunner [55] published in 2011 by Swiss Federal Research Institute WSL, Birmensdorf, with permission from the publisher.
Mycorrhizal fungi propagules are negatively affected by conventional soil tillage, while no-tillage increases fungal spore germination, mycelial network and root colonization [115,116]. The distribution and tillage effects on the richness and diversity of mycorrhizal fungi have varying effects on the species richness, as shown above. Some species thrive well in tilled soil, whereas others thrive in no-till and natural forest soil, indicating how these systems contribute to their competitiveness and adaptation or provide suitable environmental conditions for their growth. Through adaptation, some fungi able to form large spores could easily adapt to the disruption of their mycelia during soil tillage [117]. Further studies must enable us to fully understand the effects of tillage on the biology, physiology and functions of arbuscular mycorrhizal fungi.
4.5. Land Use Changes
Land use could take various forms, such as use for agriculture, forestry, parks, plantations, and grasslands or for aesthetic purposes. The form in which the land is used will determine the anthropogenic modifications or input into the soil. Lands used for agricultural purposes are mainly subjected to fertilization, tillage and the use of agrochemicals like pesticides to increase crop yield, productivity and control of diseases and pests. These chemicals and soil modifications have corresponding effects on the soil physiochemical conditions, soil enzymes, plants and soil microbiome. Forest, plantations and some grassland soils are undisturbed and are rich in soil organic matter content. Recall that soil disturbance through tillage often causes hyphal strands to break and physiological stress to mycorrhizal fungi and other soil-dwelling microbes. This perturbation inevitably negatively affects the ecological functions and symbiotic services rendered to plants. In the land use changes, forests can be converted to agricultural land or pasture land and vice versa. Each of these switches, from one form to another, will definitely affect the soil microbiome–plant interactions. Understanding the effects of these land use switches on mycorrhizal fungi is essential for conservation purposes and the maintenance of soil biodiversity. A study has shown that the shift of land use from forest to palm plantation resulted in changes in the richness and diversity of mycorrhizal fungi, with higher species richness and diversity occurring more in the forest soil than in the plantation. This clearly implies that land use changes come with pervasive consequences on the structure of soil fungi communities, and the recovery from this disturbance takes time regardless of the presence or absence of host plants [118,119,120,121]. Forest soil with healthy growing trees harbours more ectomycorrhizal fungi than arbuscular mycorrhizal fungi and vice versa [122]. The presence of trees that are unlogged and growing in a forest’s soil increases the abundance, diversity and functions of ectomycorrhizal fungi. Arbuscular mycorrhizal fungi often prevail in arable soils used for growing crops. This could be explained by the host specificity of these mycorrhizal fungi. Trees are mostly associated with ectomycorrhizal fungi, whereas crops are mostly associated mostly with arbuscular mycorrhizal fungi.
Although the conversion of farmlands to forests could have a boosting effect on the soil microbiome richness and diversity, its effects will not likely be compared with the original undisturbed forest microbial community diversity and richness [123,124]. This could be a good conservation practice for the restoration of degraded agricultural lands. The most impactful implication of these land use changes (from farmland to forest) is the increase in fungal population density and soil organic matter and the lack of effect on modulating or restoring edaphic conditions [125]. The use of land for farming results in constant disturbances through tillage, which might improve fungal diversity but without a corresponding positive outcome for farming. Soil disturbance through tillage impacts the homogeneity of the soil by reducing the dominance of most competitive microbial groups, preventing competitive exclusion and broadening environmental conditions that favour mycorrhizal fungi diversity and not richness in the disturbed soils. In fact, the degree of soil disturbance determines the indicator of mycorrhizal fungi species that will prevail in the soil. For instance, mechanically disturbed soil has an abundance of Diversisporaceae Archaeosporaceae and Claroideoglomeraceae mycorrhizal fungi taxa. The undisturbed habitats are characterized by increases in the abundance of Glomeraceae taxa [126]. Perhaps it is only the evenness component of the diversity that changed in this scenario.
Similarly, trees planted in parks or streets have a different mycorrhizal fungal structure than those growing in the forest. Forest samples have a higher abundance and diversity of mycorrhizal fungi than urban trees. Unfortunately, urban trees are often grown in soil with sealed surfaces, which exert drastic reduction effects on mycorrhizal fungal richness and diversity. However, those growing in the forest are not challenged with sealed surfaces, which alter soil moisture content, microclimate and isolation of soil or serve as a barrier between the soil and atmosphere by preventing the efficient exchange of gases between the soil and atmosphere [127]. Land use changes have damaging effects on soil biodiversity, depending on their intensity. However, this effect could be ameliorated by the presence of suitable host plants. Intensive cropping systems reduce the richness of arbuscular mycorrhizal fungi, and this can be modulated by host plants, which support the proliferation of the fungi [71]. Thus far, both land use changes and plant species have effects on the assemblages of mycorrhizal fungi in plant roots. Nonetheless, very little has been done to understand the full biochemical and physiological processes adopted by plants in modulating the effects of land use intensification or changes on the richness and diversity of mycorrhizal fungi. This area requires further study.
4.6. Seasonal Variation
Seasons determine which plant groups will thrive. C4 plants perform best in warmer climates or seasons, while C3 plants adapt well to cool or cold climates. The change in season affects the quality and quantity of photosynthates that will be allocated to the root-associated mycorrhizal fungi. This consequently influences their species abundance, colonization, diversity and ecosystem services. Colder periods increase the beta diversity of mycorrhizal fungi, and their competitive interactions are more than those during warmer periods, which increase the alpha diversity of the fungi. This could be explained by the decrease in carbon allocation of plants to the associated fungi during winter or cold periods and the increase in carbon allocation during summer or warmer seasons, leading to the dominance of a single taxon over others. Fungal spores are equally altered by seasonal variations [128,129,130]. Understanding the influence of seasonal changes on the dynamics and function of mycorrhizal fungi will guide ecologists in predicting its impact on the ecosystem functions of soil microbial communities during these seasons. Besides their resource exchange with plants (through carbon allocation and interactions), arbuscular mycorrhizal fungi are equally affected by atmospheric temperature and soil water [131]. They may be less distributed in cooler periods than in the warmer seasons. The change in seasons might lead to the extinction of less competitive arbuscular mycorrhizal fungal species, as dominant and more competitive ones during the winter season prevail. Nevertheless, microbes are constantly evolving strategies to adapt and tolerate changes in their environment, and extinction is likely not possible. These adaptation strategies of microbes (AMF) during the colder seasons are not fully understood and require further studies. The comprehension of the influence of seasons on plant–arbuscular mycorrhizal fungi interactions (especially in plants’ activation of immune defense genes) and plant phenology is required for accurate prediction of fungi dynamics during seasonal variations. Another area worth researching further is how seasonal variations affect plants’ translocation of lipids to the arbuscular mycorrhizal fungi.
In addition to the influence of seasons on plant-associated AMF community, plant phenology (leaf unfolding/development, flowering time, leaf colouring, fruiting and life cycle), which is under the influence of seasons (and/or temperature) [132], exerts direct effects on the richness, colonization, abundance and community composition of associated AMF. Typically, plants’ flowering and fruiting are influenced by seasons, and at this phase of plant development, a decrease in carbon is allocated to the roots and to the associated mycorrhizal fungi [133]. This decrease in plants’ photosynthate allocation to the associated mycorrhizal fungi occurs more often when the plants can acquire nutrients easily from the soil and, therefore, require little contribution from the fungi in meeting their nutritional needs. In this situation, the plants tend to distribute their photosynthates to the aerial parts of the plants [134] and less to the roots. In support of the foregoing, Vázquez-Santos et al. [135] observed in their study that AMF colonization of Acaena elongate plants was lowest during the rainy season and highest during the dry season. The authors also observed high species richness in the rainy season (6.15 ± 0.35 species) compared with low species richness in the dry season (4.60 ± 0.30 species), while the abundance followed the reverse pattern and positively correlated with the openness of the canopy and phosphorus availability. Phenologically, flowering and fruit formation are positively associated with light intensity/incidence and canopy openness among forest plants, which are higher during the dry season than during the rainy season [135]. The rationale behind the decrease in AMF root colonization during the rainy season could be attributed to an increase in soil water content and dissolved nutrients, which are easily absorbed by plants without the aid of the AMF symbionts. The reverse event occurs during the dry season with an increase in root colonization and plants’ dependence on the symbionts for the extraction of water and nutrients from the soil [136]. In another study, AMF species richness showed little fluctuation across the four seasons (autumn, winter, spring and summer), whereas the fungal abundance and root colonization were significantly affected by seasonal variations [137].
Furthermore, given that most AMF plant hosts are herbaceous plant species, and from these, many are annuals, the importance of short life cycle effects in root colonization under seasonal variation and plant phenology should be noted. However, AMF species richness is unaffected by plants’ phenology [138], and this is due to the presence of roots throughout the growing season in the soil despite the absence of leaves and photosynthesis activities. Herbaceous plants, the ephemeral ones, have the tendency for quick growth, nutrient assimilation, storage of carbohydrates in their roots and dying off within a short period of time [139,140].
Although plant phenology under the influence of season alters AMF community richness, colonization, abundance and diversity, AMF, on the other hand, also exert a direct effect on plant phenology. It has been uncovered that AMF increase flower mass [141], flowering stem diameter [142] and flower/inflorescence number [143]; influence the time and duration of flowering [144]; increase seed development [145]; and increase fruit production [146]. In general, AMF have significant effects on plant reproduction and overall phenology.
4.7. Effects of Soil Pollutants
Soil pollution is inevitable in this era of industrialization, commercialization of agriculture, urbanization and the wide use of chemical fertilizers and agrochemicals that add different kinds of pollutants to the soil. Among the pollutants introduced either naturally or through human actions are cadmium, lead, silver, copper, microplastics, nickel, etcetera [147,148]. Here, we will briefly discuss the effects of various soil pollutants on AMF richness.
4.7.1. Cadmium (Cd)
Cadmium, a heavy metal, has been shown to affect the co-occurrence pattern and assembly mechanisms of AMF in the soil. When present in the soil at high concentrations, this heavy metal is implicated in an increase in specialist AMF diversity and a corresponding decreasing effect on generalist AMF diversity. An increase in cadmium concentration comes with the selection of deterministic processes (involving niche-based and non-random mechanisms such as interspecific interactions among the fungal community and environmental selection) over stochastic ones (involving random alterations in the species’ relative abundance as a result of death, reproduction and dispersal), thus altering the ecological functions of the AMF community [149,150,151]. Cadmium exerts selection pressure on the heterogeneous soil ecological niche harbouring AMF and frees up the niche to accommodate more species of AMF that adapt to the metal stress, thereby stimulating an increase in AMF species richness [149,151].
4.7.2. Lead (Pb)
Lead is a toxic metal that negatively affects the biological processes of microbes when present at high concentrations. Studies have shown that it induces physiological stress and decreases the diversity/richness of arbuscular mycorrhizal fungi while having little effect on their abundance. This lead-induced selection pressure depends on the fungi’s metal tolerance capacity. AMF such as Paraglomus sp. have been found to tolerate the presence of lead and accumulate it in their biomass. The fungi abundance increases in the presence of lead contamination [148]. However, the presence of lead contaminant negatively correlates with AMF species richness but increases the abundance of AMF species belonging to Paraglomeraceae. AMF species belonging to Glomeraceae and Acaulosporaceae are negatively affected by an increase in lead concentration in the soil, whereas Paraglomeraceae, on the other hand, is unaffected [152].
4.7.3. Silver (Ag)
Silver nanoparticles, like other pollutants discussed above, have no negative effect on the alpha diversity of AMF growing in contaminated soil, but they enhance the abundance of Glomus to well over 70 percent compared with other AMF fungi taxa [147]. The next metal that constitutes a harmful effect on the AMF when present in high concentration is copper.
4.7.4. Copper (Cu)
Copper is an essential cofactor for the efficient functioning of microbial enzymes. At moderate or low concentrations (50 mg Kg−1 Cu), it has an enhancement effect on the diversity of AMF and other soil fungi. However, when the concentration exceeds (1600 mg Kg−1 Cu), its effect becomes negative on the AMF richness. An increase in the concentration of this metal equally decreases the abundance of AMF fungi [153]. In a study examining the influence of vineyard age on the richness of AMF, Betancur-Agudelo et al. [154] observed that AMF species richness was highest in the youngest vineyards and decreased with an increase in the vineyard age. It was noticed that an increase in vineyard age correlates with an increase in copper concentration in the soil, which adversely affects the AMF species richness. This increased soil copper concentration enhanced the alpha diversity or abundance of AMF belonging to the Glomeraceae family. The youngest vineyards with low soil copper concentration and decreased pH favour the proliferation of AMF species belonging to the Acaulosporaceae family. High copper concentration in the soil causes a reduction in AMF sporulation and decreased growth of extraradical mycelia, while the reverse effects occur in soil with low copper concentrations [155]. High soil copper concentrations encourage the growth of ruderal fungi (belonging to the Glomeraceae family). This group of AMF adapts well to disturbance and has the inherent ability to quickly establish their hyphal networks, increased root colonization, and spore formation. These fungi also have a high sorption capacity for metals as well as compartmentalization of copper and other metals in their spores, which promotes adaptation to copper toxicity. Glomeraceae fungi equally have high efficiency for carbon utilization and can control their metabolism and regulate their structural formation during a short supply of carbon from their associated plants [104,154,155,156]. Glomeraceae AMF fungi have been found to dominate in heavy metal-contaminated soil in different geographical locations due to their aforementioned physiological properties [157,158].
4.7.5. Nickel (Ni)
Another essential enzyme cofactor is nickel. At moderate concentrations, nickel in the soil increases the diversity/richness of AMF and other soil-dwelling fungi. An increase in the abundance of AMF at moderate Ni concentrations was recorded due to its biological role in the fungi metabolism. However, higher concentrations of Ni contamination exert detrimental effects on fungal richness and community structure [159].
4.7.6. Mercury (Hg)
Mercury is a persistent and toxic metal capable of bioaccumulation in the food chain and could be introduced into the soil through combustion of fossil fuel, mining or discharge of industrial wastes [160,161]. Studies have shown that soil mercury concentration correlates negatively with AMF diversity and richness. An increase in soil mercury concentration decreases AMF species richness but increases the alpha diversity of resistant ones. It selects for the proliferation of mercury-tolerant AMF over the intolerant ones. AMF species belonging to Glomeraceae are supported over those belonging to Paraglomeraceae, which are sensitive and inhibited by mercury [162]. In another study, Paraglomeraceae were detected to be very abundant in soil with little metal contamination [163].
4.7.7. Arsenic (As) and Antimony (Sb)
These metalloids have been found to negatively affect the richness of AMF species in contaminated soil. However, due to the physiological nature of certain species of AMF, arsenic and antimony exert a linear positive correlation with Diversisporaceae. These AMF species have a very high tolerance to the metalloids and could easily proliferate in their presence in the soil [164]. In another study, arsenic was found to reduce the species richness of AMF in mining-contaminated soil [165].
4.7.8. Microplastics
Microplastics are another emerging environmental contaminant that affects soil microbial communities. These particles are released into the soil from biosolids, plastic mulching films and organic fertilizers [166,167,168]. These nanoparticles provide surfaces for the adsorption of metal contaminants in the soil and increase their bioavailability and toxicity to plants and soil microbes [169]. Examples of microplastics are high-density polyethylene, polylactic acid, polyester, polypropylene, polyethylene terephthalate, etc. [170]. The effects of these microplastics are dependent on the microplastic type and dose present in the soil. Additionally, the abundance of AMF belonging to Glomeromycotina is enhanced by polyethylene microplastic. AMF alpha diversity is not affected by microplastics; rather, the community composition of the fungi is the one that is altered in the presence of microplastics [171]. Microplastics have also been implicated in adversely affecting plant growth, nutrient uptake and productivity. Nevertheless, these nanoparticles have little effect on the establishment of a symbiotic association between AMF and plants [172]. In another study, the presence of microplastics in the soil significantly reduced the abundance of AMF [173]. The evidence presented in the foregoing has shown that soil pollutants indeed reduce AMF richness and affect its ecological functions.
5. Future Perspective and Conclusions
It is, at the moment, necessary to strategically sustain and manage the indigenous arbuscular mycorrhizal fungi communities in the soil since not every agriculturist has adopted the commercial inoculation of crops with arbuscular mycorrhizal fungal inoculum in the field. Although some commercial producers in the USA Midwest region are practicing this, many others are not. Mycorrhizal fungi assemblage based on vegetation type (i.e., host plant) and its specificity in association could be exploited to increase the diversity and functions of naturally occurring mycorrhizal fungi. Genetic modification of plants through breeding to increase their photosynthesis ability during cold and warmer seasons and to increase their photosynthate allocation is required. Obviously, mycorrhizal fungi physiology, reproduction, colonization and nutrient uptake potential are influenced by their host plants. A strategy to adopt in this regard is through crop rotation of different species of legumes, cereals and other crops with legacy effects able to increase the diversity, richness and functions of the fungi. A study by Brigido et al. [174] has shown that this approach is effective, as they observed that wheat grown in undisturbed agricultural soil after legumes was able to acquire communities of arbuscular mycorrhizal fungi similar to that of the previous leguminous crops. However, a different outcome was found in the disturbed soil before cultivating the wheat crops. Stubble retention, cover cropping and suitable crop rotation are important in maintaining the microbial community functions and sustaining the mycorrhizal fungi activities in the soil [54]. However, we strongly consider that adopting sequential polyculture of crops is the best approach for increasing the richness and ecological function of arbuscular mycorrhizal fungi in the soil.
However, an increase in the disturbance of soil is a sure recipe for reducing the richness, diversity and functions of arbuscular mycorrhizal fungi. Conservation agricultural practices should be employed since they maintain soil ecosystem functions better than conventional systems.
Further research might focus on how fungi can play a role in climate-smart agriculture as well as on the genetic engineering of the arbuscular mycorrhizal fungi to tolerate different climatic conditions and exploit the metabolic regulation strategies and phylogenetic trait conservatism of Acaulosporaceae in improving the adaptive features of other mycorrhizal fungal species. These traits will enable the generation of climate-tolerant species of mycorrhizal fungi and increase crop yield, nutritional quality and health. As for the previously mentioned factors affecting arbuscular mycorrhizal fungal richness, the factors could be categorized into conditions that affect soil nutrients and those regarding the host. Soil chemical conditions and host susceptibility to arbuscular mycorrhizal fungi colonization are the key areas governing plant–mycorrhizal fungal interactions, fungi species richness, diversity and function. Going by this, researchers should understand the traits of the fungi and those of the plants that promote compatibility and ease of colonization. The application of organic manure for soil conditioning and promoting soil organic matter and nutrients should be encouraged, and the adoption of an organic farming system or management is required.
Author Contributions
M.C.E. and M.E. contributed equally to the planning and writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Funding was made available through the Biogeochemistry Research Infrastructure Platform (BIOGRIP), a platform within the CMBG that is funded by the Department of Science and Innovation (DSI).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge financial support from the Centre for Mineral Biogeochemistry (CMBG) and the Biogeochemistry Research Infrastructure Platform (BIOGRIP), a platform within the CMBG that is funded by the Department of Science and Innovation (DSI).
Conflicts of Interest
The authors declare no conflict of interest.
References
- McCormack, M.L.; Guo, D.; Iversen, C.M.; Chen, W.; Eissenstat, D.M.; Fernandez, C.W.; Li, L.; Ma, C.; Ma, Z.; Poorter, H. Building a better foundation: Improving root-trait measurements to understand and model plant and ecosystem processes. New Phytol. 2017, 215, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Simard, S.W.; Beiler, K.J.; Bingham, M.A.; Deslippe, J.R.; Philip, L.J.; Teste, F.P. Mycorrhizal networks: Mechanisms, ecology and modelling. Fungal Biol. Rev. 2012, 26, 39–60. [Google Scholar] [CrossRef]
- Smith, S.; Read, D. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: New York, NY, USA, 2008. [Google Scholar]
- Gomes, S.I.; Fortuna, M.A.; Bascompte, J.; Merckx, V.S. Mycoheterotrophic plants preferentially target arbuscular mycorrhizal fungi that are highly connected to autotrophic plants. New Phytol. 2022, 235, 2034–2045. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-N.; Liu, C.-C.; Zhu, A.-Q.; Niu, K.-X.; Guo, R.; Tian, L.; Wu, Y.-N.; Sun, B.; Wang, B. OsRAM2 function in lipid biosynthesis is required for arbuscular mycorrhizal symbiosis in rice. Mol. Plant-Microbe Interact. 2022, 35, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.E.; Bever, J.D. Mycorrhizal species differentially alter plant growth and response to herbivory. Ecology 2007, 88, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Gorzelak, M.A.; Asay, A.K.; Pickles, B.J.; Simard, S.W. Inter-plant communication through mycorrhizal networks mediates complex adaptive behaviour in plant communities. AoB Plants 2015, 7, plv050. [Google Scholar] [CrossRef]
- Fujita, M.; Kusajima, M.; Fukagawa, M.; Okumura, Y.; Nakajima, M.; Akiyama, K.; Asami, T.; Yoneyama, K.; Kato, H.; Nakashita, H. Response of tomatoes primed by mycorrhizal colonization to virulent and avirulent bacterial pathogens. Sci. Rep. 2022, 12, 4686. [Google Scholar] [CrossRef]
- Aseel, D.G.; Rashad, Y.M.; Hammad, S.M. Arbuscular mycorrhizal fungi trigger transcriptional expression of flavonoid and chlorogenic acid biosynthetic pathways genes in tomato against Tomato Mosaic Virus. Sci. Rep. 2019, 9, 9692. [Google Scholar] [CrossRef]
- Dowarah, B.; Gill, S.S.; Agarwala, N. Arbuscular mycorrhizal fungi in conferring tolerance to biotic stresses in plants. J. Plant Growth Regul. 2021, 41, 1429–1444. [Google Scholar] [CrossRef]
- Pickles, B.J.; Wilhelm, R.; Asay, A.K.; Hahn, A.S.; Simard, S.W.; Mohn, W.W. Transfer of 13C between paired Douglas-fir seedlings reveals plant kinship effects and uptake of exudates by ectomycorrhizas. New Phytol. 2017, 214, 400–411. [Google Scholar] [CrossRef]
- Mali, S.; Naik, S.; Jha, B.; Singh, A.; Bhatt, B. Planting geometry and growth stage linked fertigation patterns: Impact on yield, nutrient uptake and water productivity of Chilli pepper in hot and sub-humid climate. Sci. Hortic. 2019, 249, 289–298. [Google Scholar] [CrossRef]
- Ge, S.; He, L.; Jin, L.; Xia, X.; Li, L.; Ahammed, G.J.; Qi, Z.; Yu, J.; Zhou, Y. Light-dependent activation of HY5 promotes mycorrhizal symbiosis in tomato by systemically regulating strigolactone biosynthesis. New Phytol. 2022, 233, 1900–1914. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, T.; Kohlen, W.; Sasse, J.; Borghi, L.; Schlegel, M.; Bachelier, J.B.; Reinhardt, D.; Bours, R.; Bouwmeester, H.J.; Martinoia, E. A petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching. Nature 2012, 483, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Stirnemann, M.; Gübeli, C.; Egloff, S.; Courty, P.-E.; Aubry, S.; Vandenbussche, M.; Morel, P.; Reinhardt, D.; Martinoia, E. Strigolactones play an important role in shaping exodermal morphology via a KAI2-dependent pathway. IScience 2019, 17, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Rich, M.K.; Schorderet, M.; Reinhardt, D. The role of the cell wall compartment in mutualistic symbioses of plants. Front. Plant Sci. 2014, 5, 238. [Google Scholar] [CrossRef]
- Kohlen, W.; Charnikhova, T.; Liu, Q.; Bours, R.; Domagalska, M.A.; Beguerie, S.; Verstappen, F.; Leyser, O.; Bouwmeester, H.; Ruyter-Spira, C. Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in nonarbuscular mycorrhizal host Arabidopsis. Plant Physiol. 2011, 155, 974–987. [Google Scholar] [CrossRef]
- Mayzlish-Gati, E.; De-Cuyper, C.; Goormachtig, S.; Beeckman, T.; Vuylsteke, M.; Brewer, P.B.; Beveridge, C.A.; Yermiyahu, U.; Kaplan, Y.; Enzer, Y. Strigolactones are involved in root response to low phosphate conditions in Arabidopsis. Plant Physiol. 2012, 160, 1329–1341. [Google Scholar] [CrossRef]
- Ruyter-Spira, C.; Kohlen, W.; Charnikhova, T.; van Zeijl, A.; van Bezouwen, L.; De Ruijter, N.; Cardoso, C.; Lopez-Raez, J.A.; Matusova, R.; Bours, R. Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: Another belowground role for strigolactones? Plant Physiol. 2011, 155, 721–734. [Google Scholar] [CrossRef]
- Yoneyama, K.; Yoneyama, K.; Takeuchi, Y.; Sekimoto, H. Phosphorus deficiency in red clover promotes exudation of orobanchol, the signal for mycorrhizal symbionts and germination stimulant for root parasites. Planta 2007, 225, 1031–1038. [Google Scholar] [CrossRef]
- Mohammad, I. Mycorrhizae’s role in plant nutrition and protection from pathogens. Curr. Investig. Agric. Curr. Res. 2019, 8, 1037–1045. [Google Scholar]
- Hsieh, Y.H.; Wei, Y.H.; Lo, J.C.; Pan, H.Y.; Yang, S.Y. Arbuscular mycorrhizal symbiosis enhances tomato lateral root formation by modulating CEP2 peptide expression. New Phytol. 2022, 235, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Irving, T.B.; Chakraborty, S.; Ivanov, S.; Schultze, M.; Mysore, K.S.; Harrison, M.J.; Ané, J.M. KIN3 impacts arbuscular mycorrhizal symbiosis and promotes fungal colonisation in Medicago truncatula. Plant J. 2022, 110, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Wu, Y.N.; Liu, C.C.; Liu, Y.N.; Tian, L.; Cheng, J.F.; Pan, Z.; Wang, D.; Wang, B. OsADK1, a novel kinase regulating arbuscular mycorrhizal symbiosis in rice. New Phytol. 2022, 234, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, J.; Liao, D.; Ye, H.; Li, C.; Luo, Z.; Yan, A.; Zhao, Q.; Xie, K.; Li, Y. Auxin-mediated regulation of arbuscular mycorrhizal symbiosis: A role of SlGH3. 4 in tomato. Plant Cell Environ. 2022, 45, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Goh, D.M.; Cosme, M.; Kisiala, A.B.; Mulholland, S.; Said, Z.M.; Spíchal, L.; Emery, R.N.; Declerck, S.; Guinel, F.C. A stimulatory role for cytokinin in the arbuscular mycorrhizal symbiosis of pea. Front. Plant Sci. 2019, 10, 262. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.; Xie, K.; Tian, Y.; Yan, A.; Liu, J.; Huang, Y.; Wang, S.; Zhu, Y.; Chen, A. A mycorrhiza-specific H+-ATPase is essential for arbuscule development and symbiotic phosphate and nitrogen uptake. Plant Cell Environ. 2020, 43, 1069–1083. [Google Scholar] [CrossRef] [PubMed]
- Vangelisti, A.; Natali, L.; Bernardi, R.; Sbrana, C.; Turrini, A.; Hassani-Pak, K.; Hughes, D.; Cavallini, A.; Giovannetti, M.; Giordani, T. Transcriptome changes induced by arbuscular mycorrhizal fungi in sunflower (Helianthus annuus L.) roots. Sci. Rep. 2018, 8, 4. [Google Scholar] [CrossRef]
- Banasiak, J.; Jamruszka, T.; Murray, J.D.; Jasiński, M. A roadmap of plant membrane transporters in arbuscular mycorrhizal and legume–rhizobium symbioses. Plant Physiol. 2021, 187, 2071–2091. [Google Scholar] [CrossRef]
- Hooper, D.U.; Vitousek, P.M. The effects of plant composition and diversity on ecosystem processes. Science 1997, 277, 1302–1305. [Google Scholar] [CrossRef]
- Schulze, E.-D.; Mooney, H.A. Biodiversity and Ecosystem Function; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Aarssen, L.W. Ecological combining ability and competitive combining ability in plants: Toward a general evolutionary theory of coexistence in systems of competition. Am. Nat. 1983, 122, 707–731. [Google Scholar] [CrossRef]
- Brown, V.; Gange, A. Herbivory by soil-dwelling insects depresses plant species richness. Funct. Ecol. 1989, 3, 667–671. [Google Scholar] [CrossRef]
- Dobson, A.; Crawley, M. Pathogens and the structure of plant communities. Trends Ecol. Evol. 1994, 9, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Ricklefs, R.E. Environmental heterogeneity and plant species diversity: A hypothesis. Am. Nat. 1977, 111, 376–381. [Google Scholar] [CrossRef]
- Grime, J.; Mackey, J.; Hillier, S.; Read, D. Floristic diversity in a model system using experimental microcosms. Nature 1987, 328, 420–422. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.; Klironomos, J.N.; Ursic, M.; Moutoglis, P.; Streitwolf-Engel, R.; Boller, T.; Wiemken, A.; Sanders, I.R. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 1998, 396, 69–72. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G. Arbuscular mycorrhizal fungi as a determinant of plant diversity: In search of underlying mechanisms and general principles. In Mycorrhizal Ecology; Springer: Berlin, Heidelberg, 2003; pp. 243–265. [Google Scholar]
- Van der Heijden, M.G.; Boller, T.; Wiemken, A.; Sanders, I.R. Different arbuscular mycorrhizal fungal species are potential determinants of plant community structure. Ecology 1998, 79, 2082–2091. [Google Scholar] [CrossRef]
- Bartelt-Ryser, J.; Joshi, J.; Schmid, B.; Brandl, H.; Balser, T. Soil feedbacks of plant diversity on soil microbial communities and subsequent plant growth. Perspect. Plant Ecol. Evol. Syst. 2005, 7, 27–49. [Google Scholar] [CrossRef]
- Han, X.-M.; Wang, R.-Q.; Jian, L.; Wang, M.-C.; Juan, Z.; Guo, W.-H. Effects of vegetation type on soil microbial community structure and catabolic diversity assessed by polyphasic methods in North China. J. Environ. Sci. 2007, 19, 1228–1234. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Plant-soil interactions in Mediterranean forest and shrublands: Impacts of climatic change. Plant Soil 2013, 365, 1–33. [Google Scholar] [CrossRef]
- Dirks, I.; Navon, Y.; Kanas, D.; Dumbur, R.; Gruenzweig, J.M. Atmospheric water vapor as driver of litter decomposition in Mediterranean shrubland and grassland during rainless seasons. Glob. Chang. Biol. 2010, 16, 2799–2812. [Google Scholar] [CrossRef]
- Matias, L.; Castro, J.; Zamora, R. Soil-nutrient availability under a global-change scenario in a Mediterranean mountain ecosystem. Glob. Chang. Biol. 2011, 17, 1646–1657. [Google Scholar] [CrossRef]
- Barea, J.; Palenzuela, J.; Cornejo, P.; Sánchez-Castro, I.; Navarro-Fernández, C.; Lopéz-García, A.; Estrada, B.; Azcón, R.; Ferrol, N.; Azcón-Aguilar, C. Ecological and functional roles of mycorrhizas in semi-arid ecosystems of Southeast Spain. J. Arid Environ. 2011, 75, 1292–1301. [Google Scholar] [CrossRef]
- Martínez-García, L.B.; de Dios Miranda, J.; Pugnaire, F.I. Impacts of changing rainfall patterns on mycorrhizal status of a shrub from arid environments. Eur. J. Soil Biol. 2012, 50, 64–67. [Google Scholar] [CrossRef]
- Tkacz, A.; Bestion, E.; Bo, Z.; Hortala, M.; Poole, P.S. Influence of plant fraction, soil, and plant species on microbiota: A multikingdom comparison. MBio 2020, 11, e02719–e02785. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Eisenhauer, N.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Vázquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 2015, 6, 6707. [Google Scholar] [CrossRef]
- Schmid, M.W.; van Moorsel, S.J.; Hahl, T.; De Luca, E.; De Deyn, G.B.; Wagg, C.; Niklaus, P.A.; Schmid, B. Effects of plant community history, soil legacy and plant diversity on soil microbial communities. J. Ecol. 2021, 109, 3007–3023. [Google Scholar] [CrossRef]
- Carney, K.M.; Matson, P.A. Plant communities, soil microorganisms, and soil carbon cycling: Does altering the world belowground matter to ecosystem functioning? Ecosystems 2005, 8, 928–940. [Google Scholar] [CrossRef]
- Enebe, M.C.; Babalola, O.O. The influence of plant growth-promoting rhizobacteria in plant tolerance to abiotic stress: A survival strategy. Appl. Microbiol. Biotechnol. 2018, 102, 7821–7835. [Google Scholar] [CrossRef]
- Enebe, M.C.; Babalola, O.O. The impact of microbes in the orchestration of plants’ resistance to biotic stress: A disease management approach. Appl. Microbiol. Biotechnol. 2019, 103, 9–25. [Google Scholar] [CrossRef]
- Enebe, M.C.; Babalola, O.O. Functional diversity of bacterial communities in the rhizosphere of maize grown on a soil under organic and inorganic fertilization. Sci. Afr. 2022, 16, e01212. [Google Scholar] [CrossRef]
- Nwokolo, N.L.; Enebe, M.C.; Chigor, C.B.; Chigor, V.N.; Dada, O.A. The contributions of biotic lines of defence to improving plant disease suppression in soils: A review. Rhizosphere 2021, 19, 100372. [Google Scholar] [CrossRef]
- Egli, S.; Brunner, I. Mykorrhiza: Eine Faszinierende Lebensgemeinschaft im Wald; WSL: Zürich, Switzerland, 2011. [Google Scholar]
- Kaur, S.; Campbell, B.J.; Suseela, V. Root metabolome of plant–arbuscular mycorrhizal symbiosis mirrors the mutualistic or parasitic mycorrhizal phenotype. New Phytol. 2022, 234, 672–687. [Google Scholar] [CrossRef]
- Enebe, M.C.; Erasmus, M. Susceptibility and plant immune control—A case of mycorrhizal strategy for plant colonization, symbiosis, and plant immune suppression. Front. Microbiol. 2023, 14, 1178258. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Bahram, M.; Zobel, M. How mycorrhizal associations drive plant population and community biology. Science 2020, 367, eaba1223. [Google Scholar] [CrossRef]
- Janos, D.P. Mycorrhizae influence tropical succession. Biotropica 1980, 12, 56–64. [Google Scholar] [CrossRef]
- Koziol, L.; Bever, J.D. AMF, phylogeny, and succession: Specificity of response to mycorrhizal fungi increases for late-successional plants. Ecosphere 2016, 7, e01555. [Google Scholar] [CrossRef]
- Almario, J.; Fabiańska, I.; Saridis, G.; Bucher, M. Unearthing the plant–microbe quid pro quo in root associations with beneficial fungi. New Phytol. 2022, 234, 1967–1976. [Google Scholar] [CrossRef]
- Klironomos, J.N. Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 2003, 84, 2292–2301. [Google Scholar] [CrossRef]
- Honnay, O.; Helsen, K.; Van Geel, M. Plant community reassembly on restored semi-natural grasslands lags behind the assembly of the arbuscular mycorrhizal fungal communities. Biol. Conserv. 2017, 212, 196–208. [Google Scholar] [CrossRef]
- Hannula, S.E.; Heinen, R.; Huberty, M.; Steinauer, K.; De Long, J.R.; Jongen, R.; Bezemer, T.M. Persistence of plant-mediated microbial soil legacy effects in soil and inside roots. Nat. Commun. 2021, 12, 5686. [Google Scholar] [CrossRef]
- Houlden, A.; Timms-Wilson, T.M.; Day, M.J.; Bailey, M.J. Influence of plant developmental stage on microbial community structure and activity in the rhizosphere of three field crops. FEMS Microbiol. Ecol. 2008, 65, 193–201. [Google Scholar] [CrossRef]
- López-García, Á.; Varela-Cervero, S.; Vasar, M.; Öpik, M.; Barea, J.M.; Azcón-Aguilar, C. Plant traits determine the phylogenetic structure of arbuscular mycorrhizal fungal communities. Mol. Ecol. 2017, 26, 6948–6959. [Google Scholar] [CrossRef]
- López-García, Á.; Azcón-Aguilar, C.; Barea, J.M. The interactions between plant life form and fungal traits of arbuscular mycorrhizal fungi determine the symbiotic community. Oecologia 2014, 176, 1075–1086. [Google Scholar] [CrossRef]
- Cobb, A.B.; Wilson, G.W.; Goad, C.L.; Bean, S.R.; Kaufman, R.C.; Herald, T.J.; Wilson, J.D. The role of arbuscular mycorrhizal fungi in grain production and nutrition of sorghum genotypes: Enhancing sustainability through plant-microbial partnership. Agric. Ecosyst. Environ. 2016, 233, 432–440. [Google Scholar] [CrossRef]
- Kabouw, P.; van Dam, N.M.; van der Putten, W.H.; Biere, A. How genetic modification of roots affects rhizosphere processes and plant performance. J. Exp. Bot. 2012, 63, 3475–3483. [Google Scholar] [CrossRef]
- Liu, W. Do genetically modified plants impact arbuscular mycorrhizal fungi? Ecotoxicology 2010, 19, 229–238. [Google Scholar] [CrossRef]
- Ciccolini, V.; Ercoli, L.; Davison, J.; Vasar, M.; Öpik, M.; Pellegrino, E. Land-use intensity and host plant simultaneously shape the composition of arbuscular mycorrhizal fungal communities in a Mediterranean drained peatland. FEMS Microbiol. Ecol. 2016, 92, fiw186. [Google Scholar] [CrossRef]
- Reinhart, K.O.; Wilson, G.W.; Rinella, M.J. Predicting plant responses to mycorrhizae: Integrating evolutionary history and plant traits. Ecol. Lett. 2012, 15, 689–695. [Google Scholar] [CrossRef]
- Tahiri, A.-i.; Meddich, A.; Raklami, A.; Alahmad, A.; Bechtaoui, N.; Anli, M.; Göttfert, M.; Heulin, T.; Achouak, W.; Oufdou, K. Assessing the potential role of compost, PGPR, and AMF in improving tomato plant growth, yield, fruit quality, and water stress tolerance. J. Soil Sci. Plant Nutr. 2022, 22, 743–764. [Google Scholar] [CrossRef]
- Dierks, J.; Blaser-Hart, W.J.; Gamper, H.A.; Six, J. Mycorrhizal fungi-mediated uptake of tree-derived nitrogen by maize in smallholder farms. Nat. Sustain. 2022, 5, 64–70. [Google Scholar] [CrossRef]
- Qin, Z.; Peng, Y.; Yang, G.; Feng, G.; Christie, P.; Zhou, J.; Zhang, J.; Li, X.; Gai, J. Relationship between phosphorus uptake via indigenous arbuscular mycorrhizal fungi and crop response: A 32P-labeling study. Appl. Soil Ecol. 2022, 180, 104624. [Google Scholar] [CrossRef]
- Massa, N.; Cesaro, P.; Todeschini, V.; Capraro, J.; Scarafoni, A.; Cantamessa, S.; Copetta, A.; Anastasia, F.; Gamalero, E.; Lingua, G. Selected autochthonous rhizobia, applied in combination with AM fungi, improve seed quality of common bean cultivated in reduced fertilization condition. Appl. Soil Ecol. 2020, 148, 103507. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, C.; Wang, C. Vermicompost assisted arbuscular mycorrhizal fungi to transfer 15 N from crop residues to lettuce. Plant Soil 2020, 456, 175–187. [Google Scholar] [CrossRef]
- Caser, M.; Demasi, S.; Victorino, Í.M.M.; Donno, D.; Faccio, A.; Lumini, E.; Bianciotto, V.; Scariot, V. Arbuscular mycorrhizal fungi modulate the crop performance and metabolic profile of saffron in soilless cultivation. Agronomy 2019, 9, 232. [Google Scholar] [CrossRef]
- Andrzejak, R.; Janowska, B. Yield and quality of inflorescences in the Zantedeschia albomaculata (Hook.) Baill. ‘Albomaculata’ after the treatment with AMF and GA3. Agronomy 2021, 11, 644. [Google Scholar] [CrossRef]
- Deja-Sikora, E.; Werner, K.; Hrynkiewicz, K. AMF species do matter: Rhizophagus irregularis and Funneliformis mosseae affect healthy and PVY-infected Solanum tuberosum L. in a different way. Front. Microbiol. 2023, 14, 1127278. [Google Scholar] [CrossRef]
- Marro, N.; Cofre, N.; Grilli, G.; Alvarez, C.; Labuckas, D.; Maestri, D.; Urcelay, C. Soybean yield, protein content and oil quality in response to interaction of arbuscular mycorrhizal fungi and native microbial populations from mono-and rotation-cropped soils. Appl. Soil Ecol. 2020, 152, 103575. [Google Scholar] [CrossRef]
- Gao, X.; Guo, H.; Zhang, Q.; Guo, H.; Zhang, L.; Zhang, C.; Gou, Z.; Liu, Y.; Wei, J.; Chen, A. Arbuscular mycorrhizal fungi (AMF) enhanced the growth, yield, fiber quality and phosphorus regulation in upland cotton (Gossypium hirsutum L.). Sci. Rep. 2020, 10, 2084. [Google Scholar] [CrossRef]
- Saboor, A.; Ali, M.A.; Danish, S.; Ahmed, N.; Fahad, S.; Datta, R.; Ansari, M.J.; Nasif, O.; Rahman, M.H.u.; Glick, B.R. Effect of arbuscular mycorrhizal fungi on the physiological functioning of maize under zinc-deficient soils. Sci. Rep. 2021, 11, 18468. [Google Scholar] [CrossRef]
- Oliveira, T.C.; Cabral, J.S.R.; Santana, L.R.; Tavares, G.G.; Santos, L.D.S.; Paim, T.P.; Müller, C.; Silva, F.G.; Costa, A.C.; Souchie, E.L. The arbuscular mycorrhizal fungus Rhizophagus clarus improves physiological tolerance to drought stress in soybean plants. Sci. Rep. 2022, 12, 9044. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, X.; Feng, G.; Li, Z.; Zhang, Y.; Cao, N. Arbuscular mycorrhizal enhancement of phosphorus uptake and yields of maize under high planting density in the black soil region of China. Sci. Rep. 2021, 11, 1100. [Google Scholar] [CrossRef] [PubMed]
- Jie, W.; Yang, D.; Yao, Y.; Guo, N. Effects of Rhizophagus intraradices on soybean yield and the composition of microbial communities in the rhizosphere soil of continuous cropping soybean. Sci. Rep. 2022, 12, 17390. [Google Scholar] [CrossRef] [PubMed]
- Trisilawati, O.; Hartoyo, B.; Bermawie, N.; Pribadi, E. Application of AMF (Arbuscular Mycorrhizal Fungi) and organic fertilizer to increase the growth, biomass and bioactive content of Centella. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Saint Petersburg, Russia, 17–18 April 2019; p. 012067. [Google Scholar]
- Dickie, I.A.; Martínez-García, L.B.; Koele, N.; Grelet, G.-A.; Tylianakis, J.M.; Peltzer, D.A.; Richardson, S.J. Mycorrhizas and mycorrhizal fungal communities throughout ecosystem development. Plant Soil 2013, 367, 11–39. [Google Scholar] [CrossRef]
- Morriën, E.; Hannula, S.E.; Snoek, L.B.; Helmsing, N.R.; Zweers, H.; De Hollander, M.; Soto, R.L.; Bouffaud, M.-L.; Buée, M.; Dimmers, W. Soil networks become more connected and take up more carbon as nature restoration progresses. Nat. Commun. 2017, 8, 14349. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, L.B.; Richardson, S.J.; Tylianakis, J.M.; Peltzer, D.A.; Dickie, I.A. Host identity is a dominant driver of mycorrhizal fungal community composition during ecosystem development. New Phytol. 2015, 205, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Courty, P.; Buee, M.; Tech, J.; Brulé, D.; Colin, Y.; Leveau, J.; Uroz, S. Impact of soil pedogenesis on the diversity and composition of fungal communities across the California soil chronosequence of Mendocino. Mycorrhiza 2018, 28, 343–356. [Google Scholar] [CrossRef]
- Cui, X.; Hu, J.; Wang, J.; Yang, J.; Lin, X. Reclamation negatively influences arbuscular mycorrhizal fungal community structure and diversity in coastal saline-alkaline land in Eastern China as revealed by Illumina sequencing. Appl. Soil Ecol. 2016, 98, 140–149. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Waud, M.; Merckx, V.S.; Lievens, B.; Brys, R. Mycorrhizal diversity, seed germination and long-term changes in population size across nine populations of the terrestrial orchid Neottia ovata. Mol. Ecol. 2015, 24, 3269–3280. [Google Scholar] [CrossRef]
- Johnson, N.C. Can fertilization of soil select less mutualistic mycorrhizae? Ecol. Appl. 1993, 3, 749–757. [Google Scholar] [CrossRef]
- Kahiluoto, H.; Ketoja, E.; Vestberg, M. Promotion of utilization of arbuscular mycorrhiza through reduced P fertilization 1. Bioassays in a growth chamber. Plant Soil 2000, 227, 191–206. [Google Scholar] [CrossRef]
- Ma, M.; Ongena, M.; Wang, Q.; Guan, D.; Cao, F.; Jiang, X.; Li, J. Chronic fertilization of 37 years alters the phylogenetic structure of soil arbuscular mycorrhizal fungi in Chinese Mollisols. AMB Express 2018, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Phillips, L.A.; Ward, V.; Jones, M.D. Ectomycorrhizal fungi contribute to soil organic matter cycling in sub-boreal forests. ISME J. 2014, 8, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zheng, R.; Bai, S.; Bai, Y.; Wang, J. Slope aspect influences arbuscular mycorrhizal fungus communities in arid ecosystems of the Daqingshan Mountains, Inner Mongolia, North China. Mycorrhiza 2017, 27, 189–200. [Google Scholar] [CrossRef]
- Begum, F.; Bajracharya, R.M.; Sitaula, B.K.; Sharma, S. Seasonal dynamics, slope aspect and land use effects on soil mesofauna density in the mid-hills of Nepal. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2013, 9, 290–297. [Google Scholar] [CrossRef]
- Nadal-Romero, E.; Petrlic, K.; Verachtert, E.; Bochet, E.; Poesen, J. Effects of slope angle and aspect on plant cover and species richness in a humid Mediterranean badland. Earth Surf. Process. Landf. 2014, 39, 1705–1716. [Google Scholar] [CrossRef]
- Vašutová, M.; Edwards-Jonášová, M.; Baldrian, P.; Čermák, M.; Cudlín, P. Distinct environmental variables drive the community composition of mycorrhizal and saprotrophic fungi at the alpine treeline ecotone. Fungal Ecol. 2017, 27, 116–124. [Google Scholar] [CrossRef]
- Argüelles-Moyao, A.; Garibay-Orijel, R. Ectomycorrhizal fungal communities in high mountain conifer forests in central Mexico and their potential use in the assisted migration of Abies religiosa. Mycorrhiza 2018, 28, 509–521. [Google Scholar] [CrossRef]
- Looby, C.I.; Maltz, M.R.; Treseder, K.K. Belowground responses to elevation in a changing cloud forest. Ecol. Evol. 2016, 6, 1996–2009. [Google Scholar] [CrossRef]
- Chagnon, P.-L.; Bradley, R.L.; Maherali, H.; Klironomos, J.N. A trait-based framework to understand life history of mycorrhizal fungi. Trends Plant Sci. 2013, 18, 484–491. [Google Scholar] [CrossRef]
- Egan, C.P.; Callaway, R.M.; Hart, M.M.; Pither, J.; Klironomos, J. Phylogenetic structure of arbuscular mycorrhizal fungal communities along an elevation gradient. Mycorrhiza 2017, 27, 273–282. [Google Scholar] [CrossRef]
- Liu, L.; Hart, M.M.; Zhang, J.; Cai, X.; Gai, J.; Christie, P.; Li, X.; Klironomos, J.N. Altitudinal distribution patterns of AM fungal assemblages in a Tibetan alpine grassland. FEMS Microbiol. Ecol. 2015, 91, fiv078. [Google Scholar] [CrossRef] [PubMed]
- Maherali, H.; Klironomos, J.N. Phylogenetic and trait-based assembly of arbuscular mycorrhizal fungal communities. PLoS ONE 2012, 7, e36695. [Google Scholar] [CrossRef] [PubMed]
- Ritz, K.; Young, I.M. Interactions between soil structure and fungi. Mycologist 2004, 18, 52–59. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Q.; Lü, Y.; Sun, X.; Jia, S.; Zhang, X.; Liang, W. Conservation tillage positively influences the microflora and microfauna in the black soil of Northeast China. Soil Tillage Res. 2015, 149, 46–52. [Google Scholar] [CrossRef]
- Mangalassery, S.; Mooney, S.J.; Sparkes, D.; Fraser, W.; Sjögersten, S. Impacts of zero tillage on soil enzyme activities, microbial characteristics and organic matter functional chemistry in temperate soils. Eur. J. Soil Biol. 2015, 68, 9–17. [Google Scholar] [CrossRef]
- Indoria, A.; Rao, C.S.; Sharma, K.; Reddy, K.S. Conservation agriculture–a panacea to improve soil physical health. Curr. Sci. 2017, 112, 52–61. [Google Scholar] [CrossRef]
- Lohan, S.K.; Jat, H.; Yadav, A.K.; Sidhu, H.; Jat, M.; Choudhary, M.; Peter, J.K.; Sharma, P. Burning issues of paddy residue management in north-west states of India. Renew. Sustain. Energy Rev. 2018, 81, 693–706. [Google Scholar] [CrossRef]
- Giller, K.E.; Andersson, J.A.; Corbeels, M.; Kirkegaard, J.; Mortensen, D.; Erenstein, O.; Vanlauwe, B. Beyond conservation agriculture. Front. Plant Sci. 2015, 6, 870. [Google Scholar] [CrossRef]
- de Pontes, J.S.; Oehl, F.; Pereira, C.D.; de Toledo Machado, C.T.; Coyne, D.; da Silva, D.K.A.; Maia, L.C. Diversity of arbuscular mycorrhizal fungi in the Brazilian’s Cerrado and in soybean under conservation and conventional tillage. Appl. Soil Ecol. 2017, 117, 178–189. [Google Scholar] [CrossRef]
- Hu, J.; Yang, A.; Wang, J.; Zhu, A.; Dai, J.; Wong, M.H.; Lin, X. Arbuscular mycorrhizal fungal species composition, propagule density, and soil alkaline phosphatase activity in response to continuous and alternate no-tillage in Northern China. Catena 2015, 133, 215–220. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Wu, Q.S. Effects of soil tillage and planting grass on arbuscular mycorrhizal fungal propagules and soil properties in citrus orchards in southeast China. Soil Tillage Res. 2016, 155, 54–61. [Google Scholar] [CrossRef]
- Hart, M.M.; Reader, R.J. Do arbuscular mycorrhizal fungi recover from soil disturbance differently? Trop. Ecol. 2004, 45, 97–112. [Google Scholar]
- Hui, N.; Jumpponen, A.; Francini, G.; Kotze, D.J.; Liu, X.; Romantschuk, M.; Strömmer, R.; Setälä, H. Soil microbial communities are shaped by vegetation type and park age in cities under cold climate. Environ. Microbiol. 2017, 19, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Kerfahi, D.; Tripathi, B.M.; Lee, J.; Edwards, D.P.; Adams, J.M. The impact of selective-logging and forest clearance for oil palm on fungal communities in Borneo. PLoS ONE 2014, 9, e111525. [Google Scholar] [CrossRef] [PubMed]
- Peay, K.G.; Schubert, M.G.; Nguyen, N.H.; Bruns, T.D. Measuring ectomycorrhizal fungal dispersal: Macroecological patterns driven by microscopic propagules. Mol. Ecol. 2012, 21, 4122–4136. [Google Scholar] [CrossRef]
- Verbruggen, E.; Röling, W.F.; Gamper, H.A.; Kowalchuk, G.A.; Verhoef, H.A.; van der Heijden, M.G. Positive effects of organic farming on below-ground mutualists: Large-scale comparison of mycorrhizal fungal communities in agricultural soils. New Phytol. 2010, 186, 968–979. [Google Scholar] [CrossRef]
- Ishaq, L.; Barber, P.A.; Hardy, G.E.S.J.; Dell, B. Diversity of fungi associated with roots of Eucalyptus gomphocephala seedlings grown in soil from healthy and declining sites. Australas. Plant Pathol. 2018, 47, 155–162. [Google Scholar] [CrossRef]
- Chazdon, R.L. Beyond deforestation: Restoring forests and ecosystem services on degraded lands. science 2008, 320, 1458–1460. [Google Scholar] [CrossRef]
- Connell, J.H. Diversity in tropical rain forests and coral reefs: High diversity of trees and corals is maintained only in a nonequilibrium state. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef]
- Hui, N.; Liu, X.; Jumpponen, A.; Setälä, H.; Kotze, D.J.; Biktasheva, L.; Romantschuk, M. Over twenty years farmland reforestation decreases fungal diversity of soils, but stimulates the return of ectomycorrhizal fungal communities. Plant Soil 2018, 427, 231–244. [Google Scholar] [CrossRef]
- Moora, M.; Davison, J.; Öpik, M.; Metsis, M.; Saks, Ü.; Jairus, T.; Vasar, M.; Zobel, M. Anthropogenic land use shapes the composition and phylogenetic structure of soil arbuscular mycorrhizal fungal communities. FEMS Microbiol. Ecol. 2014, 90, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Martinová, V.; Van Geel, M.; Lievens, B.; Honnay, O. Strong differences in Quercus robur-associated ectomycorrhizal fungal communities along a forest-city soil sealing gradient. Fungal Ecol. 2016, 20, 88–96. [Google Scholar] [CrossRef]
- Dumbrell, A.J.; Ashton, P.D.; Aziz, N.; Feng, G.; Nelson, M.; Dytham, C.; Fitter, A.H.; Helgason, T. Distinct seasonal assemblages of arbuscular mycorrhizal fungi revealed by massively parallel pyrosequencing. New Phytol. 2011, 190, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Dumbrell, A.J.; Nelson, M.; Helgason, T.; Dytham, C.; Fitter, A.H. Idiosyncrasy and overdominance in the structure of natural communities of arbuscular mycorrhizal fungi: Is there a role for stochastic processes? J. Ecol. 2010, 98, 419–428. [Google Scholar] [CrossRef]
- Oehl, F.; Sieverding, E.; Ineichen, K.; Maeder, P.; Wiemken, A.; Boller, T. Distinct sporulation dynamics of arbuscular mycorrhizal fungal communities from different agroecosystems in long-term microcosms. Agric. Ecosyst. Environ. 2009, 134, 257–268. [Google Scholar] [CrossRef]
- Gavito, M.E.; Schweiger, P.; Jakobsen, I. P uptake by arbuscular mycorrhizal hyphae: Effect of soil temperature and atmospheric CO2 enrichment. Glob. Chang. Biol. 2003, 9, 106–116. [Google Scholar] [CrossRef]
- Cleland, E.E.; Chuine, I.; Menzel, A.; Mooney, H.A.; Schwartz, M.D. Shifting plant phenology in response to global change. Trends Ecol. Evol. 2007, 22, 357–365. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Fresh perspectives on the roles of arbuscular mycorrhizal fungi in plant nutrition and growth. Mycologia 2012, 104, 1–13. [Google Scholar] [CrossRef]
- Johnson, N.C. Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol. 2010, 185, 631–647. [Google Scholar] [CrossRef]
- Vázquez-Santos, Y.; Castillo-Argüero, S.; Martínez-Orea, Y.; Sánchez-Gallen, I.; Vega-Frutis, R.; Camargo-Ricalde, S.L.; Hernández-Cuevas, L.V. The reproductive phenology of Acaena elongata and its relation with arbuscular mycorrhizal fungi. Symbiosis 2019, 79, 129–140. [Google Scholar] [CrossRef]
- Vega-Frutis, R.; Guevara, R. Greater mycorrhizal colonization of unisexual morphs than of hermaphroditic morphs of Jacaratia mexicana during flowering and fruiting in central Mexico. Symbiosis 2013, 59, 173–181. [Google Scholar] [CrossRef]
- Bencherif, K.; Boutekrabt, A.; Dalpé, Y.; Sahraoui, A.L.-H. Soil and seasons affect arbuscular mycorrhizal fungi associated with Tamarix rhizosphere in arid and semi-arid steppes. Appl. Soil Ecol. 2016, 107, 182–190. [Google Scholar] [CrossRef]
- Hewins, C.R.; Carrino-Kyker, S.R.; Burke, D.J. Seasonal variation in mycorrhizal fungi colonizing roots of Allium tricoccum (wild leek) in a mature mixed hardwood forest. Mycorrhiza 2015, 25, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Rawlik, M.; Jagodziński, A.M. Seasonal dynamics of shoot biomass of dominant clonal herb species in an oak–hornbeam forest herb layer. Plant Ecol. 2020, 221, 1133–1142. [Google Scholar] [CrossRef]
- Zubek, S.; Rola, K.; Rożek, K.; Błaszkowski, J.; Stanek, M.; Chmolowska, D.; Chowaniec, K.; Zalewska-Gałosz, J.; Stefanowicz, A.M. Experimental assessment of forest floor geophyte and hemicryptophyte impact on arbuscular mycorrhizal fungi communities. Plant Soil 2022, 480, 651–673. [Google Scholar] [CrossRef]
- Saini, I.; Aggarwal, A.; Kaushik, P. Inoculation with mycorrhizal fungi and other microbes to improve the morpho-physiological and floral traits of Gazania rigens (L.) Gaertn. Agriculture 2019, 9, 51. [Google Scholar] [CrossRef]
- Tognon, G.B.; Sanmartín, C.; Alcolea, V.; Cuquel, F.L.; Goicoechea, N. Mycorrhizal inoculation and/or selenium application affect post-harvest performance of snapdragon flowers. Plant Growth Regul. 2016, 78, 389–400. [Google Scholar] [CrossRef]
- Lazzara, S.; Militello, M.; Carrubba, A.; Napoli, E.; Saia, S. Arbuscular mycorrhizal fungi altered the hypericin, pseudohypericin, and hyperforin content in flowers of Hypericum perforatum grown under contrasting P availability in a highly organic substrate. Mycorrhiza 2017, 27, 345–354. [Google Scholar] [CrossRef]
- Xie, M.-M.; Wu, Q.-S. Arbuscular mycorrhizal fungi regulate flowering of hyacinths orientalis l. Anna marie. Emir. J. Food Agric. 2018, 30, 144–149. [Google Scholar]
- Liu, S.; Guo, H.; Xu, J.; Song, Z.; Song, S.; Tang, J.; Chen, X. Arbuscular mycorrhizal fungi differ in affecting the flowering of a host plant under two soil phosphorus conditions. J. Plant Ecol. 2018, 11, 623–631. [Google Scholar] [CrossRef]
- Bona, E.; Lingua, G.; Manassero, P.; Cantamessa, S.; Marsano, F.; Todeschini, V.; Copetta, A.; D’Agostino, G.; Massa, N.; Avidano, L. AM fungi and PGP pseudomonads increase flowering, fruit production, and vitamin content in strawberry grown at low nitrogen and phosphorus levels. Mycorrhiza 2015, 25, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xiao, Y. Silver nanoparticles and arbuscular mycorrhizal fungi influence Trifolium repen root-associated AMF community structure and its co-occurrence pattern. Sci. Hortic. 2023, 320, 112232. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, J.; Fan, Z.; Fang, M.; Xu, Z.; Ban, Y. The function and community structure of arbuscular mycorrhizal fungi in ecological floating beds used for remediation of Pb contaminated wastewater. Sci. Total Environ. 2023, 872, 162233. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, X.; Wang, Z.; Su, X.; Liu, F.; Tian, X.; Ye, Y.; Shao, Y.; Yuan, Z. Cd contamination determined assembly processes and network stability of AM fungal communities in an urban green space ecosystem. Sci. Total Environ. 2023, 899, 166372. [Google Scholar] [CrossRef]
- Chase, J.M.; Myers, J.A. Disentangling the importance of ecological niches from stochastic processes across scales. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2351–2363. [Google Scholar] [CrossRef]
- Jiao, S.; Zhang, B.; Zhang, G.; Chen, W.; Wei, G. Stochastic community assembly decreases soil fungal richness in arid ecosystems. Mol. Ecol. 2021, 30, 4338–4348. [Google Scholar] [CrossRef] [PubMed]
- Faggioli, V.; Menoyo, E.; Geml, J.; Kemppainen, M.; Pardo, A.; Salazar, M.J.; Becerra, A.G. Soil lead pollution modifies the structure of arbuscular mycorrhizal fungal communities. Mycorrhiza 2019, 29, 363–373. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, G.; Xing, S.; Fu, W.; Liu, X.; Wu, H.; Zhou, X.; Ma, Y.; Zhang, X.; Chen, B. Structure and diversity of fungal communities in long-term copper-contaminated agricultural soil. Sci. Total Environ. 2022, 806, 151302. [Google Scholar] [CrossRef]
- Betancur-Agudelo, M.; Meyer, E.; Lovato, P.E. Arbuscular mycorrhizal fungus richness in the soil and root colonization in vineyards of different ages. Rhizosphere 2021, 17, 100307. [Google Scholar] [CrossRef]
- Gonzalez-Guerrero, M.; Melville, L.H.; Ferrol, N.; Lott, J.N.; Azcon-Aguilar, C.; Peterson, R.L. Ultrastructural localization of heavy metals in the extraradical mycelium and spores of the arbuscular mycorrhizal fungus Glomus intraradices. Can. J. Microbiol. 2008, 54, 103–110. [Google Scholar] [CrossRef]
- Staddon, P.L.; Thompson, K.; Jakobsen, I.; Grime, J.P.; Askew, A.P.; Fitter, A.H. Mycorrhizal fungal abundance is affected by long-term climatic manipulations in the field. Glob. Chang. Biol. 2003, 9, 186–194. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.; Kim, C.-G.; Subramanian, P.; Kim, K.-Y.; Selvakumar, G.; Sa, T.-M. Arbuscular mycorrhizal fungi community structure, abundance and species richness changes in soil by different levels of heavy metal and metalloid concentration. PLoS ONE 2015, 10, e0128784. [Google Scholar] [CrossRef] [PubMed]
- Long, L.K.; Yao, Q.; Guo, J.; Yang, R.H.; Huang, Y.H.; Zhu, H.H. Molecular community analysis of arbuscular mycorrhizal fungi associated with five selected plant species from heavy metal polluted soils. Eur. J. Soil Biol. 2010, 46, 288–294. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, B.; Yin, R.; Xing, S.; Fu, W.; Wu, H.; Hao, Z.; Ma, Y.; Zhang, X. Long-term nickel contamination increased soil fungal diversity and altered fungal community structure and co-occurrence patterns in agricultural soils. J. Hazard. Mater. 2022, 436, 129113. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Luo, G.; Zhang, Q.; Cui, W.; Li, Z.; Zhang, S. Potential hazards of novel waste-derived sorbents for efficient removal of mercury from coal combustion flue gas. J. Hazard. Mater. 2021, 412, 125226. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhang, Z.; Zhang, M.; Rinklebe, J.; Ma, Y.; Hou, D. Effect of production temperature and particle size of rice husk biochar on mercury immobilization and erosion prevention of a mercury contaminated soil. J. Hazard. Mater. 2021, 420, 126646. [Google Scholar] [CrossRef]
- Mi, Y.; Bai, X.; Li, X.; Zhou, M.; Liu, X.; Wang, F.; Su, H.; Chen, H.; Wei, Y. Soil Mercury Pollution Changes Soil Arbuscular Mycorrhizal Fungal Community Composition. J. Fungi 2023, 9, 395. [Google Scholar] [CrossRef]
- Ban, Y.; Jiang, Y.; Li, M.; Zhang, X.; Zhang, S.; Wu, Y.; Xu, Z. Homogenous stands of a wetland grass living in heavy metal polluted wetlands harbor diverse consortia of arbuscular mycorrhizal fungi. Chemosphere 2017, 181, 699–709. [Google Scholar] [CrossRef]
- Mi, Y.; Xu, C.; Li, X.; Zhou, M.; Cao, K.; Dong, C.; Li, X.; Ji, N.; Wang, F.; Su, H. Arbuscular mycorrhizal fungi community analysis revealed the significant impact of arsenic in antimony-and arsenic-contaminated soil in three Guizhou regions. Front. Microbiol. 2023, 14, 1189400. [Google Scholar] [CrossRef]
- Schneider, J.; Stürmer, S.L.; Guilherme, L.R.G.; de Souza Moreira, F.M.; de Sousa Soares, C.R.F. Arbuscular mycorrhizal fungi in arsenic-contaminated areas in Brazil. J. Hazard. Mater. 2013, 262, 1105–1115. [Google Scholar] [CrossRef]
- Zhu, F.; Zhu, C.; Wang, C.; Gu, C. Occurrence and ecological impacts of microplastics in soil systems: A review. Bull. Environ. Contam. Toxicol. 2019, 102, 741–749. [Google Scholar] [CrossRef]
- Rillig, M.C.; de Souza Machado, A.A.; Lehmann, A.; Klümper, U. Evolutionary implications of microplastics for soil biota. Environ. Chem. 2018, 16, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Nizzetto, L.; Langaas, S.; Futter, M. Pollution: Do microplastics spill on to farm soils? Nature 2016, 537, 488. [Google Scholar] [CrossRef] [PubMed]
- Hodson, M.E.; Duffus-Hodson, C.A.; Clark, A.; Prendergast-Miller, M.T.; Thorpe, K.L. Plastic bag derived-microplastics as a vector for metal exposure in terrestrial invertebrates. Environ. Sci. Technol. 2017, 51, 4714–4721. [Google Scholar] [CrossRef] [PubMed]
- de Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics can change soil properties and affect plant performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, X.; Zhang, S.; Zhang, S.; Sun, Y. Interactions of microplastics and cadmium on plant growth and arbuscular mycorrhizal fungal communities in an agricultural soil. Chemosphere 2020, 254, 126791. [Google Scholar] [CrossRef]
- Giambalvo, D.; Amato, G.; Ingraffia, R.; Porto, A.L.; Mirabile, G.; Ruisi, P.; Torta, L.; Frenda, A.S. Nitrogen fertilization and arbuscular mycorrhizal fungi do not mitigate the adverse effects of soil contamination with polypropylene microfibers on maize growth. Environ. Pollut. 2023, 334, 122146. [Google Scholar] [CrossRef]
- Fan, P.; Tan, W.; Yu, H. Effects of different concentrations and types of microplastics on bacteria and fungi in alkaline soil. Ecotoxicol. Environ. Saf. 2022, 229, 113045. [Google Scholar] [CrossRef]
- Brigido, C.; Van Tuinen, D.; Brito, I.; Alho, L.; Goss, M.J.; Carvalho, M. Management of the biological diversity of AM fungi by combination of host plant succession and integrity of extraradical mycelium. Soil Biol. Biochem. 2017, 112, 237–247. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).