Abstract
Periods of heat and water deficit often occur together and are especially dangerous for plants grown in pots, where the substrate volume for roots is limited. The purpose of the present research was to understand the response of shrubs planted in containers to the addition of a starch-based superabsorbent to their growing medium. The growth parameters, physiological conditions, and oxidative stress of Cornus alba ’Aurea’, Hydrangea paniculata ‘Limelight’, and Physocarpus opulifolius ‘Red Baron’ were assessed by adding a hydrogel (1, 2, or 3 g·dm−3) to their growing medium. The use of the superabsorbent improved the stomatal conductance and photosynthetic rate, resulting in better growth parameters. The application of 1 g·dm−3 hydrogel increased the chlorophyll content in hydrangea and ninebark leaves (8%) and increased the content of total soluble sugars in these plants (12% and 15%, respectively). The highest increase in reducing sugars was caused by a dosage of 3 g·dm−3. The lowest dose of hydrogel resulted in a decrease in hydrogen peroxide content in the leaves of all the taxa. The relationship between the contents of biologically active components and oxidative stress proved ambiguous for all the taxa. Oxidative stress was reduced, as evidenced by lower hydrogen peroxide and an increase in pigment content. In summary, a hydrogel dosage of 2 g·dm−3 in the medium could be optimal in pot nursery production using 3 dm3 pots.
1. Introduction
The most important existing and predicted threats from climate change to horticulture production and crop security worldwide seem to be unexpected fluctuations and variations, including periodic water deficits and prolonged droughts [1]. Recently, periods with scarce rainfall have occurred frequently, especially in the summer, and are additionally linked to increases in temperature and record-breaking heat waves. Droughts have become common even in the mild climate of Middle Europe, where sufficient amounts of rainwater used to fall earlier [2]. Dry periods endanger horticultural production, as they affect not only plants in gardens and parks but also those produced in containers, where the substrate volume, i.e., nutrient and water amounts available for roots, is limited [3].
Stress resulting from water deficits or droughts has been widely investigated. It decreases nutrient uptake, thus limiting growth and metabolic processes [4,5]. Insufficient water availability results in morphological and physiological changes in plants, such as limited rates of cell division and cell expansion; decreases in leaf area, stem elongation, and root system size; and the decreased formation and opening of stomata. Woody plants have developed several morphological adaptations to cope with drought, including limiting shoot length, foliage area, and the ratio of the above-soil plant area to that underground while enhancing root system development [6]. Prunus sargentii and Larix kaempferi seedlings cultivated in drought conditions showed a significant decrease in leaf size and branch growth, while there was an increase in leaf mass [7]. The physiological parameters assessed in this study revealed direct proof of the existence of a water deficit through, e.g., a decrease in the photosynthetic rate, including stomatal conductance [7,8]. However, water deficits and droughts affect most plant life processes and thus have effects on plant production.
The sustainable development of modern agriculture requires a combination of possibly eco-friendly methods to significantly improve plant productivity and environmental quality [4]. Moreover, in order to increase the safety and quality of plant production, the influence of extreme weather events, including prolonged water deficits, should be minimized as much as possible. In container production, the stress resulting from water deficits may be limited by the application of superabsorbents (hydroabsorbents, hydrogels, and supergels) [9]. The most commonly available plant polysaccharides, such as starch, can be used as hydrogels. Starch can be chemically modified to meet a variety of requirements for medicinal [10], pharmaceutical, and personal care products or cosmetic applications [11]. This is an alternative solution to the use of non-eco-friendly hydrogels prepared from synthetic polymers and petrochemicals [11]. New technologies have been introduced to synthesize starch-based hydrogels, including zero-waste ideas, such as rice-cooked wastewater [12].
Due to their unique physicochemical properties, starch-based hydrogels can accumulate and give back soil water, store it in the vicinity of the roots, decrease water losses by evaporation or leaching, increase soil permeability, and protect the soil from degradation. These effects make hydrogels increasingly popular in agri- and horticulture [13]. Hydrogels are able to absorb water in volumes of up to one thousand times their dry weight [11,14]. However, their ability to absorb water or other substances varies in relation to many factors regarding their chemical and biochemical transformation [11,15,16], size, porosity, surface structure [17], and environmental conditions, including soil properties [18]. Their presence in the soil increases the efficiency of plants’ use of water and prolongs plant activities, especially during dry periods [19,20]. Research conducted on polysaccharide cellulose-based hydrogels has indicated the role of various factors affecting the behavior of superabsorbents, e.g., temperature, relative humidity, and soil type [21].
Starch-based sustainable hydrogels (SHs) are especially noteworthy. Starch is hydrophilic in nature, non-toxic, biocompatible, completely biodegradable in different environments, and cheap. Due to these benefits, it may be a good solution instead of synthetic hydrogels to address several potential multidimensional applications [15,16,17,18,20]. The addition of superabsorbents similar to sugars to the growing medium has been shown to increase the amount of macronutrient (nitrogen, potassium, and phosphorus) absorption in the leaves, stems, and roots of acacia plants growing under drought stress conditions [20], but the mechanism of this reaction was not described by the authors of the publication [20] and seems to still be unknown.
In our experiment, we used a starch-based commercial preparation, Zeba SP, that contains maize starch modified by side chains of propenamide and acryl acid copolymers in the form of potassium salts. Zeba SP is a completely biodegradable maize-starch-based superabsorbent in the form of micro-granules. This hydrogel based on starch polymers expands by absorbing 400 times more water relative to its mass and can release this water later on according to plant demand. Water is absorbed when the soil is well hydrated and released during periods of water deficit. Hydration and dehydration can occur repeatedly, thus limiting soil water losses during the whole vegetative period. As the hydrogel improves air–water relations in the soil, it enhances rhizogenesis in cuttings and improves the growth of the above-soil plant parts. With time, Zeba SP is degraded and used by soil microorganisms [13].
Prolonged agricultural droughts (deficits in soil moisture) have been observed to occur with accompanying periods of high temperatures and hydrological (grid-scale runoff) droughts [2] that indicate the necessity of water saving. The problem is especially prevalent in the container production and cultivation of woody plants, where the medium’s capacity is limited. Additionally, natural products are preferred in plant production and cultivation, according to international legislation [22]. Due to the above arguments and previous research related to the benefits of the use of starch-based hydrogels [23] like Zeba SP [13] in forestry, our experiments were conducted on woody ornamentals planted in containers mimicking nursery production. Few-year-old ornamental shrubs are the leading group in the nursery production of plant material for green areas in cities and gardens. We deduced that the varieties selected for research (dogwood, Cornus alba ‘Aurea’; hydrangea, Hydrangea paniculata ‘Limelight’; and ninebark, Physocarpus opulifolius ‘Red Baron’) are among the most popular shrubs in cultivation.
The aim of this research was to evaluate the growth and physiological condition parameters of ornamental deciduous woody plants growing in containers using a medium with the addition of a starch-based superabsorbent. We hypothesized that the starch-based superabsorbent decreases the oxidative stress in plants cultivated in pots and that adding it in the proper dose enhances the quality of the nursery plant material at the final stage of production before planting them in greeneries.
2. Materials and Methods
2.1. Plant Material and Pattern of Conducted Experiment
Experiments were performed on two-year-old deciduous shrubs of dogwood, Cornus alba ‘Aurea’; hydrangea, Hydrangea paniculata ‘Limelight’; and ninebark, Physocarpus opulifolis ‘Red Baron’. On the first day of the experiment, they were repotted from P9 pots (volume 0.5 dm3) to C3 containers (3 dm3) and placed on a nursery bed provided with an automatic watering system. Watering was switched on every day for 20 min between 7 and 8 a.m., with 300 mL of water per pot.
The growing medium in P9 and C3 was composed of peat, bark, and sand (5:4:1 volume) at pH 6.7–7.0. The medium was supplemented with Osmocote 5–6 M in a dose of 4 g/dm3.
In the experiment, the medium was mixed with a given amount of a starch-based hydrogel. The experiment consisted of (i) control—without hydrogel; (ii) 1 g·dm−3; (iii) 2 g·dm−3; (iv) 3 g·dm−3. The starch-based Zeba SP (UPL Limited, Gujarat, India), which consists of starch modified with side chains of propenamide and acrylic acid copolymers in the form of potassium salts (88%), was used.
The experiment lasted 20 weeks, from 5th May to 22nd September 2022, in the research plant nursery of the Section of Ornamental Plants, Institute of Horticultural Sciences, WULS, Warsaw. The climatic conditions in the year 2022 compared with the mean of the average temperatures in the years 1951–2021 indicated a tendency for increased temperature in the year 2022. Attention is drawn to the high-temperature extrema (Figure 1). An increase in average temperature in recent years in Warsaw was indicated in the authors’ previous publication [24].
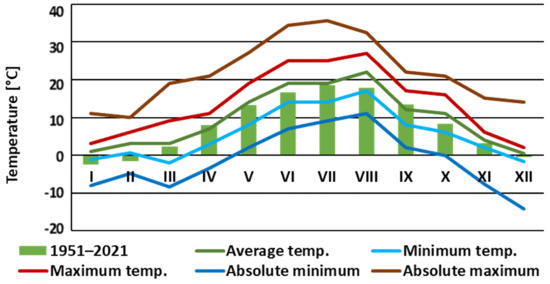
Figure 1.
Means of monthly temperatures (°C) (average, minimum, maximum; absolute minimum and maximum) in the year 2022 and the mean of average temperatures in the years 1951–2021 in Warsaw [25]. The numbers I–XII denote the months from January to December.
The effectiveness of the starch-based superabsorbent was determined with the measurement of medium moisture around the plant roots. The relative soil humidity around the roots (%RH) was determined once a week, with measurements taken twice a day at 1 p.m. and 7 p.m. at 20 °C, with an Elmetron PWT-105 thermo-hygrometer (Elmetron, Poland).
In the afternoons and evenings, the growing medium humidity around the roots was measured in all treatments. The calculated means for all measurements made in the experimental combinations in the afternoon hours are given in Figure 2. The lowest mean medium humidity was determined in the controls, while all of the treatments with growing medium supplemented with the starch-based superabsorbent showed higher values of this parameter, regardless of the preparation dosage applied. A similar tendency was observed for the evening humidity measurements. Soil humidity (%RH) was always higher in containers with growing medium supplemented with the hydrogel, regardless of its dosage (Figure 2). The medium dried out more slowly when the starch-based superabsorbent was added, independently of the dose and regardless of the taxon. The significant decrease in medium moisture in the evening suggests that the plants used more water to grow in the medium with the addition of the superabsorbent. The correlation analysis indicated that the dosage of hydrogel significantly influenced the stability of moisture in the evening (Figure 3).
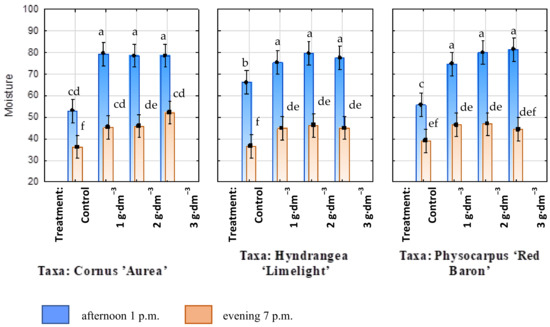
Figure 2.
The relative soil humidity (%RH) around root ball measured in the afternoon (1 p.m.) and evening (7 p.m.) with starch-based superabsorbent and control. Bars followed by the same letter are not significantly different according to Duncan’s multiple range test at α = 0.05. Vertical bars denote 95% confidence intervals for the mean of counts (three-way ANOVA).
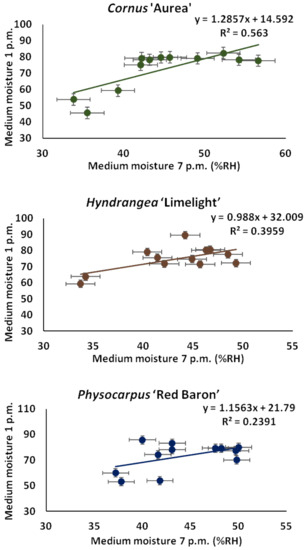
Figure 3.
The linear regression analysis between the relative soil humidity around the root ball (%RH) measured at afternoon (1 p.m.) and in the evening (7 p.m.) with starch-based superabsorbent and control. Data represent the mean ± standard error (n = 3).
2.2. Growth Parameters of Plants
On the first and last days of the experiment, plant height was measured (from the medium level to the top of the highest shoot).
Root system development was assessed on the basis of an evaluation scale on the last day of the experiment. All of the shrubs were assessed using the following 5-point scale: 1—single, poorly formed, and visible roots; 2—at least a dozen poorly formed roots; 3—a few longer and clearly visible roots; 4—roots visibly developed, differentiated in length, starting to form a root ball; 5—roots strong, forming a well-developed root ball. The evaluation scale is shown in Figure 4.
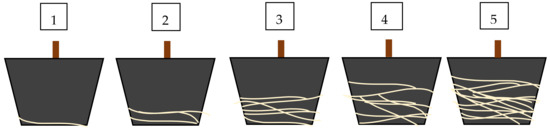
Figure 4.
The evaluation scale for the root systems (root balls) of plants used in the assessment at the end of the experiment.
Randomly selected leaves from the middle parts of shoots were measured with a leaf area meter (AM 300, ADC BioScientific Ltd., Hoddesdon, UK) at the end of summer, which was the end of the experiment.
2.3. Epidermis Stomata
The epidermis stomata observations were conducted in Hydrangea paniculata ‘Limelight’. Five leaves were collected in the middle parts of five shoots on a sunny day at 12 a.m. on 16 August. The leaves were protected with clear tape before sampling and immediately taken to the laboratory. The epidermis for microscopic observations of stomata was isolated with clear tape and was then placed on microscopic slides and dyed with toluidine blue solution (0.1%). Each preparation was then protected with a glycerin (pure, 92.09 g·mol−1, Sigma-Aldrich, St. Louis, MO, USA, no. G2289) drop and covered with a cover glass. Observations were carried out under an Olympus CX43 Standard light microscope associated with a camera and image analysis software. Stomatal pore width (Wp) was measured in several fields of view with the program dedicated to the Olympus camera (Olympus America Inc., New York, NY, USA).
2.4. Photosynthetic Rate and Stomatal Conductance
Measurements of the net photosynthetic rate (A, µmol CO2 m−2·s−1) were made in three leaves from each of three replications (nine records for each data point) at midday (12 a.m.; 16 August) under a natural photosynthetic photon flux of 1000–1400 µmol, photosynthetically active radiation (PAR) m−2·s−1 at 25 °C, RH of 50–60%, and CO2 concentration of 300–350 µmol·mol−1 air. Stomatal conductance (gs), as a parameter of gas exchange, was measured using a CIRAS-2 gas analyzer (PP System Inc., Amesbury, MA, USA) and is given in mol H2O m−2·s−1 in the same manner and on the same day as above.
2.5. Determination of Chlorophylls and Carotenoids
The plant material for analysis was harvested on the last day of the experiment. The plant material from each treatment was finely chopped and mixed, and 0.5 g samples were weighed. Freshly harvested leaves from the middle parts of shoots were sampled for chlorophyll a/b and carotenoids content analysis [18]. The plant material was ground in a mortar with 80% acetone cooled to 4 °C degrees. The supernatant developed the pigments were determined spectrophotometrically at 663 nm (chlorophyll a), 646 nm (chlorophyll b), and 652 nm (carotenoids) (spectrophotometer UV-1601 PC, Shimadzu, Kyoto, Japan) against acetone. Pigment contents were calculated according to the formula in [26] and are given as mg·g−1 DW (DW—dry weight). The data on chlorophyll a and b were added together and joined as a single result.
2.6. Determination of Bioactive Components
Fresh leaves were harvested for analysis from plants on the last day of tests. For the dry matter content, 1 g of leaves was dried at 105 °C to constant weight [27].
Total soluble and reducing sugar analyses were performed [28] spectrophotometrically. For the determination of sugars, the samples were homogenized in 80% boiling ethanol. After reacting with phenol and 96% sulfuric acid, the absorbance of the colorful reaction mixture was read at 490 nm. The contents were calculated from a standard (3 cm3 of glucose in 100 cm3), and the values were noted in milligrams of C6H12O6 mg−1 of dry weight (DW).
Hydrogen peroxide was determined spectrophotometrically [29]. The reaction mixture consisted of the extract, 0.1 M potassium-phosphoric buffer pH 7.0, and 1 M potassium iodide. After 1 h incubation in darkness, solution absorbance was measured by the UV-1601 PC spectrophotometer (Shimadzu) at 390 nm against a blank sample. Hydrogen peroxide content is expressed in µg·g−1 DW.
2.7. Statistical Analysis
Each treatment of the experiment had 3 replicates containing 5 pots. To sum up, 180 pots were used, with 60 plants from each taxon. The experiment was designed as a randomized complete block design (RCBD) for each taxon separately [30].
The relative soil humidity (%RH) around the root system was analyzed by three-factors analysis of variance (ANOVA). The results of analytical measurements and growth parameters were subjected to one- or two-factors analysis of variance (ANOVA), followed by Duncan’s Honest Significant Difference test at α = 0.05 [30,31]. Linear regression analysis by the method of least squares was conducted for (i) the relative medium humidity around the root ball (%RH) in the morning and in the evening; (ii) increments in shoot length (cm) and leaf area (cm2); (iii) stomatal conductance (gs) (mol H2O m−2·s−1) and photosynthetic rate (A) (µmol CO2 m−2·s−1).
Correlations between (i) stomatal conductance (gs) (mol H2O m−2·s−1), photosynthetic rate (A) (µmol CO2 m−2·s−1), and stomata pore width (Wp) (µm) in Hyndrangea paniculata ‘Limelight’ and (ii) chlorophyll (a + b), carotenoids, reducing and total carbohydrates, and hydrogen peroxide were measured. The analyses were performed separately for each combination and taxon.
STATISTICA 13.3 (StatSoft, Cracow, Poland) and Excel (Microsoft Professional Office Plus 2016) software were used.
3. Results
3.1. Starch-Based Superabsorbent Added to Medium Encourages Woody Plant Growth
The application of the starch-based superabsorbent positively affected the increase in shoot length in all taxa under study. As compared to the control treatments, the shoots in plants of Cornus alba ‘Aurea’, Hydrangea paniculata ‘Limelight’, and Physocarpus opulifolius ‘Red Baron’ growing in the substrate containing 1 g·dm−3 of the preparation had shoots that were 24, 30, and 19% longer, respectively. Increasing the preparation dosage to 2 and 3 g·dm−3 did not further enhance shoot elongation in Cornus alba ‘Aurea’ or Physocarpus opulifolius ‘Red Baron’. In the case of hydrangea ‘Limelight’, each used dosage of the hydrogel influenced the shoot increase as compared to the control (Figure 5).
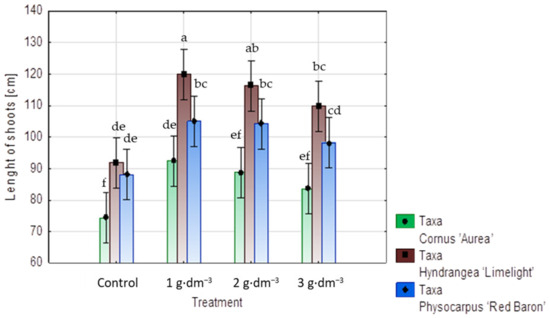
Figure 5.
Means of shoot length increments (cm) in shrubs taxa planted in medium with starch-based superabsorbent and control. Bars followed by the same letter are not significantly different according to Duncan’s multiple range test at α = 0.05. Vertical bars denote 95% confidence intervals for the mean of counts (two-way ANOVA).
The means of the leaf blade areas of shrubs of all taxa growing in media supplemented with the starch-based superabsorbent were larger for each used dosage than those of shrubs planted in the control medium (Figure 6 and Figure 7A–C). The mean leaf blade areas of dogwood plants provided with 1, 2, or 3 g·dm−3 superabsorbent were 1.7, 1.5, and 1.2 times larger than those of control plants, respectively. In hydrangea, this increase in leaf area ranged between 1.2 times with 2 or 3 g·dm−3 doses and 1.5 times with a 1 g·dm−3 dose. A smaller area increase occurred in ninebark, ranging between 17% for 1 and 2 g·dm−3 superabsorbent in the medium and 14% with a 3 g·dm−3 hydrogel dosage (Figure 6).
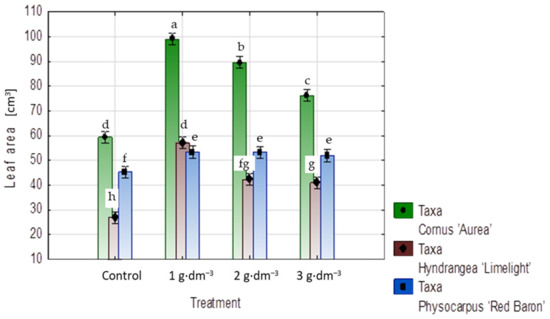
Figure 6.
Means of leaf blade area (cm2) in shrubs taxa planted in medium with starch-based superabsorbent and control. Bars followed by the same letter are not significantly different according to Duncan’s multiple range test at α = 0.05. Vertical bars denote 95% confidence intervals for the mean of counts (two-way ANOVA).
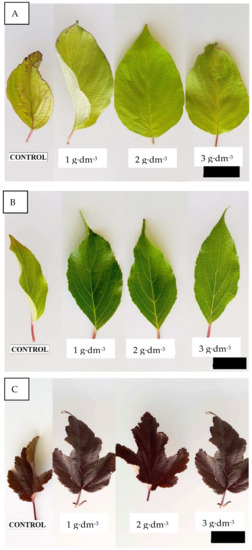
Figure 7.
Leaf blades of shrubs growing in a substrate enriched with one of three doses of starch-based superabsorbent in Cornus alba ’Aurea’ (A), Hyndrangea paniculata ‘Limelight’ (B), and Physocarpus opulifolius ‘Red Baron’ (C). Scale bars—10 cm.
The increment in shoot length was positively correlated with the leaf area blade in Cornus alba ‘Aurea’ and Hydrangea paniculata ‘Limelight’. This result indicates that a higher value of the shoot length increment is linked to a larger mean leaf area, which contributes to increases in growth parameters and the quality of the shrubs. This correlation was not observed in Physocarpus opulifolius ‘Red Baron’ (Figure 8).
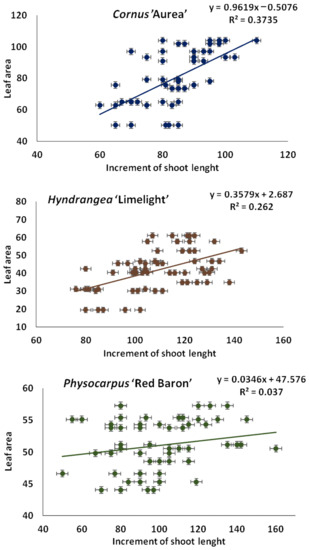
Figure 8.
Linear regression analysis between increments in shoot length (cm) and leaf area (cm2) for the taxa planted in medium with starch-based superabsorbent and control. Data represent the means of fifteen measurements ± standard error (n = 15).
The application of the starch-based hydrogel also positively affected the root development of plants evaluated on a scale (Figure 9). Even the lowest dosage significantly increased root system development as compared to the respective controls. Only in ninebark in a concentration of 3 g·dm−3 was root growth not better on the evaluation scale than that of plants grown without the hydrogel.
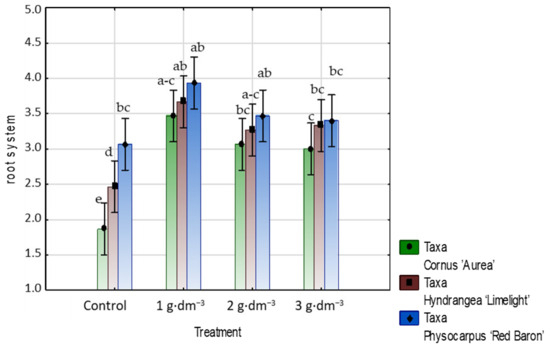
Figure 9.
The quality of root system in evaluation scale of taxa planted in medium with starch-based superabsorbent and control. The following 5-point scale was used: 1—single, poorly formed, and visible roots; 2—at least a dozen poorly formed roots; 3—a few longer and clearly visible roots; 4—roots visibly developed, differentiated in length, starting to form a root ball; 5—roots strong, forming a well-developed root ball (Figure 4). Bars followed by the same letter are not significantly different according to Duncan’s multiple range test at α = 0.05. Vertical bars denote 95% confidence intervals for the mean of counts (two-way ANOVA).
3.2. Starch-Based Superabsorbent Added to Medium Differentially Affects Changes in Photosynthetic Rate and Stomatal Conductance
The mean photosynthetic rate (A) ranged between 2.0 and 2.2 μmol CO2 m−2·s−1 in the control. The results after treatment indicated that the presence of the starch-based hydrogel in the growing medium positively affected the photosynthetic rate; however, the proper dosage for the highest values varied between taxa. The mean increase was 1.5–1.6 times in dogwood and 1.3–1.9 times in hydrangea in all treatments of the hydrogel relative to the control, while in ninebark, the photosynthetic rate was 1.3 times higher with the highest preparation dosage and 1.9 times higher with 2 g·dm−3. The photosynthetic rate was higher in ninebark with a 3 g·dm−3 dosage compared to 1 and 2 g·dm−3 (Figure 10).
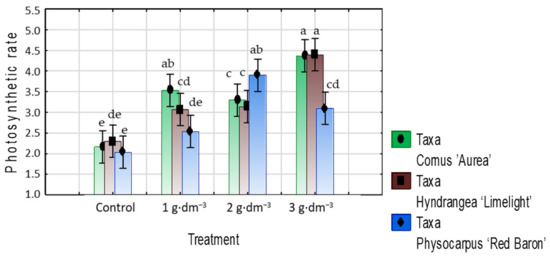
Figure 10.
Photosynthetic rate (A) (µmol CO2 m−2·s−1) of taxa planted in medium with starch-based superabsorbent and control. Bars followed by the same letter are not significantly different according to Duncan’s multiple range test at α = 0.05. Vertical bars denote 95% confidence intervals for the mean of counts (two-way ANOVA).
Stomatal conductance (gs) (mol H2O m−2·s−1) in the control varied between the studied taxa. In plants growing in medium supplemented with the hydrogel, stomatal conductance was higher than in the respective controls: 33–54% higher in dogwood, 34–42% higher in hydrangea, and 40–70% higher in ninebark (Figure 11).
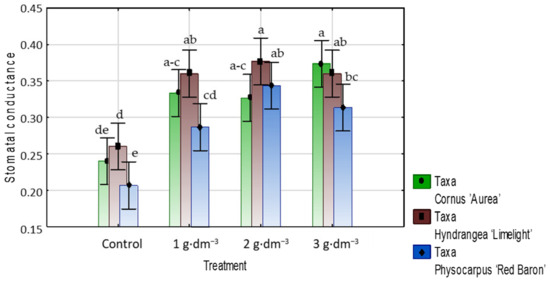
Figure 11.
Stomatal conductance (gs) (mol H2O m−2·s−1) of taxa planted in medium with starch-based superabsorbent and control. Bars followed by the same letter are not significantly different according to Duncan’s multiple range test at α = 0.05. Vertical bars denote 95% confidence intervals for the mean of counts (two-way ANOVA).
The correlation analysis between the photosynthetic rate (A) and stomatal conductance (gs) (mol H2O m−2·s−1) showed a strictly positive relationship between the traits. These results indicate that higher maximum and minimum stomatal conductance is linked to a higher dynamism of photosynthesis (Figure 12).
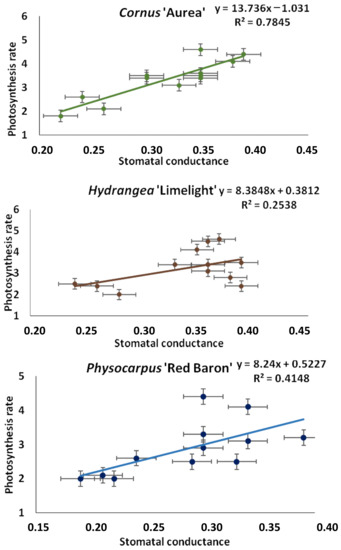
Figure 12.
Linear regression analysis between stomatal conductance (gs) (mol H2O m−2·s−1) and photosynthetic rate (A) (µmol CO2 m−2·s−1) of taxa planted in medium with starch-based superabsorbent and control. Data represent the mean ± standard error (n = 3).
An additional correlation analysis was performed for hydrangea ‘Limelight’. The width of the stomatal opening in Hydrangea ‘Limelight’ was always greater in plants growing in the presence of the hydrogel. The means were almost twice as high with the addition of 2 g·dm−3 superabsorbent compared to leaves from the control medium (Figure 13). Moreover, stomatal conductance (gs) was negatively correlated with the photosynthetic rate (A) in the control and the group with 1 g·dm−3 superabsorbent in the medium, while the use of 2 and 3 g·dm−3 showed a positive correlation. This result indicates a water deficit. A dosage of 2 g·dm−3 seems to be optimal, with increased stomatal conductance (gs), a higher photosynthetic rate (A), and simultaneously, a wider stomata pore width (Wp). Moreover, stomatal conductance was positively correlated with the photosynthetic rate with a dosage of 3 g·dm−3, while the stomata pore width (Wp) correlated negatively with the photosynthetic rate (A) and stomatal conductance (gs) (Table 1).
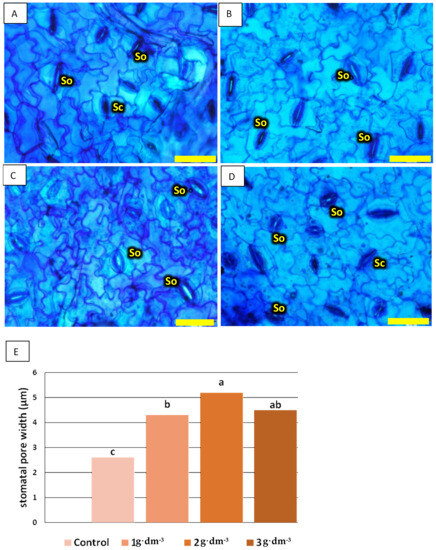
Figure 13.
Epidermis stomata width in Hyndrangea paniculata ‘Limelight’ in (A) control and (B–D) plants treated with starch-based superabsorbent (respectively: (B) 1 g·dm−3; (C) 2 g·dm−3; (D) g·dm−3) and (E) means of stomatal pore width (Wp) (µm). Designations: Sc—stomata closed; So—stomata open. Bar = 60 µm. Means followed by the same letter are not significantly different according to Duncan‘s multiple range test at α = 0.05.

Table 1.
Parts of correlation matrices between results of stomatal conductance (gs) (mol H2O m−2·s−1), photosynthetic rate (A) (µmol CO2 m−2·s−1), and stomata pore width (Wp) (µm) in Hyndrangea paniculata ‘Limelight’, with separate results for added starch-based superabsorbent. The correlations are significant at p < 0.05, marked in color scale: 0.1–0.29—very low; 0.30–0.49—low; 0.50–0.69—restrained; 0.70–0.89—high; >0.90—very high.
3.3. Starch-Based Superabsorbent Added to Medium Changes the Content of Photosynthetically Active Pigments and Carbohydrates in Leaves of Woody Plants
The application of 1 or 2 g·dm−3 hydrogel to the growing medium resulted in a nearly 9% increase in chlorophylls content in dogwood leaves, while with the highest preparation dosage, the chlorophylls did not differ from that in the control. In hydrangea and ninebark, even the lowest hydrogel dose increased the chlorophyll concentration by nearly 8% relative to the controls, while with increased hydrogel contents (2 and 3 g·dm−3 in hydrangea ‘Limelight’ and 2 g·dm−3 in ninebark) in the growing medium, the means of chlorophylls content decreased in value as compared to the control treatment (Figure 14).
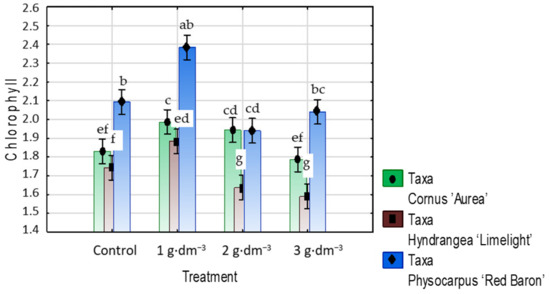
Figure 14.
The chlorophylls content (mg·g−1 DW) in leaves of taxa planted in medium with starch-based superabsorbent and control. Bars followed by the same letter are not significantly different according to Duncan’s multiple range test at α = 0.05. Vertical bars denote 95% confidence intervals for the mean of counts (two-way ANOVA).
The carotenoids level was little affected by the addition of the hydrogel to the growing medium. In C. alba ‘Aurea’ and P. opulifolius ‘Red Baron’, only a dose of 1 g·dm−3 slightly but significantly increased the contents of these pigments. In hydrangea with 2 g·dm−3 hydrogel, an increase in carotenoids content was found (Figure 15).
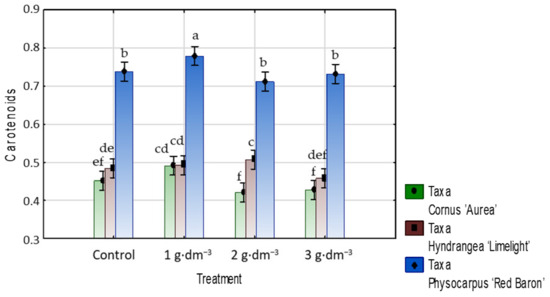
Figure 15.
The carotenoids content (mg·g−1 DW) in leaves of taxa planted in medium with starch-based superabsorbent and control. Bars followed by the same letter are not significantly different according to Duncan’s multiple range test at α = 0.05. Vertical bars denote 95% confidence intervals for the mean of counts (two-way ANOVA).
In the case of total soluble carbohydrates content, the results varied according to the taxa. The values were higher in C. alba ‘Aurea’ and P. opulifolius ‘Red Baron’ in the presence of 1 g·dm−3 hydrogel, while in H. paniculata ‘Limelight’, they were higher with 2 and 3 g·dm−3 (Figure 16).
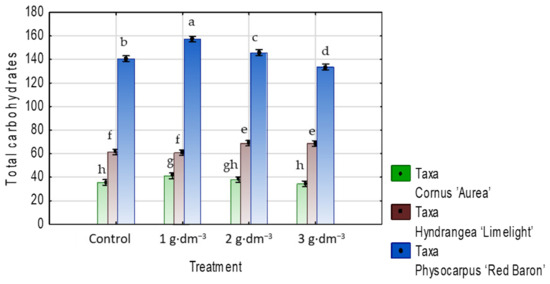
Figure 16.
The total carbohydrates content (mg·g−1 DW) in leaves of taxa planted in medium with starch-based superabsorbent and control. Bars followed by the same letter are not significantly different according to Duncan’s multiple range test at α = 0.05. Vertical bars denote 95% confidence intervals for the mean of counts (two-way ANOVA).
The starch-based superabsorbent differentially affected the content of reducing carbohydrates. In the case of C. alba ‘Aurea’, a dosage of 2 or 3 g·dm−3 contributed to a decrease in reducing carbohydrates, while in H. paniculata ‘Limelight’, there was an increase in this component as compared to the control. In the case of P. opulifolius ‘Red Baron’, a dosage of 1 g·dm−3 contributed to an increase, while with 1 or 2 g·dm−3, there was a decrease in reducing carbohydrates content (Figure 17).
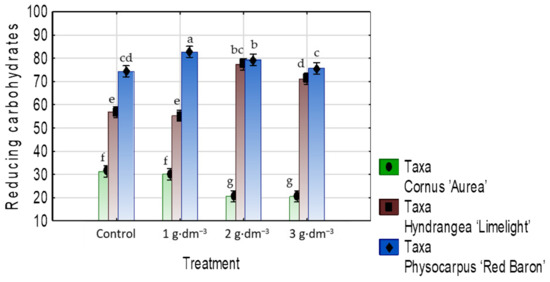
Figure 17.
The reducing carbohydrates content (mg·g−1 DW) in leaves of taxa planted in medium with starch-based superabsorbent and control. Bars followed by the same letter are not significantly different according to Duncan’s multiple range test at α = 0.05. Vertical bars denote 95% confidence intervals for the mean of counts (two-way ANOVA).
3.4. Starch-Based Superabsorbent Added to Medium Differentially Determines the Level of Hydrogen Peroxide in Leaves
The contents of hydrogen peroxide in the leaves of shrubs growing in the presence of the starch-based superabsorbent decreased, even with the lowest dose of 1 g·dm−3 in the growing medium, which reduced it by 7%, 38%, and 16% in dogwood, hydrangea, and ninebark, respectively, as compared to the controls. In hydrangea, the two higher hydrogel doses also decreased the H2O2 level by 10–18% while increasing it in dogwood and ninebark (Figure 18).
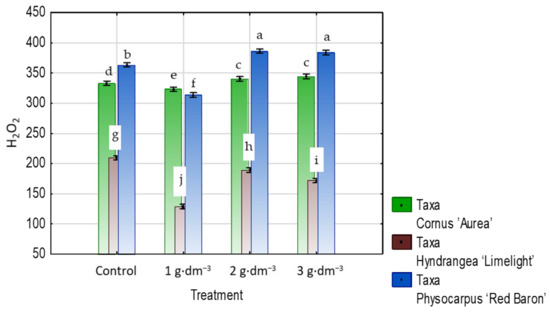
Figure 18.
The hydrogen peroxide (H2O2) content (µg·g−1 DW) in leaves of taxa planted in medium with starch-based superabsorbent and control. Bars followed by the same letter are not significantly different according to Duncan’s multiple range test at α = 0.05. Vertical bars denote 95% confidence intervals for the mean of counts (two-way ANOVA).
3.5. Correlations between Biologically Active Components Change with the Use of Various Doses of Starch-Based Superabsorbent
The correlation analysis performed for the dogwood ‘Aurea’, hydrangea ‘Limelight’, and ninebark ‘Red Baron’ growing in media with the addition of 1, 2, and 3 g·dm−3 starch-based superabsorbent showed multiple relationships between chlorophylls, carotenoids, reducing and total carbohydrates, and hydrogen peroxide in leaves (Table 2, Table 3 and Table 4).

Table 2.
Parts of correlation matrices between results of chlorophyll, carotenoids (mg·g−1 DW), total and reducing carbohydrates (mg·g−1 DW), and hydrogen peroxide (H2O2) (µg·g−1 DW) in Cornus ‘Aurea’. The correlations are significant at p < 0.05, marked in color scale: 0.1–0.29—very low; 0.30–0.49—low; 0.50–0.69—restrained; 0.70–0.89—high; >0.90—very high.

Table 3.
Parts of correlation matrices between results of chlorophylls, carotenoids (mg·g−1 DW), total and reducing carbohydrates (mg·g−1 DW), and hydrogen peroxide (H2O2) (µg·g−1 DW) in Hydrangea paniculata ‘Limelight’. The correlations are significant at p < 0.05, marked in color scale: 0.1–0.29—very low; 0.30–0.49—low; 0.50–0.69—restrained; 0.70–0.89—high; >0.90—very high.

Table 4.
Parts of correlation matrices between results of chlorophylls, carotenoids (mg·g−1 DW), total and reducing carbohydrates (mg·g−1 DW), and hydrogen peroxide (H2O2) (µg·g−1 DW) in Physocarpus opulifolius ‘Red Baron’. The correlations are significant at p < 0.05, marked in color scale: 0.1–0.29—very low; 0.30–0.49—low; 0.50–0.69—restrained; 0.70–0.89—high; >0.90—very high.
In the case of Cornus ‘Aurea’ in control plants and after the addition of 1 and 2 g·dm−3 hydrogel to the medium, the rise in chlorophyll content was correlated with an increase in hydrogen peroxide (H2O2) content in leaves. The correlation analysis for plants with 1 g·dm−3 hydrogel added to the medium showed an interaction between the increase in the level of chlorophylls and the increase in carotenoids, total carbohydrates, and hydrogen peroxide (H2O2) contents. The decrease in the level of total carbohydrates in the control and in groups with the addition of 1 or 3 g·dm−3 correlated with an increase in the level of hydrogen peroxide (H2O2) in leaves. A relationship was shown between the rise in total and reducing carbohydrates and the decrease in hydrogen peroxide (H2O2) in the leaves of the control and plants growing in media with the addition of the 2 or 3 g·dm−3 hydrogel dose (Table 2).
The correlation analysis of control hydrangea ‘Limelight’ plants showed decreases in carotenoids, reducing carbohydrates, and hydrogen peroxide in leaves through the increase in chlorophyll content. The addition of 1 and 2 g·dm−3 hydrogel to the medium contributed to changing these relations. In the case of the 1 g·dm−3 dose, the increase in chlorophylls correlated with the increase in total and reducing carbohydrate contents, while in the case of the 2 g·dm−3 dose, there was also a rise in carotenoids content (Table 3).
In the case of Physocarpus opulifolius ‘Red Baron’, the correlation analysis showed a relationship between the increase in chlorophyll content and the decrease in carotenoids content in plants growing in the control medium and in the medium with the addition of a 1 g·dm−3 dose of the hydrogel. Meanwhile, the carotenoids content increased after the addition of a 2 or 3 g·dm−3 dose. A relationship between the increase in hydrogen peroxide (H2O2) in leaves and the increase in pigments and total carbohydrates was proved for the 2 g·dm−3 dose of the hydrogel, while a decrease in hydrogen peroxide (H2O2) was observed for the 3 g·dm−3 dose (Table 4).
4. Discussion
The plant response to drought is a well-known research topic. Plant responses to soil water deficit are diverse and interlinked. Adaptive abilities may differ even within a given species [8,32]. Water deficit provokes different anatomical changes [13], e.g., reductions in the xylem vessel area, vessel density, and vessel diameter [33]. Other consequences may be morphological and physiological effects on plants, such as reduced shoot elongation and leaf area; a smaller number of stomata and a lower degree of their opening, crucial for gas exchange; increased epidermis thickness [6,34]; and reductions in the midday leaf water potential (ΨMD), stem sap flow, hydraulic conductance (Ks), and net photosynthetic rate (Pn) [33]. Our research was conducted mimicking the third and simultaneously last year of nursery production before distribution to the consumer. In this period, plants should be in their best physiological condition so that they adapt more easily to their final place of cultivation in green areas.
Enhanced plant growth in field conditions where periodic drought often occurs may be achieved by applying different water absorbents that improve air–water relations in soil [3,8]. The starch-based superabsorbent, commercially named Zeba SP, belongs to the group of eco-friendly plant production inputs [11] and enables water to be delivered to plants under water stress conditions: the hydrogel absorbs water when it is abundant in the soil and releases it when needed [8,12,14,15,16,17,21]. In our experiment, this effect was checked and proved in pot production and cultivation for three taxa (dogwood, Cornus alba ‘Aurea’; hydrangea, Hydrangea paniculata ‘Limelight’; and ninebark, Physocarpus opulifolis ‘Red Baron’) under study, where relative soil humidity (RH%) was always higher in the pots filled with the growing substrate supplemented with the hydrogel. A dose of substrate as small as 1 g·dm−3 was sufficient to significantly increase the water content at both measurement hours. In the evening (7 p.m.), the relative soil humidity (%RH) in control treatments fell to 35–39% on average, while the addition of the hydrogel allowed it to be maintained at 45–52%. The higher %RH at afternoon (1 p.m.) in the medium for hydrangea ‘Limelight’ could be the effect of this taxon’s waved water use in a 24 h period (Figure 2). These results indicate the limiting of water deficit around the roots for all of the taxa and simultaneously confirm the applicability of starch-based hydrogels in nursery container production.
Given that plant responses to water stress are multiple, we studied them at the morphological, anatomical, physiological, and biochemical levels. As shown by the results, the effect of the hydrogel’s presence in the growing medium was positive in every case. The application of the preparation to the growth medium in containers—even in its lowest dose —positively affected root development (Figure 9) in all three taxa; with the addition of 2 or 3 g·dm−3, root growth was further enhanced in hydrangea ‘Limelight’ and dogwood ‘Aurea’. The positive effect of starch-based hydrogels added to the soil was proved [18]. Similarly, in the study in [14] on maize (Zea mays cv. ‘tri-hybrid 311’) grown in sandy soil, a positive effect of different hydrogels on the root system was observed under drought: the more hydrogel provided, the more vigorous the plants, and the soil remained more humid. However, the plant responses differed in terms of growth parameters such as plant height, root length, and fresh and dry weights of shoots and roots, depending on the hydrogels. The starch-based superabsorbent (commercially named Was, Nippon Starch Chemicals) in a dose of 0.4% soil caused an increase of 147% in plant height and 190.6% in root length compared to the control. Moreover, the water content amounted to 137.9% in roots and 162.3% in comparison to the control [14]. El-Asmar et al. [35] and Farzi [36] stated that the application of hydrogel polymers to the soil improves the availability of water in the substrate, increases the leaf water content, improves root development and plant growth, minimizes nutrient losses by leaching, and contributes to improving soil penetration, decreasing the adverse effects of water stress. With higher soil humidity and a better-developed root system, the shrubs in this experiment showed more vigorous growth of shoot length and leaf area parts. The mean increases in shoot length were significantly larger in shrubs growing in the presence of the hydrogel at 1 and 2 g·dm−3. Hadam et al. [37] arrived at similar conclusions when studying the response of legumes to hydrogel use. The soil application of a hydrogel also positively affected grass growth under drought conditions [38].
Shrubs growing in the substrate supplemented with the hydrogel had larger leaf blades than those produced in the control medium. Similarly, Barakat et al. [38] also reported increased growth of banana plants and their parameters, like height (254.7 to 257.0 cm), leaf area (1.43 m2), and the number of green leaves (11.82 to 11.87), after using 150 g hydrogel per plant, with irrigation at 80% of the irrigation requirement (IR). Improved plant growth in the hydrogel-provided shrubs resulted from enhanced photosynthesis due to an increased foliage area.
The increased stomata opening improved gas exchange [39] and elevated the chlorophyll content. Our practice indicated that hydrangea ‘Limelight’ is sensitive to a low water level in the medium. The epidermis stomata width had higher values after adding the hydrogel to the medium in all concentrations. Meanwhile, the pigment concentration in leaves increased with as low as 1 g hydrogel per liter of growing medium in all taxa. Chlorophylls are responsible for absorbing light and transforming it into the chemical energy needed to produce assimilates. Chlorophylls content affects plant vigor and condition, as well as stress resistance [40]. Kenawy et al. [41] used superabsorbent nanocomposites (SANCs) in a drought-affected bean culture and proved that SANCs alleviated water stress and increased the contents of chlorophyll b and carotenoids, thus improving plant growth. According to Sayed et al. [42], with increasing doses of hydrogel (polyurethane with ethyl glycol), chlorophyll concentrations were elevated in the leaves of cotton (Gossypium hirsutum) and maize (Zea mays) grown under controlled soil water deficit. A similar plant response was confirmed by applying a hydrogel to maize grown under salinity stress [43]. In our experiment, a starch-based hydrogel maintained a higher relative soil humidity and simultaneously higher chlorophylls content in the leaves of all three taxa. Drought stress reduces the chlorophylls content [33]. The physiological performance, especially the photosynthetic rate (A) and stomatal conductance (Gs), increased due to the increase in chlorophylls content, which helps to better capture light. Then, the photosynthetic rate (A) was increased through the conversion of light energy into chemical energy [33].
With enhanced photosynthesis in the hydrogel-provided shrubs, their carbohydrates production increased, as shown in the example of soluble sugars, both total and reducing, in plants growing in a dose as low as 1 g·dm−3. A high sugars level was shown to affect gene expression, thus controlling vegetative and generative plant growth, while a low sugar concentration enhanced the photosynthetic rate and the accumulation of storage materials [44]. However, the use of the hydrogel TerraCottem in a culture of a mixture of Trifolium sp. grasses negatively affected carbohydrates metabolism, stimulating cellulose degradation and increasing carbon dioxide release [45].
Under stress conditions, reactive oxygen species (ROS) may be produced. Hydrogen peroxide, which is transformed into the hydroxyl radical, is one such species. By reacting with cell components, it damages them and produces mutations that may eventually lead to cell death [46,47,48]. ROS are generated in the cells and removed when the plants are exposed to stress conditions, e.g., drought [49]. ROS production is accompanied by a higher level of various antioxidants, flavonoids, and secondary metabolites, which play a role in protecting the plant by detoxifying ROS. In plant life, dynamically generated and removed ROS regulate development, from seed germination to senescence, and allow the plant to adapt to various environmental conditions. ROS levels should be maintained between the cytostatic and cytotoxic level boundaries [50]. Contents of H2O2 were lower in the leaves of shrubs growing in the presence of the preparation used in our study, proving that hydrogel application may be a non-conventional and efficient method to mitigate the effects of water stress on plants. This may also occur due to enhanced antioxidant production by the plants themselves. Carotenoids are natural plant antioxidants classified as one of the non-enzymatic defense systems among ROS-scavenging mechanisms generated during photosynthetic processes. The synthesis of carotenoids is induced by ROS under stress conditions [51]. As yellow-orange pigments, they also participate in photosynthesis [51,52]. In our experiment, their contents increased due to hydrogel application—1 g·dm−3 in ninebark and 2 g·dm−3 in hydrangea. In previous research, it was found that the contents of carotenoids in tomato, cucumber, and lettuce leaves increased with increasing doses of the polymer hydrogel used in their culture [53].
In these experiments, the shrubs growing in the medium supplemented with the hydrogel had better conditions, which enabled them to develop several morphological adaptations to cope with periodic water deficit, i.e., increases in leaf area and stomatal conductance, which resulted in enhanced photosynthesis, increased carbohydrates production, and more vigorous plant growth. As shown by our results, the consequences of stress in container shrub production may be alleviated by the application of a starch-based hydrogel. Such an effect would be desirable for all plants growing under water stress [54].
5. Conclusions
Supplementing the growing medium with a superabsorbent based on maize starch polymers resulted in increased relative humidity in the vicinity of the root ball in three container-grown deciduous ornamental shrubs. The equalized moisture around the roots and probably the reduction in water deficit caused higher physiological and growth parameters in all the taxa. The presence of the hydrogel in the growing medium reduced oxidative stress, as shown by the decreased contents of hydrogen peroxide and elevated concentrations of carotenoids in the leaves of two shrub species. Plants growing in the presence of the starch-based hydrogel (1 and 2 g·dm−3) had well-developed root systems, higher increments in shoot length, and larger leaf blade areas than control plants. Growing in a medium with the added hydrogel provided the shrubs of H. paniculata ‘Limelight’ with more widely open stomata. Stomatal conductance and chlorophyll contents were elevated, thus enhancing photosynthesis as compared to controls growing without the hydrogel. The contents of soluble carbohydrates—total and reducing sugars—were higher in the plants provided with the hydrogel. The results indicate improved plant tolerance to possible fluctuations in medium humidity and more stable growth conditions, reducing the possible water deficit occurring in nurseries, i.a., as a result of changing climactic conditions. This research may contribute to the more eco-friendly cultivation and production of plants in containers both by limiting water use and due to the biodegradability of starch. However, the next topic of research may be the utilization of starch-based hydrogels in the soil and their effects on soil ecosystems. The physiological condition of nursery plant material planted in greeneries is as important as the first period after planting them.
Author Contributions
Conceptualized and conducted the research, collected results, and wrote the article, A.P.; conducted some of the measurements, performed statistical analyses, and wrote part of research paper, K.N.; performed visualization and supervision, conducted statistical analyses, contributed to data analyses and the presentation of the results, and commented on and revised the manuscript, M.J.M. All authors have read and agreed to the published version of the manuscript.
Funding
The publication was financed by the Science Development Fund of the Warsaw University of Life Sciences—SGGW.
Institutional Review Board Statement
This study did not require ethical approval and did not involve humans or animals.
Data Availability Statement
The data supporting the conclusions of this article will be made available by the authors without undue reservation.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A Review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Hanel, M.; Rakovec, O.; Markonis, Y.; Máca, P.; Samaniego, L.; Kyselý, J.; Kumar, R. Revisiting the recent European droughts from a long-term perspective. Sci. Rep. 2018, 1, 9499. [Google Scholar] [CrossRef] [PubMed]
- Rydlová, J.; Püschel, D. Arbuscular mycorrhiza, but not hydrogel, alleviates drought stress of ornamental plants in peat-based substrate. Appl. Soil Ecol. 2020, 146, 103394. [Google Scholar] [CrossRef]
- Shao, H.B.; Chu, L.Y.; Jaleel, C.; Zhao, C.X. Water deficit stress-induced anatomical changes in higher plants. C. R. Biol. 2008, 331, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.N.; Yadav, N. Stress-adaptive microbes for plant growth promotion and alleviation of drought stress in plants. Acta Sci. Agric. 2018, 2, 85–88. [Google Scholar]
- Elansary, H.O.; Salem, M.Z.M. Morphological and physiological responses and drought resistance enhancement of ornamental shrubs by trinexapac-ethyl application. Sci. Hort. 2015, 189, 1–11. [Google Scholar] [CrossRef]
- Bhusal, N.; Lee, M.; Han, A.R.; Kim, H.S. Responses to drought stress in Prunus sargentii and Larix kaempferi seedlings using morphological and physiological parameters. For. Ecol. Manag. 2020, 465, 118099. [Google Scholar] [CrossRef]
- Urban, L.; Aarrouf, J.; Bidel, L.P.R. Assessing the effects of water deficit on photosynthesis using parameters derived from measurements of leaf gas exchange and of chlorophyll a fluorescence. Front. Plant Sci. 2017, 8, 2068. [Google Scholar] [CrossRef]
- Ghebru, G.M.; Du Toit, E.S.; Steyn, M.J. Water and nutrient retention by Aquasoil and Stockosorb polymers. S. Afr. J. Plant Soil 2007, 24, 32–36. [Google Scholar] [CrossRef]
- Masina, N.; Choonara, Y.E.; Kumar, P.; Toit, L.C.; Govender, M.; Indermun, S.; Pillay, V. A review of the chemical modification techniques of starch. Carbohydr. Polym. 2017, 157, 1226–1236. [Google Scholar] [CrossRef]
- Qamruzzaman, M.; Ahmed, F.; Mondal, I. An Overview on Starch-Based Sustainable Hydrogels: Potential Applications and Aspects. J. Polym. Environ. 2022, 30, 19–50. [Google Scholar] [CrossRef]
- Kolya, H.; Kang, C.-W. Synthesis of starch-based smart hydrogel derived from rice-cooked wastewater for agricultural use. Int. J. Biol. Macromol. 2022, 226, 1477–1489. [Google Scholar] [CrossRef] [PubMed]
- Chiorescu, E.; Clinciu-Radu, A.R.; Chiorescu, D.; Teliban, G.C.; Robu, T. Research on the influence of hydrogels Zeba SP and Terracottem on the development of some aromatic plant species. Res. J. Agric. Sci. 2017, 49, 385–391. [Google Scholar]
- Mazen, M.M.; Eldeen, D.; Radwan, M.; Ahmed, F.A. Growth responses of maize plants cultivated in sandy soil amended by different superabsorbant hydrogels. J. Plant Nutr. 2015, 38, 325–337. [Google Scholar] [CrossRef]
- Khlestkin, V.K.; Peltek, S.E.; Kolchanov, N.A. Review of direct chemical and biochemical transformations of starch. Carbohydr. Polym. 2018, 181, 460–476. [Google Scholar] [CrossRef]
- Luo, H.; Dong, F.; Wang, Q.; Li, Y.; Xiong, Y. Construction of porous starch-based hydrogel via regulating the ratio of amylopectin/amylose for enhanced water-retention. Molecules 2021, 26, 3999. [Google Scholar] [CrossRef]
- Omidian, H.; Rocca, J.G.; Park, K. Advances in superporous hydrogels. J. Control. Release 2005, 102, 3–12. [Google Scholar] [CrossRef]
- Ostrand, M.S.; DeSutter, T.M.; Daigh, A.L.M.; Limb, R.F.; Steele, D.D. Superabsorbent polymer characteristics, properties and applications. Agrosyst. Geosci. Environ. 2020, 3, e20074. [Google Scholar] [CrossRef]
- Luo, Z.; Li, K.; Jiang, X.; Polle, A. Ectomycorrhizal fungus (Paxillus involutus) and hydrogels affect performance of Populus euphratica exposed to drought stress. Ann. For. Sci. 2009, 66, 106–116. [Google Scholar] [CrossRef]
- Moallemi, N.; Khaleghii, E.; Danaeifar, A. Investigating the effect of using three types of superabsorbent polymers on N.P.K absorption by acacia under drought stress conditions. Agric. Eng. 2022, 2, 153–167. [Google Scholar]
- Ranganathan, N.; Bensingh, R.J.; Kader, M.A.; Nayak, S.K. Cellulose-based hydrogels for agricultures. In Polymers and Polymeric Composites: A Reference Series; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–21. [Google Scholar]
- Official Journal of the European Union. Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. Off. J. Eur. Union 2009, 309, 1–50. [Google Scholar]
- Lertsarawut, P.; Rattanawongwiboon, T.; Tangthong, T.; Laksee, S.; Kwamman, T.; Phuttharak, B.; Romruensukharom, P.; Suwanmala, P.; Hemvichian, K. Starch-based super water absorbent: A promising and sustainable way to increase survival rate of trees planted in arid areas. Polymers 2021, 13, 1314. [Google Scholar] [CrossRef] [PubMed]
- Monder, M.J.; Bąbelewski, P.; Szperlik, J. The adjustment of China endemic Heptacodium miconioides Rehd. to temperate zone of Poland. BMC Plant Biol. 2023, 23, 184. [Google Scholar] [CrossRef] [PubMed]
- Climate. 2022. Available online: https://meteomodel.pl/dane/srednie-miesieczne/?imgwid=352200375&par=%28tx%2Btn%29%2F2&max_empty=2 (accessed on 1 June 2023).
- Lichtenthaler, K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Strzelecka, H.; Kamińska, J.; Kowalski, J. Chemiczne Metody Badań Roślinnych Surowców Leczniczych; PZWL: Warsaw, Poland, 1982; pp. 56–57. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 35–356. [Google Scholar] [CrossRef]
- Pick, E.; Keisari, Y.A. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J. Immunol. Methods 1980, 38, 161–170. [Google Scholar] [CrossRef]
- Laudański, Z.; Mańkowski, D.R. Statistical Methods for the Preparation of Results; IHAR: Radzików, Poland, 2007; Chapter 5; pp. 126–140. [Google Scholar]
- Compton, M.E. Statistical methods suitable for the analysis of plant tissue culture data. Plant Cell Tissue Organ Cult. 1994, 37, 217–242. [Google Scholar] [CrossRef]
- Torrecillas, A.; Rodríguez, P.; Sánchez-Blanco, M.J. Comparison of growth, leaf water relations and gas exchange of Cistus albidus and Cistus monspeliensis plants irrigated with water of different NaCl salinity levels. Sci. Hort. 2003, 97, 353–368. [Google Scholar] [CrossRef]
- Bhusal, N.; Han, S.-G.; Yoon, T.-M. Impact of drought stress on photosynthetic response, leaf water potential, and stem sap flow in two cultivars of bi-leader apple trees (Malus × domestica Borkh.). Sci. Hort. 2019, 246, 535–543. [Google Scholar] [CrossRef]
- Parida, A.K.; Veerabathini, S.K.; Kumari, A.; Agarwal, P.K. Physiological, anatomical and metabolic implications of salt tolerance in the halophyte Salvadora persica under hydroponic culture condition. Front. Plant Sci. 2016, 7, 351. [Google Scholar] [CrossRef]
- El-Asmar, J.; Jaafar, H.; Bash, I. Hydrogel banding improves plant, survival, and water use efficiency in two calcareous soils. Clean Soil Air Water 2017, 47, 1700251. [Google Scholar] [CrossRef]
- Farzi, R.; Gholami, M.; Baninasab, B. Water-retention additives’ effects on plant water status and some physiological parameters of two olive cultivars under reduced irrigation regimes. Acta Physiol. Plant. 2017, 3, 126. [Google Scholar] [CrossRef]
- Hadam, A.; Wrochna, M.; Karaczun, Z. Effect of hydrogel on the turf grass species growing under salt stress. Ann. WULS 2011, 43, 47–55. [Google Scholar]
- Barakat, M.R.; El-Kosary, S.; Borham, T.I. Effect of hydrogel soil addition under different irrigation levels on Grand Nain banana plants. J. Hort. Sci. Ornam. Plants 2015, 7, 19–28. [Google Scholar]
- Jamnická, G.; Ditmarová, L.; Kurjak, D.; Kme, J.; Pšidová, E.; Macková, M.; Gömöry, D.; Střelcová, K. The soil hydrogel improved photosynthetic performance of beech seedlings treated under drought. Plant Soil Environ. 2013, 59, 446–451. [Google Scholar] [CrossRef]
- Woodward, A.J.; Bennett, I.J. The effect of salt stress and abscisic acid on proline production, chlorophyll content and growth of in vitro propagated shoots of Eucalyptus camaldulensis. Plant Cell Tissue Organ Cult. 2005, 82, 189–200. [Google Scholar] [CrossRef]
- Kenawy, E.; Rashad, M.; Hosny, A.; Shendy, S.; Gad, D.; Saad-Allah, K.M. Enhancement of growth and physiological traits under drought stress in Faba bean (Vicia faba L.) using nanocomposite. J. Plant Interact. 2022, 17, 404–418. [Google Scholar] [CrossRef]
- Sayed, E.H.; Kirkwood, C.R.; Graham, B.N. Studies on the effect of salinity and hydrogel polymer treatments on the growth. yield production and solute accumulation in cotton and maize. Agric. Sci. 1995, 7, 129–333. [Google Scholar]
- Sayed, H.; Sayed, A. Influence of salinity stress on growth parameters photosynthetic activity and cytological studies of Zea mays. L. plant using hydrogel polymer. Agric. Biol. J. N. Am. ABJNA 2011, 2, 907–920. [Google Scholar]
- Essmann, J.; Bones, P.; Weis, E.; Scharte, J. Leaf carbohydrate metabolism during defense. Intracellular sucrose-cleaving enzymes do not compensate repression of cell wall invertase. Plant Signal. Behav. 2008, 3, 885–887. [Google Scholar] [CrossRef]
- Šarapatka, B.; Rak, I.; Bubenikova, I. The effect of hydroabsorbent on selected soil biological and biochemical characteristics and its possible use in revitalization. Ekológia 2006, 25, 422–429. [Google Scholar]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species. oxidative damage and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species. abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Mehla, N.; Sindhi, V.; Josula, D.; Bisht, P.; Wani, S.H. An introduction to antioxidants and their roles in plant stress tolerance. In Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress; Khan, M., Khan, N., Eds.; Springer: Singapore, 2017; pp. 1–23. [Google Scholar]
- Lee, S.; Seo, P.J.; Lee, H.-J.; Park, C.-M. A NAC transcription factor NTL4 promotes reactive oxygen species production during drought-induced leaf senescence in Arabidopsis. Plant J. 2012, 70, 831–844. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Xi, Y.; Kong, F.; Chi, Z. ROS induce β-carotene biosynthesis caused by changes of photosynthesis efficiency and energy metabolism in Dunaliella salina under stress conditions. Front. Bioeng. Biotechnol. 2021, 8, 613768. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Sayed, E.H.; Kirkwood, C.R.; Graham, B.N. The effects of a hydrogel polymer on the growth of certain horticultural crops under saline conditions. J. Exp. Bot. 1991, 42, 891–899. [Google Scholar] [CrossRef]
- Elsheery, N.I.; Cao, K.F. Gas exchange, chlorophyll fluorescence, and osmotic adjustment in two mango cultivars under drought stress. Acta Physiol. Plant. 2008, 30, 69–777. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).