Abstract
Drought is one of the most important factors that limit crop yield. In this study, the growth promotive activity of terrein, a microbial metabolite, on four selected agricultural plants (Vigna radiata, Brassica chinensis, Triticum aestivum and Sorghum bicolor) under drought conditions was assessed via pot experiment. Terrein effectively stimulated the seedling growth of tested species and increased their fresh and dry weight at low concentrations (2 and 10 μg/mL), either applied as a seed soaking agent or a spray solution, especially on root growth. The maximum stimulatory effect on root growth was observed on B. chinensis seedlings (99.20%), on fresh weight was found on T. aestivum seedlings (97.23%), and on dry weight was discovered on V. radiata seedlings (58.33%), implying that this stimulatory effect was species-specific. Further study revealed that the application of terrein significantly raised the contents of GA3, IAA and CTK; meanwhile, MDA content declined significantly, whereas the activity of POD, CAT and SOD was boosted significantly, suggesting that terrein can stimulate plant growth by reducing the production of ROS. Our work is the first study focusing on terrein’s plant growth promotive activity, indicating terrein has the potential to be further explored as an environment-friendly growth regulator.
1. Introduction
As the world population continues to grow, both the quantity and quality of agricultural production need to be improved to meet the increasing demand [1]. However, with the global climate anomaly and the destruction of ecological balance, various abiotic stresses have led to the reduction of important crops worldwide and seriously restricted the development of agriculture [1,2]. Abiotic stress mainly includes salinity, temperature, drought, strong light, heavy metals, pH changes, mineral nutrient deficiency and ultraviolet irradiation [3,4]. Drought is one of the main factors that limit crop yield and cause physical damage and physiological, biochemical and molecular changes in plants, thus seriously affecting plant growth and development [5,6]. Most crops, including legumes and cereal plants, are susceptible to abiotic stress such as drought [7,8,9]. Therefore, searching for appropriate strategies to minimize the effects of abiotic stress and improve crop yield has become a research hotspot [3].
Nowadays, chemical fertilizers are extensively used on a large scale worldwide as an important input in agricultural production to meet the world’s food and feed needs [10,11]. However, while chemical fertilizers increase crop yields, they also bring about many negative effects [11]. Long-term application of chemical fertilizers not only leads to the deterioration of the water environment but also adversely affects the soil and the diversity of natural microorganisms in the environment, thereby reducing soil fertility and crop quality [12,13]. In addition, excessive use of chemical fertilizers is one of the important reasons for agricultural carbon emissions. Data collected from 30 provinces and cities in China from 2000 to 2019 showed that the impact of chemical fertilizer consumption on agricultural emissions was positively correlated [14]. In another study, by comparing the greenhouse gas emissions after applying seven kinds of fertilizers to 9 crops in China from 1998 to 2016, it was found that urea produced the largest greenhouse gas emissions, accounting for about 60% of the total greenhouse gas emissions [15]. Therefore, to ensure the healthy growth of crops, reduce the pollution of chemical fertilizers to the environment and harm to human and animal health, the development and application of environmentally friendly biological fertilizers and plant growth regulators is increasingly urgent [10].
Biofertilizers are products of fermentation processes that can promote plant growth by providing easy-to-use nutrients [16]. Moreover, biofertilizers are cost-effective and eco-friendly compared with chemical fertilizers, and their application has been recognized as a better option for soil improvement and agricultural sustainability [17,18,19]. Both higher plants and microorganisms can be resources of effective biofertilizers. For instance, Garcia-Gonzalez and Sommerfeld [20] showed that the extract of green algae (Acutodesmus dimorphus) cells could be used as a biofertilizer to accelerate the germination of Roma tomato (Solanum lycopersicum) seeds, promote plant growth and increase flower yield. Bakonyi et al. [21] found that a living bacterium containing biofertilizers produced in Hungary increased the germination rate of maize (Zea mays) seedlings by 20% and the dry weight by 7%. Shang et al. [22] found that the new microbial fertilizer containing Bacillus thuringiensis and Phanerochaete chrysosporium could regulate soil nitrogen supply, thereby reducing the amount of nicotine in tobacco (Nicotiana tabacum) leaves and improving the quality of tobacco leaves. In another study, Bandopadhyay [23] reported that the protein content, soluble sugar content and phosphorus content of soybean (Glycine max) increased by 34%, about 66% and more than 75% when cultured with rhizobacterial strain Bacillus thuringiensis A5-BRSC as carbon-based biofertilizer.
Besides the direct application of live microorganisms, their secondary substances have also been found to possess plant growth regulatory activity. For example, alternariol and altenuisol produced by Alternaria sp. were found to promote root growth of the monocot plant Pennisetum alopecuroides by 11.1% and 75.2% at 10 μg/mL, respectively [24]. Terrein is one of the main secondary metabolites produced by the soil fungus Aspergillus terreus [25]. Terrein is a kind of small molecular polyketone with a simple structure with a variety of biological activities such as anticancer, anti-inflammatory, antibacterial, insecticidal, etc., and has potential application prospects in the fields of beauty, medicine, agriculture and so on [26,27]. Buachan et al. [28] found that terrein showed different degrees of toxicity to human lung cancer cells and could inhibit all the major metastasis processes of such cells from achieving the purpose of cancer treatment. Nakagawa et al. [29] reported that 10 μM terrein inhibited osteoclast formation and resorption induced by receptor activator of nuclear factor—Kappa B ligand (RANKL), thus has a potential effect on the treatment of periodontal disease. Furthermore, terrein also has strong phytotoxic activity. Lu et al. [30] found that the median inhibitory concentration (IC50 values) of terrein on the growth of Amaranthus retroflexus and Echinochloa crusgalli were 11.2 μg/mL and 3.1 μg/mL, respectively, and the inhibitory effect increased with the increase of terrein concentration. However, there is no study focusing on the promotive activity of terrein on receiver plants so far. In our previous work, we found that terrein was able to stimulate plants’ growth when applied concentrations were low, especially under drought conditions. Based on this, the objectives of the current study include the (i) determination of the plant growth promotive effect of terrein on four agricultural plants, i.e., pakchoi (Brassica chinensis), mungbean (Vigna radiata), sorghum (Sorghum bicolor) and wheat (Triticum aestivum), under drought conditions, and the (ii) investigation of terrein’s effect on phytohormones and antioxidant systems of the most sensitive tested plant, B. chinensis. Our results will provide evidence on whether this compound has the potential to be utilized as a plant growth promotive agent.
2. Materials and Methods
2.1. Materials and Reagents
Seeds of B. chinensis, V. radiata, S. bicolor and T. aestivum were purchased from Shandong Weier Seed Co., Ltd. (Shandong, China) Terrein was previously isolated and purified from the broth of A. terreus by our lab with purity >95% (checked by HPLC). Methanol, petroleum ether, ethyl acetate, acetic acid and NaOH were all purchased from Tianjin Fuyu Fine Chemical Co., Ltd. (Tianjin, China). Trichloroacetic acid, 2-thiobarbituric acid, polyvinyl polypyrrolidone (PVPP), hydrogen peroxide and phosphate buffer (pH = 6.8) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). All other reagents were of analytical grade.
2.2. Plant Growth Regulatory Effect of Terrein on Selected Agricultural Plants via Pot Experiments
The plant growth regulatory effect of terrein on tested species was investigated via a pot experiment conducted at the greenhouse of Xinjiang Institute of Ecology and Geography (XIEG), Chinese Academy of Sciences (CAS), during May and June 2021. In our preliminary work, we found that terrein mainly exhibited a phytotoxic effect when applied concentrations were relatively high (50 and 250 μg/mL), whereas promotive effects were observed only when applied concentrations were below 10 μg/mL, especially under drought conditions. Therefore, a control (distilled water) and terrein at 2 and 10 μg/mL (dissolved in distilled water) were prepared for the pot experiment (filled with soils collected from a nearby corn farm). We designed two different ways of applying terrein on the receiver plants: seed soaking and seedling spray. For the seed soaking treatment, seeds of tested species were soaked in the corresponding aqueous terrein solutions for 6 h, with the distilled water being used as the blank control. Then, 20 soaked seeds were sowed into each pot (diameter 20 cm, height 20 cm) with 5 replicates. Seedlings were thinned after germination to allow 10 seedlings to grow in each pot. For the seedling spray treatment, seedlings were sprayed thoroughly with terrein solutions (2 mL for each pot) when they reached 10 cm in height (only one time). Pots were watered normally using tap water in the beginning. When the seedling’s height reached 10 cm, drought treatment began. That is, every week, each pot received 200 mL of water, which lasted for 4 weeks to maintain a drought condition (in total, 800 mL of water was applied, and the water content of each pot was maintained between 9~12%); our preliminary experiment indicated that to maintain normal moisture of the soils, 2400 mL of water was needed for each pot. After 4 weeks, all seedlings were harvested for measurement of root and shoot length, fresh and dry weight. Altogether, 50 seedlings were measured for each treatment (n = 50).
2.3. Phytohormone Content Determination
B. chinensis was chosen for evaluating the impacts of terrein on the phytohormone contents as well as the antioxidant system due to the fact that it was the most sensitive tested species. According to the method described by Fang et al. [31], the fresh leaves of B. chinensis seedlings from the above pot experiment were used to determine the phytohormone levels, with slight modifications. The specific operation conditions were as follows: 2.0 g of plant samples were weighed and ground. Then, 2 mL of precooled methanol (80%) was added to the plant homogenate and mixed for 5 min. After sealing with fresh-keeping film, the samples were soaked overnight at 4 °C in the dark and then centrifuged at 5031× g for 10 min. The supernatant was then collected. The residue was further extracted with 80% methanol, sonicated at 4 °C for 30 min, and the operation was repeated twice. The supernatant was subsequently combined, and then 2 mL of petroleum ether was added for decolorization 3 times. The aqueous phase was then extracted with 5 mL of ethyl acetate 3 times. Finally, the ethyl acetate phase was combined and was blown dry with 4 °C nitrogen, and then the acetic acid solution with pH = 3.5 was added. The ethyl acetate extract was then dissolved in 0.5 mL methanol (the initial mobile phase) and detected under the wavelength of 254 nm using a Hitachi Primaide HPLC system on a HypersilTM BDS C18 column (5 μm, 250 mm × 4.6 mm) with a mobile phase consisting of methanol (eluent A) and acetic acid aqueous solution (eluent B, pH = 3.6) at a ratio of 20:80 (v/v), with the flow rate at 1.0 mL/min, and the injection volume of 20 μL. All solutions were filtrated by a 0.22 μm membrane filter prior to HPLC analysis [31].
2.4. Antioxidant System Determination
Fresh leaves of B. chinensis were used to evaluate the antioxidant system, and the determination indicators mainly included the content of malondialdehyde (MDA), the activities of peroxidase (POD), catalase (CAT) and superoxide dismutase (SOD) [32]. MDA content was determined by the MDA kits (YX-W-A401, Sino Best Biological Technology Co., Ltd., Shanghai, China). The specific steps were as follows: 3.0 g of frozen leaves was crashed in liquid nitrogen, 5 mL of 10% trichloroacetic acid was then added, and the mixture was centrifuged at 10,000× g for 25 min at 4 °C. Subsequently, 1 mL of supernatant was added to 2 mL of 0.67% 2-thiobarbituric acid (prepared in 0.05 mM NaOH) and mixed evenly. The resulting mixture was incubated in a boiling water bath for 15 min and then cooled to room temperature. Finally, the absorbance of the obtained sample was measured at 25 °C. The MDA content in the sample was expressed as nmol/g fresh weight.
POD activity was determined using the POD kits (YX-W-A502, Sino Best Biological Technology Co., Ltd., Shanghai, China). Samples were prepared according to the protocol, and the absorbance was measured at 25 °C. The POD activity in the sample was shown as U/g fresh weight. CAT activity was assayed according to the CAT kit (YX-W-A501, Sino Best Biological Technology Co., Ltd., Shanghai, China). In detail, a 3 g frozen plant sample was crushed in liquid nitrogen containing 1% PVPP. Subsequently, 0.1 g of the ground sample was transferred to 1 mL of extraction buffer and incubated in a 37 °C water bath for 3~5 min. Then, 0.1 mL of 20 mmol/L hydrogen peroxide in 50 mmol/L phosphate buffer was added to the above reaction solution and was subjected to eddy current treatment and centrifuged at 8000× g at 4 °C for 10 min. Finally, the absorbance of the supernatant was measured at 25 °C. The CAT activity in the sample was expressed as U/g fresh weight.
SOD activity was measured according to the SOD kit (YX-W-A500, Sino Best Biological Technology Co., Ltd., Shanghai, China). Three g of frozen plant samples were crushed in liquid nitrogen containing 1% PVPP. Then, 0.1 g of the ground sample was transferred to 1 mL of extraction buffer with homogeneous agitation for 3 min and centrifuged at 8000× g at 4 °C for 10 min. In the end, the absorbance of the supernatant of the sample was surveyed at 25 °C. The SOD activity was expressed as U/g fresh weight.
2.5. Statistical Analysis
All bioassay results were analyzed by one-way ANOVA (p < 0.05) using SPSS 13.0 (SPSS Inc., Chicago, IL, USA) for Windows to examine whether the plant growth regulatory activity of terrein on seedling growth was significant. Then, Fisher’s LSD test was performed on the above data at the level of p < 0.05 to compare the differences between treatments.
3. Results
3.1. Plant Growth Regulatory Effect
The plant growth regulatory activity of terrein on different agricultural plants was investigated via pot experiment. The chemical structure of terrein is shown in Figure 1. For B. chinensis, when its seeds were soaked in terrein solutions, its seedling growth was significantly enhanced (p < 0.05). At 2 μg/mL, terrein promotes the root length, shoot length, fresh weight and dry weight of B. chinensis by 76.91%, 9.00%, 9.77% and 33.33%, respectively, compared with the control. Terrein showed a more significant effect (p < 0.05) on the growth of B. chinensis when applied concentration was increased to 10 μg/mL, boosting the root length, shoot length, fresh weight and dry weight of B. chinensis by 99.20%, 15.73%, 40.80% and 50.00%, respectively. When terrein was sprayed on seedlings, its effect diminished greatly. Although terrein significantly (p < 0.05) promotes the root length and shoot length of B. chinensis by 32.57% and 8.06% at 2 μg/mL, respectively, it has no prominent effect on the fresh and dry weight of B. chinensis. When the concentration reached 10 μg/mL, terrein significantly (p < 0.05) increased the root length of B. chinensis by 16.46%; however, it did not exert any significant effect on the shoot length, fresh weight and dry weight (Figure 2).
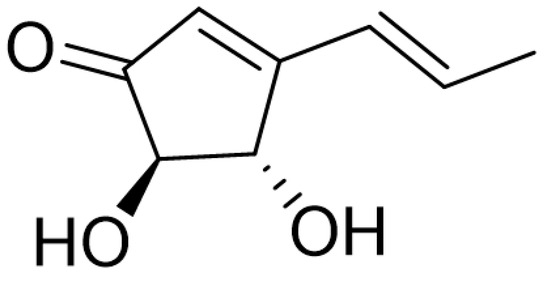
Figure 1.
Chemical structure of terrein.
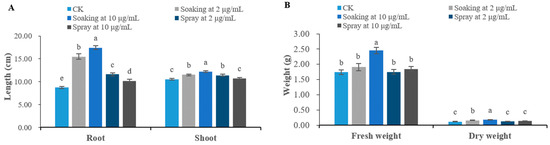
Figure 2.
Growth regulatory activity of terrein on B. chinensis seedlings. (A) Effects on the root length and shoot length of B. chinensis. (B) Effects on the fresh weight and dry weight of B. chinensis. CK represents the control group treated with distilled water. Each value is the mean of 50 seedlings (n = 50). The bar represents the standard error. Means with different letters (a, b, c, etc.) indicate significant differences at p < 0.05 level according to Fisher’s LSD test.
Similarly, terrein promoted seedling growth of V. radiata, and its effects were always significant on root growth and fresh and dry weight regardless of how terrein was applied and which concentration was applied; on the other hand, terrein’s effect on shoot growth was relatively weak. When applied as a seed soaking agent at 2 μg/mL, terrein stimulates the root length, fresh weight and dry weight of V. radiata by 44.72%, 32.26% and 26.99%, respectively; when the concentration reached 10 μg/mL, they are promoted by 30.92%, 18.29% and 35.83%. Spraying seemed to be more effective, which increases root length, fresh weight and dry weight of V. radiata by 61.94%, 24.12% and 25.00% at 2 μg/mL, and 57.19%, 57.18% and 58.33% at 10 μg/mL, respectively (Figure 3).
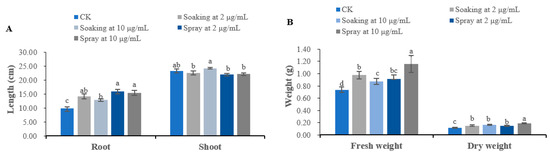
Figure 3.
Growth regulatory activity of terrein on V. radiata seedlings. (A) Effects on the root length and shoot length of V. radiata. (B) Effects on the fresh weight and dry weight of V. radiata. CK represents the control group treated with distilled water. Each value is the mean of 50 seedlings (n = 50). The bar represents the standard error. Means with different letters (a, b, c, etc.) indicate significant differences at p < 0.05 level according to Fisher’s LSD test.
In addition, terrein also significantly (p < 0.05) promotes the growth of S. bicolor seedlings, especially root length and fresh and dry weight, whereas it has no significant effect on shoot growth. Using 2 μg/mL terrein as seed soaking solution, the root length and fresh and dry weight of S. bicolor are promoted by 10.72%, 6.83% and 11.76%, respectively. When the concentration reached 10 μg/mL, the promotion rates were 5.59%, 16.77% and 23.53%, respectively. When applied as a spraying agent at 2 μg/mL, the root length and fresh and dry weight of S. bicolor increased by 18.52%, 19.25% and 23.53%, respectively; at 10 μg/mL, the effect is most significant, where the root length and fresh and dry weight of S. bicolor was enhanced by 30.51%, 19.88% and 26.47%, respectively (Figure 4).
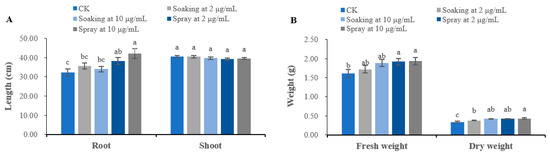
Figure 4.
Growth regulatory activity of terrein on S. bicolor seedlings. (A) Effects on the root length and shoot length of S. bicolor. (B) Effects on the fresh weight and dry weight of S. bicolor. CK represents the control group treated with distilled water. Each value is the mean of 50 seedlings (n = 50). The bar represents the standard error. Means with different letters (a, b, c, etc.) indicate significant differences at p < 0.05 level according to Fisher’s LSD test.
The seedling growth of T. aestivum seeds was stimulated by different concentrations of terrein solution. When seeds are immersed in 2 μg/mL terrein solution, shoot length and fresh and dry weight of T. aestivum seedlings increase by 6.15%, 59.29% and 21.90% compared with control, respectively. When the application concentration was increased to 10 μg/mL, the effect of terrein solution on T. aestivum growth was more significant (p < 0.05), and the root length and fresh and dry weight of T. aestivum was promoted by 25.08%, 76.42% and 21.96%, respectively. When terrein was sprayed on T. aestivum seedlings, the effect is significantly (p < 0.05) enhanced. At 2 μg/mL, terrein stimulates root length and fresh and dry weight of T. aestivum seedlings by 12.26%, 61.03% and 14.29%, respectively. When the concentration reached 10 μg/mL, terrein significantly (p < 0.05) increased root length and fresh and dry weight of T. aestivum by 67.37%, 97.23% and 37.62%; however, it had no significant effect on shoot length (Figure 5). The growth status of the four plants is shown in Figure 6.
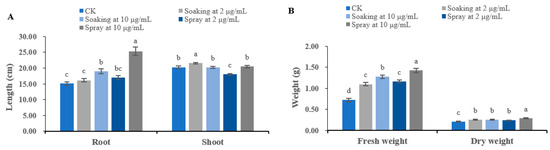
Figure 5.
Growth regulatory activity of terrein on T. aestivum seedlings. (A) Effects on the root length and shoot length of T. aestivum. (B) Effects on the fresh weight and dry weight of T. aestivum. CK represents the control group treated with distilled water. Each value is the mean of 50 seedlings (n = 50). The bar represents the standard error. Means with different letters (a, b, c, etc.) indicate significant differences at p < 0.05 level according to Fisher’s LSD test.

Figure 6.
Effects of terrein on selected agricultural plants. (A) V. radiata; (B) B. chinensis; (C) T. aestivum; and (D) S. bicolor. Treatments from left to right (A–D): control (distilled water), soaking at 2 μg/mL, soaking at 10 μg/mL, spray at 2 μg/mL and spray at 10 μg/mL.
3.2. Phytohormone Content Determination
The above experimental results showed that terrein showed the strongest effect on B. chinensis; therefore, B. chinensis was chosen as the representative species of the tested plants for phytohormone determination and the subsequent antioxidant system evaluation. The phytohormone contents in B. chinensis seedlings under different terrein treatment conditions are shown in Figure 7. After seed soaking with terrein at 2 μg/mL, the content of gibberellic acid 3 (GA3) was significantly (p < 0.05) reduced by 42.92% compared with the control, in contrast, indole-3-acetic acid (IAA) content was significantly (p < 0.05) enhanced by 94.66%, whereas the contents of cytokinin (CTK) and abscisic acid (ABA) were not significantly changed. When the concentration of terrein was increased to 10 μg/mL, the content of GA3 was significantly (p < 0.05) cut down by 20.65%, whereas that of IAA and CTK was raised by 209.23% and 11.44%, respectively. Meanwhile, the content of ABA remained unchanged. When B. chinensis seedlings were sprayed with terrein at 2 μg/mL and 10 μg/mL, only the content of GA3 was significantly (p < 0.05) reduced by 37.72% and 33.77%, respectively; however, the amounts of IAA, CTK and ABA were not significantly different from the control. In addition, the content of ABA could be detected only when B. chinensis seedlings were sprayed, which ranged from 3.6 to 3.9 ng/g, compared with when ABA was not detected in the other treatments.
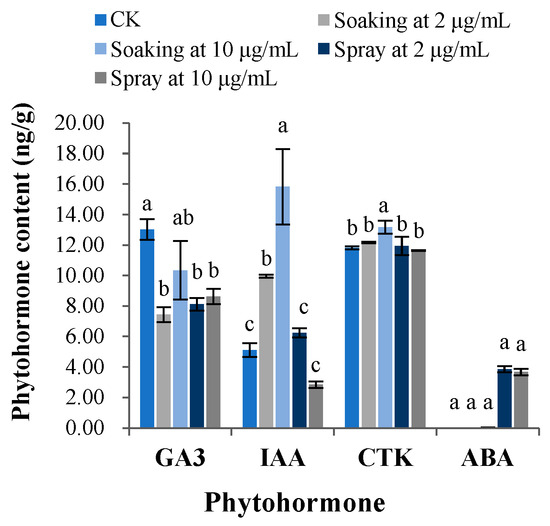
Figure 7.
Changes of the phytohormone content in B. chinensis seedlings under different terrein treatments. CK was the control group treated with distilled water under drought conditions. Each value is the mean of 50 seedlings (n = 50). The bar represents the standard error. Means with different letters (a, b, c, etc.) indicate significant differences at p < 0.05 level according to Fisher’s LSD test.
3.3. Antioxidant System Determination
Effects of terrein on MDA, CAT, POD and SOD in B. chinensis seedlings are shown in Figure 8. When seedlings were soaked in terrein solutions at 2 μg/mL, the amount of MDA was significantly (p < 0.05) reduced by 14.49%. Meanwhile, the activities of POD, CAT and SOD were increased by 51.74%, 29.07% and 39.35%, respectively. At 10 μg/mL, the amount of MDA was significantly (p < 0.05) decreased by 14.10%, whereas the activities of POD, CAT and SOD were enhanced by 53.44%, 34.85% and 44.13%, respectively. When B. chinensis seedlings were sprayed with terrein, the above-mentioned parameters exhibited a similar trend. At 2 μg/mL, the content of MDA declined significantly (p < 0.05) by 14.49%, whereas the activities of POD, CAT and SOD raised greatly by 58.41%, 34.84% and 54.97%, respectively. When the concentration reached 10 μg/mL, the amount of MDA was significantly (p < 0.05) lowered by 13.54%, while the activities of POD, CAT and SOD were enhanced by 68.47%, 44.09% and 61.98%, respectively.
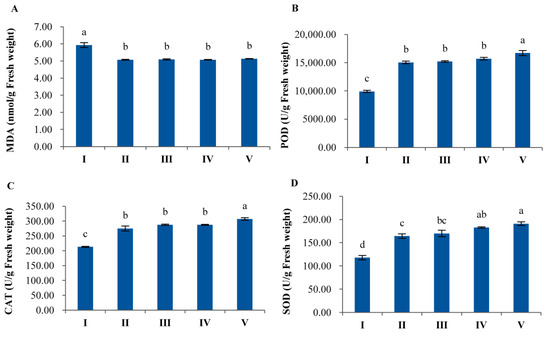
Figure 8.
Changes of MDA (A), CAT (B), POD (C) and SOD (D) of B. chinensis seedlings under different terrein treatments. CK represents the control group treated with distilled water. The letters I–V represent the control, seed soaking treatment at 2 μg/mL, seed soaking treatment at 10 μg/mL, seedling spray at 2 μg/mL and seedling spray at 10 μg/mL. Each value is the mean of 5 replicates (n = 5). The bar represents the standard error. Means with different letters (a, b, c, etc.) indicate significant differences at p < 0.05 level according to Fisher’s LSD test.
4. Discussion
Plant growth regulators are chemical substances that can regulate the physiological, biochemical and developmental processes of plants and are widely used in agriculture [33,34]. Plant growth regulators can improve the physiological characteristics of crops by improving the hormone balance and other ways so as to enhance the tolerance of crops to abiotic stress and increase crop yield [33]. Li et al. [35] showed that promalin (1.8% 6-BA and 1.8% GA3) diluted 6000 times could improve the texture, quality and berry weight of grapes (Vitis vinifera) effectively. Baron et al. [36] found that the use of plant growth regulators alone or in combination with mineral elements was beneficial to the activity of antioxidant enzymes in grapevines, the change of photosynthetic pigment content in leaves and the transport and accumulation of reserves. In another study, Ji et al. [37] proposed that sodium nitroprusside (SNP) and melatonin (MT) could improve the tolerance of tomatoes in a high nitrate environment and promote the growth of tomatoes compared with salicylic acid (SA) and humic acid (HA). In our study, we found that root development was much more sensitive to terrein treatment than shoot growth, despite whether terrein was used as a seed soaking agent or spraying agent; shoot growth responded very weak to terrein application regardless of the species. As roots are responsible for the water and nutrient uptake of plants, it can be concluded that improved root development will certainly benefit greatly from plant growth. In general, although terrein always exerted a stimulatory effect on the tested plants, the degree was different: it possessed better promotive activity on B. chinensis seedlings when being applied as a seed soaking agent, whereas for T. aestivum, S. bicolor and V. radiata, using terrein as a seed soaking agent acted more effectively than as a soaking solution.
Our experiments were conducted in Xinjiang province, the largest province located in northwest China, suffering from severe water shortage. Indeed, drought is the major abiotic stress caused by climate change, causing huge crop losses worldwide [38]. The solutions reported so far include increased irrigation, the use of conventional breeding and genetic engineering to develop drought-resistant crops and the direct use of naturally occurring bacteria that boost plant growth [38,39]. Among them, the use of plant growth-promoting bacteria (PGPB) increased plant sugar production, relative water content, leaf number, ascorbic acid level and photosynthetic pigment number, which proved to be the preferred method to alleviate drought stress [40]. In addition, Van et al. [41] found for the first time that maize treated with copper nanoparticles showed higher biomass and leaf water content under drought conditions and exhibited greater drought tolerance compared with water-treated plants, which could be used as a promising method for producing drought-resistant crops. However, there is a shortcoming of utilizing live microorganisms to promote plant growth: they first need to be established in the environment, and drought might limit this. Therefore, natural compounds are more efficient and convenient from this perspective of purpose. However, the current study is limited to pot experiments, whereas the application of terrein in the practice of agriculture may be complicated and influenced by various factors such as plant species/varieties, growth conditions, geographical locations, etc. Therefore, future work is needed to testify whether the promotive effect of terrein will persist in field conditions, which will allow the technology to be scaled up.
Our results indicated that terrein altered the phytohormones of B. chinensis seedlings. Phytohormones are active molecules that play an important role in embryogenesis, organ size regulation, reproductive development and plant pathogen defense [42]. They have long been considered important endogenous molecules that regulate plant development under various abiotic stresses [43]. Studies have shown that melatonin, as a plant regulatory factor first discovered in animal tissues, can cope with biotic and abiotic stresses, thereby regulating the metabolism of plant hormones (biosynthesis and catabolism) and other pathways to increase plant tolerance [44]. Demirkol’s [45] study showed that the application of exogenous PopW (a harpin protein from Ralstonia solanacearum) could significantly increase the contents of GA, jasmonic acid (JA), sialic acid (SA) and IAA in alfalfa (Medicago sativa) under drought stress and enhance the drought resistance of alfalfa. Our results were similar: terrein promoted the amount of IAA and CTK in B. chinensis seedlings, especially when it was used as a soaking agent at 10 μg/mL.
The effects of terrein on the antioxidant system were also evaluated in this study. Our results showed that MDA content in B. chinensis seedlings treated with terrein was significantly reduced compared with that in the control group. Increased MDA content is often used as an indicator of cell membrane degradation, dysfunction and reactive oxygen species (ROS) [46]. ROS are produced by organelles and compartments with high electron transport rates, such as mitochondria, chloroplasts and peroxisomes, and play a role in cell messenger and redox regulation in a variety of plant biological processes [47]. Various stresses usually lead to excessive production of ROS in plant cells, resulting in oxidative stress and cell damage [48]. In this study, terrein treatment reduced MDA content in recipient plants, suggesting that ROS was not generated in excess owing to the presence of terrein.
The plant antioxidant system can protect plants from oxidative stress damage, which has very efficient enzymes, including SOD, CAT, glutathione reductase (GR), etc., working together to protect plant cells from oxidative damage by removing ROS [49,50]. In this study, the activities of POD, CAT and SOD in the receiver plants treated with terrein were all significantly stronger than those in the control group, indicating that terrein could help plants to remove ROS triggered by drought stress, thus promoting plant growth and development. This is consistent with the study of Li et al. [51] that the activities of SOD, CAT, POD and other antioxidant enzymes in tomato plants treated with a bioactive flavonoid (epigallocatechin-3-gallate, EGCG) were enhanced, which reinforced the tolerance of tomato to salt stress. In addition, Tian et al. [52] showed that under saline-alkali stress of 0–150 mmol/L, the activities of SOD, CAT and POD of Elytrigia elongata increased significantly; however, when the stress continued to strengthen, POD activities in plants decreased. This indicated that the plant’s antioxidant system could no longer cope with the adverse effects of saline-alkali stress. Dai et al. [53] found that after salt and drought treatment, the tolerance and survival rate of Malus domestica (MdBBX1) transgenic plants were higher than those of wild-type (WT) plants, which were manifested as decreased MDA levels, increased proline (PRO) content, increased activities of POD, CAT, SOD and APX and decreased ROS accumulation in MdBBX1 plants. Our results were consistent with the above-mentioned reports, implying that terrein may also play a key role in regulating drought stress tolerance by reducing ROS production. It is noteworthy to mention that further work is needed to elucidate the underlying mechanisms driving the observed changes of the phytohormones and enzyme activities, for example, the comparison of expression levels of related regulatory genes before and after the treatment with terrein.
5. Conclusions
Our results indicated that terrein, either applied as a seed soaking agent or a spray solution, can effectively promote the growth of four selected agricultural plants at low concentrations (2 and 10 μg/mL) under drought conditions by improving their seedling growth as well as fresh and dry weight, especially root growth. It is noteworthy to mention that such a promotive effect was species-specific; therefore, the best-applying condition differed with the species. Further study revealed that terrein significantly (p < 0.05) increased the contents of the phytohormones GA3, IAA and CTK in the receiver plants; meanwhile, MDA content was significantly (p < 0.05) decreased, whereas the activities of POD, CAT and SOD were significantly (p < 0.05) higher than the control, indicating that terrein can stimulate plant growth by reducing the production of ROS under drought condition. Our results indicated that terrein can reinforce plants’ ability to endure drought stress so as to promote their growth, implying that it has the potential to be further explored as an environment-friendly plant growth regulator.
Author Contributions
Investigation, L.L.; formal analysis, L.L.; methodology, writing—original draft, L.L.; software, L.L.; writing—review and editing, H.S.; conceptualization, H.S.; supervision, H.S.; project administration, H.S.; validation, H.S.; resources, H.S.; visualization, H.S.; data curation, H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Xinjiang Uygur Autonomous Region (2022D01D02) and the Third Xinjiang Scientific Expedition Program (2022xjkk1505).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alptekin, B.; Langridge, P.; Budak, H. Abiotic stress miRNomes in the Triticeae. Funct. Integr. Genom. 2017, 17, 145–170. [Google Scholar] [CrossRef]
- Gechev, T.; Petrov, V. Reactive Oxygen species and abiotic stress in plants. Int. J. Mol. Sci. 2020, 21, 7433. [Google Scholar] [CrossRef]
- Rhaman, M.S.; Imran, S.; Karim, M.M.; Chakrobortty, J.; Hasanuzzaman, M. 5-aminolevulinic acid-mediated plant adaptive responses to abiotic stress. Plant Cell Rep. 2021, 40, 1451–1469. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Takasaki, H.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. NAC transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta Gene Regul. Mech. 2012, 1819, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.Y.; Wallrad, L.; Bader, O.; Kudla, J. Ca2+ signaling in plant responses to abiotic stresses. J. Integr. Plant Biol. 2022, 64, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Lu, C.; Guo, J.; Qiao, Z.; Wang, B. C2H2 zinc finger proteins: Master regulators of abiotic stress responses in plants. Front Recent. Dev. Plant Sci. 2020, 11, 115. [Google Scholar] [CrossRef]
- Hossain, Z.; Khatoon, A.; Komatsu, S. Soybean proteomics for unraveling abiotic stress response mechanism. J. Proteome Res. 2013, 12, 4670–4684. [Google Scholar] [CrossRef]
- Hinojosa, L.; Gonzalez, J.A.; Barrios-Masias, F.H.; Fuentes, F.; Murphy, K.M. Quinoa abiotic stress responses: A review. Plants 2018, 7, 106. [Google Scholar] [CrossRef]
- Rane, J.; Singh, A.K.; Kumar, M.; Boraiah, K.M.; Meena, K.K.; Pradhan, A.; Prasad, P.V.V. The adaptation and tolerance of major cereals and legumes to important abiotic stresses. Int. J. Mol. Sci. 2021, 22, 12970. [Google Scholar] [CrossRef]
- Singh, K.; Gera, R.; Sharma, R.; Maithani, D.; Chandra, D.; Bhat, M.A.; Kumar, R.; Bhatt, P. Mechanism and application of Sesbania root-nodulating bacteria: An alternative for chemical fertilizers and sustainable development. Arch. Microbiol. 2021, 203, 1259–1270. [Google Scholar] [CrossRef]
- Guo, Y.Z.; Wang, J.Y. Spatiotemporal changes of chemical fertilizer application and its environmental risks in China from 2000 to 2019. Int. J. Environ. Res. Public Health 2021, 18, 11911. [Google Scholar] [CrossRef]
- Wang, J.Q.; Qin, L.J.; Cheng, J.R.; Shang, C.C.; Li, B.; Dang, Y.C.; He, H.S. Suitable chemical fertilizer reduction mitigates the water footprint ofmaize production: Evidence from Northeast China. Environ. Sci. Pollut. Res. 2022, 29, 22589–22601. [Google Scholar] [CrossRef] [PubMed]
- Zainuddin, N.; Keni, M.F.; Ibrahim, S.A.S.; Masri, M.M. Effect of integrated biofertilizers with chemical fertilizers on the oil palm growth and soilmicrobial diversity. Biocatal. Agric. Biotechnol. 2022, 39, 102237. [Google Scholar] [CrossRef]
- Guo, L.L.; Guo, S.H.; Tang, M.Q.; Su, M.Y.; Li, H.J. Financial support for agriculture, chemical fertilizer use, and carbon emissions from agricultural production in China. Int. J. Environ. Res. Public Health 2022, 19, 7155. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; MacDonald, G.K.; Galloway, J.N.; Zhang, L.; Gao, L.M.; Yang, L.; Yang, J.X.; Li, X.L.; Li, H.R.; Yang, T. The influence of crop and chemical fertilizer combinations on greenhouse gas emissions: A partial life-cycle assessment of fertilizer production and use in China. Resour. Conserv. Recycl. 2021, 168, 105303. [Google Scholar] [CrossRef]
- Suthar, H.; Hingurao, K.; Vaghashiya, J.; Parmar, J. Fermentation: A process for biofertilizer production. Microorg. Green Revolut. 2017, 6, 229–252. [Google Scholar] [CrossRef]
- Gu, Y.B.; Meng, D.L.; Yang, S.; Xiao, N.W.; Li, Z.Y.; Liu, Z.H.; Li, L.Z.; Zeng, X.X.; Zeng, S.R.; Yin, H.Q. Invader-resident community similarity contribute to the invasion process and regulate biofertilizer effectiveness. J. Cleaner. Prod. 2019, 241, 118278. [Google Scholar] [CrossRef]
- Raimi, A.; Roopnarain, A.; Adeleke, R. Biofertilizer production in Africa: Current status, factors impeding adoption and strategies for success. Sci. Afr. 2021, 11, e00694. [Google Scholar] [CrossRef]
- Xue, L.X.; Sun, B.; Yang, Y.H.; Jin, B.; Zhuang, G.Q.; Bai, Z.H.; Zhuang, X.L. Efficiency and mechanism of reducing ammonia volatilization in alkaline farmland soil using Bacillus amyloliquefaciens biofertilizer. Environ. Res. 2021, 202, 111672. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, J.; Sommerfeld, M. Biofertilizer and biostimulant properties of the microalga Acutodesmus dimorphus. J. Appl. Phycol. 2016, 28, 1051–1061. [Google Scholar] [CrossRef]
- Bakonyi, N.; Bott, S.; Gajdos, E.; Szabo, A.; Jakab, A.; Toth, B.; Makleit, P.; Veres, S. Using biofertilizer to improve seed germination and early development of maize. Pol. J. Environ. Stud. 2013, 22, 1595–1599. [Google Scholar]
- Shang, C.; Chen, A.W.; Chen, G.Q.; Li, H.K.; Guan, S.; He, J.M. Microbial biofertilizer decreases nicotine content by improving soil nitrogen supply. Appl. Biochem. Biotechnol. 2017, 181, 1–14. [Google Scholar] [CrossRef]
- Bandopadhyay, S. Application of plant growth promoting Bacillus thuringiensis as biofertilizer on Abelmoschus esculentus plants under field condition. J. Pure Appl. Microbiol. 2020, 14, 1287–1294. [Google Scholar] [CrossRef]
- Tang, J.; Huang, L.; Liu, Y.; Toshmatov, Z.; Zhang, C.; Shao, H. Two phytotoxins isolated from the pathogenic fungus of the invasive weed Xanthium italicum. Chem. Biodivers. 2020, 17, e2000043. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.S.; Bai, X.F.; Zhang, B.X.; Wang, L.; Chen, S.B.; Wang, Z.H. Enhanced production of terrein in marine-derived Aspergillus terreus by refactoring both global and pathway-specific transcription factors. Microb. Cell Fact. 2022, 21, 136. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Yang, J.N.; Li, C.; Hui, Y.; Chen, W.H. Recent advances in isolation, synthesis and biological evaluation of terrein. Chem. Biodivers. 2021, 18, e2100594. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Choudhary, M.; Singh, B.; Singh, R.; Dhar, M.K.; Kaul, S. Diversity and biological activity of fungal endophytes of Zingiber officinale Rosc. with emphasis on Aspergillus terreus as a biocontrol agent of its leaf spot. Biocatal. Agric. Biotechnol. 2022, 39, 102234. [Google Scholar] [CrossRef]
- Buachan, P.; Namsa-Aid, M.; Sung, H.K.; Peng, C.; Sweeney, G.; Tanechpongtamb, W. Inhibitory effects of terrein on lung cancer cell metastasis and angiogenesis. Oncol. Rep. 2021, 45, 94. [Google Scholar] [CrossRef]
- Nakagawa, S.; Omori, K.; Nakayama, M.; Mandai, H.; Yamamoto, S.; Kobayashi, H.; Sako, H.; Sakaida, K.; Ibaragi, S.; Hirai, K.; et al. The fungal metabolite (+)-terrein abrogates osteoclast differentiation via suppression of the RANKL signalingpathway through NFATc1. Int. Immunopharmacol. 2020, 83, 106429. [Google Scholar] [CrossRef]
- Lu, Y.H.; Jin, L.P.; Kong, L.C.; Zhang, Y.L. Phytotoxic, antifungal and immunosuppressive metabolites from Aspergillus terreus QT122 isolated from the gut of dragonfly. Curr. Microbiol. 2017, 74, 84–89. [Google Scholar] [CrossRef]
- Fang, S.; Gao, K.; Hu, W.; Snider, J.L.; Wang, S.S.; Chen, B.L.; Zhou, Z.G. Chemical priming of seed alters cotton floral bud differentiation by inducing changes in hormones, metabolites and gene expression. Plant Physiol. Biochem. 2018, 130, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.J.; Zhao, Y.; Shi, K.; Liu, Y.; Hu, Y.X.; Shao, H. Phytotoxic activity of alkaloids in the desert plant Sophora alopecuroides. Toxins 2021, 13, 706. [Google Scholar] [CrossRef]
- Desta, B.; Amare, G. Paclobutrazol as a plant growth regulator. Chem. Biol. Technol. Agric. 2021, 8, 1. [Google Scholar] [CrossRef]
- Shah, S.H.; Islam, S.; Parrey, Z.A.; Mohammad, F. Role of exogenously applied plant growth regulators in growth and development of edible oilseed crops under variable environmental conditions: A review. J. Soil Sci. Plant Nutr. 2021, 21, 3284–3308. [Google Scholar] [CrossRef]
- Li, L.; Yan, W.J.; Yao, H.D.; Li, H.; Guo, X.Z.; Cheng, D.W.; Sun, J.L.; Chen, J.Y. Influences of two plant growth regulators on the fruit quality of the ‘Crimson Seedless’ grapes. J. Plant Growth Regul. 2023, 42, 771–779. [Google Scholar] [CrossRef]
- Baron, A.C.E.A.; Baron, D.; Souza, E.R.; Moreira, L.S.; Ono, E.O.; Rodrigues, J.D. Effects of the plant growth regulators, cobalt and molybdenum on the physiology of ‘Crimson Seedless’ grapevines. Acta Physiol. Plant 2022, 44, 63. [Google Scholar] [CrossRef]
- Ji, R.T.; Min, J.; Wang, Y.; Kronzucker, H.J.; Shi, W.M. The role of plant growth regulators in modulating root architecture and tolerance to high-nitrate stress in tomato. Front. Recent Dev. Plant Sci. 2022, 13, 864285. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. Recent advances in bacterial amelioration of plant drought and salt stress. Biology 2022, 11, 437. [Google Scholar] [CrossRef]
- Riseh, R.S.; Ebrahimi-Zarandi, M.; Vazvani, M.G.; Skorik, Y.A. Reducing drought stress in plants by encapsulating plant growth-promoting bacteriawith polysaccharides. Int. J. Mol. Sci. 2021, 22, 12979. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Santoyo, G.; Yadav, A.N.; Babalola, O.O. Efforts towards overcoming drought stress in crops: Revisiting the mechanisms employed byplant growth-promoting bacteria. Front. Microb. 2022, 13, 962427. [Google Scholar] [CrossRef]
- Van Nguyen, D.; Nguyen, H.M.; Le, N.T.; Nguyen, K.H.; Nguyen, H.T.; Le, H.M.; Nguyen, A.T.; Dinh, N.T.T.; Hoang, S.A.; Van Ha, C. Copper nanoparticle application enhances plant growth and grain yield in maize under drought stress conditions. J. Plant Growth Regul. 2022, 41, 364–375. [Google Scholar] [CrossRef]
- Bhandari, S.; Nailwal, T.K. Role of brassinosteroids in mitigating abioticstresses in plants. Biologia 2020, 75, 2203–2230. [Google Scholar] [CrossRef]
- Ryu, H.; Cho, Y.G. Plant hormones in salt stress tolerance. J. Plant Biol. 2015, 58, 147–155. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernandez-Ruiz, J. Melatonin as a regulatory hub of plant hormone levels and action in stress situations. Plant Biol. 2021, 23, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Demirkol, G. PopW enhances drought stress tolerance of alfalfa via activating antioxidative enzymes, endogenous hormones, drought related genes and inhibiting senescence genes. Plant Physiol. Biochem. 2021, 166, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.X.; Huang, F.; Cheng, H.; Song, H.N.; Yu, D.Y. Overexpression of the GmNAC2 gene, an NAC transcription factor, reduces abiotic stress tolerance in tobacco. Plant Mol. Biol. Rep. 2013, 31, 435–442. [Google Scholar] [CrossRef]
- Raja, V.; Majeed, U.; Kang, H.; Andrabi, K.I.; John, R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ. Exp. Bot. 2017, 137, 142–157. [Google Scholar] [CrossRef]
- Amir, R.; Hussain, S.; Noor-ul-Ain, H.; Hussain, A.; Yun, B.W. ROS mediated plant defense against abioticstresses. Plant Biotechnol. 2019, 2019, 481–515. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Aleem, M.; Aleem, S.; Sharif, I.; Wu, Z.Y.; Aleem, M.; Tahir, A.; Atif, R.M.; Cheema, H.M.N.; Shakeel, A.; Lei, S.; et al. Characterization of SOD and GPX gene families in the soybeans in response to drought and salinity stresses. Antioxidants 2022, 11, 460. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Ahammed, G.J.; Zhang, X.N.; Ying, L.; Zhang, L.; Yan, P.; Zhang, L.P.; Li, Q.Y.; Han, W.Y. RBOH1-dependent apoplastic H2O2 mediates epigallocatechin-3-gallate-induced abiotic stress tolerance in Solanum lycopersicum L. Environ. Exp. Bot. 2019, 161, 357–366. [Google Scholar] [CrossRef]
- Tian, X.X.; Mao, P.C.; Zheng, M.L.; Meng, Q.Y.; Meng, L. Seed germination and biochemical responses of two Elytrigia elongata accessions exposed to abiotic stresses. Grassl. Sci. 2021, 67, 369–379. [Google Scholar] [CrossRef]
- Dai, Y.Q.; Lu, Y.; Zhou, Z.; Wang, X.Y.; Ge, H.J.; Sun, Q.H. B-box containing protein 1 from Malus domestica (MdBBX1) is involved in the abiotic stress response. PeerJ 2022, 10, e12852. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).