Morphology and SSR Markers-Based Genetic Diversity Analysis of Sesame (Sesamum indicum L.) Cultivars Released in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Morpho-Physiological Investigation
2.3. Total DNA Extraction
2.4. Simple Sequence Repeat (SSR) Detection
2.5. Data Analysis
3. Results
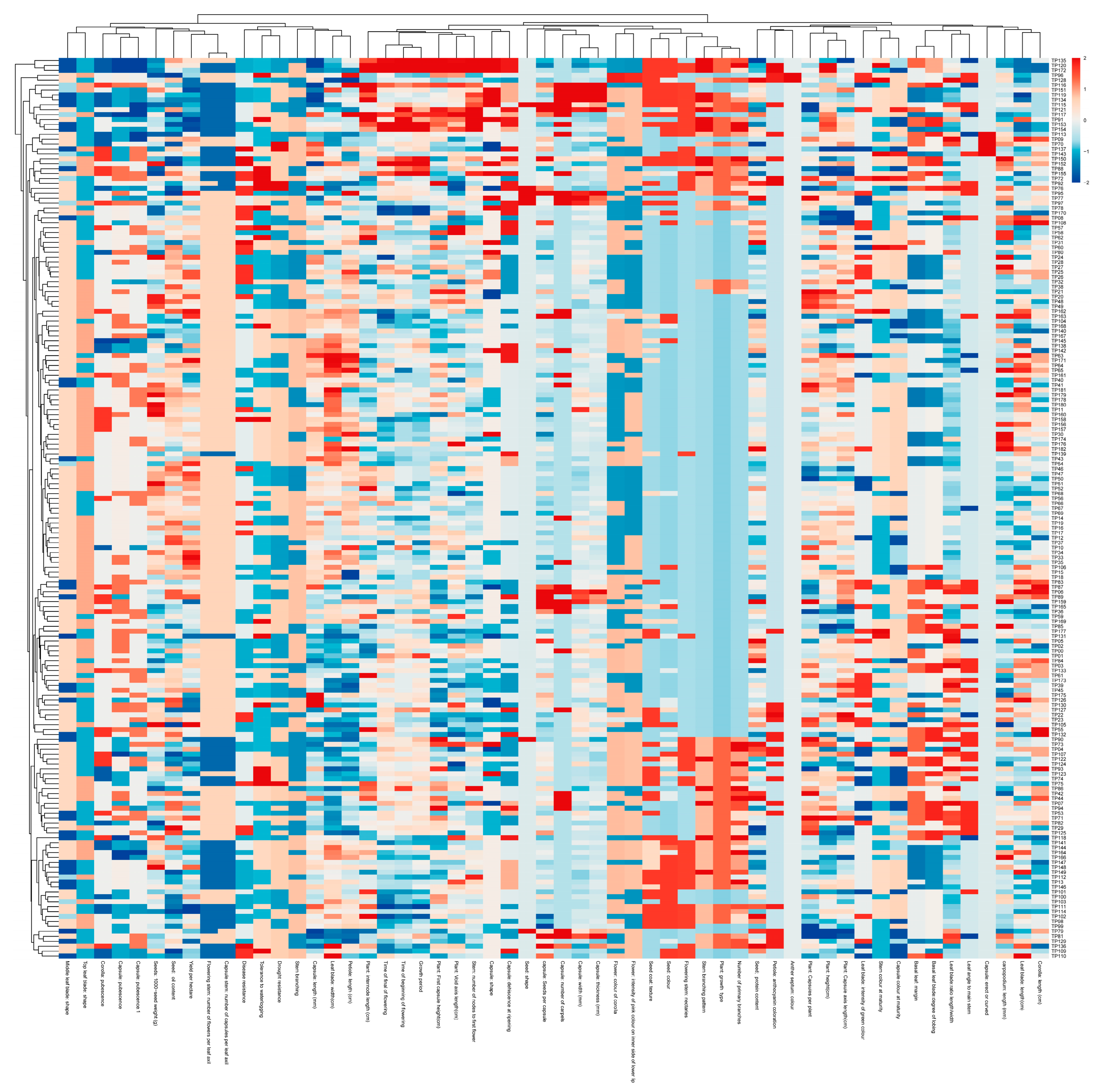
3.1. Morphological and Agronomical Diversity of the Sesame Accessions
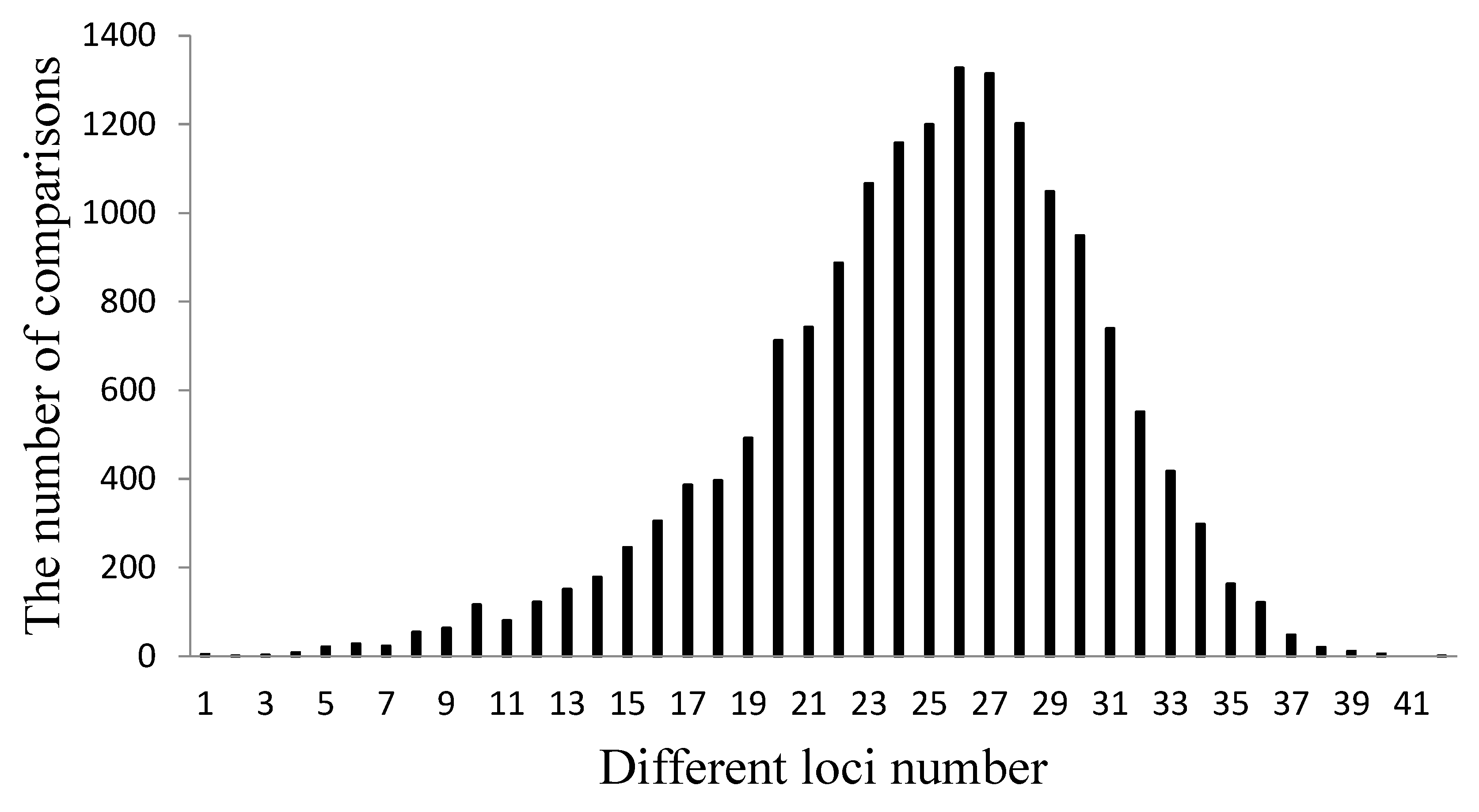
3.2. Polymorphism of the SSR Markers
3.3. DNA Fingerprinting of 183 Accessions
3.4. Genetic Diversity Analysis of Sesame Accessions with Different Origins
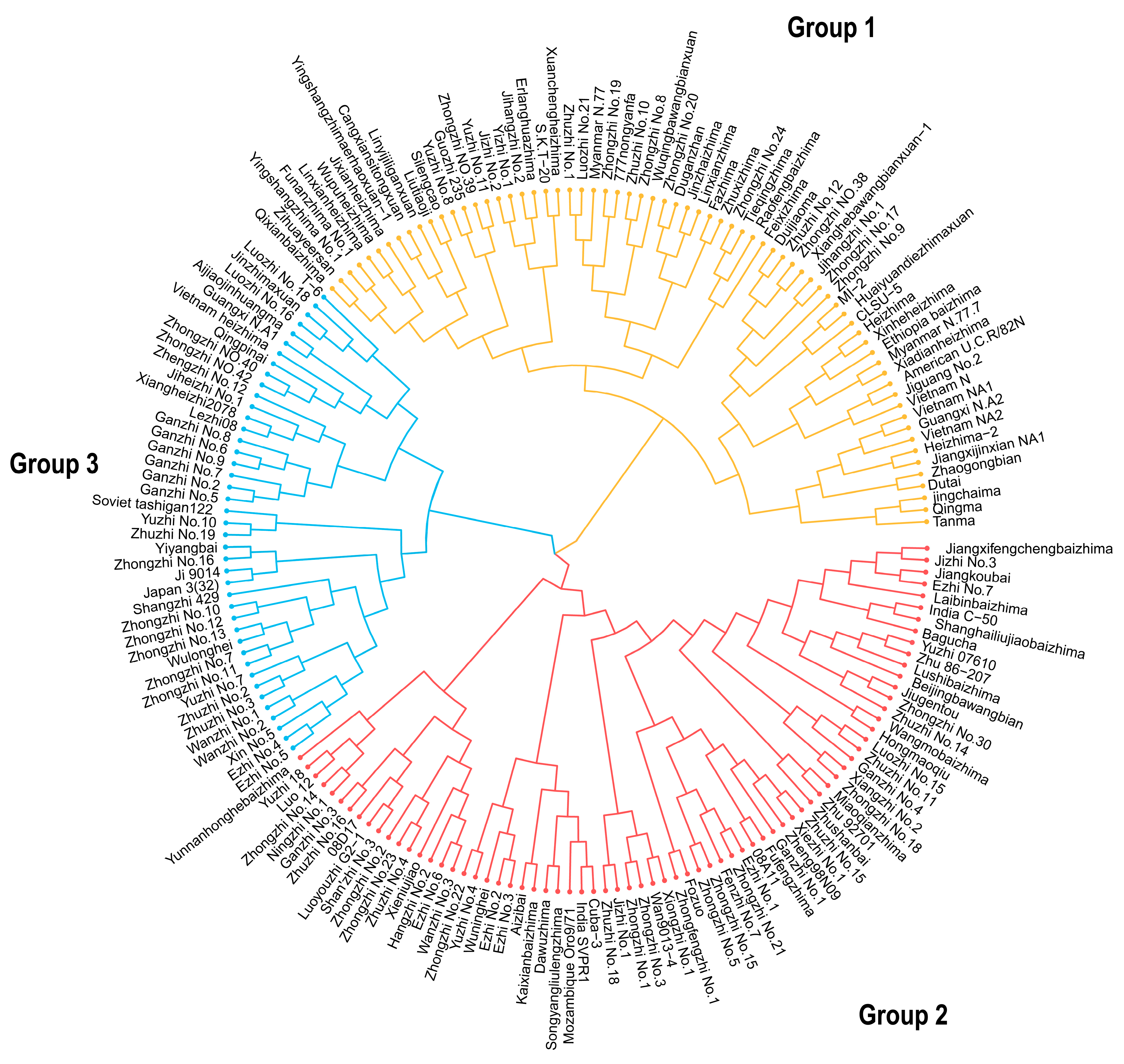
3.5. Systematic Clustering of 183 Sesame Accessions
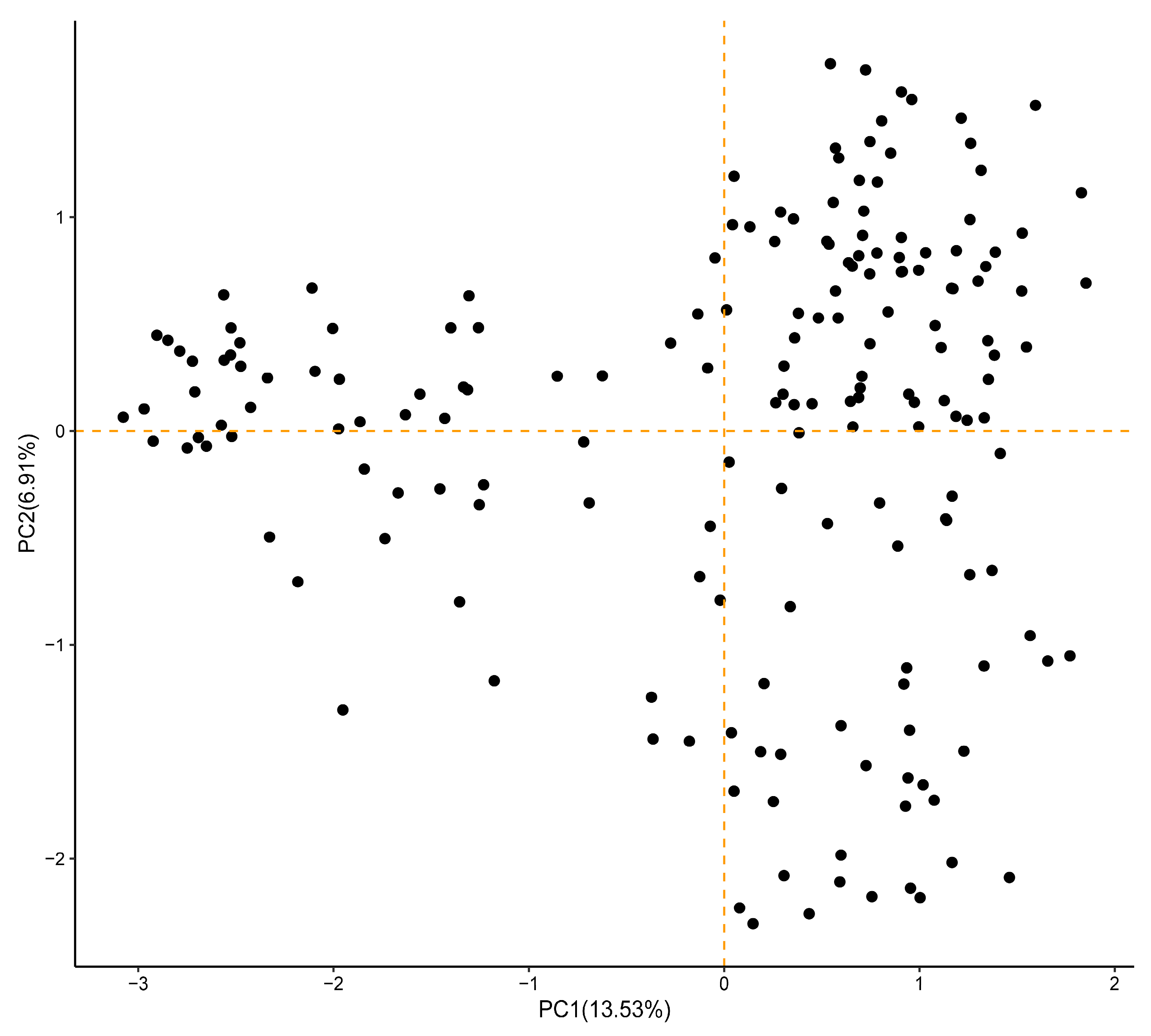
3.6. Principal Coordinate Analysis of 183 Sesame Accessions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kobayashi, T.; Kinoshita, M.; Hattori, S.; Ogawa, T.; Tsuboi, Y.; Ishida, M.; Ogawa, S.; Saito, H. Development of the sesame metallic fuel performance code. Nucl. Technol. 1990, 89, 183–193. [Google Scholar] [CrossRef]
- Nayar, N.M.; Mehra, K.L. Sesame-its uses botany, cytogenetics, and origin. Econ. Bot. 1970, 24, 20–31. [Google Scholar] [CrossRef]
- Ashri, A. Sesame. In Oil Crops of the World; Roebblen, G., Downey, R.K., Ashri, A., Eds.; McGraw Hill: New York, NY, USA, 1989; pp. 375–387. ISBN 0070530815. [Google Scholar]
- Bhat, K.V.; Babrekar, P.P.; Lakhanpaul, S. Study of genetic diversity in Indian and exotic sesame (Sesamum indicum L.) germplasm using random amplified polymorphic DNA (RAPD) markers. Euphytica 1999, 110, 21–33. [Google Scholar] [CrossRef]
- Zhou, F.; Zhao, Y.Z.; Zhou, T.; Yang, Y.X.; Liu, H.Y. Progress and suggestions of national and regional trials of new sesame varieties in China in the past 40 years. Mol. Plant Breed. 2022, 20, 1383–1392. [Google Scholar]
- Suh, M.C.; Kim, M.J.; Hur, C.G.; Bae, J.M.; Park, Y.I.; Chung, C.H.; Kang, C.W.; Ohlrogge, J.B. Comparative analysis of expressed sequence tags from Sesamum indicum and Arabidopsis thaliana developing seeds. Plant Mol. Biol. 2003, 52, 1107–1123. [Google Scholar]
- Liu, H.Y.; Zhou, F.; Zhou, T.; Yang, Y.X.; Zhao, Y.Z. A novel wrinkled-leaf sesame mutant as a potential edible leafy vegetable rich in nutrients. Sci. Rep. 2022, 12, 18478. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.L.; Park, J.H.; Lee, C.W.; Ra, W.H.; Chung, J.W.; Lee, J.R.; Ma, K.H.; Lee, S.Y.; Lee, K.S.; Lee, M.C.; et al. Evaluation of the genetic diversity and population structure of sesame (Sesamum indicum L.) using microsatellite markers. Genes Genom. 2011, 33, 187–195. [Google Scholar] [CrossRef]
- Uzun, B.; Lee, D.; Donini, P.; Cagirgan, M.I. Identification of a molecular marker linked to the closed capsule mutant trait in sesame using AFLP. Plant Breed. 2003, 122, 95–99. [Google Scholar] [CrossRef]
- Liu, H.Y. Fresh sesame flowers are wonderful for treating warts and chilblains. Chin. Community Physician. 2005, 21, 36. [Google Scholar]
- Bedigian, D.; Smyth, C.; Harlan, J. Patterns of morphological variation in sesame. Econ Bot. 1986, 40, 353–365. [Google Scholar] [CrossRef]
- Agarwal, M.; Shrivastava, N.; Padh, H. Advances in molecular marker techniques and their applications in plant sciences. Plant Cell Rep. 2008, 27, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.G.; Kubelik, A.R.; Livak, K.J.; Rafalski, J.A.; Tingey, S.V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990, 18, 6531–6535. [Google Scholar] [CrossRef]
- Safhi, F.A.; Alshamrani, S.M.; Alshaya, D.S.; Hussein, M.A.A.; Abd El-Moneim, D. Genetic diversity analysis of banana cultivars (Musa sp.) in Saudi Arabia based on AFLP marker. Curr. Issues Mol. Biol. 2023, 45, 1810–1819. [Google Scholar] [CrossRef] [PubMed]
- Abd-Dada, H.; Bouda, S.; Khachtib, Y.; Bella, Y.A.; Haddioui, A. Use of ISSR markers to assess the genetic diversity of an endemic plant of Morocco (Euphorbia resinifera O. Berg). J. Genet. Eng. Biotechnol. 2023, 21, 91. [Google Scholar] [CrossRef]
- Hamm, T.P.; Boggess, S.L.; Kandel, J.S.; Staton, M.E.; Huff, M.L.; Hadziabdic, D.; Shoemaker, D.; Adamczyk, J.J., Jr.; Nowicki, M.; Trigiano, R.N. Development and characterization of 20 genomic SSR markers for ornamental cultivars of weigela. Plants 2022, 11, 1444. [Google Scholar] [CrossRef]
- Feng, S.; Jiao, K.; Zhang, Z.; Yang, S.; Gao, Y.; Jin, Y.; Shen, C.; Lu, J.; Zhan, X.; Wang, H. Development of chloroplast microsatellite markers and evaluation of genetic diversity and population structure of cutleaf groundcherry (Physalis angulata L.) in China. Plants 2023, 12, 1755. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Xu, M.; Li, T.; Sun, R. Development of EST-SSRs based on the transcriptome of Castanopsis carlesii and cross-species transferability in other Castanopsis species. PLoS ONE 2023, 18, e0288999. [Google Scholar] [CrossRef]
- Azizi, M.M.F.; Lau, H.Y.; Abu-Bakar, N. Integration of advanced technologies for plant variety and cultivar identification. J. Biosci. 2021, 46, 91. [Google Scholar] [CrossRef]
- Uncu, A.Ö.; Gultekin, V.; Allmer, J.; Frary, A.; Doganlar, S. Genomic simple sequence repeat markers reveal patterns of genetic relatedness and diversity in sesame. Plant Genome. 2015, 8, 1–12. [Google Scholar] [CrossRef]
- Wei, W.; Qi, X.; Wang, L.; Zhang, Y.; Hua, W.; Li, D.; Lv, H.; Zhang, X. Characterization of the sesame (Sesamum indicum L.) global transcriptome using Illumina paired-end sequencing and development of EST-SSR markers. BMC Genomics. 2011, 12, 451. [Google Scholar] [CrossRef]
- Wu, K.; Yang, M.; Liu, H.; Tao, Y.; Mei, J.; Zhao, Y. Genetic analysis and molecular characterization of Chinese sesame (Sesamum indicum L.) cultivars using insertion-deletion (InDel) and simple sequence repeat (SSR) markers. BMC Genet. 2014, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Teklu, D.H.; Shimelis, H.; Tesfaye, A.; Mashilo, J.; Zhang, X.; Zhang, Y.; Dossa, K.; Shayanowako, A.I.T. Genetic variability and population structure of Ethiopian sesame (Sesamum indicum L.) germplasm assessed through phenotypic traits and simple sequence repeat markers. Plants 2021, 10, 1129. [Google Scholar] [CrossRef] [PubMed]
- Stavridou, E.; Lagiotis, G.; Kalaitzidou, P.; Grigoriadis, I.; Bosmali, I.; Tsaliki, E.; Tsiotsiou, S.; Kalivas, A.; Ganopoulos, I.; Madesis, P. Characterization of the genetic diversity present in a diverse sesame landrace collection based on phenotypic traits and EST-SSR markers coupled with an HRM analysis. Plants 2021, 10, 656. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.P.; Ren, G.X.; Wen, F. Genetic diversity of sesame (Sesamum indicum L.) germplasm from Shanxi and the major planting areas in China. Chin. J. Oil Crop Sci. 2013, 35, 539–545. [Google Scholar]
- Yue, W.D.; Wei, L.B.; Zhang, T.D.; Li, C.; Miao, H.M.; Zhang, H.Y. Genetic diversity and population structure of germplasm resources in sesame (Sesamum indicum L.) by SSR markers. Acta Agron. Sin. 2012, 38, 2286–2296. [Google Scholar] [CrossRef]
- Wang, L.; Yu, S.; Tong, C.; Zhao, Y.; Liu, Y.; Song, C.; Zhang, Y.; Zhang, X.; Wang, Y.; Hua, W.; et al. Genome sequencing of the high-oil crop sesame provides insight into oil biosynthesis. Genome Biol. 2014, 15, R39. [Google Scholar] [CrossRef]
- Guo, G.; Pan, B.; Yi, X.; Khan, A.; Zhu, X.; Ge, W.; Liu, J.; Diao, W.; Wang, S. Genetic diversity between local landraces and current breeding lines of pepper in China. Sci. Rep. 2023, 13, 4058. [Google Scholar] [CrossRef]
- Pozharskiy, A.; Kostyukova, V.; Khusnitdinova, M.; Adilbayeva, K.; Nizamdinova, G.; Kapytina, A.; Kerimbek, N.; Taskuzhina, A.; Kolchenko, M.; Abdrakhmanova, A.; et al. Genetic diversity of the breeding collection of tomato varieties in Kazakhstan assessed using SSR, SCAR and CAPS markers. PeerJ 2023, 11, e15683. [Google Scholar] [CrossRef]
- Pardeshi, P.; Jadhav, P.; Sakhare, S.; Zunjare, R.; Rathod, D.; Sonkamble, P.; Saroj, R.; Varghese, P. Morphological and microsatellite marker-based characterization and diversity analysis of novel vegetable soybean [Glycine max (L.) Merrill]. Mol. Biol. Rep. 2023, 50, 4049–4060. [Google Scholar] [CrossRef]
- Zhang, X.R.; Feng, X.Y. Descriptors and Data Standard for Sesame (Sesamum indicum L.); China Agriculture Press: Beijing, China, 2006; pp. 30–47. ISBN 9787109110151. [Google Scholar]
- Agbagwa, I.O.; Datta, S.; Patil, P.G.; Singh, P.; Nadarajan, N. A protocol for high-quality genomic DNA extraction from legumes. Genet. Mol. Res. 2012, 11, 4632–4639. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, H.Y.; Guo, W.Z.; Zheng, Y.Z.; Wei, L.B.; Zhang, T.Z. Analysis of genetic diversity of sesame germplasm resources by SRAP and EST-SSR markers. Crop Sci. 2007, 33, 1696–1702. [Google Scholar]
- Liu, H.Y.; Zhou, F.; Zhou, T.; Yang, Y.X.; Zhao, Y.Z. Cytological characterization and molecular mapping of a novel recessive genic male sterility in sesame (Sesamum indicum L.). PLoS ONE 2018, 13, e0204034. [Google Scholar] [CrossRef] [PubMed]
- Ning, W.; Wei, Y.; Gao, L.; Han, C.; Gou, Y.; Fu, S.; Liu, D.; Zhang, C.; Huang, X.; Wu, S.; et al. HemI 2.0: An online service for heatmap illustration. Nucleic Acids Res. 2022, 50, W405–W411. [Google Scholar] [CrossRef] [PubMed]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using principal component analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- Murtagh, F.; Legendre, P. Ward’s hierarchical agglomerative clustering method: Which algorithms implement ward’s criterion? J. Classif. 2014, 31, 274–295. [Google Scholar] [CrossRef]
- Yeh, F.C. Population genetic analysis of co-dominant and dominant marker and quantitative traits. Belgian J. Bot. 1997, 130, 129–157. [Google Scholar]
- Chikh-Rouhou, H.; Mezghani, N.; Mnasri, S.; Mezghani, N.; Garcés-Claver, A. Assessing the genetic diversity and population structure of a tunisian melon (Cucumis melo L.) collection using phenotypic traits and SSR molecular markers. Agronomy 2021, 11, 1121. [Google Scholar] [CrossRef]
- Bakir, M.; Dumanoglu, H.; Aygun, A.; Erdogan, V.; Dost, S.E.; Gülsen, O.; Serdar, U.; Kalkisim, O.; Bastas, K. Genetic diversity and population structure of apple germplasm from Eastern Black Sea region of Turkey by SSRs. Sci. Hortic. 2022, 294, 10793. [Google Scholar] [CrossRef]
- Cao, S.S.; Stringer, S.; Gunawan, G.; McGregor, C.; Conner, P.J. Genetic diversity and pedigree analysis of muscadine grape using SSR markers. J. Am. Soc. Hortic. Sci. 2020, 145, 143–151. [Google Scholar] [CrossRef]
- Singh, S.; Mahato, A.K.; Jayaswal, P.K.; Singh, N.; Dheer, M.; Goel, P.; Raje, R.S.; Yasin, J.K.; Sreevathsa, R.; Rai, V.; et al. A 62K genic-SNP chip array for genetic studies and breeding applications in pigeonpea (Cajanus cajan L. Millsp.). Sci. Rep. 2020, 10, 4960. [Google Scholar] [CrossRef]
- Sun, J.; Yan, T.X.; Yue, M.W.; Rao, Y.L.; Yan, X.W.; Zhou, H.Y. Study on breeding characteristics of sesame (Sesamum indicum L.) IV: Spontaneous outcrossing rate of sesame in winter multiplication in Hainan. Jiangxi Acta Agric. 2017, 29, 17–20. [Google Scholar]


| SSR Marker | Polymorphic Bands | SI | He | HeFr | PIC | No. of Alleles | DP | Major Allele Size and Frequency | h |
|---|---|---|---|---|---|---|---|---|---|
| SB393 | 3 | 0.498 | 0.342 | 0.237 | 0.573 | 1.23 | 0.719 | 130, 0.24 | 0.331 |
| LG03 | 3 | 0.640 | 0.008 | 0.414 | 0.616 | 1.81 | 0.813 | 227, 0.41 | 0.448 |
| LG21 | 3 | 0.554 | 0.339 | 0.284 | 0.590 | 1.65 | 0.768 | 156, 0.28 | 0.374 |
| LG22 | 3 | 0.592 | 0.314 | 0.361 | 0.590 | 1.74 | 0.775 | 198, 0.36 | 0.407 |
| LG28 | 3 | 0.571 | 0.316 | 0.282 | 0.595 | 1.67 | 0.760 | 155, 0.28 | 0.387 |
| LG31 | 3 | 0.554 | 0.359 | 0.270 | 0.601 | 1.62 | 0.762 | 148, 0.27 | 0.370 |
| LG56 | 3 | 0.635 | 0.012 | 0.337 | 0.626 | 1.80 | 0.763 | 185, 0.34 | 0.443 |
| LG57 | 3 | 0.634 | 0.017 | 0.363 | 0.636 | 1.80 | 0.782 | 199, 0.36 | 0.443 |
| LG40 | 3 | 0.631 | 0.017 | 0.384 | 0.629 | 1.79 | 0.791 | 211, 0.38 | 0.439 |
| LG46 | 3 | 0.555 | 0.332 | 0.266 | 0.617 | 1.62 | 0.749 | 146, 0.27 | 0.371 |
| LG64 | 3 | 0.564 | 0.312 | 0.302 | 0.572 | 1.65 | 0.763 | 166, 0.30 | 0.380 |
| LG108 | 3 | 0.273 | 0.680 | 0.257 | 0.086 | 1.26 | 0.456 | 141, 0.26 | 0.166 |
| LG111 | 3 | 0.581 | 0.332 | 0.419 | 0.573 | 1.73 | 0.790 | 230, 0.42 | 0.400 |
| LG115 | 3 | 0.450 | 0.430 | 0.293 | 0.386 | 1.49 | 0.681 | 161, 0.29 | 0.295 |
| LG116 | 3 | 0.407 | 0.376 | 0.290 | 0.357 | 1.49 | 0.669 | 159, 0.29 | 0.280 |
| LG123 | 3 | 0.496 | 0.336 | 0.293 | 0.510 | 1.62 | 0.729 | 161, 0.29 | 0.338 |
| LG141 | 3 | 0.650 | 0.006 | 0.437 | 0.633 | 1.85 | 0.698 | 240, 0.44 | 0.457 |
| LG131 | 3 | 0.506 | 0.334 | 0.352 | 0.507 | 1.67 | 0.760 | 193, 0.35 | 0.351 |
| LG144 | 3 | 0.541 | 0.442 | 0.290 | 0.515 | 1.61 | 0.712 | 159, 0.29 | 0.360 |
| LG139 | 3 | 0.611 | 0.130 | 0.321 | 0.631 | 1.75 | 0.803 | 176, 0.32 | 0.421 |
| LG140 | 3 | 0.526 | 0.540 | 0.259 | 0.570 | 1.58 | 0.717 | 142, 0.26 | 0.347 |
| LG148 | 3 | 0.371 | 0.795 | 0.390 | 0.296 | 1.29 | 0.522 | 214, 0.39 | 0.219 |
| LG147 | 3 | 0.543 | 0.373 | 0.432 | 0.474 | 1.60 | 0.752 | 237, 0.43 | 0.361 |
| LG151 | 3 | 0.385 | 0.638 | 0.492 | 0.478 | 1.42 | 0.553 | 270, 0.49 | 0.251 |
| LG153 | 3 | 0.465 | 0.335 | 0.370 | 0.479 | 1.61 | 0.728 | 203, 0.37 | 0.324 |
| Accession Type | Number of Accessions | Number of Alleles | Heterozygous Genotype Frequency | Gene Diversity |
|---|---|---|---|---|
| Chinese cultivars (CC) | 101 | 120 | 0.3379 | 0.3454 |
| Chinese landraces (CL) | 62 | 121 | 0.3219 | 0.3515 |
| Exotic accessions (EA) | 20 | 117 | 0.3667 | 0.3499 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhou, F.; Tang, X.; Yang, Y.; Zhou, T.; Liu, H. Morphology and SSR Markers-Based Genetic Diversity Analysis of Sesame (Sesamum indicum L.) Cultivars Released in China. Agriculture 2023, 13, 1885. https://doi.org/10.3390/agriculture13101885
Wang Z, Zhou F, Tang X, Yang Y, Zhou T, Liu H. Morphology and SSR Markers-Based Genetic Diversity Analysis of Sesame (Sesamum indicum L.) Cultivars Released in China. Agriculture. 2023; 13(10):1885. https://doi.org/10.3390/agriculture13101885
Chicago/Turabian StyleWang, Zhen, Fang Zhou, Xuehui Tang, Yuanxiao Yang, Ting Zhou, and Hongyan Liu. 2023. "Morphology and SSR Markers-Based Genetic Diversity Analysis of Sesame (Sesamum indicum L.) Cultivars Released in China" Agriculture 13, no. 10: 1885. https://doi.org/10.3390/agriculture13101885
APA StyleWang, Z., Zhou, F., Tang, X., Yang, Y., Zhou, T., & Liu, H. (2023). Morphology and SSR Markers-Based Genetic Diversity Analysis of Sesame (Sesamum indicum L.) Cultivars Released in China. Agriculture, 13(10), 1885. https://doi.org/10.3390/agriculture13101885





