The Influence of Short-Term Tillage, Compost, and Beneficial Microbes on Soil Properties and the Productivity of Wheat and Cowpea Crops
Abstract
:1. Introduction
2. Materials and Methods
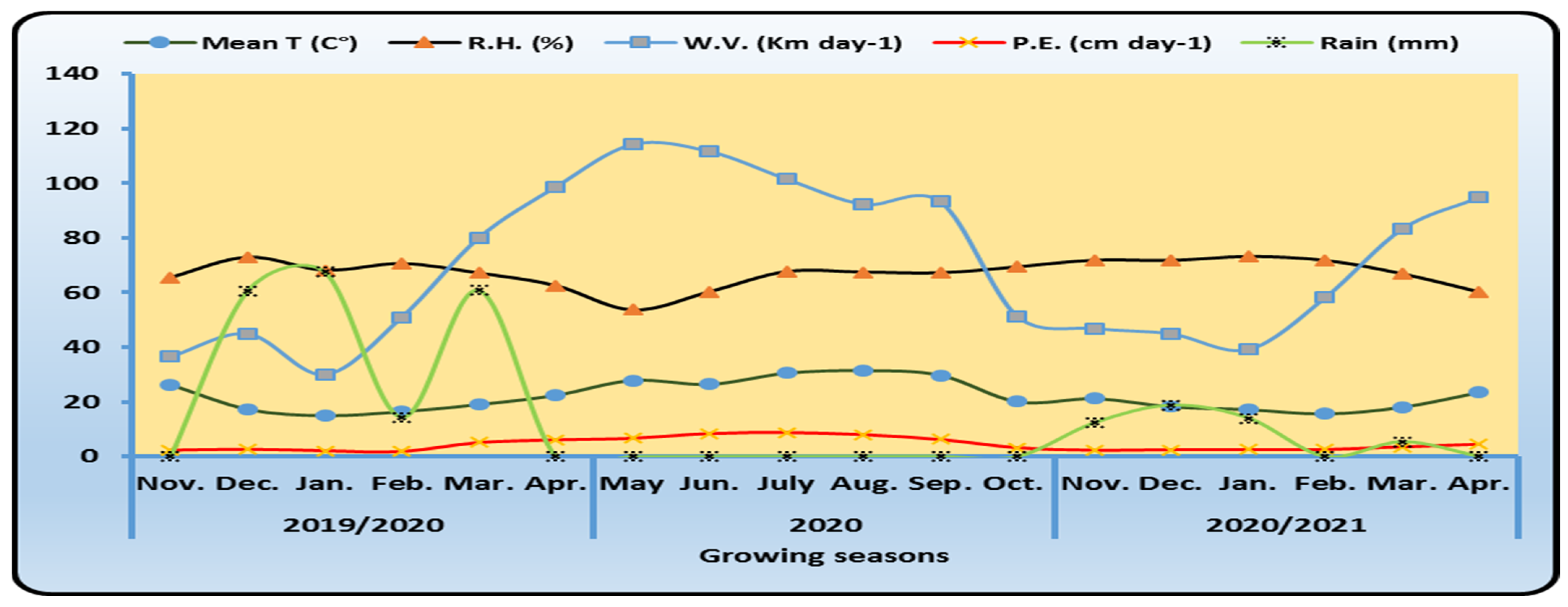
2.1. Location
2.2. Compost and Beneficial Microorganisms
2.3. Field Experiment
2.4. Measurements and Analyses
2.4.1. Estimations of the Microbial Community and Soil Enzymes
2.4.2. Chemical and Physical Properties of Soil
2.5. Statistical Analysis
3. Results
3.1. Biological Properties
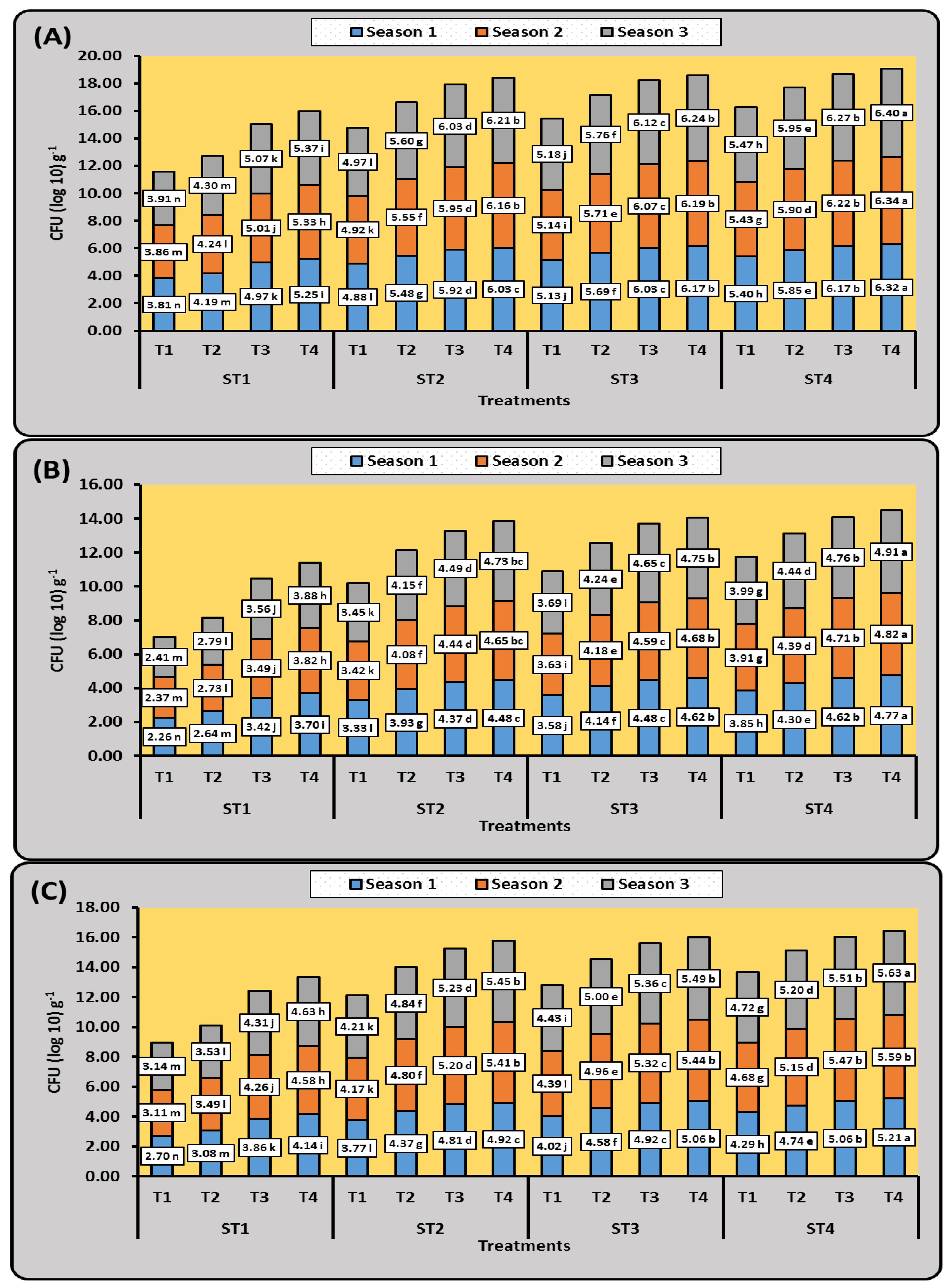
3.1.1. Microbial Community
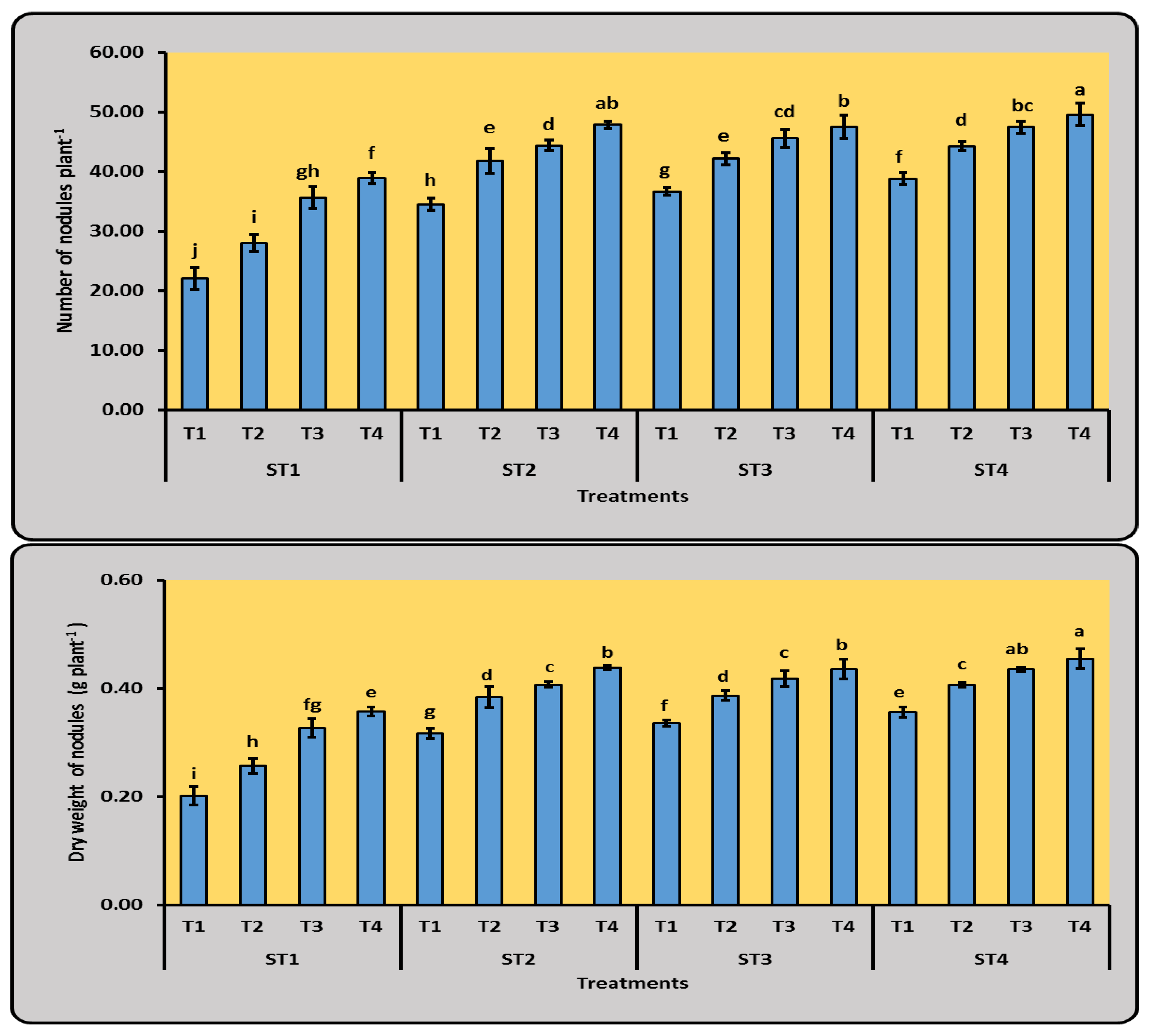
3.1.2. Number and Dry Weight of Nodules of Cowpea Plants
3.1.3. Soil Enzymes Activities
3.2. Chemical Properties of Soil
ECe and ESP
3.3. Physical Properties of Soil
3.3.1. Soil bulk Density and Soil Porosity
3.3.2. Soil Aggregates
3.3.3. Soil Penetration Resistance (SPRa) and Soil Infiltration Rate (IR)
3.4. Yield of Wheat and Cowpea
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janke, R.R.; Papendick, R.I. Preface. In Defining Soil Quality for a Sustainable Environment; Soil Science Society of America and American Society of Agronomy; Doran, J.W., Coleman, D.C., Bezdicek, D.F., Stewart, B.A., Eds.; Madison: New York, NY, USA, 1994. [Google Scholar]
- Fuentes, M.; Govaerts, B.; De Leon, F.; Hidalgo, C.; Dendooven, L.; Sayre, K.D.; Etchevers, J. Fourteen years of applying zero and conventional tillage, crop rotation and residue management systems and its effect on physical and chemical soil quality. Eur. J. Agron. 2009, 30, 228–237. [Google Scholar] [CrossRef]
- So, H.B.; Grabski, A.; Desborough, P. The impact of 14 years of conventional and no-till cultivation on the physical properties and crop yields of a loam soil at Grafton NSW, Australia. Soil Tillage Res. 2009, 104, 180–184. [Google Scholar] [CrossRef]
- Govaerts, B.; Mezzalama, M.; Sayre, K.D.; Crossa, J.; Lichter, K.; Troch, V.; Vanherck, K.; De Corte, P.; Decker, J. Long-term consequences of tillage, residue management, and crop rotation on selected soil micro-flora groups in the subtropical highlands. Appl. Soil Ecol. 2008, 38, 197–210. [Google Scholar] [CrossRef]
- Kandeler, E.; Tscherko, D.; Spiegel, H. Long-term monitoring of microbial biomass, N mineralisation and enzyme activities of a Chernozem under different tillage management. Biol. Fertil. Soils 1999, 28, 343–351. [Google Scholar] [CrossRef]
- Bekele, D. The Effect of Tillage on Soil Moisture Conservation: A Review. Int. J. Res. Stud. Comput. 2020, 6, 30–41. [Google Scholar]
- Page, K.L.; Dang, Y.P.; Dalal, R.C. The ability of conservation agriculture to conserve soil organic carbon and the subsequent impact on soil physical, chemical, and biological properties and yield. Front. Sustain. Food Syst. 2020, 4, 31. [Google Scholar] [CrossRef]
- Mitchell, J.P.; Carter, L.M.; Reicosky, D.C.; Shrestha, A.; Pettygrove, G.S.; Klonsky, K.M.; Marcum, D.B.; Chessman, D.; Roy, R.; Hogan, P.; et al. A history of tillage in California’s Central Valley. Soil Tillage Res. 2016, 157, 52–64. [Google Scholar] [CrossRef]
- Khurshid, K.; Iqbal, M.; Arif, M.S.; Nawaz, A. Effect of tillage and mulch on soil physical properties and growth of maize. Int. J. Agric. Biol. 2006, 8, 593–596. [Google Scholar]
- Adugna, O. Effects of tillage practices and cropping system on soil physical properties and in-situ water conservation in clay loam of Assosa, Ethiopia. Int. J. Agric. Res. Rev. 2019, 7, 794–802. [Google Scholar]
- Ulrich, S.; Tischer, S.; Hofmann, B.; Christen, O. Biological soil properties in a long-term tillage trial in Germany. J. Plant Nutr. Soil Sci. 2010, 173, 483–489. [Google Scholar] [CrossRef]
- Mohammadi, K.; Heidari, G.; Javaheri, M.; Karimi Nezhad, M.T. Soil microbial response to tillage systems and fertilization in a sunflower rhizosphere. Arch. Geonomy Soil Sci. 2013, 59, 899–910. [Google Scholar] [CrossRef]
- Szostek, M.; Szpunar-Krok, E.; Pawlak, R.; Stanek-Tarkowska, J.; Ilek, A. Effect of different tillage systems on soil organic carbon and enzymatic activity. Agronomy 2022, 12, 208. [Google Scholar] [CrossRef]
- Aiad, M. Productivity of heavy clay soils as affected by some soil amendments combined with irrigation regime. Environ. Biodivers. Soil Secur. 2019, 3, 147–162. [Google Scholar] [CrossRef]
- Duong, T.T.; Penfold, C.; Marschner, P. Amending soils of different texture with six compost types: Impact on soil nutrient availability, plant growth and nutrient uptake. Plant Soil 2012, 354, 197–209. [Google Scholar] [CrossRef]
- Brown, S.; Cotton, M. Changes in soil properties and carbon content following compost application: Results of on-farm sampling. Compost. Sci. Util. 2011, 19, 87–96. [Google Scholar] [CrossRef]
- Chang, E.H.; Wang, C.H.; Chen, C.L.; Chung, R.S. Effects of long-term treatments of different organic fertilizers complemented with chemical N fertilizer on the chemical and biological properties of soils. Soil Sci. Plant. Nutr. 2014, 60, 499–511. [Google Scholar] [CrossRef]
- Omonode, R.A.; Smith, D.R.; Gal, A.; Vyn, T.J. Soil nitrous oxide emissions in corn following three decades of tillage and rotation treatments. Soil Sci. Soc. Am. J. 2011, 75, 152–163. [Google Scholar] [CrossRef]
- Chavarría, D.N.; Verdenelli, R.A.; Serri, D.L.; Restovich, S.B.; Andriulo, A.E.; Meriles, J.M.; Vargas-Gil, S. Effect of cover crops on microbial community structure and related enzyme activities and macronutrient availability. Eur. J. Soil Biol. 2016, 76, 74–82. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, X.; Wang, J.; Gao, L. Soil microbial communities associated with the rhizosphere of cucumber under different summer cover crops and residue management: A 4-year field experiment. Sci. Hortic. 2013, 150, 100–109. [Google Scholar] [CrossRef]
- Capo-Bauca, S.; Marques, A.; Llopis-Vidal, N.; Bota, J.; Baraza, E. Long-term establishment of natural green cover provides agroecosystem services by improving soil quality in a Mediterranean vineyard. Ecol. Eng. 2019, 127, 285–291. [Google Scholar] [CrossRef]
- Saikia, R.; Sharma, S.; Thind, H.S.; Sidhu, H.S.; Yadvinder, S. Temporal changes in biochemical indicators of soil quality in response to tillage, crop residue and green manure management in a rice-wheat system. Ecol. Indic. 2019, 103, 383–394. [Google Scholar] [CrossRef]
- Nunes, M.R.; Karlen, D.L.; Veum, K.S.; Moorman, T.B.; Cambardella, C.A. Biological soil health indicators respond to tillage intensity: A US metaanalysis. Geoderma 2020, 369, 114335. [Google Scholar] [CrossRef]
- Sharma, S.; Dhaliwal, S.S. Conservation agriculture based practices enhanced micronutrients transformation in earthworm cast soil under rice-wheat cropping system. Ecol. Eng. 2021, 163, 106195. [Google Scholar] [CrossRef]
- Wortman, S.E.; Drijber, R.A.; Francis, C.A.; Lindquist, J.L. Arable weeds, cover crops, and tillage drive soil microbial community composition in organic cropping systems. Appl. Soil Ecol. 2013, 72, 232–241. [Google Scholar] [CrossRef]
- Vincent, J.M. A Manual for the Practical Study of Root-Nodule Bacteria; Blackwell: Oxford, UK, 1970. [Google Scholar]
- Döbereiner, J.; Day, J.M. Associative Symbiosis in Tropical Grasses: Characterization of Microorganisms and Dinitrogen Fixing Sites in Sysposium on Nitrogen Fixation; Washington State University Press: Washington, WA, USA, 1976. [Google Scholar]
- Allen, O.N. Experiments in Soil Bacteriology; Wisconsin Univeristy Press: Madison, WI, USA, 1959; p. 202. [Google Scholar]
- Casida, L.E.; Klein, D.A.; Snatoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Pancholy, S.K.; Rice, E.L. Soil enzymes in relation to old succession: Amylase, invertase, cellulose, dehydrogenase and urease. Soil Sci. Soc. Am. Proc. 1973, 37, 47–50. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil enzymes. In Methods of Soil Analysis. Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 903–948. [Google Scholar]
- Page, A.L.R.; Miller, H.; Keeney, D.R. Methods of Soil Analysis. Part 2: Chemical and Microbiological Properties, 2nd ed.; Agronomy Monograph No. 9; ASA, CSSA & SSSA: Madison, WI, USA, 1982. [Google Scholar]
- Campbell, D.J. Determination and use of bulk density in relation to soil compaction. In Soil Compaction in Crop Production; Soane, B.D., van Ouwerkerk, C., Eds.; Elsevier: London, UK; Amsterdam, The Netherlands, 1994. [Google Scholar]
- Garcia, G. Soil Water Engineering Laboratory Manual; Colorado State University Department of Agriculture and Chemical Engineering: Fortcollins, CO, USA, 1978. [Google Scholar]
- Anderson, G.; Pidgeon, J.D.; Spencer, H.B.; Parks, R. A new hand-held recording penetrometer for soil studies. J. Soil Sci. 1980, 31, 279–296. [Google Scholar] [CrossRef]
- Tricker, A.S. The infiltration cylinder: Some comments on its use. J. Hydrol. 1978, 36, 383–391. [Google Scholar] [CrossRef]
- Kemper, W.D.; Rosenau, R.C. Aggregate stability and size distribution. In Methods of Soil Analysis. 1986, Part I (425–442); Klute, A., Campbell, G.S., Jacson, R.D., Mortland, M.M., Nielsen, D.R., Eds.; ASA, CSSA & SSSA: Madison, WI, USA.
- Gomez, K.A.; Gomez, A. Statistical Procedure for Agricultural Research—Hand Book; John Wiley & Sons: New York, NY, USA, 1984. [Google Scholar]
- Nyambo, P.; Thengeni, B.; Chiduza, C.; Araya, T. Tillage, crop rotation, residue management and biochar influence on soil chemical and biological properties. S. Afr. J. Plant Soil 2021, 38, 390–397. [Google Scholar] [CrossRef]
- Minoshima, H.; Jackson, L.E.; Cavagnaro, T.R.; Sánchez-Moreno, S.; Ferris, H.; Temple, S.R.; Goyal, S.; Mitchell, J.P. Soil food webs and carbon dynamics in response to conservation tillage in California. Soil Sci. Soc. Am. J. 2007, 71, 952–963. [Google Scholar] [CrossRef]
- Taylor, T.S.; Titshall, L.W.; Hughes, J.C.; Thibaud, G.R. Effect of tillage systems and nitrogen application rates on selected physical and biological properties of a clay loam soil in KwaZulu-Natal, South Africa. S. Afr. J. Plant Soil 2012, 29, 47–52. [Google Scholar] [CrossRef]
- Xing, P.F.; Wu, X.S.; Gao, S.C.; Li, H.J.; Zhao, T.K.; Zhou, X.L.; Shen, D.L.; Sun, J.D. Effects of different fertilization on soil microbial community and functional diversity in maize-wheat crop rotation. J. Microbiol. 2016, 36, 22–29. [Google Scholar]
- Zhang, D.; Yang, X.; Wang, Y.; Zong, J.; Ma, J.; Li, C. Changes in soil organic carbon fractions and bacterial community composition under different tillage and organic fertiliser application in a maize−wheat rotation system. Acta Agric. Scand. Sect. B Soil Plant Sci. 2020, 70, 457–466. [Google Scholar] [CrossRef]
- Roldán, A.; Caravaca, F.; Hernández, M.T.; Garcıa, C.; Sánchez-Brito, C.; Velásquez, M.; Tiscareno, M. No-tillage, crop residue additions, and legume cover cropping effects on soil quality characteristics under maize in Patzcuaro watershed (Mexico). Soil Tillage Res. 2003, 72, 65–73. [Google Scholar] [CrossRef]
- Dey, J.K.; Saren, B.K.; Debnath, A.; Gudade, B.A.; Singh, S.; Kumar, A.; Palai, J.B.; Gaber, A.; Hossain, A. Productivity and Nutrient Dynamic of Legume in a Maize-legume Cropping System are Influenced by Biomulches Under No-tillage System. Int. J. Plant Prod. 2022, 16, 531–545. [Google Scholar] [CrossRef]
- Chen, C.R.; Condron, L.M.; Davis, M.R.; Sherlock, R.R. Seasonal changes in soil phosphorus and associated microbial properties under adjacent grassland and forest in New Zealand. For. Ecol. Manag. 2003, 177, 539–557. [Google Scholar] [CrossRef]
- Bieli´nska, E.; Mocek-Płóciniak, A. Impact of the Tillage System on the Soil Enzymatic Activity. Arch. Environ. Prot. 2012, 38, 75–82. [Google Scholar] [CrossRef]
- Tomkowiak, A.; Starzyk, J.; Kosicka-Dziechciarek, D.; Karwatka, K. The influence of tillage systems on the microbiological conditions of soil. Nauka Przyr. Technol. 2017, 11, 355–364. [Google Scholar]
- Yang, L.; Li, T.; Li, F.; Lemcoff, J.H.; Cohen, S. Fertilization regulates soil enzymatic activity and fertility dynamics in a cucumber field. Sci. Hortic. 2008, 116, 21–26. [Google Scholar] [CrossRef]
- Shirani, H.; Hajabbasi, M.A.; Afyuni, M.; Hemmat, A. Effects of farmyard manure and tillage systems on soil physical properties and corn yield in central Iran. Soil Tillage Res. 2002, 68, 101–108. [Google Scholar] [CrossRef]
- Shi, Y.; Lalande, R.; Hamel, C.; Ziadi, N.; Gagnon, B.; Hu, Z. Seasonal variation of microbial biomass, activity, and community structure in soil under different tillage and phosphorus management practices. Biol. Fertil. Soils 2013, 49, 803–818. [Google Scholar] [CrossRef]
- Alijani, K.; Bahrani, M.; Kazemeini, A. Short-term responses of soil and wheat yield to tillage, corn residue management and nitrogen fertilization. Soil Tillage Res. 2017, 124, 78–82. [Google Scholar] [CrossRef]
- Perez-Brandán, C.; Arzeno, J.L.; Huidobro, J.; Grümberg, B.; Conforto, C.; Hilton, S.; Bending, G.D.; Meriles, J.M.; Vargas-Gil, S. Long-term effect of tillage systems on soil microbiological, chemical and physical parameters and the incidence of charcoal rot by Macrophomina phaseolina (Tassi) Goid in soybean. Crop Prot. 2012, 40, 73–82. [Google Scholar] [CrossRef]
- Naderi, R.; Ghadiri, H. Nitrogen, manure and municipal waste compost effects on yield and photosynthetic characteristics of corn (Zea mays L.) under weedy conditions. J. Biol. Environ. Sci. 2013, 7, 141–151. [Google Scholar]
- Moinoddini, S.S.; Koocheki, A.; Mahalati, M.N.; Borzooei, A. Tillage and N application effects on crop yield, N uptake and soil properties in a corn-based rotation. Arch. Agron. Soil Sci. 2017, 63, 1150–1162. [Google Scholar] [CrossRef]
- Balesdent, J.; Chenu, C.; Balabane, M. Relationship of soil organic matter dynamics to physical protection and tillage. Soil Tillage Res. 2000, 53, 215–230. [Google Scholar] [CrossRef]
- Ozpinar, S.; Ozpinar, A. Tillage effects on soil properties and maize productivity in western Turkey. Arch. Agron. Soil Sci. 2015, 61, 1029–1040. [Google Scholar] [CrossRef]
- Franchini, J.C.; Crispino, C.C.; Souza, R.A.; Torres, E.; Hungria, M. Microbiological parameters as indicators of soil quality under various soil management and crop rotation systems in southern Brazil. Soil Tillage Res. 2007, 92, 18–29. [Google Scholar] [CrossRef]
- Gil, V.S.; Becker, A.; Oddino, C.; Zuza, M.; Marinelli, A.; March, G. Field trial assessment of biological, chemical, and physical responses of soil to tillage intensity, fertilization, and grazing. Environ. Manag. 2009, 44, 378–386. [Google Scholar]
- Hati, K.M.; Swarup, A.; Dwivedi, A.K.; Misra, A.K.; Bandyopadhyay, K.K. Changes in soil physical properties and organic carbon status at the topsoil horizon of a vertisol of central India after 28 years of continuous cropping, fertilization and manuring. Agric. Ecosyst. Environ. 2007, 119, 127–134. [Google Scholar] [CrossRef]
- Kay, B.D.; VandenBygaart, A.J. Conservation tillage and depth stratification of porosity and soil organic matter. Soil Tillage Res. 2002, 66, 107–118. [Google Scholar] [CrossRef]
- Al-Kaisi, M.M.; Yin, X.; Licht, M.A. Soil carbon and nitrogen changes as affected by tillage system and crop biomass in a cornsoybean rotation. Appl. Soil Ecol. 2005, 30, 174–191. [Google Scholar] [CrossRef]
- Osunbitan, J.A.; Oyedele, D.J.; Adekalu, K.O. Tillage effects on bulk density, hydraulic conductivity and strength of a loamy sand soil in southwestern Nigeria. Soil Tillage Res. 2005, 82, 57–64. [Google Scholar] [CrossRef]
- Ishaq, M.; Ibrahim, M.; Lal, R. Tillage effects on soil properties at different levels of fertilizer application in Punjab, Pakistan. Soil Tillage Res. 2002, 68, 93–99. [Google Scholar] [CrossRef]
- Halvorson, A.D.; Black, A.L.; Krupinsky, J.M.; Merrill, S.D. Dryland winter wheat response to tillage and nitrogen within an annual cropping system. Agron. J. 1999, 91, 702–707. [Google Scholar] [CrossRef]
- Liu, K.; Wiatrak, P. Corn production response to tillage and nitrogen application in dry-land environment. Soil Tillage Res. 2012, 124, 138–143. [Google Scholar] [CrossRef]
- Archer, D.W.; Halvorson, A.D.; Reule, C.A. Economics of irrigated continuous corn under conventional-till and no-till in northern Colorado. Agron J. 2008, 100, 1166–1172. [Google Scholar] [CrossRef]
- Alvaresz, R.; Steinbach, H.S. A review of the effects of tillage systems on some soil physical properties, water content, nitrate availability and crops yield in the Argentine pampas. Soil Tillage Res. 2009, 104, 1–15. [Google Scholar] [CrossRef]
| Soil Depth (cm) | Sand | Silt | Clay | Tx | pH | CaCO3 (%) | EC (dS m−1) | ESP (%) | OM (g Kg−1) |
|---|---|---|---|---|---|---|---|---|---|
| (%) | |||||||||
| 0–20 | 15.65 | 26.31 | 58.04 | clay | 8.02 | 1.85 | 4.12 | 13.11 | 17.4 |
| 20–40 | 18.75 | 25.10 | 56.15 | clay | 8.11 | 1.98 | 4.14 | 13.15 | 16.9 |
| 40–60 | 18.95 | 24.95 | 56.10 | clay | 8.13 | 2.10 | 4.16 | 13.18 | 15.5 |
| Soil Depth (cm) | CEC (cmole kg−1) | FC | WP | AW | TSA | OSA | BD (kg m−3) | PR (Mpa) | IR (cm h−1) |
| (%) | |||||||||
| 0–20 | 31.45 | 44.15 | 21.91 | 22.24 | 37.11 | 19.78 | 1.370 | 1.240 | 0.75 |
| 20–40 | 30.11 | 41.19 | 20.08 | 21.11 | 36.61 | 20.05 | 1.372 | 1.241 | |
| 40–60 | 29.55 | 39.25 | 19.03 | 20.22 | 35.45 | 18.77 | 1.375 | 1.242 | |
| Soil Depth (cm) | TCB | TCF | TCA | DHA (mg TPF g−1 Soil Day−1) | URE (NH4+-N g−1 Soil Day−1) | PHOS (μg pnp g−1 Soil h−1) |
|---|---|---|---|---|---|---|
| 0–20 | 109 × 106 | 66 × 103 | 81 × 105 | 78.92 | 45.28 | 3.19 |
| Parameters | Value |
|---|---|
| pH | 8.26 |
| EC (dS m−1) | 4.55 |
| Organic carbon (%) | 20.71 |
| Bulk density (kg m3) | 560 |
| Moisture content (%) | 37 |
| C/N ratio | 15.11 |
| N (%) | 1.42 |
| P (%) | 0.55 |
| K (%) | 1.73 |
| Germination (%) | 92.45 |
| TCB | 102 × 106 |
| TCF | 34 × 103 |
| TCA | 69 × 105 |
| E. coli | 0.0 |
| Salmonella sp. | 0.0 |
| Shegella sp. | 0.0 |
| Main Plot | Subplot | |||
|---|---|---|---|---|
| Soil Tillage | First Season (Wheat) | Second Season (Cowpea) | Third Season (Wheat) | Soil Supplements |
| ST1 | Tillage | Tillage | Tillage | Control |
| C | ||||
| BM | ||||
| C + BM | ||||
| ST2 | Tillage | Tillage | No-tillage | Control |
| C | ||||
| BM | ||||
| C + BM | ||||
| ST3 | Tillage | No-tillage | No-tillage | Control |
| C | ||||
| BM | ||||
| C + BM | ||||
| ST4 | No-tillage | No-tillage | No-tillage | Control |
| C | ||||
| BM | ||||
| C + BM | ||||
| Treatments | DHA (mg TPF g−1 Soil Day−1) | URA (mg NH4+-N g−1 Soil Day−1) | PHOS (μg pnp g−1 Soil h−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| First Season | Second Season | Third Season | First Season | Second Season | Third Season | First Season | Second Season | Third Season | ||
| ST1 | T1 | 50.00 ± 4.58 l | 58.02 ± 4.62 n | 65.41 ± 6.01 n | 45.33 ± 1.53 k | 50.18 ± 0.58 j | 55.12 ± 0.53 j | 2.65 ± 0.14 n | 3.74 ± 0.15 l | 4.55 ± 0.32 j |
| T2 | 74.00 ± 2.65 k | 80.35 ± 2.65 m | 89.00 ± 2.16 m | 58.33 ± 3.51 j | 62.18 ± 2.52 i | 67.03 ± 2.11 i | 3.03 ± 0.04 m | 4.15 ± 0.14 k | 5.15 ± 0.24 i | |
| T3 | 84.00 ± 4.00 j | 91.68 ± 4.73 l | 100.62 ± 6.87 l | 71.33 ± 3.79 i | 74.84 ± 3.79 h | 79.74 ± 3.95 h | 3.81 ± 0.16 k | 4.95 ± 0.14 i | 5.93 ± 0.14 g | |
| T4 | 80.00 ± 3.00 jk | 86.35 ± 3.00 lm | 96.94 ± 1.00 l | 89.00 ± 3.61 f | 93.51 ± 3.61 e | 98.32 ± 3.46 e | 4.09 ± 0.03 i | 5.23 ± 0.05 g | 6.21 ± 0.15 f | |
| ST2 | T1 | 104.67 ± 5.51 i | 111.35 ± 4.58 k | 118.94 ± 3.99 k | 57.00 ± 2.65 j | 60.18 ± 3.21 i | 64.95 ± 3.31 i | 3.72 ± 0.09 l | 4.81 ± 0.16 j | 5.43 ± 0.37 h |
| T2 | 147.33 ± 5.69 f | 153.68 ± 5.69 h | 162.40 ± 7.09 h | 71.00 ± 2.00 i | 76.18 ± 3.06 h | 81.08 ± 2.82 h | 4.32 ± 0.05 g | 5.44 ± 0.15 f | 6.48 ± 0.12 de | |
| T3 | 169.33 ± 5.51 d | 175.02 ± 5.13 f | 183.46 ± 5.94 f | 82.67 ± 3.21 g | 87.18 ± 3.21 f | 92.02 ± 3.44 f | 4.76 ± 0.14 d | 5.85 ± 0.26 d | 6.85 ± 0.05 b | |
| T4 | 184.33 ± 4.16 c | 190.02 ± 3.79 d | 198.06 ± 3.51 cd | 97.00 ± 2.00 d | 102.18 ± 3.06 d | 107.21 ± 3.20 d | 4.87 ± 0.05 c | 6.09 ± 0.28 bc | 7.13 ± 0.16 a | |
| ST3 | T1 | 122.33 ± 5.51 h | 128.68 ± 5.51 j | 136.79 ± 4.46 j | 68.67 ± 2.52 i | 72.51 ± 1.73 h | 77.44 ± 1.65 h | 3.97 ± 0.04 j | 5.07 ± 0.14 h | 6.12 ± 0.14 fg |
| T2 | 158.33 ± 4.04 e | 164.68 ± 4.04 g | 173.57 ± 5.23 g | 77.67 ± 3.51 h | 82.18 ± 3.51 g | 86.94 ± 3.44 g | 4.53 ± 0.13 f | 5.60 ± 0.18 e | 6.58 ± 0.18 cd | |
| T3 | 176.00 ± 3.61 d | 183.35 ± 2.00 e | 192.06 ± 3.56 de | 92.67 ± 3.51 e | 98.84 ± 4.16 d | 103.62 ± 4.22 d | 4.87 ± 0.09 c | 6.01 ± 0.14 c | 7.00 ± 0.15 ab | |
| T4 | 201.33 ± 3.51 b | 212.35 ± 1.73 b | 220.26 ± 3.34 b | 124.33 ± 3.51 b | 129.84 ± 3.21 b | 134.75 ± 3.60 b | 5.01 ± 0.13 b | 6.10 ± 0.22 bc | 7.13 ± 0.16 a | |
| ST4 | T1 | 134.33 ± 4.16 g | 140.68 ± 4.16 i | 147.61 ± 4.12 i | 76.33 ± 2.08 h | 80.51 ± 2.00 g | 85.49 ± 2.14 g | 4.24 ± 0.15 h | 5.31 ± 0.18 g | 6.32 ± 0.05 ef |
| T2 | 172.00 ± 4.58 d | 178.35 ± 4.58 ef | 186.82 ± 4.71 ef | 89.67 ± 3.06 ef | 94.18 ± 3.06 e | 99.25 ± 2.86 e | 4.69 ± 0.03 e | 5.79 ± 0.14 d | 6.80 ± 0.18 bc | |
| T3 | 190.00 ± 3.61 c | 196.35 ± 3.61 c | 205.19 ± 4.97 c | 106.00 ± 4.36 c | 111.84 ± 4.93 c | 116.53 ± 4.94 c | 5.01 ± 0.15 b | 6.11 ± 0.23 b | 7.11 ± 0.17 a | |
| T4 | 216.00 ± 5.57 a | 224.02 ± 5.51 a | 232.86 ± 6.40 a | 138.00 ± 4.58 a | 143.51 ± 5.29 a | 148.33 ± 5.39 a | 5.16 ± 0.16 a | 6.21 ± 0.13 a | 7.20 ± 0.14 a | |
| F-test | ||||||||||
| Main | ** | ** | ** | ** | ** | ** | ** | ** | ** | |
| Sub main | ** | ** | ** | ** | ** | ** | ** | ** | ** | |
| Interaction | ** | ** | ** | ** | ** | ** | ** | ** | ** | |
| Treatments | ECe (dSm−1) | ESP (%) | |||||
|---|---|---|---|---|---|---|---|
| First Season | Second Season | Third Season | First Season | Second Season | Third Season | ||
| ST1 | T1 | 4.12 ± 0.01 b | 4.19 ± 0.02 c | 4.25 ± 0.02 c | 13.07 ± 0.5 b | 12.46 ± 0.09 c | 11.06 ± 0.03 h |
| T2 | 2.86 ± 0.01 i | 2.88 ± 0.01 m | 2.95 ± 0.02 L | 12.36 ± 0.05 b | 12.01 ± 0.13 e | 11.47 ± 0.16 e | |
| T3 | 3.22 ± 0.02 f | 3.23 ± 0.02 k | 3.21 ± 0.01 j | 12.23 ± 0.03 b | 12.21 ± 0.01 d | 11.85 ± 0.01 c | |
| T4 | 2.45 ± 0.01 j | 2.46 ± 0.01n | 2.49 ± 0.02 n | 10.21 ± 0.10 c | 11.02 ± 0.07 h | 10.59 ± 0.07 i | |
| ST2 | T1 | 4.11 ± 0.01 b | 4.14 ± 0.02 d | 4.23 ± 0.02 c | 13.20 ± 0.09 b | 12.67 ± 0.06 b | 12.11 ± 0.06 b |
| T2 | 2.90 ± 0.01 h | 2.98 ± 0.02 L | 3.19 ± 0.02 k | 12.53 ± 0.05 b | 11.99 ± 0.11 d | 11.14 ± 0.05 g | |
| T3 | 3.21 ± 0.01 f | 3.25 ± 0.02 k | 3.35 ± 0.02 i | 12.25 ± 0.03 b | 12.23 ± 0.01 d | 11.80 ± 0.01 c | |
| T4 | 2.41 ± 0.03 k | 2.47 ± 0.02 n | 2.81 ± 0.02 m | 10.08 ± 0.02 c | 10.79 ± 0.09 i | 9.64 ± 0.11 k | |
| ST3 | T1 | 4.12 ± 0.01 b | 4.30 ± 0.04 b | 4.45 ± 0.02 b | 12.91 ± 0.08 b | 12.15 ± 0.10 d | 11.52 ± 0.01 de |
| T2 | 2.87 ± 0.01 i | 3.70 ± 0.04 h | 3.80 ± 0.04 f | 12.39 ± 0.03 b | 10.36 ± 0.04 j | 9.86 ± 0.04 j | |
| T3 | 3.26 ± 0.02 e | 3.85 ± 0.06 f | 3.92 ± 0.04 e | 12.15 ± 0.02 b | 11.85 ± 0.04 f | 11.40 ± 0.04 f | |
| T4 | 2.41 ± 0.03 k | 3.41 ± 0.02 j | 3.56 ± 0.02 g | 9.97 ± 0.03 c | 8.77 ± 0.03 k | 8.25 ± 0.03 l | |
| ST4 | T1 | 4.26 ± 0.03 a | 4.41 ± 0.06 a | 4.55 ± 0.02 a | 15.08 ± 0.08 a | 14.33 ± 0.20 a | 13.62 ± 0.08 a |
| T2 | 3.31 ± 0.03 d | 3.78 ± 0.02 g | 3.80 ± 0.04 f | 13.07 ± 0.09 b | 11.96 ± 0.04 e | 11.54 ± 0.08 d | |
| T3 | 3.46 ± 0.03 c | 3.95 ± 0.02 e | 4.11 ± 0.02 d | 13.60 ± 0.05 ab | 12.50 ± 0.04 c | 11.81 ± 0.04 c | |
| T4 | 3.12 ± 0.04 g | 3.45 ± 0.04 i | 3.50 ± 0.04 h | 12.32 ± 0.08 b | 11.58 ± 0.15 g | 11.11 ± 0.06 gh | |
| F-test | |||||||
| Main | ** | ** | ** | ** | ** | ** | ** |
| Sub main | ** | ** | ** | ** | ** | ** | ** |
| Interaction | ** | ** | ** | ** | ** | ** | ** |
| Treatments | Soil Bulk Density (kg m−3) | Soil Porosity (%) | |||||
|---|---|---|---|---|---|---|---|
| First Season | Second Season | Third Season | First Season | Second Season | Third Season | ||
| ST1 | T1 | 1.383 ± 0.006 b | 1.372 ± 0.004 c | 1.362 ± 0.004 d | 47.78 ± 0.23 g | 48.22 ± 0.15 f | 48.60 ± 0.15 e |
| T2 | 1.362 ± 0.004 e | 1.352 ± 0.004 e | 1.332 ± 0.004 g | 48.59 ± 0.15 b | 48.97 ± 0.15 d | 49.72 ± 0.15 b | |
| T3 | 1.383 ± 0.006 b | 1.361 ± 0.003 d | 1.352 ± 0.004 e | 47.78 ± 0.23 g | 48.61 ± 0.11 e | 48.98 ± 0.15 d | |
| T4 | 1.333 ± 0.005 g | 1.331 ± 0.002 h | 1.322 ± 0.005 h | 49.69 ± 0.20 b | 49.77 ± 0.07 a | 50.1 ± 0.19 a | |
| ST2 | T1 | 1.381 ± 0.002 c | 1.372 ± 0.005 c | 1.383 ± 0.005 b | 47.88 ± 0.07 f | 48.22 ± 0.20 f | 47.81 ± 0.19 g |
| T2 | 1.371 ± 0.002 d | 1.363 ± 0.005 d | 1.362 ± 0.005 d | 48.26 ± 0.07 d | 48.56 ± 0.20 e | 48.59 ± 0.19 e | |
| T3 | 1.382 ± 0.004 c | 1.372 ± 0.004 c | 1.383 ± 0.005 b | 47.84 ± 0.15 fg | 48.20 ± 0.17 f | 47.81 ± 0.19 g | |
| T4 | 1.331 ± 0.002 gh | 1.333 ± 0.005 g | 1.342 ± 0.005 f | 49.77 ± 0.07 a | 49.69 ± 0.20 b | 49.34 ± 0.18 c | |
| ST3 | T1 | 1.380 ± 0.002 c | 1.392 ± 0.005 a | 1.382 ± 0.005 b | 47.89 ± 0.08 f | 47.47 ± 0.20 h | 47.84 ± 0.15 g |
| T2 | 1.371 ± 0.003 d | 1.362 ± 0.005 d | 1.373 ± 0.005 c | 48.25 ± 0.11 e | 48.59 ± 0.19 e | 48.18 ± 0.20 f | |
| T3 | 1.381 ± 0.003 c | 1.392 ± 0.005 a | 1.382 ± 0.005 b | 47.88 ± 0.12 f | 47.46 ± 0.19 h | 47.83 ± 0.15 g | |
| T4 | 1.332 ± 0.004 gh | 1.341 ± 0.004 f | 1.353 ± 0.004 e | 49.73 ± 0.15 ab | 49.37 ± 0.15c | 48.94 ± 0.20 d | |
| ST4 | T1 | 1.392 ± 0.004 a | 1.393 ± 0.005 a | 1.392 ± 0.005 a | 47.45 ± 0.17 h | 47.43 ± 0.20 h | 47.47 ± 0.15 h |
| T2 | 1.391 ± 0.004 a | 1.393 ± 0.005 b | 1.383 ± 0.005 b | 48.23 ± 0.15 e | 48.18 ± 0.20 f | 47.81 ± 0.19 g | |
| T3 | 1.372 ± 0.004 d | 1.373 ± 0.005 c | 1.383 ± 0.005 b | 47.45 ± 0.17 h | 47.81 ± 0.19 g | 47.81 ± 0.19 g | |
| T4 | 1.351 ± 0.002 f | 1.353 ± 0.005 e | 1.362 ± 0.005 d | 44.02 ± 0.08 c | 48.94 ± 0.20 d | 48.59 ± 0.19 e | |
| F-test | |||||||
| Main | ** | ** | ** | ** | ** | ** | ** |
| Sub main | ** | ** | ** | ** | ** | ** | ** |
| Interaction | ** | ** | ** | ** | ** | ** | ** |
| Treatments | Total Stable Aggregates (%) | Optimal-Sized Aggregates (%) | |||||
|---|---|---|---|---|---|---|---|
| First Season | Second Season | Third Season | First Season | Second Season | Third Season | ||
| ST1 | T1 | 36.39 ± 1.39 fg | 36.91 ± 1.38 f | 37.43 ± 1.37 f | 19.53 ± 1.10 f | 19.79 ± 1.10 h | 20.05 ± 1.01 e |
| T2 | 41.32 ± 1.31 b | 41.88 ± 1.30 b | 42.44 ± 1.29 b | 23.59 ± 1.03 b | 23.87 ± 1.02 b | 24.15 ± 1.01 b | |
| T3 | 37.71 ± 0.48 e | 38.27 ± 0.47 e | 38.83 ± 0.45 e | 20.76 ± 0.40 e | 21.04 ± 0.41 ef | 21.32 ± 0.40 d | |
| T4 | 42.92 ± 1.05 a | 43.55 ± 1.01 a | 44.41 ± 0.98 a | 24.73 ± 0.34 a | 25.10 ± 0.34 a | 25.47 ± 0.31 a | |
| ST2 | T1 | 34.32 ± 1.09 i | 34.84 ± 1.08 h | 35.24 ± 1.02 h | 18.27 ± 1.06 g | 18.53 ± 1.05 i | 18.73 ± 1.03 f |
| T2 | 39.04 ± 1.39 d | 39.60 ± 1.30 d | 39.60 ± 1.28 d | 22.56 ± 1.02 c | 22.84 ± 1.01 c | 22.84 ± 0.98 c | |
| T3 | 35.71 ± 1.45 gh | 36.27 ± 1.40 fg | 36.27 ± 1.35 g | 19.97 ± 1.00 f | 20.25 ± 0.99 gh | 20.25 ± 0.94 e | |
| T4 | 39.91 ± 2.00 c | 40.66 ± 2.0 c | 40.66 ± 1.98 c | 23.48 ± 0.84 b | 23.86 ± 0.81 b | 23.86 ± 0.80 b | |
| ST3 | T1 | 36.31 ± 0.39 fg | 35.91 ± 0.38 g | 35.47 ± 0.39 h | 20.67 ± 0.13 e | 20.47 ± 0.12 fgh | 20.23 ± 0.12 e |
| T2 | 38.74 ± 1.20 d | 38.34 ± 1.11 e | 37.90 ± 1.01 f | 21.81 ± 0.65 d | 21.61 ± 0.61 de | 21.39 ± 0.60 d | |
| T3 | 38.45 ± 0.69 d | 38.05 ± 0.68 e | 37.57 ± 0.66 f | 20.76 ± 1.14 e | 20.56 ± 1.11 fg | 20.34 ± 1.01 e | |
| T4 | 38.90 ± 2.31 d | 38.50 ± 2.28 e | 38.02 ± 2.18 f | 21.99 ± 0.79 d | 21.79 ± 0.75 d | 21.55 ± 0.74 d | |
| ST4 | T1 | 30.06 ± 1.21 k | 29.66 ± 1.12 j | 29.22 ± 1.01 k | 15.87 ± 1.12 h | 15.67 ± 1.01 k | 15.45 ± 0.98 h |
| T2 | 35.10 ± 2.41 h | 34.70 ± 2.31 h | 34.26 ± 2.01 i | 20.53 ± 1.85 e | 20.33 ± 1.83 gh | 20.11 ± 1.80 e | |
| T3 | 32.12 ± 1.27 j | 31.72 ± 1.25 i | 31.28 ± 1.04 j | 17.83 ± 0.44 g | 17.63 ± 0.43 j | 17.41 ± 0.41 g | |
| T4 | 36.71 ± 2.04 f | 36.31 ± 1.95 fg | 35.87 ± 1.41 gh | 21.67 ± 0.66 d | 21.47 ± 0.65 de | 21.25 ± 0.64 d | |
| F-test | |||||||
| Main | ** | ** | ** | ** | ** | ** | ** |
| Sub main | ** | ** | ** | ** | ** | ** | ** |
| Interaction | ** | ** | ** | ** | ** | ** | ** |
| Treatments | SPR (Mpa) | IR (cmh−1) | |||||
|---|---|---|---|---|---|---|---|
| First Season | Second Season | Third Season | First Season | Second Season | Third Season | ||
| ST1 | T1 | 1.242 ± 0.004 a | 1.131 ± 0.003 h | 1.060 ± 0.020 l | 0.753 ± 0.004 g | 0.761 ± 0.003 e | 0.782 ± 0.004 f |
| T2 | 1.130 ± 0.020 d | 0.983 ± 0.005 l | 0.892 ± 0.004 m | 0.853 ± 0.005 c | 0.862 ± 0.004 b | 0.912 ± 0.004 c | |
| T3 | 1.231 ± 0.002 a | 1.122 ± 0.005 i | 1.050 ± 0.010 l | 0.763 ± 0.005 f | 0.761 ± 0.004 e | 0.793 ± 0.005 e | |
| T4 | 1.050 ± 0.020 f | 0.952 ± 0.004 m | 0.801 ± 0.002 n | 0.982 ± 0.004 b | 1.016 ± 0.041 a | 1.086 ± 0.011 a | |
| ST2 | T1 | 1.241 ± 0.004 a | 1.184 ± 0.007 e | 1.243 ± 0.005 f | 0.753 ± 0.004 g | 0.761 ± 0.003 e | 0.752 ± 0.005 h |
| T2 | 1.151 ± 0.101 d | 1.073 ± 0.010 j | 1.173 ± 0.005 j | 0.853 ± 0.005 c | 0.862 ± 0.004 b | 0.853 ± 0.005 d | |
| T3 | 1.231 ± 0.003 a | 1.173 ± 0.005 f | 1.232 ± 0.003 g | 0.763 ± 0.005 f | 0.761 ± 0.003 e | 0.761 ± 0.003 g | |
| T4 | 1.050 ± 0.020 f | 1.040 ± 0.020 k | 1.124 ± 0.007 k | 0.982 ± 0.004 b | 1.016 ± 0.041 a | 0.982 ± 0.004 b | |
| ST3 | T1 | 1.241 ± 0.002 a | 1.213 ± 0.006 d | 1.293 ± 0.005 c | 0.752 ± 0.005 g | 0.702 ± 0.004 f | 0.683 ± 0.005 j |
| T2 | 1.150 ± 0.103 cd | 1.133 ± 0.005 h | 1.222 ± 0.004 h | 0.853 ± 0.005 c | 0.782 ± 0.004 d | 0.753 ± 0.005 h | |
| T3 | 1.231 ± 0.004 a | 1.223 ± 0.005 c | 1.255 ± 0.006 e | 0.752 ± 0.005 g | 0.702 ± 0.004 f | 0.682 ± 0.004 j | |
| T4 | 1.050 ± 0.020 ef | 1.123 ± 0.005 i | 1.192 ± 0.004 i | 0.993 ± 0.005 a | 0.803 ± 0.005 c | 0.782 ± 0.004 f | |
| ST4 | T1 | 1.180 ± 0.020 bc | 1.232 ± 0.004 b | 1.334 ± 0.007 a | 0.702 ± 0.004 h | 0.683 ± 0.005 g | 0.653 ± 0.005 l |
| T2 | 1.123 ± 0.006 d | 1.183 ± 0.007 e | 1.273 ± 0.005 d | 0.782 ± 0.004 e | 0.753 ± 0.005 e | 0.712 ± 0.005 i | |
| T3 | 1.186 ± 0.010 b | 1.253 ± 0.005 a | 1.323 ± 0.008 b | 0.702 ± 0.004 h | 0.683 ± 0.005 g | 0.663 ± 0.005 k | |
| T4 | 1.089 ± 0.011 e | 1.152 ± 0.004 g | 1.253 ± 0.006 e | 0.803 ± 0.005 d | 0.781 ± 0.002 d | 0.752 ± 0.004 h | |
| F-test | |||||||
| Main | ** | ** | ** | ** | ** | ** | ** |
| Sub main | ** | ** | ** | ** | ** | ** | ** |
| Interaction | ** | ** | ** | ** | ** | ** | ** |
| Treatments | First Season (Wheat, Ton ha−1) | Second Season (Cowpea, Ton ha−1) | Third Season (Wheat, Ton ha−1) | ||||
|---|---|---|---|---|---|---|---|
| Grain | Straw | Grain | Straw | Grain | Straw | ||
| ST1 | T1 | 4.897 ± 0.003 g | 4.920 ± 0.010 i | 3.816 ± 0.022 i | 4.017 ± 0.012 g | 5.171 ± 0.004 j | 5.177 ± 0.011 k |
| T2 | 4.935 ± 0.005 d | 5.252 ± 0.016 c | 4.106 ± 0.008 c | 4.462 ± 0.012 e | 5.214 ± 0.007 f | 5.220 ± 0.005 g | |
| T3 | 4.922 ± 0.008 e | 5.065 ± 0.010 g | 3.940 ± 0.004 e | 4.335 ± 0.014 f | 5.197 ± 0.003 g | 5.202 ± 0.006 i | |
| T4 | 4.983 ± 0.007 b | 5.313 ± 0.004 a | 3.861 ± 0.010 g | 3.645 ± 0.008 i | 5.266 ± 0.004 c | 5.309 ± 0.006 c | |
| ST2 | T1 | 4.892 ± 0.003 g | 4.920 ± 0.010 i | 3.816 ± 0.027 i | 4.0128 ± 0.016 h | 4.857 ± 0.003 l | 4.943 ± 0.007 l |
| T2 | 4.937 ± 0.008 d | 5.248 ± 0.004 c | 4.099 ± 0.010 d | 4.461 ± 0.016 d | 5.230 ± 0.005 e | 5.242 ± 0.007 f | |
| T3 | 4.920 ± 0.010 e | 5.061 ± 0.020 g | 3.926 ± 0.006 f | 4.329 ± 0.004 f | 5.183 ± 0.007 h | 5.215 ± 0.004 h | |
| T4 | 4.990 ± 0.060 a | 5.288 ± 0.004 b | 4.11 ± 0.012 b | 4.507 ± 0.004 c | 5.259 ± 0.001 d | 5.283 ± 0.007 d | |
| ST3 | T1 | 4.893 ± 0.006 g | 4.924 ± 0.012 i | 3.232 ± 0.010 l | 3.468 ± 0.008 j | 4.619 ± 0.006 m | 4.634 ± 0.005 m |
| T2 | 4.937 ± 0.003 d | 5.233 ± 0.012 d | 3.926 ± 0.020 f | 4.351 ± 0.012 e | 5.196 ± 0.004 g | 5.245 ± 0.005 e | |
| T3 | 4.922 ± 0.008 e | 5.059 ± 0.004 g | 3.837 ± 0.006 h | 4.015 ± 0.006 gh | 5.181 ± 0.004 hi | 5.214 ± 0.006 h | |
| T4 | 4.993 ± 0.006 a | 5.286 ± 0.008 b | 4.186 ± 0.006 a | 4.788 ± 0.006 a | 5.306 ± 0.003 b | 5.346 ± 0.004 a | |
| ST4 | T1 | 4.123 ± 0.007 h | 4.269 ± 0.012 j | 3.357 ± 0.004 k | 3.367 ± 0.006 m | 4.400 ± 0.010 n | 4.450 ± 0.005 n |
| T2 | 4.954 ± 0.006 c | 5.133 ± 0.014 f | 3.840 ± 0.010 h | 3.528 ± 0.008 j | 5.180 ± 0.005 i | 5.220 ± 0.005 g | |
| T3 | 4.907 ± 0.003 f | 5.049 ± 0.004 h | 3.782 ± 0.005 j | 3.489 ± 0.004 l | 5.156 ± 0.010 k | 5.189 ± 0.001 j | |
| T4 | 4.991 ± 0.004 a | 5.214 ± 0.012 e | 4.188 ± 0.010 a | 4.513 ± 0.008 b | 5.325 ± 0.005 a | 5.338 ± 0.002 b | |
| F-test | |||||||
| Main | ** | ** | ** | ** | ** | ** | ** |
| Sub main | ** | ** | ** | ** | ** | ** | ** |
| Interaction | ** | ** | ** | ** | ** | ** | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amer, M.M.; Elbagory, M.; Aiad, M.; Omara, A.E.-D. The Influence of Short-Term Tillage, Compost, and Beneficial Microbes on Soil Properties and the Productivity of Wheat and Cowpea Crops. Agriculture 2023, 13, 1857. https://doi.org/10.3390/agriculture13101857
Amer MM, Elbagory M, Aiad M, Omara AE-D. The Influence of Short-Term Tillage, Compost, and Beneficial Microbes on Soil Properties and the Productivity of Wheat and Cowpea Crops. Agriculture. 2023; 13(10):1857. https://doi.org/10.3390/agriculture13101857
Chicago/Turabian StyleAmer, Megahed M., Mohssen Elbagory, Mahmoud Aiad, and Alaa El-Dein Omara. 2023. "The Influence of Short-Term Tillage, Compost, and Beneficial Microbes on Soil Properties and the Productivity of Wheat and Cowpea Crops" Agriculture 13, no. 10: 1857. https://doi.org/10.3390/agriculture13101857
APA StyleAmer, M. M., Elbagory, M., Aiad, M., & Omara, A. E.-D. (2023). The Influence of Short-Term Tillage, Compost, and Beneficial Microbes on Soil Properties and the Productivity of Wheat and Cowpea Crops. Agriculture, 13(10), 1857. https://doi.org/10.3390/agriculture13101857






