Bioactive Compounds in Malanto (Kalimeris indica) Leaves and Their Antioxidant Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Samples
2.3. Extraction of Total Flavonoids from Malanto
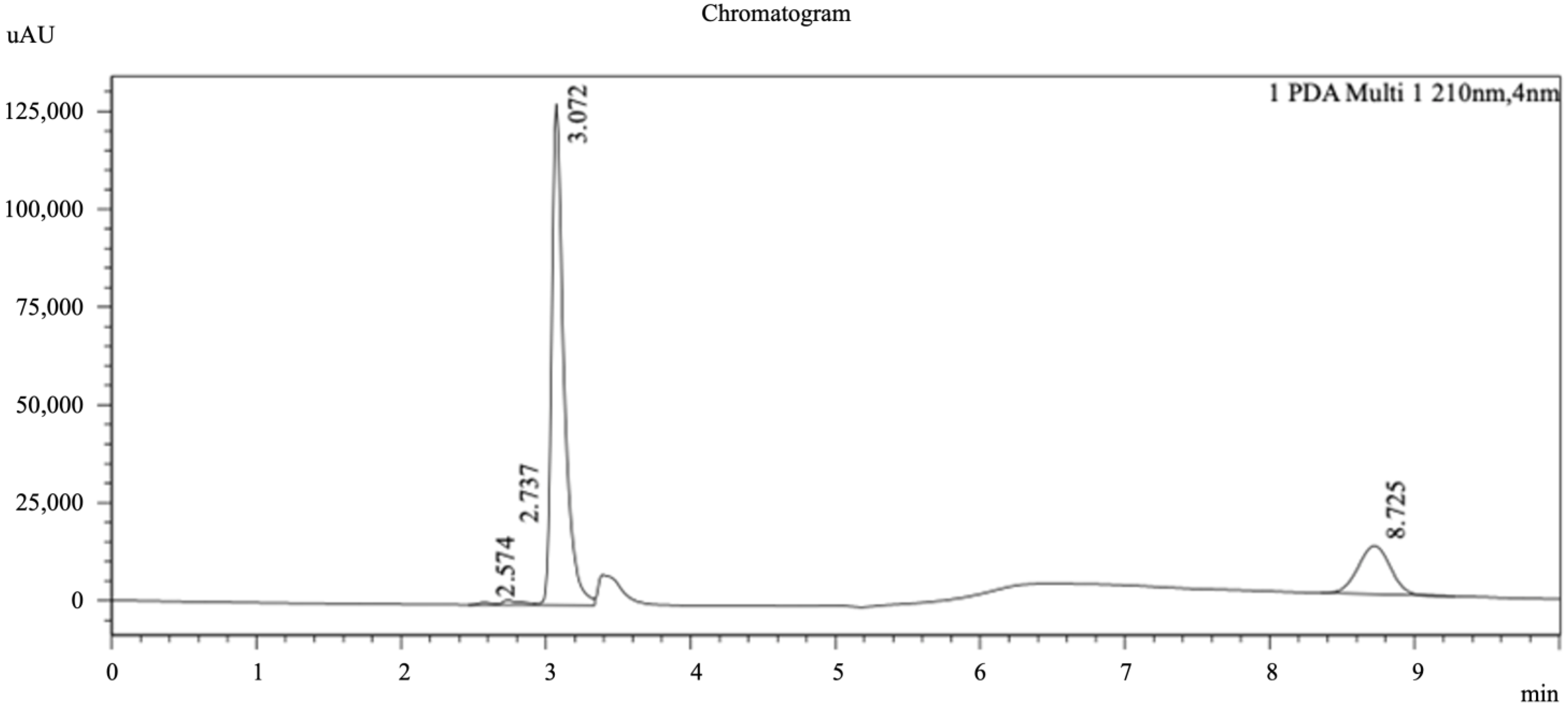
2.4. HPLC Photo-Diode Array (PDA) Chromatogram
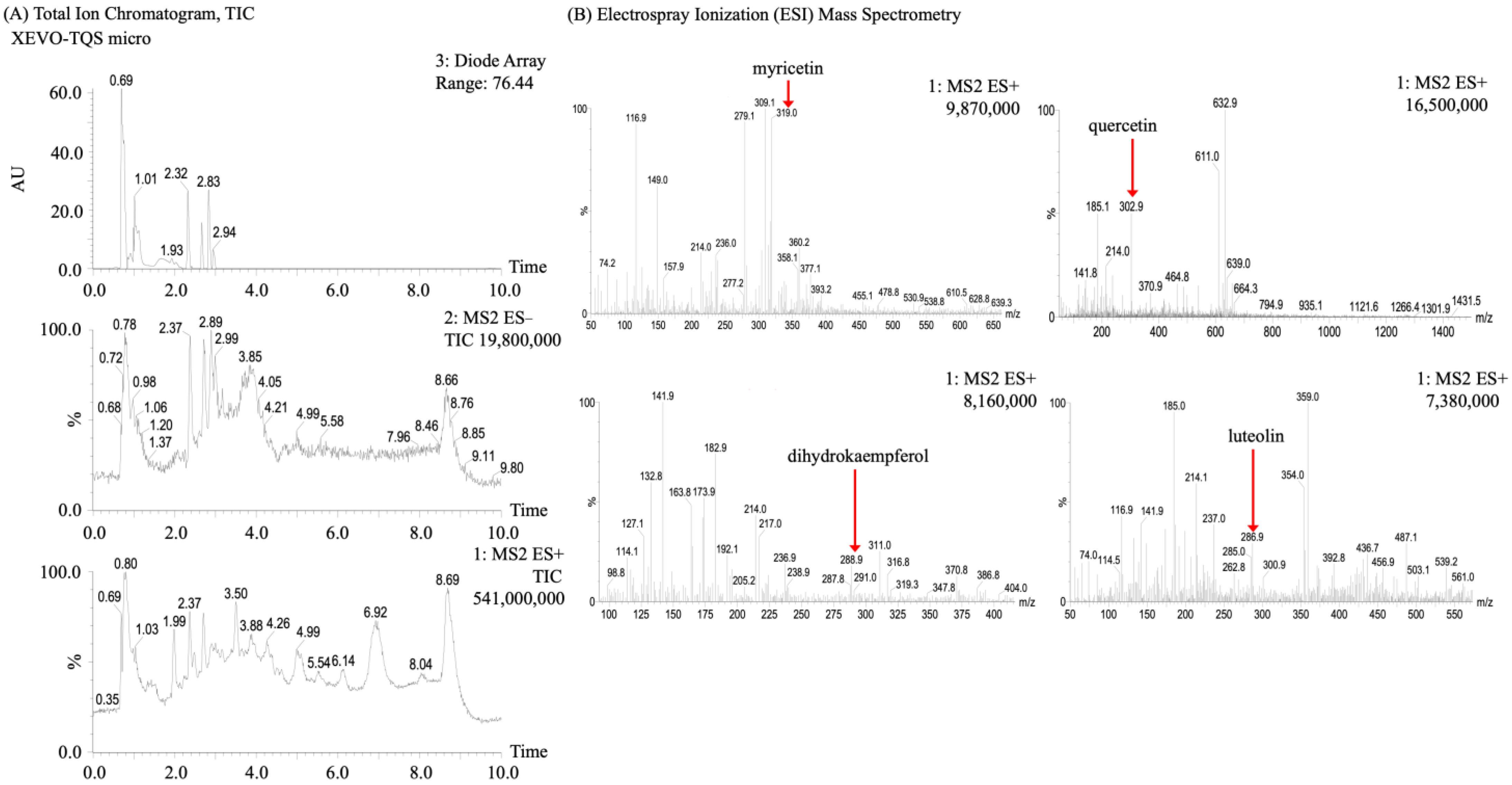
2.5. LC-MS Electron Spray Ionization (ESI+)
2.6. Quantification of Total Flavonoids from Malanto by HPLC
2.7. Molecular Weight Determination of Malanto Extract
2.8. Determination of Total Flavonoid Content (TFC)
2.9. Measured DPPH Free Radical Scavenging Ability
2.10. Analysis of Hydrogen Peroxide Scavenging Capacity
2.11. Determination of ·OH Radical Scavenging Ability
2.12. Measurement of Reducing Power
2.13. Oxidative Stability
2.14. Statistical Analysis
3. Results
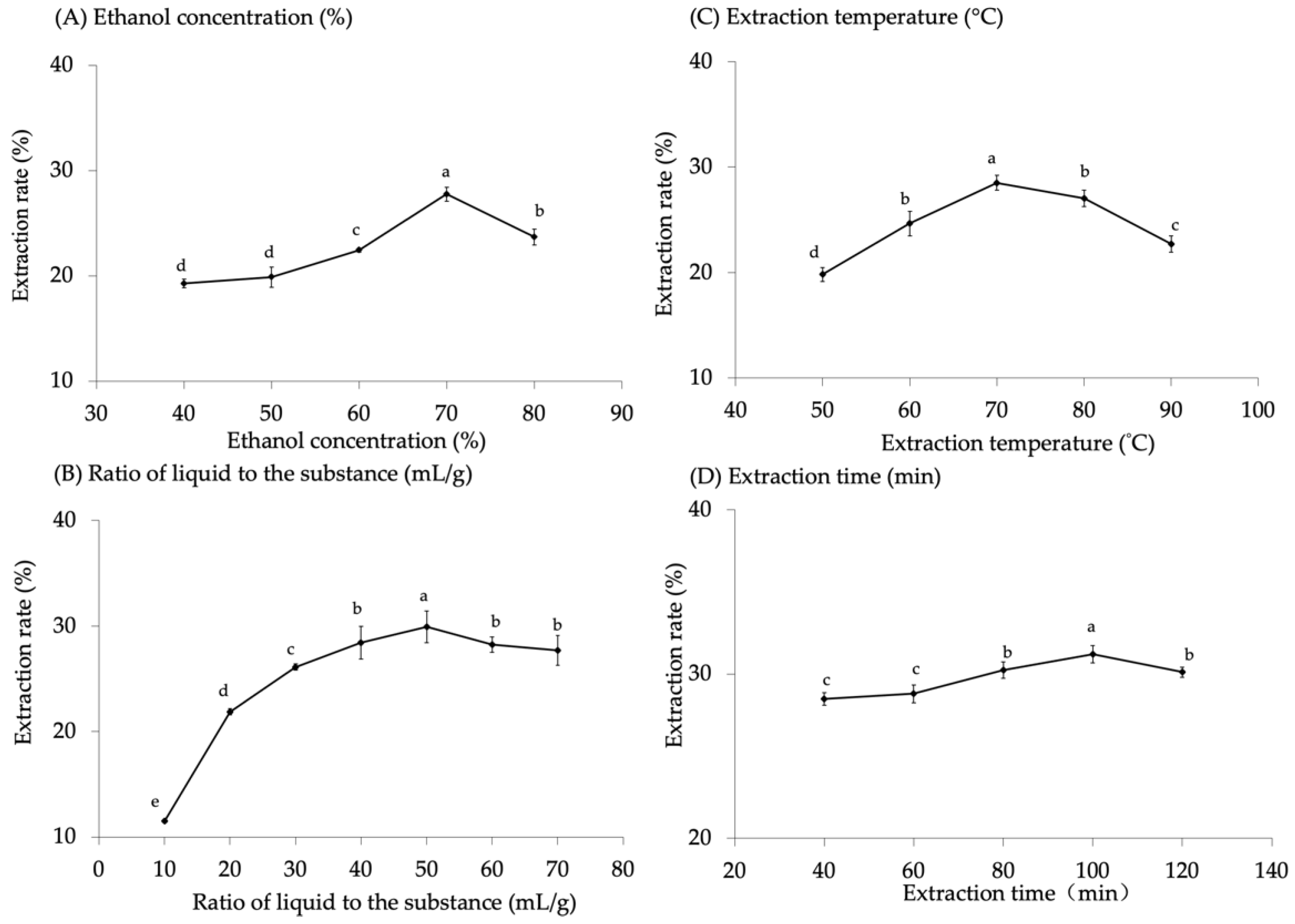
3.1. Extraction Factor Results of Total Flavonoids from Malanto
3.2. Analysis of Flavonoids in Malanto
3.2.1. Qualitative Analysis of Flavonoids in Malanto
3.2.2. Flavonoid Content and Molecular Weight Distribution in Malanto
3.3. In Vitro Antioxidant Capacity of Bioactive Properties
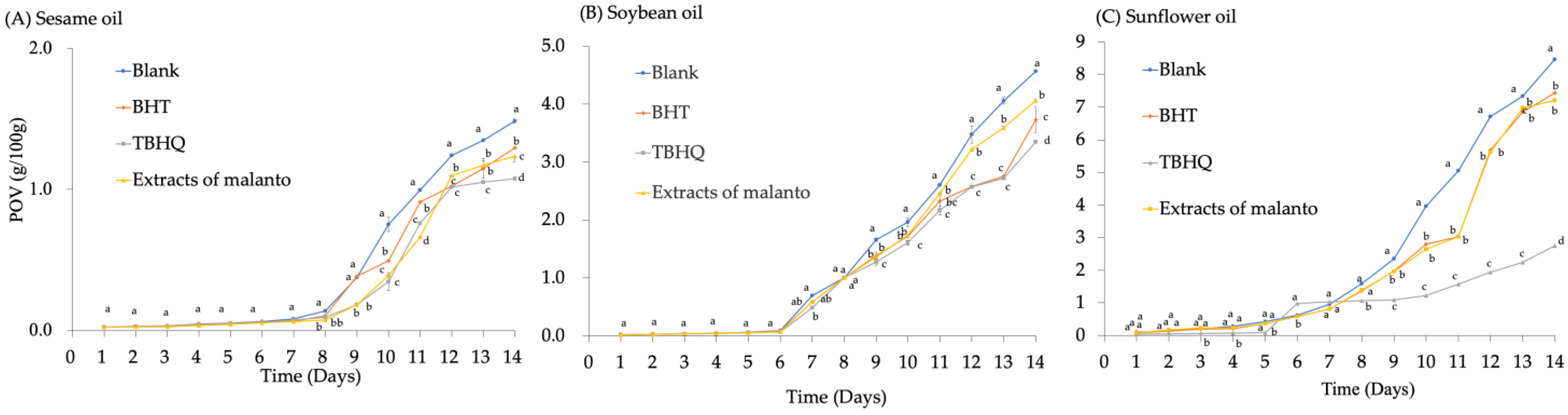
3.4. Effect of Malanto Crude Extracts on Oxidative Stability
4. Conclusions
Highlights
- Natural antioxidants are popular due to their apparent effects and high safety.
- The antioxidant effect of malanto on edible oils was similar to that of BHT.
- The addition of malanto at 0.02% provided an antioxidant effect to edible oils.
- The simple extraction process obtained flavonoids from malanto.
- Malanto flavonoids retard the autoxidation of edible oils for 6–8 days.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Xie, L.; Lin, Q.; Guo, K.; Tong, C.; Shi, S.; Shi, F. HPLC-DAD-QTOF-MS/MS based comprehensive metabolomic profiling of phenolic compounds in Kalimeris indica anti-inflammatory fractions. Ind. Crop. Prod. 2019, 140, 111636. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Huang, P.-H.; Tsai, Y.-H.; Jiang, C.-M.; Hou, C.-Y. Effects of limonene on the PAHS mutagenicity risk in roasted fish skin. J. Food Process. Technol. 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Franco-Vega, A.; Reyes-Jurado, F.; Cardoso-Ugarte, G.A.; Sosa-Morales, M.E.; Palou, E.; López-Malo, A. Chapter 89—Sweet orange (Citrus sinensis) oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 783–790. [Google Scholar]
- Martínez, L.; Bastida, P.; Castillo, J.; Ros, G.; Nieto, G. Green alternatives to synthetic antioxidants, antimicrobials, nitrates, and nitrites in clean label spanish chorizo. Antioxidants 2019, 8, 184. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Q.; Si, F.; Wu, C.; Wang, J.; Zhang, A.; Tao, J.; Zhang, L.; Liu, Y.; Feng, Z. Transcriptome sequencing and flavonoid metabolism analysis in the leaves of three different cultivars of Acer truncatum. Plant Physiol. Biochem. 2021, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, C.; Huang, Z.; Lyu, L.; Li, W.; Wu, W. Integrative analysis of the metabolome and transcriptome provides insights into the mechanisms of flavonoid biosynthesis in blackberry. Food Res. Int. 2022, 153, 110948. [Google Scholar] [CrossRef]
- Rha, C.-S.; Jeong, H.W.; Park, S.; Lee, S.; Jung, Y.S.; Kim, D.-O. Antioxidative, anti-inflammatory, and anticancer effects of purified flavonol glycosides and aglycones in green tea. Antioxidants 2019, 8, 278. [Google Scholar] [CrossRef]
- Zhang, X.-K.; Lan, Y.-B.; Huang, Y.; Zhao, X.; Duan, C.-Q. Targeted metabolomics of anthocyanin derivatives during prolonged wine aging: Evolution, color contribution and aging prediction. Food Chem. 2020, 339, 127795. [Google Scholar] [CrossRef]
- Khan, W.A.; Hu, H.; Cuin, T.A.; Hao, Y.; Ji, X.; Wang, J.; Hu, C. Untargeted metabolomics and comparative flavonoid analysis reveal the nutritional aspects of pak choi. Food Chem. 2022, 383, 132375. [Google Scholar] [CrossRef]
- Moura, C.F.H.; Oliveira, L.S.; de Souza, K.O.; da Franca, L.G.; Ribeiro, L.B.; de Souza, P.A.; de Miranda, M.R.A. Acerola—Malpighia emarginata. In Exotic Fruits; Rodrigues, S., de Oliveira Silva, E., de Brito, E.S., Eds.; Academic Press: San Diego, CA, USA, 2018; pp. 7–14. [Google Scholar]
- Zou, Q.; Feng, J.; Li, T.; Cheng, G.; Wang, W.; Rao, G.; He, H.; Li, Y. Antioxidation and anti-inflammatory actions of the extract of Nitraria Tangutorum Bobr. fruits reduce the severity of ulcerative colitis in a dextran sulphate sodium-induced mice model. J. Funct. Foods 2022, 91, 105005. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, Y.; Zhao, Y.; Hong, Y.; Cai, S.; Pang, M. Phenolic composition, antioxidant and pancreatic lipase inhibitory activities of Chinese sumac (Rhus chinensis Mill.) fruits extracted by different solvents and interaction between myricetin-3-O-rhamnoside and quercetin-3-O -rhamnoside. Int. J. Food Sci. Technol. 2017, 53, 1045–1053. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.K.S.; Barros, R.G.C.; Pereira, U.C.; Nogueira, J.P.; Gualberto, N.C.; de Oliveira, C.S.; Shanmugam, S.; Narain, N. Bioaccessibility of bioactive compounds after in vitro gastrointestinal digestion and probiotics fermentation of Brazilian fruits residues with antioxidant and antidiabetic potential. LWT 2022, 153, 112469. [Google Scholar] [CrossRef]
- García-Lafuente, A.; Guillamón, E.; Villares, A.; Rostagno, M.A.; Martínez, J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef]
- Victoria-Campos, C.I.; Ornelas-Paz, J.D.J.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Failla, M.L.; Pérez-Martínez, J.D.; Rios-Velasco, C.; Ibarra-Junquera, V. Gastrointestinal metabolism and bioaccessibility of selected anthocyanins isolated from commonly consumed fruits. Food Chem. 2022, 383, 132451. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Nunes, A.R.; Flores-Félix, J.D.; Alves, G.; Silva, L.R. Cherries and blueberries-based beverages: Functional foods with antidiabetic and immune booster properties. Molecules 2022, 27, 3294. [Google Scholar] [CrossRef]
- Manasa, V.; Tumaney, A.W. Evaluation of the anti-dyslipidemic effect of spice fixed oils in the in vitro assays and the high fat diet-induced dyslipidemic mice. Food Biosci. 2022, 46, 101574. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Q.; Zhang, C.; Ni, L. Comparative study of the anti-obesity and gut microbiota modulation effects of green tea phenolics and their oxidation products in high-fat-induced obese mice. Food Chem. 2021, 367, 130735. [Google Scholar] [CrossRef]
- Chen, M.-L.; Yang, D.-J.; Liu, S.-C. Effects of drying temperature on the flavonoid, phenolic acid and antioxidative capacities of the methanol extract of citrus fruit (Citrus sinensis (L.) Osbeck) peels. Int. J. Food Sci. Technol. 2011, 46, 1179–1185. [Google Scholar] [CrossRef]
- Echegaray, N.; Pateiro, M.; Nieto, G.; Rosmini, M.R.; Munekata, P.E.S.; Sosa-Morales, M.E.; Lorenzo, J.M. Chapter 7—Lipid oxidation of vegetable oils. In Food Lipids; Lorenzo, J.M., Munekata, P.E.S., Pateiro, M., Barba, F.J., Domínguez, R., Eds.; Academic Press: San Diego, CA, USA, 2022; pp. 127–152. [Google Scholar]
- Galano, J.-M.; Lee, Y.Y.; Durand, T.; Lee, J.C.-Y. ecial issue on “analytical methods for oxidized biomolecules and antioxidants” the use of isoprostanoids as biomarkers of oxidative damage, and their role in human dietary intervention studies. Free. Radic. Res. 2015, 49, 583–598. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Wang, G.-K.; Jin, W.-F.; Zhang, N.; Wang, G.; Cheng, Y.-Y.; Morris-Natschke, S.L.; Goto, M.; Zhou, Z.-Y.; Liu, J.-S.; Lee, K.-H. Kalshiolin A, new lignan from Kalimeris shimadai. J. Asian Nat. Prod. Res. 2019, 22, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-K.; Zhang, N.; Yao, J.-N.; Yu, Y.; Wang, G.; Hung, C.-C.; Cheng, Y.-Y.; Morris-Natschke, S.L.; Zhou, Z.-Y.; Liu, J.-S.; et al. Kalshinoids A–F, Anti-inflammatory Sesquiterpenes from Kalimeris shimadae. J. Nat. Prod. 2019, 82, 3372–3378. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, N.; Wang, Y.; Liu, J.; Wang, G.; Zhou, Z.; Lu, C.; Yang, J. The hepatoprotective activities of Kalimeris indica ethanol extract against liver injury in vivo. Food Sci. Nutr. 2019, 7, 3797–3807. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.-F.; Xu, G.-B.; Wang, Z.; Wang, A.-M.; Guan, H.-Y.; Li, J.; He, X.; Liu, J.-H.; Zhou, M.; Li, Y.-J.; et al. Identification of anti-inflammatory constituents from Kalimeris indica with UHPLC-ESI-Q-TOF-MS/MS and GC–MS. J. Ethnopharmacol. 2015, 165, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-K.; Yu, Y.; Wang, Z.; Cai, B.-X.; Zhou, Z.-Y.; Wang, G.; Liu, J.-S. Two new terpenoids from Kalimeris indica. Nat. Prod. Res. 2017, 31, 2348–2353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Jin, W.-F.; Sun, Y.-P.; Wang, G.; Yu, Y.; Zhou, Z.-Y.; Wang, G.-K.; Liu, J.-S. Indole and flavonoid from the herbs of Kalimeris shimadai. Phytochem. Lett. 2018, 28, 135–138. [Google Scholar] [CrossRef]
- Wang, G.-K.; Wang, Z.; Yu, Y.; Zhang, N.; Zhou, Z.-Y.; Wang, G.; Liu, J.-S. A new sesquiterpene from Kalimeris integrifolia. Nat. Prod. Res. 2017, 32, 1004–1009. [Google Scholar] [CrossRef]
- Figueirinha, A.; Paranhos, A.; Pérez-Alonso, J.J.; Santos-Buelga, C.; Batista, M.T. Cymbopogon citratus leaves: Characterization of flavonoids by HPLC–PDA–ESI/MS/MS and an approach to their potential as a source of bioactive polyphenols. Food Chem. 2008, 110, 718–728. [Google Scholar] [CrossRef]
- Ibrahime Sinan, K.; Aktumsek, A.; de la Luz Cádiz-Gurrea, M.; Leyva-Jiménez, F.J.; Fernández-Ochoa, Á.; Segura-Carretero, A.; Glamocilja, J.; Sokovic, M.; Nenadić, M.; Zengin, G. A prospective of multiple biopharmaceutical activities of procyanidins-rich uapaca togoensis pax extracts: HPLC-ESI-TOF-MS coupled with bioinformatics analysis. Chem. Biodivers. 2021, 18, e2100299. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, Y.; Jiang, Z.; Hu, C.; Gao, Y.; Zhou, Q. Efficacy of total flavonoids of Rhizoma drynariae on the blood vessels and the bone graft in the induced membrane. Phytomedicine 2022, 99, 153995. [Google Scholar] [CrossRef]
- Choi, K.-C.; Son, Y.-O.; Hwang, J.-M.; Kim, B.-T.; Chae, M.; Lee, J.-C. Antioxidant, anti-inflammatory and anti-septic potential of phenolic acids and flavonoid fractions isolated from Lolium multiflorum. Pharm. Biol. 2016, 55, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Obmann, A.; Zehl, M.; Purevsuren, S.; Narantuya, S.; Reznicek, G.; Kletter, C.; Glasl, S. Quantification of flavonoid glycosides in an aqueous extract from the traditional mongolian medicinal plant dianthus versicolorfisch. J. Sep. Sci. 2011, 34, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.-H.; Cheng, Y.-T.; Chan, Y.-J.; Lu, W.-C.; Li, P.-H. Effect of heat treatment on nutritional and chromatic properties of mung bean (Vigna radiata L.). Agronomy 2022, 12, 1365. [Google Scholar] [CrossRef]
- Huang, P.-H.; Lu, H.-T.; Wang, Y.-T.; Wu, M.-C. Antioxidant activity and emulsion-stabilizing effect of pectic enzyme treated pectin in soy protein isolate-stabilized Oil/Water emulsion. J. Agric. Food Chem. 2011, 59, 9623–9628. [Google Scholar] [CrossRef] [PubMed]
- James, A.; Yao, T.; Ma, G.; Gu, Z.; Cai, Q.; Wang, Y. Effect of hypobaric storage on Northland blueberry bioactive compounds and antioxidant capacity. Sci. Hortic. 2021, 291, 110609. [Google Scholar] [CrossRef]
- Boulebd, H. Radical scavenging behavior of butylated hydroxytoluene against oxygenated free radicals in physiological environments: Insights from DFT calculations. Int. J. Chem. Kinet. 2021, 54, 50–57. [Google Scholar] [CrossRef]
- Li, Q.; Li, H.-T.; Bai, Y.-P.; Zhu, K.-R.; Huang, P.-H. Effect of thermal treatment on the physicochemical, ultrastructural, and antioxidant characteristics of Euryale ferox seeds and flour. Foods 2022, 11, 2404. [Google Scholar] [CrossRef]
- Crowe, T.D.; White, P.J. Adaptation of the AOCS official method for measuring hydroperoxides from small-scale oil samples. J. Am. Oil Chem. Soc. 2001, 78, 1267–1269. [Google Scholar] [CrossRef]
- Lu, W.-C.; Chiu, C.-S.; Hsieh, C.-W.; Chan, Y.-J.; Liang, Z.-C.; Wang, C.-C.R.; Mulio, A.T.; Le, D.H.T.; Li, P.-H. Calcined Oyster Shell powder as a natural preservative for maintaining quality of white shrimp (Litopenaeus vannamei). Biology 2022, 11, 334. [Google Scholar] [CrossRef]
- Anouar, E.H.; Gierschner, J.; Duroux, J.-L.; Trouillas, P. UV/Visible spectra of natural polyphenols: A time-dependent density functional theory study. Food Chem. 2012, 131, 79–89. [Google Scholar] [CrossRef]
- Mabry, T.J.; Markham, K.R.; Thomas, M.B. The ultraviolet spectra of flavones and flavonols. In The Systematic Identification of Flavonoids; Mabry, T.J., Markham, K.R., Thomas, M.B., Eds.; Springer: Berlin/Heidelberg, Germany, 1970; pp. 41–164. [Google Scholar]
- Liu, R.; Cai, Z.; Xu, B. Characterization and quantification of flavonoids and saponins in adzuki bean (Vigna angularis L.) by HPLC–DAD–ESI–MSn analysis. Chem. Central J. 2017, 11, 93. [Google Scholar] [CrossRef]
- Cai, J.; Sweeney, A.M. The Proof Is in the Pidan: Generalizing Proteins as Patchy Particles. ACS Central Sci. 2018, 4, 840–853. [Google Scholar] [CrossRef] [PubMed]
- Monobe, M.; Nomura, S.; Ema, K.; Matsunaga, A.; Nesumi, A.; Yoshida, K.; Maeda-Yamamoto, M.; Horie, H. Quercetin Glycosides-rich Tea Cultivars (Camellia sinensis L.) in Japan. Food Sci. Technol. Res. 2015, 21, 333–340. [Google Scholar] [CrossRef]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A dietary molecule with diverse biological activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wen, Z.; Wang, Q.; Chen, R.; Li, Z.; Feng, Y.; Yang, S. The chemical profile of active fraction of Kalimeris indica and its quantitative analysis. Biomed. Chromatogr. 2020, 34, e4828. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.-W.; Zhu, B.T. Myricetin and quercetin are naturally occurring co-substrates of cyclooxygenases in vivo. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Ma, R.; Chen, L.; Shi, S.; Cai, P.; Zhang, S.; Xiang, H. Antioxidant profiling of vine tea (Ampelopsis grossedentata): Off-line coupling heart-cutting HSCCC with HPLC–DAD–QTOF-MS/MS. Food Chem. 2017, 225, 55–61. [Google Scholar] [CrossRef]
- Park, K.-S.; Chong, Y.; Kim, M.K. Myricetin: Biological activity related to human health. Appl. Biol. Chem. 2016, 59, 259–269. [Google Scholar] [CrossRef]
- Kim, D.-O.; Lee, C.Y. Comprehensive study on vitamin c equivalent antioxidant capacity (VCEAC) of various polyphenolics in scavenging a free radical and its structural relationship. Crit. Rev. Food Sci. Nutr. 2004, 44, 253–273. [Google Scholar] [CrossRef]
- Subotin, I.; Druta, R.; Popovici, V.; Covaci, E.; Sturza, R. Kinetic of forced oxidation of grape seeds, walnuts and corn germs oils in the presence of antioxidants. Food Nutr. Sci. 2021, 12, 1326–1339. [Google Scholar] [CrossRef]
- Matthäus, B. 6—Oxidation of edible oils. In Oxidation in Foods and Beverages and Antioxidant Applications; Decker, E.A., Elias, R.J., McClements, D.J., Eds.; Woodhead Publishing: Philadelphia, PA, USA, 2010; pp. 183–238. [Google Scholar]
- Shi, S.; Guo, K.; Tong, R.; Liu, Y.; Tong, C.; Peng, M. Online extraction–HPLC–FRAP system for direct identification of antioxidants from solid Du-zhong brick tea. Food Chem. 2019, 288, 215–220. [Google Scholar] [CrossRef] [PubMed]
| Standard | Amount (μg/mL) |
|---|---|
| Dihydromyricetin | 0.59 |
| Taxifolin | 1.98 |
| Dihydrokaempferol | 9.33 |
| Myricetin | 0.73 |
| Eriodictyol | N.D. (<0.20) |
| Quercetin | 1.06 |
| Naringenin | N.D. (<0.20) |
| Luteolin | 0.28 |
| Kaempferol | N.D. (<0.20) |
| Apigenin | N.D. (<0.20) |
| Antioxidant Capacity, IC50 | Extracts of Malanto | Vitamin C |
|---|---|---|
| DPPH free radical scavenging ability | 0.31 ± 0.05 a | 0.15 ± 0.02 b |
| Hydrogen peroxide scavenging capacity | 4.27 ± 0.03 a | 2.15 ± 0.01 b |
| ·OH radical scavenging ability | 0.50 ± 0.01 a | 0.27 ± 0.02 b |
| Reducing power | 0.04 ± 0.01 a | 0.06 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhao, Y.-T.; Lu, W.-C.; Huang, P.-H.; Song, T.-Y.; Li, P.-H. Bioactive Compounds in Malanto (Kalimeris indica) Leaves and Their Antioxidant Characteristics. Agriculture 2023, 13, 211. https://doi.org/10.3390/agriculture13010211
Liu J, Zhao Y-T, Lu W-C, Huang P-H, Song T-Y, Li P-H. Bioactive Compounds in Malanto (Kalimeris indica) Leaves and Their Antioxidant Characteristics. Agriculture. 2023; 13(1):211. https://doi.org/10.3390/agriculture13010211
Chicago/Turabian StyleLiu, Jie, Yu-Ting Zhao, Wen-Chien Lu, Ping-Hsiu Huang, Tuzz-Ying Song, and Po-Hsien Li. 2023. "Bioactive Compounds in Malanto (Kalimeris indica) Leaves and Their Antioxidant Characteristics" Agriculture 13, no. 1: 211. https://doi.org/10.3390/agriculture13010211
APA StyleLiu, J., Zhao, Y.-T., Lu, W.-C., Huang, P.-H., Song, T.-Y., & Li, P.-H. (2023). Bioactive Compounds in Malanto (Kalimeris indica) Leaves and Their Antioxidant Characteristics. Agriculture, 13(1), 211. https://doi.org/10.3390/agriculture13010211








