Abstract
The global demand for clean water has become increasingly important in the past decade as a result of the growing world population, civilization, and the increase in sources of contaminations. Aerogels are an exceptional form of porous materials with extraordinary unique properties. The aerogel has been fabricated from different inorganic and organic materials and incorporated with a variety of novel compounds for specific applications and to enhance its performance in the desired application. Activated carbon is well known for its water-pollutant adsorption, it has been prepared from several organic materials including agricultural wastes and used to treat water from organic dyes, heavy metals, oils, and toxic chemicals. However, as a powder form, activated carbon must be incorporated either into a filter or undergo a post-treatment step to remove the adsorbent from treated water. This review highlighted the development of agricultural waste-based carbon and activated carbon loaded nano-structured aerogels. A review of the types of aerogels and the properties based on the precursor materials was conducted to extensively discuss the potential use of agricultural waste-based carbon and activated carbon loaded nano-structured aerogels in wastewater treatment applications. We also discussed the challenges and future prospects of carbon and activated carbon nano-structured aerogels for wastewater treatment applications.
1. Introduction
Aerogels are three-dimensional (3D) nano-porous structures of non-fluid colloidal inter-connected polymeric or non-polymeric networks [1]. Aerogels exhibit several unique properties, such as ultra-low density, high porosity and extremely high surface area, which make them suitable in water treatment applications [2,3]. They are normally fabricated from silica through the conventional sol–gel method followed by a drying method such as ambient pressure drying, supercritical drying or freeze drying [4]. More recently, other forms of aerogels have been developed, including carbon aerogels, biopolymeric aerogels, synthetic polymers aerogels and metal aerogels, etc. The fabrication approaches have been also widely developed to become more facile, eco-friendly and unexpensive. Over the past few years, many applications have been identified for aerogels that suit their unique properties, including: thermal and non-thermal insulation [5], absorption [6], packaging [7], supercapacitors [8], catalysts [9], energy storage [10], filtration [11], conduction [12], sensing [13], and the cleaning and adsorption of wastes [14]. Hybrid aerogels were the key to enhance the properties and performance of the pure aerogel, as integration of new substances into a pure aerogel both significantly enhance and gave the material new properties for use in wider applications [15]. The past ten years have witnessed accelerated and widespread use of different aerogels in several applications, including water treatment applications. Figure 1 shows the accelerated grow of scientific publications related to aerogels in general and water treatment applications.
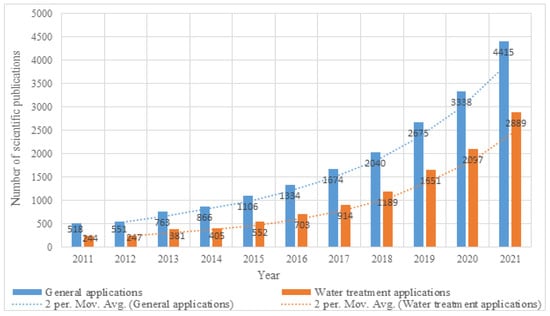
Figure 1.
Illustration of the accelerated growth of scientific publications in the past decade related to aerogel and its general and wastewater treatment applications (The search done on 1 November 2022 through Science Direct database).
Activated carbon is a unique form of adsorbent material that has been extensively used for the treatment and purification of different aqueous solutions [16]. However, activated carbon is also linked with some drawbacks, including its separation from the water after it performs the adsorption, in addition to its limited dispersion in water [16,17,18]. Several scientists have worked to overcome this issue, and facilitate its integration into the aerogels to incorporate the magnificent properties of the two materials [19,20]. Carbon aerogels are a unique class of aerogels that have extensive potential applications in water treatment applications due to the combined effect of both materials. Carbon and activated carbon aerogels have been recently used in several wastewater treatment applications including the removal of organic and inorganic dyes, the elimination of heavy metals, pesticides, herbicides, and oil/water separation [21,22,23]. So far, there are no or only a limited number of review articles discussing the use of carbon and activated carbon nanostructured aerogels in wastewater treatment applications. Several review articles either discuss general carbon aerogels [24], single precursor aerogels [25], or the adsorption of specific materials from water [26]. Other works address aerogels and their applications in water treatment without specifying carbon and activated carbon aerogels [27]. The aim of this review was to present an introduction to aerogels and classify their types, including organic, inorganic and composite aerogels. We also discussed the preparation of agricultural waste-based carbon and activated carbon nano-structured aerogels in water treatment applications. Agricultural waste-based activated carbon is also discussed as a key player in functional materials, and we presented its main properties and preparations. Finally, we discussed the most recent research on the utilization of carbon and activated carbon nano-structured aerogels in wastewater treatment applications, including organic dyes adsorption, heavy metals removal, oil/water separation, water deionization, and the removal of toxic chemicals.
2. Development of Agricultural-Based Nano-Structured Aerogels
Aerogels are open-celled and mesoporous forms of materials composed of inter-connected nanostructured networks exhibiting a porosity of more than 50% [28]. Aerogels have steadily developed since S. Kistler fabricated aerogels for the first time from silica gel in 1931 [29]. Abdul Khalil et al. [30] presented the chronological development of aerogel materials from inorganic silica aerogel until the current forms of nano-structured aerogels. The term nano is applied to materials that have at least one dimension in the nano-meter range (1 to 100 nm). Nano-structured aerogels are said to be materials with pores of less than 50 nm in diameter [31]. Having such a character, nano-structured aerogels possess an ultra-light weight and extremely high surface area, making them attractive materials for several advanced functionalities.
2.1. Classification and Properties of Nano-Structured Aerogels
The history of aerogel materials commenced with the fabrication of a silica aerogel by Kistler in 1931, who replaced the liquid in “Hydrogel” with gas without inducing any shrinkage [32]. Owing to the unique properties that suit particular applications, these materials have been widely utilized in several applications, including acting as: carriers for the delivery of different drugs [3]; electrodes in batteries [33]; filters for wastewater treatment [34]; and catalyst supports in fuel cells [35]. Several methods have been used to classify aerogels since there is no standard IUPAC classification yet for them [36]. The most convenient classification for aerogels is based on the precursor/s and/or the additives, which can be classified into three main groups, including inorganic, organic and composites [1]. Inorganic aerogels are the initial form of the materials prepared from metal alkoxides and/or metal salts to form metal oxide aerogels, chalcogenide aerogels and metallic aerogels. Organic aerogels are derived from several form of carbon, including biopolymers, phenol formaldehyde resin, etc to form biopolymeric aerogels, carbon nanotubes, graphene aerogels and other polymeric aerogels [37]. However, the third form of aerogels is formed by combining both inorganic and organic precursors to form composite aerogels (Table 1).

Table 1.
Classification of aerogels based on the precursor material/s.
Aerogels have been developed from almost every material, which determines the properties of that aerogel. However, the lack of novel properties in a single material limits the multifunctionality of many pure aerogels. Therefore, composite aerogels provide a solution for many potential applications to enhance, introduce and develop novel materials for many new applications. Nanoparticles of silica were impregnated with a network of polymers and loaded with model drugs [51]. This composite aerogel possesses an excellent drug release properties, which can be a potential drug carrier for many medical applications, such as wound dressing [51]. A silica–gelatin aerogel hybrid was fabricated and incorporated with the anticancer drug methotrexate [52]. The incorporation of the drug to the composite aerogel provided an excellent control for drug release. Other composite aerogels have been also used in the field of adsorption, separation and filtration, such as the removal of antibiotics from polluted water using nanocellulose/graphene oxide hybrid aerogel [53], and the same composite aerogel has been used for water purification [54]. Gonçalves and co-workers developed a composite aerogel based on alginate by incorporation of an alginate biopolymer with some drugs in the form of microparticles for mucosal administration. The authors reported that drug release from the alginate-based hybrid was faster than in previous polymer hybrids such as alginate/pectin [55].
The silica aerogel is the first prepared inorganic aerogel characterized with extraordinary properties, which opened a new pathway to many potential applications. The high cross-linked structure, high porosity and ultra-low density of silica-based aerogels raised a lot of interest in many applications [56]. They are mainly composed of air (more than 95%) and only the rest are silica, therefore, it exhibits poor thermal conductivity, and a high surface area [57,58], and being a novel material with unusual properties, has a promising and bright future in many important fields. Several precursors have been used for the preparation of silica aerogels including; Na2SiO3, Si(OR)4, MTMS, TEOS, and TMOS [59]. The production expenses of silica aerogels minimized their usage at the time they were discovered. However, in recent years, silica aerogels have been prepared from cheaper precursors, such as bamboo leaf [60], fly ash [61], oil shale ash [62], water glass [63], agricultural wastes, such as wheat husk [64] and bagasse ash [65] instead of with expensive organoalkoxysilanes [64]. Moreover, instead of using expensive, health risky supercritical drying in the large-scale production of aerogels, which is expensive may pose a risk to health, numerous researches have used alternative methods such as ambient pressure [66], freeze drying [67] and microwave drying [68]. The fabrication of flexible, smokeless, super thermal insulators have been always a novel objective for humankind. However, Kim, Y.-G., et al. [69] developed a silica-based aerogel which exhibited an ultra-low thermal conductivity compared to previous fabrications. Similarly, an unexpansive silica aerogel was developed from rice husk and incorporated into cement for thermal insulation purposes [70]. The authors observed an excellent thermal insulation in their aerogels, which may potentially be used as green materials for building applications.
2.2. Fabrication of Agricultral Carbon Nano-Structured Aerogels
Several polymeric materials can easily form gels when they are suspended in water, such as carrageenan, gelatine, and starch, etc., but others, such as cellulose and synthetic polymers require a cross-linker for this purpose [30]. However, aerogels can be simply prepared from both types of materials, with or without the formation of a wet-gel of the material. The shape of particles can be preservative and fixed during the drying phase either by the formation of wet-gel or by freezing of the suspension. The fabrication of an aerogel can be initiated by dissolving or dispersing the precursor material/s in distilled water or any other liquid solvent. The homogenization of precursor materials are required for a non-solvable material to achieve homogeneous suspension; the solution then requires some time for the aging process to form a wet-gel [71]. The precursor particles are linked together during the aging process, forming a viscous semi-solid material known as wet-gel. This material is formed by the network of precursor/s surrounded by the solvent. The next step is to remove the solvent without shrinking or disturbing the structure of that network. In order to achieve this, the wet-gel is frozen to keep the structure integrity intact, and then the frozen material is either freeze-dried or supercritical-dried. Freezing of the wet-gel will result in removing the liquid from the system, leaving the precursor network, which appears as a porous 3D material known as aerogel [72]. Li et al. [73] fabricated cocoon-based carbon aerogel by pyrolyzing the initially prepared aerogel at 800 °C for two hours. The authors were able to achieve a nano-porous structure with excellent catalytic activity for their carbon aerogel (Figure 2). The porosity, pore size and volume of the aerogel can be determined by the type and concentration of the precursor material/s. The fabrication technique and the conditions of preparation also highly affect the physical, chemical, and mechanical properties of the aerogels.
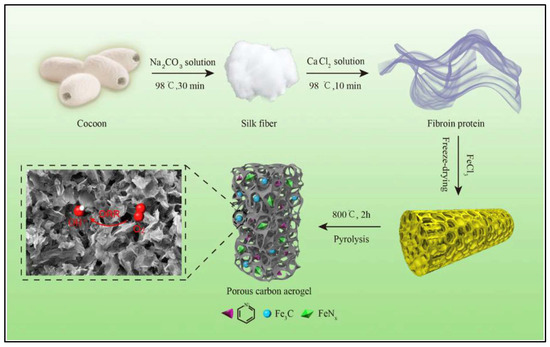
Figure 2.
Schematic representation of agricultural carbon aerogel fabrication using cocoon as precursor material. Adapted from ref. [73].
The preparation techniques of aerogels are divided into two major classes; conventional preparation techniques, which include those techniques that do not involve computer aid and follow fully manual lab-based routes. Advanced preparation techniques (rapid prototyping techniques), include those techniques that involve the aid of a computer in the fabrication process, as described below [74].
Conventional preparation techniques of aerogels such as supercritical CO2 drying, freeze-drying and thermal-induced phase separation, etc., do not involve any computer aid and are fully controlled by technicians. Since they are fully manmade materials, these techniques have the drawback of preparing accurate shapes for particular applications. However, due to their cost effectiveness and simplicity, many of these approaches are still in use even now. These techniques are associated with several issues, including time consumption and manpower requirements; thus, the past two decades have witnessed the development in the computer-aided design of aerogels in term of composition ratios and the final shape of the material. Such fabrication techniques are referred to as rapid prototyping techniques, due to the ease of preparation compared with traditional techniques. Moreover, using the computer in mixing the precursor material also helps in determining the optimal combination of each hybrid, in addition to the control of the physical, morphological and mechanical properties of the aerogels [75]. Table 2 highlights the main differences between traditional and advanced bioaerogel fabrication techniques.

Table 2.
Comparison between conventional and advanced bioaerogel fabrication techniques.
3. Agricultural Activated Carbon and Nano-Structured Aerogels
Activated carbon is a carbonaceous organic material produced by pyrolysis process and mostly used as a remarkable adsorbent material [76]. Nanostructured aerogels have been also utilized in wastewater treatment applications. Powdered activated carbon has been immobilized into several types of filters to overcome the drawbacks associated with the post-treatment stage. Activated carbon has been synthesized from a variety of plant-based precursors including rice husk, cotton shell, straw, corn stalk, palm shell, grass, and other plant-based biomass materials. Generally, any plant-based biomass can be transformed into carbonaceous material, which is then activated by chemical and/or physical activator to result in activated carbon of the particular material. Conditions of high temperatures and a limited or absent amount of oxygen is used during the pyrolysis process, such conditions play an essential role in the properties of the resulted activated carbon [77]. Figure 3 presents the fabrication process of activated carbon from different biomass materials.
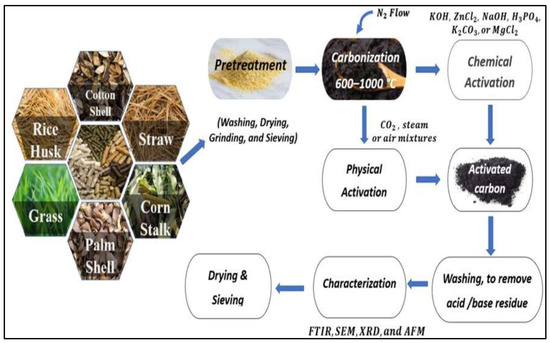
Figure 3.
Fabrication routes of agricultural-based-activated carbon from different precursors. Adapted from [78].
The activation of carbonaceous material can be done by several physical activators such as carbon dioxide, air, steam, or a combination of two or more of these agents, which is more preferable to the chemical ones due to their non-toxicity and eco-friendliness. However, chemical activators, particularly KOH-based activators were found to be more effective than physical activation as reported in [79]. The authors reported that such activation resulted in higher porosity, larger pore volume and increased specific surface areas. Huang and co-workers confirmed the results of Song and used KOH activation of their activated carbon at 800 °C and reported significant increases in specific surface areas, which led to better adsorption performance [80]. Several researches have reported the ability of biomass to perform adsorption of many pollutants such as heavy metals, pesticide and organic dyes, etc., [81,82]. Thus, the activation is only enhancing the adsorption performance of the precursor material by altering its physical, chemical and morphological properties [83].
3.1. Activated Carbon Aerogel
Activated carbon aerogel is a unique class of aerogels with 3D porous networks and extremely high specific surface areas resulting from the double porosity of activated carbon particles and the whole aerogel itself. Therefore, it has extensive potential as an adsorbent material in water treatment and purification. Several studies have reported high performance of activated carbon aerogel in the adsorption of dyes [84], heavy metals and other organic pollutants [24]. Gan and co-workers reported that biopolymers can be a sustainable precursor for the preparation of activated carbon aerogels [24]. Owing to their cost-effectiveness, sustainability and easy scale up, biopolymer-based activated carbon aerogels have great potential as an advanced functional material. Unlike toxic precursors such as formaldehyde, furfural and resorcinol and the expensive process of conventional aerogel fabrication that hamper its large-scale production, biopolymer-activated carbon aerogel is characterized by its promising adsorption capacity but the hydrophilicity of many biopolymers remains challenging and thus, chemical modification is mostly required. Yang and co-workers compared the electrochemical performances of both the commercial activated carbon and their prepared activated carbon aerogel and reported that the activated carbon aerogel had significantly better performance due to the higher specific surface area [85]. Activated carbons only have micropores, which limitate their adsorption; unlike activated carbon aerogel that has both the porosity of the activated carbon particles and that of the aerogel.
3.2. Applications of Activated Carbon Aerogels
The desire of scientists all over the world to design materials with controlled characteristics has significantly grown, with rapid and accelerated advances in materials science and technology. Since its development in 1989, carbon aerogels have been developed from several carbon precursors, such as plant biomass, graphene and carbon nano-tubes, etc., and investigated for multiple applications. Despite the unique advantages of carbon aerogels, impurities in some natural carbon as well as the difficulty of structural control are considered major issues in the application of high-quality materials. The properties of carbon and activated carbon aerogels have led to their employment in a wide range of applications, including water treatment filters, energy storage materials, sound insulators, chemical adsorbents, catalysts, thermal insulators, and catalytic supports (Figure 4).
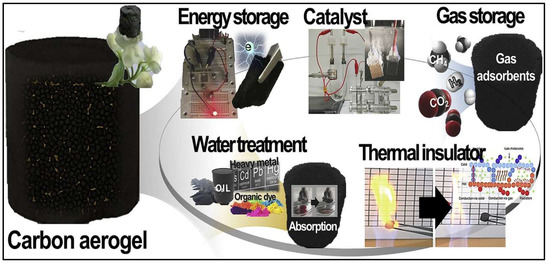
Figure 4.
Illustration of carbon and activated carbon aerogel fields with applications. Adapted from [86].
4. Agricultural Activated Carbon Nano-Structured Aerogels for Wastewater Treatment
Water quality is a general term used to describe the physical (such as turbidity, color, suspended solids, etc.), chemical (such as organic contaminant, inorganic contaminant, heavy metals, etc.), and biological (such as bacteria, plankton, algae, fungi, etc.) parameters that may be present in water [87,88]. The consideration of water quality in most cases corresponds to the origin and the purpose of water. In this section we discuss the utilization of activated carbon loaded nano-structured aerogels in the adsorption of organic and inorganic dyes, the removal of heavy metals from water, removal of harmful toxins and toxic materials, in addition to the separation of pharmaceutical compounds.
4.1. Organic Dye Adsorption
In recent years, great developments have been made in all industrial sectors, which has resulted in the excessive use of different chemical compounds that have polluted surface water bodies [89]. As part of the chemical compounds, organic and inorganic dyes that pollute the water have caused a serious threat to humans, animals and aquatic ecosystems due to their toxic nature. Several studies have linked artificial food and non-food dyes with many major health problems including cancer, hyperactivity, hives and asthma, in addition to behavioral changes such as irritability and depression in children and adults [90,91]. Activated carbon is known for its high adsorption ability, it has been widely incorporated with several forms of materials including aerogels [16]. In one study, Yu and co-workers developed an eco-friendly approach for the fabrication of nano-structured aerogel from sodium carboxymethyl cellulose using sol–gel processing and freeze-drying [84]. The authors pyrolyze their obtained aerogel and activated it via a KOH activation. The aerogel exhibited an extremely high surface area as a result of its nano-porosity and connected 3D nanostructures. The same authors reported 249.6 and 245.3 mg/g adsorption capacity of their optimum aerogel for the two tested organic dyes, which recommends this material for further water treatment applications. In different work, Wang and co-workers compared unattached activated carbon prepared from trichosanthes kirilowii maxim shell, nickel alginate-graphene oxide-based aerogels and nickel alginate-activated carbon aerogels for the adsorption of methylene blue dye [21]. The authors reported that the two aerogels loaded with the activated carbon were found to be more effective in the adsorption and to control the dispersion state. The adsorption of methylene blue dye on the nickel alginate-graphene oxide-based aerogels was 505.050 mg/g, compared with nickel alginate-activated carbon aerogel was 465.12 mg/g. The production of activated carbon is relatively expensive, and many researchers have developed approaches to reduce the cost of production. Wang and co-workers developed nano-structured carbon aerogels from agarose biomass [92]. The authors further enhanced their aerogels by introducing zeolitic imidazolate framework-8 to increase the surface area (up to 516 m2/g) and improve its adsorption capacity for different organic pollutants. In a different study, Li et al. [93] prepared carbon aerogels using an easy, cost-effective and eco-friendly fabrication approach. The authors used glucose as a precursor material, which was activated by potassium hydroxide, which built interconnected coral-like micro-structure during the sol–gel and activation processes. These structures made the aerogel more efficient, enhanced its porosity, and increased its surface area. The authors reported superior adsorption capacity of their preparation toward wide range of organic pollutant including phenols, antibiotics, and dyes; the adsorption of these compounds varied, depending on the material, from 194.07 to 1030.05 mg/g. In the same manner, Huang et al. [94] developed modified cellulose carbon aerogel using wet ball-milling and TEMPO mediated oxidation approaches followed by pyrolysis (Figure 5). The authors reported that the cellulosic fibres effectively turned into plane or wrinkle structures due to treatment conditions. These graphite-like structures made the aerogel exhibit high specific surface areas of more than 2825 m2/g, which resulted in maximum adsorption capacities to organic dyes of 644 mg/g and 1078 mg/g for alizarin reds and methylene blue, respectively. The adsorption mechanism of dyes for the prepared aerogel was found to be via pore-filling, hydrophobic partition, p/π-π interactions of electron donor–acceptor and H-bonding. For the methylene blue as a cationic dye, the adsorption was reinforced by electrostatic attraction, compared with the anionic alizarin reds that exhibited weakened electrostatic repulsion due to the high salt level [95].
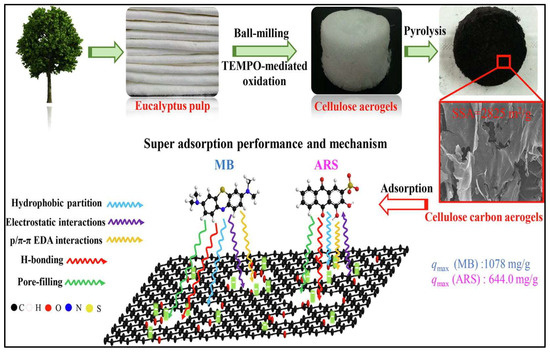
Figure 5.
Illustration of cellulose carbon aerogel for enhanced organic dyes adsorption from an aqueous solution. Adapted from Huang et al. [94].
4.2. Deionization of Water
Several studies have warned of the global depletion of freshwater resources, which will result in difficulties in accessing clean and fresh water [96,97]. Remarkable achievements in desalination technology have been recently made, but this technology has yet to meet the social and global demand for water capacitive deionization. Aerogels have been utilized in water deionization as a revolutionary solution derived from renewable and green precursors. Many aerogels have been made from biomass- derived carbon and investigated as active materials for capacitive deionization electrodes [98]. Zhang et al. [99] developed nanostructured activated carbon aerogel via ambient pressure-drying for capacitive deionization (Figure 6a). The authors reported that their aerogel was able to adsorb salts from water at a capacity of 10.34 mg/g. In a recent study, Liu et al. [100] developed a cost-effective and one-step process to develop a leather wast- based carbon aerogel and use it for water deionization (Figure 6c). The low-cost and eco-friendly aerogel was mainly fabricated from leather wastes, which is rich in N, O and S doping. The authors used one-step activated pyrolysis to reduce the construction costs; the aerogels had a significantly enhanced adsorption capacity to salts due to the extremely high specific surface area (2523 m2/g), which is highly favorable to salt ions absorption and storage. The same authors were able to achieve a maximum salt adsorption of up to 20.92 mg/g with a high adsorption rate compared to conventional active carbon.
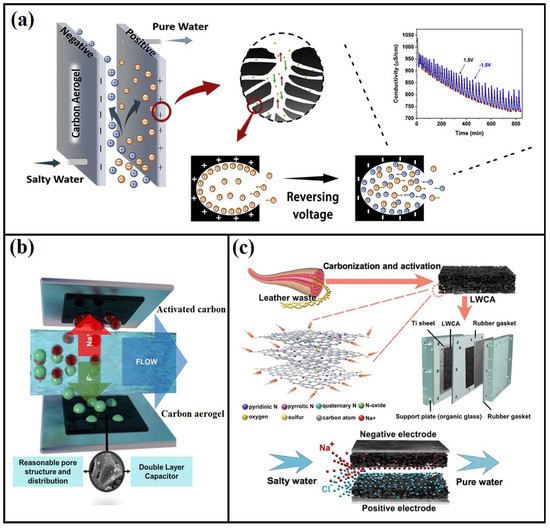
Figure 6.
Schematic illustration of carbon aerogels for water deionization; (a) nanostructured carbon aerogel for capacitive deionization, adapted from Zhang et al. [99]. (b) Schematic diagram of carbon aerogel’s electrode and its defluorination, adapted from Zhang et al. [101]. (c) leather waste-based carbon aerogel using one-step pyrolysis for enhanced capacitive water deionization. Adapted from Liu et al. [100].
4.3. Removal of Heavy Metals
Heavy metals are those metals that exhibit high density which can cause advert health effect even at very tiny amounts. There are about 23 known heavy metals that are of concern, including arsenic, cadmium, chromium, copper, iron and lead, etc. In recent years and with industrial development, most surface water bodies have been polluted with various amounts of heavy metals, which require smart and sustainable solutions. Despite the excellent adsorption performance of activated carbon, the requirements of the post-treatment stage increase the process costs in the case of using activated carbon particles. Thus, it has been incorporated with filtering membranes and aerogels to overcome the issues related to the post-treatment stage. In the research of Chen and co-workers, the authors fabricated nano-aerogels from cotton-derived porous carbon oxide and investigated its ability in heavy metal elimination as well as the removal of organic pollutants [102]. The aerogel was synthesized from natural cotton waste, the authors reported extremely heigh surface area of 1160 m2/g, which induced superior sorption capacities for heavy metal ions including strontium (II) (33.3 mg/g), lead (II) (111.1 mg/g) copper (II) (71.4 mg/g) and cadmium (II) (40.2 mg/g). In a different study, the citrate sol-gel method was used to fabricate an Fe3C/carbon aerogel for arsenic removal from water [103]. The authors reported an extremely high surface area and average pore size of 290 m2/g and 2.7 nm, respectively. The carbon aerogel had a maximum adsorption capacity for arsenic of 56.2 mg/g at pH 7.0. Such excellent performance makes it a potentially attractive material for the removal of hazardous substances from water. Li et al. [104] fabricated biochar-loaded aerogels for heavy metals elimination. Owing to the biochar loading and high surface area of the aerogel, the aerogel had excellent adsorption capacity with a maximum removal of 205.07, 137.89 and 105.56 mg/g for Pb(II), Zn(II) and Cd(II) respectively. Cao and co-workers developed a high porose carbon aerogel with a huge specific surface area using the sol-gel approach and atmospheric drying, and used it for Co (II) in water [105]. The authors reported the promising potential of their aerogel in copper removal in addition to its potential use in water deionization. Wang and co-workers fabricated nano-structured aerogel from carbon quantum dot combined with graphene and investigated its ability to reduce chromium (VI) from an aqueous solution [106]. The authors reported that their aerogel possessed superior photocatalyst activity and was further enhanced by the combination of graphene aerogel with carbon quantum dot, which immobilized the carbon dots and made the liquid phase reaction reusable. This novel aerogel was able to reduce up to 91% of Cr(VI) from the aqueous solution within only 40 min by photocatalytic reaction under the UV–Vis light irradiation (Figure 7).
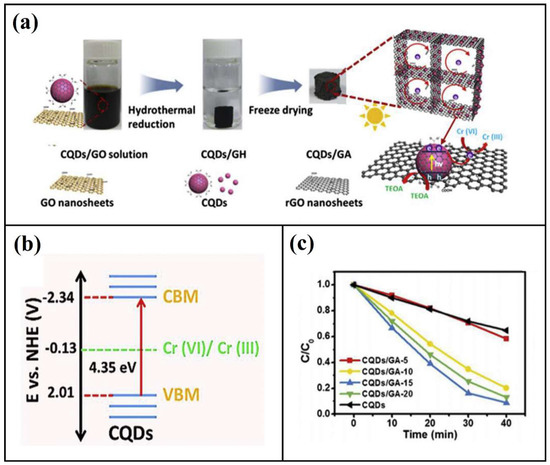
Figure 7.
Carbon quantum dot graphene nano-aerogel: (a) the fabrication process; (b) band structure of carbon quantum dot; and (c) photocatalytic performance of chromium (VI) reduction during UV–Vis light irradiation. Adapted with permission from Wang et al. [106] and Lee et al. [86].
In a different study, a magnetic carbon aerogel was synthesized using sodium alginate as a precursor material for carbon and gelatine as a cross-linker as well as a secondary carbon source [107]. The authors loaded their carbon aerogel with Fe3O4 nanoparticles as magnetic components to facilitate the separation and removal of aerogel after heavy metal adsorption. The carbon aerogel had an extremely high surface area of 145 m2/g with a variety of surface functional groups, which resulted in outstanding adsorption performance (143.88 mg/g) to Cd(II) from aqueous solutions [107]. Refer to Table 3 for the summary of using carbon and activated carbon nano-structured aerogels for heavy metals removal.

Table 3.
Characteristics and adsorptive capacities of carbon and activated carbon nano-structured aerogels for heavy metal removal.
4.4. Oil/Water Separation
Nano-structured aerogels have been also utilized in oil/water separation due to their facile, rapid, selectivity, reusability, and recyclability advantages. Different forms of aerogels possess different adsorption capacities; Qu and co-workers displayed the ability of their prepared N-doped graphene framework compared with several previous works [112]. The authors reported that their aerogel was able to adsorb oil up to 200–600 times its weight, compared with previous works; conventional graphene aerogel (10–37 times) [113], carbon nanofiber aerogels (51–139 times) [114] and carbon-nanotube aerogel (80–180 times) [115]. Bi and co-workers developed an easy approach for developing cost effective nano-structured carbon micro-belt aerogels using waste papers as a precursor material [116]. The authors reported superior properties for their aerogel including hydrophobicity, low density and a high specific surface area, which was able to adsorb organic liquids including pump oil; (up to 188 g/g), and chloroform (up to 151 g/g). Using such an aerogel opens many doors for largescale production of advanced adsorption materials, and the same authors stated that their aerogel can be regenerated and reused many times without any significant decrease in sorption performance by distillation or squeezing [116]. A mesoporous silica aerogel membrane was recently fabricated by Wang and co-workers for oil adsorption applications [117]. As an inorganic material, the aerogel exhibited high hydrophobicity, and was able to adsorb up to 99.9% of surfactant water-in-oil. A carbon nanotube aerogel was also used for oil adsorption due to its oil absorption capacity and excellent mechanical properties [118,119]. In one study done by Gui and co-workers, the authors reported an oil absorption capacity of up to 180 times for a carbon nanotube aerogel [115]. Despite the excellent performance of this aerogel, it is still associated with high production costs, which may not be applicable in at large-scale levels. To solve this issue, several studies have used biopolymers as a sustainable precursor material for aerogels. Cervin and co-workers developed a hydrophobic cellulose-based aerogel and reported encouraging performance in the separation of oil from water [120]. Eom et al. [121] fabricated an octylamine reduced graphene oxide aerogel able to effectively separate oil from water (Figure 8). The authors also modified Mg2(dobpdc) by using monoamines of several alkyl chain lengths, which led to long-term stabilization of the mixture and facile fine-tuning of its wettability. The same authors investigated the potential of their aerogel in absorbing other organic solvents and reported the promising potential of their materials (Figure 8c).
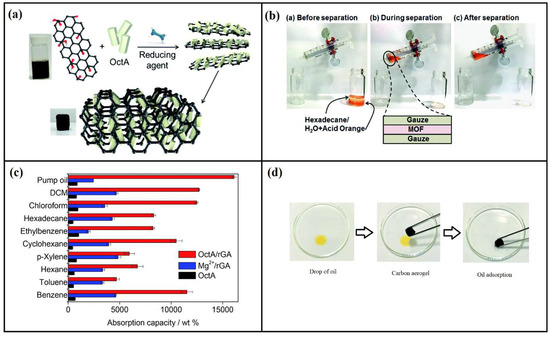
Figure 8.
Illustration of octylamine reduced graphene oxide aerogel fabrication for oil/water separation: (a) the fabrication process; (b) organic solvent separation experiment; (c) absorption of different organic solvents and dyes; and (d) absorption performance of the aerogel. Reproduced with permission from Eom et al. [121].
4.5. Removal of Toxic Chemicals
A huge amount of toxic chemicals are deposited in surface and ground water everyday as a result of industrial and agricultural processes. Although these chemicals have brought significant benefits and aid to our industries and agricultural production, the excessive use, and misuse, of such chemicals has polluted the environment including the water and soil, which becomes a serious threat to our lives and to the entire ecosystem [122]. In a recent study, a 3D graphene-based nano-aerogel able to detect and remove six different pesticides was developed through a chemical reduction process [123]. The aerogel was able to detect these toxic chemicals even at a tiny amount of 0.12 to 0.58 μg/L depending on the type. Such innovations could have great potential for environmental screening and other monitoring applications. In a different study, a carbon nanotube-based aerogels was prepared and investigated for the removal of herbicides from water [124]. The aerogel was able to absorb both chipton and alachlor herbicides at a high efficiency rate of 227.3 mg/g. The addition of metal–organic framework nanoparticles to the aerogel improved the adsorption performance of both compounds. Li and co-workers used a facile and eco-friendly approach to develop carbon aerogels from glucose [93]. The authors reported that the sol–gel process built the carbon interconnected and coral-like microstructure, which then developed microporosity and mesoporosity after the potassium hydroxide activation process. The authors reported extremely a high specific surface area of 2413 m2/g, which resulted in a high adsorption capacity ranging from 194.07 to 1030.05 mg/g for phenols, antibiotics, and even dyes. In a different study, Ahamad et al. [125] developed an N/S doped carbon aerogel by using sugarcane bagasse as a source of cellulose for the elimination of bisphenol-A toxic compound from an aqueous solution (Figure 9). The authors were able to fabricate the aerogel with a high yield and high purity and reported a maximum removal of the bisphenol-A of 98 to 99% at natural pH and initial bisphenol-A of 100 ppm. The same authors also investigated the adsorption kinetics and isotherms and reported that it followed pseudo-second-order and Langmuir for the kinetics and isotherms, respectively.
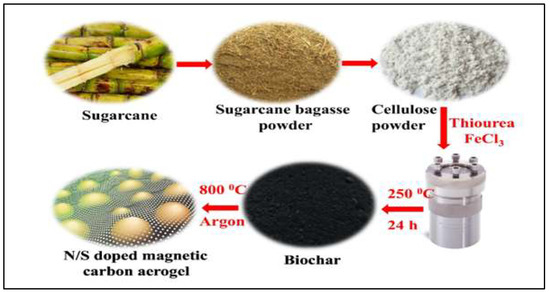
Figure 9.
The fabrication of N/S doped carbon aerogel by using sugarcane bagasse for bisphenol-A absorption. Adapted from Ahamad et al. [125].
4.6. Other Applications
Water treatment is a vital process for our daily life use including drinking, cleaning and agricultural use. The presence of a certain amount of one material could make the water undrinkable or even unusable for specific purposes [126]. Dassanayake and co-workers developed an activated carbon-loaded aerogel from chitin and KOH activation [127]. The aerogel exhibited high CO2 adsorption at different temperature. Owing to the KOH activation, the authors reported about a 37-fold increase in the aerogel’s surface area and a more than 95-fold increase in the micropore volume. This activated carbon aerogel suggests further applications for these materials. In a different study, Aylaz et al. [128] developed carbon aerogel from waste paper sources for the effective adsorption of hygromycin B, gentamicin, and vancomycin antibiotics from water. The authors reported that their aerogel had a porosity of more than 90.80% and a surface area of 795.15 mm2, thus it had an adsorption capacity of 104.1, 107.5 and 81.3 mg/g for hygromycin B, vancomycin and gentamicin, respectively. The authors claim that their study represents the first study in terms of antibiotic adsorption based on carbon aerogels obtained from waste paper. Such waste utilization to develop functional materials will help to ensure the world’s sustainable development. Fluoride contamination in groundwater has been taking a heavy toll on human life, with only about 2.5 billion people having access to safe and consumable water [129]. To solve this issue, Zhang et al. [101] prepared a carbon aerogel for excellent fluoride removal; the authors were able to achieve high removal performance of up to 24.44 mg/g. Ling et al. [130] developed a novel carbon aerogel to carry molybdenum trioxide (MoO3) for the removal of gaseous elemental mercury (Hg0) using an impregnation approach. The huge specific surface area and high porosity of the carbon aerogel, the authors stated that molybdenum trioxide (the active compound) was well distributed in the aerogel scaffold, which led to enhanced Hg0 removal performance. The maximum Hg0 removal capacity was found to be 74%, which reported for the carbon aerogel at 300 °C. Higher temperatures (500–700 °C) was found to reduce the adsorption performance till around 60% [130]. Zhang et al. recently developed carbon aerogel based microbial fuel cell able to generate electricity from wastewater by the oxidation of its organic substrates [131]. The authors used anodic exoelectrogenic bacteria for the oxidation at a neutral pH and were able to achieve 1.7 times higher maximum power density (2300 mW m−2) than conventional used Pt/C air cathodes. The carbon aerogel is a cost-efficient catalyst and can be effectively used for harvesting electrical energy from different organic polluted wastewater.
5. Challenges and Future Prospective
Despite the various encouraging advantages of carbon and activated carbon nanostructured aerogels, they are still in their initial research and evaluation stages, and not yet ready for commercialization and industrial applications. Sam et al. [132] stated that the reasons for the delay in the commercialization of carbon aerogels are the long and time-consuming preparation processes, which mostly involve a sol-gel polymerization stage. The same authors mentioned that using high capillary tension such as supercritical CO2 drying or freeze-drying techniques during the preparation raises the preparation cost and make the large-scale industry of these materials challenging. Thus, the operational costs of preparing carbon and activated carbon nanostructured aerogels need to be reduced by minimizing the processing steps or using alternative approaches that could also minimize the required time and speed of the preparation process. The use of agricultural waste materials, including grass clippings, bamboo fibres, cocoa shells, rice husk waste, wood chips, sawdust waste, and horse manure as sources of activated carbon and aerogel preparations can significantly lower the production costs of the adsorption materials. It is expected that the global market size and development of carbon aerogel will grow in the next few years, due to the high demand for such materials. The precursor materials of carbon and activated carbon nanostructured aerogels can be made from low-cost and sustainable material such as biomasses and plant waste. Although these aerogels may exhibit lower performance in term of water treatment applications compared to those nano aerogels prepared from graphene and carbon nanotubes [133], green modification may enhance their performance to reach and even exceed the performance of unsustainable materials. The control or design of carbon and activated carbon nanostructured aerogels porose structures is still a challenge, and several factors have been found to affect these characteristics but it also either reduce or affect other characters [134], which deserves extensive explorations. Using advance techniques such as 3D printing of carbon and activated carbon nanostructured aerogels may solve this issue and lead to the fabrication of materials with the desired architecture [135]. The use of such advanced techniques could also solve other issues such as the long preparation time and high preparation costs. The large-scale production of carbon and activated carbon nanostructured aerogels should benefit from these techniques to overcome such issues.
Author Contributions
Conceptualization, S.M., H.P.S.A.K. and E.B.Y.; validation, Y.M.A. investigation, E.B.Y.; resources, S.M. and H.P.S.A.K.; data curation, Y.M.A. and M.M.; writing—original draft preparation, E.B.Y.; writing—review and editing, E.B.Y. and H.P.S.A.K.; visualization, M.M. and H.P.S.A.K.; supervision, H.P.S.A.K.; project administration, S.M. and H.P.S.A.K.; funding acquisition, Y.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by International Research Grant, No: 304/PTEKIND/6501194.A158 and by LPPM-Universitas Syiah Kuala, grant number 345/UN11.2.1/PT.01.03PNBP/2021.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the collaboration between Universiti Sains Malaysia, Penang 11800, Malaysia and Universitas Syiah Kuala, Banda Aceh 23111, Indonesiathat made this work possible. Also, thanks to Lembaga Penelitian dan Pengabdian Masyarakat (LPPM) Universitas Syiah Kuala for supporting this work and providing the required resources.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alwin, S.; Sahaya Shajan, X. Aerogels: Promising nanostructured materials for energy conversion and storage applications. Mater. Renew. Sustain. Energy 2020, 9, 7. [Google Scholar] [CrossRef]
- Iskandar, M.; Yahya, E.B.; Abdul Khalil, H.P.S.; Rahman, A.; Ismail, M. Recent Progress in Modification Strategies of Nanocellulose-Based Aerogels for Oil Absorption Application. Polymers 2022, 14, 849. [Google Scholar] [CrossRef] [PubMed]
- Yahya, E.B.; Jummaat, F.; Amirul, A.; Adnan, A.; Olaiya, N.; Abdullah, C.; Rizal, S.; Mohamad Haafiz, M.; Abdul Khalil, H.P.S. A review on revolutionary natural biopolymer-based aerogels for antibacterial delivery. Antibiotics 2020, 9, 648. [Google Scholar] [CrossRef] [PubMed]
- Abdul Khalil, H.P.S.; Jummaat, F.; Yahya, E.B.; Olaiya, N.; Adnan, A.; Abdat, M.; NAM, N.; Halim, A.S.; Kumar, U.S.U.; Bairwan, R. A review on micro-to nanocellulose biopolymer scaffold forming for tissue engineering applications. Polymers 2020, 12, 2043. [Google Scholar] [CrossRef]
- Ren, S.; Li, X.; Fan, J.; Liang, J. Preparation and characterization of organic-inorganic hybrid ZrOC/PF aerogel used as high-temperature insulator. Ceram. Int. 2020, 46, 6326–6332. [Google Scholar] [CrossRef]
- Talebi, Z.; Soltani, P.; Habibi, N.; Latifi, F. Silica aerogel/polyester blankets for efficient sound absorption in buildings. Constr. Build. Mater. 2019, 220, 76–89. [Google Scholar] [CrossRef]
- de Oliveira, J.P.; Bruni, G.P.; el Halal, S.L.M.; Bertoldi, F.C.; Dias, A.R.G.; da Rosa Zavareze, E. Cellulose nanocrystals from rice and oat husks and their application in aerogels for food packaging. Int. J. Biol. Macromol. 2019, 124, 175–184. [Google Scholar] [CrossRef]
- Wang, C.; Guan, Z.; Wei, M.; Yu, S.; Sun, R.; Wong, C.-P. Synthesis and characterization of 3D CoMoO 4/rGO aerogel for supercapacitor electrodes. In Proceedings of the 2018 19th International Conference on Electronic Packaging Technology (ICEPT), Shanghai, China, 8–11 August 2018; pp. 1297–1300. [Google Scholar] [CrossRef]
- Arfaoui, J.; Ghorbel, A.; Petitto, C.; Delahay, G. Novel V2O5-CeO2-TiO2-SO42− nanostructured aerogel catalyst for the low temperature selective catalytic reduction of NO by NH3 in excess O2. Appl. Catal. B Environ. 2018, 224, 264–275. [Google Scholar] [CrossRef]
- Xia, W.; Qu, C.; Liang, Z.; Zhao, B.; Dai, S.; Qiu, B.; Jiao, Y.; Zhang, Q.; Huang, X.; Guo, W. High-performance energy storage and conversion materials derived from a single metal–organic framework/graphene aerogel composite. Nano Lett. 2017, 17, 2788–2795. [Google Scholar] [CrossRef]
- Dai, J.; Tian, Q.; Sun, Q.; Wei, W.; Zhuang, J.; Liu, M.; Cao, Z.; Xie, W.; Fan, M. TiO2-alginate composite aerogels as novel oil/water separation and wastewater remediation filters. Compos. Part B Eng. 2019, 160, 480–487. [Google Scholar] [CrossRef]
- An, F.; Li, X.; Min, P.; Li, H.; Dai, Z.; Yu, Z.-Z. Highly anisotropic graphene/boron nitride hybrid aerogels with long-range ordered architecture and moderate density for highly thermally conductive composites. Carbon 2018, 126, 119–127. [Google Scholar] [CrossRef]
- Dolai, S.; Bhunia, S.K.; Jelinek, R. Carbon-dot-aerogel sensor for aromatic volatile organic compounds. Sens. Actuators B Chem. 2017, 241, 607–613. [Google Scholar] [CrossRef]
- Zhang, H.; Lyu, S.; Zhou, X.; Gu, H.; Ma, C.; Wang, C.; Ding, T.; Shao, Q.; Liu, H.; Guo, Z. Super light 3D hierarchical nanocellulose aerogel foam with superior oil adsorption. J. Colloid Interface Sci. 2019, 536, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Yahya, E.B.; Abdul Khalil, H.P.S.; Ahmad, M.I.; Rizal, S.; Muhammad, S. Cleaner approach of preparing antibacterial bioaerogel scaffolds using oil palm waste nanocellulose. Ind. Crops Prod. 2023, 191, 115897. [Google Scholar] [CrossRef]
- Mariana, M.; Abdul Khalil, H.P.S.; Mistar, E.; Yahya, E.B.; Alfatah, T.; Danish, M.; Amayreh, M. Recent advances in activated carbon modification techniques for enhanced heavy metal adsorption. J. Water Process Eng. 2021, 43, 102221. [Google Scholar] [CrossRef]
- Gao, Y.; Yue, Q.; Gao, B.; Li, A. Insight into activated carbon from different kinds of chemical activating agents: A review. Sci. Total Environ. 2020, 746, 141094. [Google Scholar] [CrossRef]
- Al-Amshawee, S.; Yunus, M.Y.B.M.; Azoddein, A.A.M.; Hassell, D.G.; Dakhil, I.H.; Hasan, H.A. Electrodialysis desalination for water and wastewater: A review. Chem. Eng. J. 2020, 380, 122231. [Google Scholar] [CrossRef]
- Muhammad, S.; Abdul Khalil, H.P.S.; Abd Hamid, S.; Albadn, Y.M.; Suriani, A.; Kamaruzzaman, S.; Mohamed, A.; Allaq, A.A.; Yahya, E.B. Insights into Agricultural-Waste-Based Nano-Activated Carbon Fabrication and Modifications for Wastewater Treatment Application. Agriculture 2022, 12, 1737. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, Y.-S.; Kwac, L.K.; Shin, H.K. Characterization of activated carbon paper electrodes prepared by rice husk-isolated cellulose fibers for supercapacitor applications. Molecules 2020, 25, 3951. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, J.; Li, Y.; Zhang, P.; Li, M.; Zheng, H.; Zhang, X.; Li, H.; Du, Q. Methylene blue adsorption by activated carbon, nickel alginate/activated carbon aerogel, and nickel alginate/graphene oxide aerogel: A comparison study. J. Mater. Res. Technol. 2020, 9, 12443–12460. [Google Scholar] [CrossRef]
- Peydayesh, M.; Vogt, J.; Chen, X.; Zhou, J.; Donat, F.; Bagnani, M.; Müller, C.R.; Mezzenga, R. Amyloid-based carbon aerogels for water purification. Chem. Eng. J. 2022, 449, 137703. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Thomas, A.; Liao, Y. Ultra-high surface area nitrogen-doped carbon aerogels derived from a schiff-base porous organic polymer aerogel for CO2 storage and supercapacitors. Adv. Funct. Mater. 2019, 29, 1904785. [Google Scholar] [CrossRef]
- Gan, G.; Li, X.; Fan, S.; Wang, L.; Qin, M.; Yin, Z.; Chen, G. Carbon Aerogels for Environmental Clean-Up. Eur. J. Inorg. Chem. 2019, 2019, 3126–3141. [Google Scholar] [CrossRef]
- Li, W.; Chen, Z.; Yu, H.; Li, J.; Liu, S. Wood-Derived Carbon Materials and Light-Emitting Materials. Adv. Mater. 2021, 33, 2000596. [Google Scholar] [CrossRef] [PubMed]
- Sabzehmeidani, M.M.; Mahnaee, S.; Ghaedi, M.; Heidari, H.; Roy, V.A. Carbon based materials: A review of adsorbents for inorganic and organic compounds. Mater. Adv. 2021, 2, 598–627. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatami, M. Application of three dimensional porous aerogels as adsorbent for removal of heavy metal ions from water/wastewater: A review study. Adv. Colloid Interface Sci. 2020, 284, 102247. [Google Scholar] [CrossRef] [PubMed]
- Mariana, M.; Abdul Khalil, H.P.S.; Yahya, E.B.; Olaiya, N.; Alfatah, T.; Suriani, A.; Mohamed, A. Recent trends and future prospects of nanostructured aerogels in water treatment applications. J. Water Process Eng. 2022, 45, 102481. [Google Scholar] [CrossRef]
- Ziegler, C.; Wolf, A.; Liu, W.; Herrmann, A.K.; Gaponik, N.; Eychmüller, A. Modern inorganic aerogels. Angew. Chem. Int. Ed. 2017, 56, 13200–13221. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Adnan, A.; Yahya, E.B.; Olaiya, N.; Safrida, S.; Hossain, M.; Balakrishnan, V.; Gopakumar, D.A.; Abdullah, C.; Oyekanmi, A. A review on plant cellulose nanofibre-based aerogels for biomedical applications. Polymers 2020, 12, 1759. [Google Scholar] [CrossRef]
- Wang, H.-L.; Hsu, C.-Y.; Wu, K.C.; Lin, Y.-F.; Tsai, D.-H. Functional nanostructured materials: Aerosol, aerogel, and de novo synthesis to emerging energy and environmental applications. Adv. Powder Technol. 2020, 31, 104–120. [Google Scholar] [CrossRef]
- Yahya, E.B.; Alzalouk, M.M.; Alfallous, K.A.; Abogmaza, A.F. Antibacterial cellulose-based aerogels for wound healing application: A review. Biomed. Res. Ther. 2020, 7, 4032–4040. [Google Scholar] [CrossRef]
- Dong, X.; Zheng, X.; Deng, Y.; Wang, L.; Hong, H.; Ju, Z. SiO2/N-doped graphene aerogel composite anode for lithium-ion batteries. J. Mater. Sci. 2020, 55, 13023–13035. [Google Scholar] [CrossRef]
- Yu, Y.; Shi, X.; Liu, L.; Yao, J. Highly compressible and durable superhydrophobic cellulose aerogels for oil/water emulsion separation with high flux. J. Mater. Sci. 2021, 56, 2763–2776. [Google Scholar] [CrossRef]
- Öner, E.; Öztürk, A.; Yurtcan, A.B. Utilization of the graphene aerogel as PEM fuel cell catalyst support: Effect of polypyrrole (PPy) and polydimethylsiloxane (PDMS) addition. Int. J. Hydrogen Energy 2020, 45, 34818–34836. [Google Scholar]
- Salimian, S.; Zadhoush, A.; Naeimirad, M.; Kotek, R.; Ramakrishna, S. A review on aerogel: 3D nanoporous structured fillers in polymer-based nanocomposites. Polym. Compos. 2018, 39, 3383–3408. [Google Scholar] [CrossRef]
- Yahya, E.B.; Amirul, A.; Abdul Khalil, H.P.S.; Olaiya, N.G.; Iqbal, M.O.; Jummaat, F.; AK, A.S.; Adnan, A. Insights into the role of biopolymer aerogel scaffolds in tissue engineering and regenerative medicine. Polymers 2021, 13, 1612. [Google Scholar] [CrossRef]
- Wang, C.-T. Photocatalytic activity of nanoparticle gold/iron oxide aerogels for azo dye degradation. J. Non-Cryst. Solids 2007, 353, 1126–1133. [Google Scholar] [CrossRef]
- Zhang, H.; Han, W.; Xu, K.; Zhang, Y.; Lu, Y.; Nie, Z.; Du, Y.; Zhu, J.; Huang, W. Metallic sandwiched-aerogel hybrids enabling flexible and stretchable intelligent sensor. Nano Lett. 2020, 20, 3449–3458. [Google Scholar] [CrossRef]
- Ahmed, E.; Rothenberger, A. Enhancement in CO2 adsorption capacity and selectivity in the chalcogenide aerogel CuSb2S4 by post-synthetic modification with LiCl. Microporous Mesoporous Mater. 2016, 220, 247–252. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, X.; Wang, J.; Liu, Z.; Zhang, K.; Ji, X.; You, Y.; Zhang, X. Reaction-spun transparent silica aerogel fibers. ACS Nano 2020, 14, 11919–11928. [Google Scholar] [CrossRef]
- Gu, W.; Sheng, J.; Huang, Q.; Wang, G.; Chen, J.; Ji, G. Environmentally friendly and multifunctional shaddock peel-based carbon aerogel for thermal-insulation and microwave absorption. Nano-Micro Lett. 2021, 13, 102. [Google Scholar]
- Yang, L.; Li, N.; Guo, C.; He, J.; Wang, S.; Qiao, L.; Li, F.; Yu, L.; Wang, M.; Xu, X. Marine biomass-derived composite aerogels for efficient and durable solar-driven interfacial evaporation and desalination. Chem. Eng. J. 2021, 417, 128051. [Google Scholar] [CrossRef]
- Chen, P.; Bai, D.; Tang, H.; Liu, H.; Wang, J.; Gao, G.; Li, L. Polylactide aerogel with excellent comprehensive performances imparted by stereocomplex crystallization for efficient oil-water separation. Polymer 2022, 255, 125128. [Google Scholar] [CrossRef]
- Rizal, S.; Yahya, E.B.; Abdul Khalil, H.P.S.; Abdullah, C.; Marwan, M.; Ikramullah, I.; Muksin, U. Preparation and Characterization of Nanocellulose/Chitosan Aerogel Scaffolds Using Chemical-Free Approach. Gels 2021, 7, 246. [Google Scholar] [CrossRef] [PubMed]
- Dang, N.H.; Tu, T.H.; Linh, V.N.P.; Thy, L.T.M.; Nam, H.M.; Phong, M.T.; Hieu, N.H. Preparation of magnetic iron oxide/graphene aerogel nanocomposites for removal of bisphenol A from water. Synth. Met. 2019, 255, 116106. [Google Scholar]
- Batista, M.; Gonçalves, V.S.; Gaspar, F.; Nogueira, I.; Matias, A.A.; Gurikov, P. Novel alginate-chitosan aerogel fibres for potential wound healing applications. Int. J. Biol. Macromol. 2020, 156, 773–782. [Google Scholar] [CrossRef]
- Tang, R.; Hong, W.; Srinivasakannan, C.; Liu, X.; Wang, X.; Duan, X. A novel mesoporous Fe-silica aerogel composite with phenomenal adsorption capacity for malachite green. Sep. Purif. Technol. 2022, 281, 119950. [Google Scholar] [CrossRef]
- Zuo, K.; Wu, J.; Chen, S.; Ji, X.; Wu, W. Superamphiphobic nanocellulose aerogels loaded with silica nanoparticles. Cellulose 2019, 26, 9661–9671. [Google Scholar] [CrossRef]
- Mo, L.; Pang, H.; Tan, Y.; Zhang, S.; Li, J. 3D multi-wall perforated nanocellulose-based polyethylenimine aerogels for ultrahigh efficient and reversible removal of Cu (II) ions from water. Chem. Eng. J. 2019, 378, 122157. [Google Scholar]
- Follmann, H.D.; Oliveira, O.N.; Lazarin-Bidóia, D.; Nakamura, C.V.; Huang, X.; Asefa, T.; Silva, R. Multifunctional hybrid aerogels: Hyperbranched polymer-trapped mesoporous silica nanoparticles for sustained and prolonged drug release. Nanoscale 2018, 10, 1704–1715. [Google Scholar] [CrossRef]
- Nagy, G.; Király, G.; Veres, P.; Lázár, I.; Fábián, I.; Bánfalvi, G.; Juhász, I.; Kalmár, J. Controlled release of methotrexate from functionalized silica-gelatin aerogel microparticles applied against tumor cell growth. Int. J. Pharm. 2019, 558, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Fan, B.; Xiong, Y.; Jin, C.; Sun, Q.; Sheng, C. 3D assembly based on 2D structure of cellulose nanofibril/graphene oxide hybrid aerogel for adsorptive removal of antibiotics in water. Sci. Rep. 2017, 7, 45914. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Gui, S.-H.; Wu, J.-H.; Xu, D.-D.; Sun, Y.; Dong, X.-Y.; Dai, Y.-Y.; Li, Y.-F. Nanocellulose-Graphene Oxide Hybrid Aerogel to Water Purification. Appl. Environ. Biotechnol. 2019, 4, 11–17. [Google Scholar] [CrossRef]
- Gonçalves, V.S.; Gurikov, P.; Poejo, J.; Matias, A.A.; Heinrich, S.; Duarte, C.M.; Smirnova, I. Alginate-based hybrid aerogel microparticles for mucosal drug delivery. Eur. J. Pharm. Biopharm. 2016, 107, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Iswar, S.; Malfait, W.J.; Balog, S.; Winnefeld, F.; Lattuada, M.; Koebel, M.M. Effect of aging on silica aerogel properties. Microporous Mesoporous Mater. 2017, 241, 293–302. [Google Scholar] [CrossRef]
- Chandradass, J.; Kang, S.; Bae, D.-S. Synthesis of silica aerogel blanket by ambient drying method using water glass based precursor and glass wool modified by alumina sol. J. Non-Cryst. Solids 2008, 354, 4115–4119. [Google Scholar] [CrossRef]
- Lee, K.-J.; Choe, Y.-J.; Kim, Y.H.; Lee, J.K.; Hwang, H.-J. Fabrication of silica aerogel composite blankets from an aqueous silica aerogel slurry. Ceram. Int. 2018, 44, 2204–2208. [Google Scholar] [CrossRef]
- Gurav, J.L.; Jung, I.-K.; Park, H.-H.; Kang, E.S.; Nadargi, D.Y. Silica aerogel: Synthesis and applications. J. Nanomater. 2010, 2010, 409310. [Google Scholar] [CrossRef]
- Kow, K.-W.; Yusoff, R.; Aziz, A.A.; Abdullah, E. From bamboo leaf to aerogel: Preparation of water glass as a precursor. J. Non-Cryst. Solids 2014, 386, 76–84. [Google Scholar] [CrossRef]
- Shi, F.; Liu, J.-X.; Song, K.; Wang, Z.-Y. Cost-effective synthesis of silica aerogels from fly ash via ambient pressure drying. J. Non-Cryst. Solids 2010, 356, 2241–2246. [Google Scholar] [CrossRef]
- Gao, G.-M.; Liu, D.-R.; Zou, H.-F.; Zou, L.-C.; Gan, S.-C. Preparation of silica aerogel from oil shale ash by fluidized bed drying. Powder Technol. 2010, 197, 283–287. [Google Scholar] [CrossRef]
- He, P.; Gao, X.-D.; Li, X.-M.; Jiang, Z.-W.; Yang, Z.-H.; Wang, C.-L.; Gu, Z.-Y. Highly transparent silica aerogel thick films with hierarchical porosity from water glass via ambient pressure drying. Mater. Chem. Phys. 2014, 147, 65–74. [Google Scholar] [CrossRef]
- Liu, S.-W.; Wei, Q.; Cui, S.-P.; Nie, Z.-R.; Du, M.-H.; Li, Q.-Y. Hydrophobic silica aerogel derived from wheat husk ash by ambient pressure drying. J. Sol-Gel Sci. Technol. 2016, 78, 60–67. [Google Scholar] [CrossRef]
- Nazriati, N.; Setyawan, H.; Affandi, S.; Yuwana, M.; Winardi, S. Using bagasse ash as a silica source when preparing silica aerogels via ambient pressure drying. J. Non-Cryst. Solids 2014, 400, 6–11. [Google Scholar] [CrossRef]
- Shi, F.; Wang, L.; Liu, J. Synthesis and characterization of silica aerogels by a novel fast ambient pressure drying process. Mater. Lett. 2006, 60, 3718–3722. [Google Scholar] [CrossRef]
- Hyun, S.; Kim, T.; Kim, G.; Park, H.-H. Synthesis of low-k porous silica films via freeze drying. J. Mater. Sci. Lett. 2000, 19, 1863–1866. [Google Scholar] [CrossRef]
- Nocentini, K.; Achard, P.; Biwole, P.; Stipetic, M. Hygro-thermal properties of silica aerogel blankets dried using microwave heating for building thermal insulation. Energy Build. 2018, 158, 14–22. [Google Scholar] [CrossRef]
- Kim, Y.-G.; Kim, H.S.; Jo, S.M.; Kim, S.Y.; Yang, B.; Cho, J.; Lee, S.; Cha, J.E. Thermally insulating, fire-retardant, smokeless and flexible polyvinylidene fluoride nanofibers filled with silica aerogels. Chem. Eng. J. 2018, 351, 473–481. [Google Scholar] [CrossRef]
- Abbas, N.; Khalid, H.R.; Ban, G.; Kim, H.T.; Lee, H.-K. Silica aerogel derived from rice husk: An aggregate replacer for lightweight and thermally insulating cement-based composites. Constr. Build. Mater. 2019, 195, 312–322. [Google Scholar] [CrossRef]
- Zheng, Q.; Tian, Y.; Ye, F.; Zhou, Y.; Zhao, G. Fabrication and application of starch-based aerogel: Technical strategies. Trends Food Sci. Technol. 2020, 99, 608–620. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Yahya, E.B.; Jummaat, F.; Adnan, A.; Olaiya, N.; Rizal, S.; Abdullah, C.; Pasquini, D.; Thomas, S. Biopolymers based Aerogels: A review on revolutionary solutions for smart therapeutics delivery. Prog. Mater. Sci. 2022, 131, 101014. [Google Scholar]
- Li, C.; Sun, F.; Lin, Y. Refining cocoon to prepare (N, S, and Fe) ternary-doped porous carbon aerogel as efficient catalyst for the oxygen reduction reaction in alkaline medium. J. Power Sources 2018, 384, 48–57. [Google Scholar] [CrossRef]
- Smirnova, I.; Gurikov, P. Aerogel production: Current status, research directions, and future opportunities. J. Supercrit. Fluids 2018, 134, 228–233. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, W.; Qi, W.; Chen, S.; Tan, Q.; Wei, Z.; Gong, L.; Chen, J.; Zhou, W. The comprehensive evaluation model and optimization selection of activated carbon in the O3-BAC treatment process. J. Water Process Eng. 2021, 40, 101931. [Google Scholar] [CrossRef]
- Tan, X.-F.; Liu, S.-B.; Liu, Y.-G.; Gu, Y.-L.; Zeng, G.-M.; Hu, X.-J.; Wang, X.; Liu, S.-H.; Jiang, L.-H. Biochar as potential sustainable precursors for activated carbon production: Multiple applications in environmental protection and energy storage. Bioresour. Technol. 2017, 227, 359–372. [Google Scholar] [CrossRef]
- Naji, S.Z.; Tye, C.T. A review of the synthesis of activated carbon for biodiesel production: Precursor, preparation, and modification. Energy Convers. Manag. X 2022, 13, 100152. [Google Scholar] [CrossRef]
- Song, M.; Jin, B.; Xiao, R.; Yang, L.; Wu, Y.; Zhong, Z.; Huang, Y. The comparison of two activation techniques to prepare activated carbon from corn cob. Biomass Bioenergy 2013, 48, 250–256. [Google Scholar] [CrossRef]
- Huang, F.-C.; Lee, C.-K.; Han, Y.-L.; Chao, W.-C.; Chao, H.-P. Preparation of activated carbon using micro-nano carbon spheres through chemical activation. J. Taiwan Inst. Chem. Eng. 2014, 45, 2805–2812. [Google Scholar] [CrossRef]
- Alkherraz, A.M.; Ali, A.K.; Elsherif, K.M. Removal of Pb (II), Zn (II), Cu (II) and Cd (II) from aqueous solutions by adsorption onto olive branches activated carbon: Equilibrium and thermodynamic studies. Chem. Int. 2020, 6, 11–20. [Google Scholar]
- Guo, J.; Song, Y.; Ji, X.; Ji, L.; Cai, L.; Wang, Y.; Zhang, H.; Song, W. Preparation and characterization of nanoporous activated carbon derived from prawn shell and its application for removal of heavy metal ions. Materials 2019, 12, 241. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.; Othman, F.; Yusof, N.; Matsuura, T.; Lau, W.; Jaafar, J.; Ismail, A.; Salleh, W.; Aziz, F. Preparation of nanocomposite activated carbon nanofiber/manganese oxide and its adsorptive performance toward leads (II) from aqueous solution. J. Water Process Eng. 2020, 37, 101430. [Google Scholar] [CrossRef]
- Yu, M.; Li, J.; Wang, L. KOH-activated carbon aerogels derived from sodium carboxymethyl cellulose for high-performance supercapacitors and dye adsorption. Chem. Eng. J. 2017, 310, 300–306. [Google Scholar] [CrossRef]
- Yang, I.; Kwon, D.; Kim, M.-S.; Jung, J.C. A comparative study of activated carbon aerogel and commercial activated carbons as electrode materials for organic electric double-layer capacitors. Carbon 2018, 132, 503–511. [Google Scholar] [CrossRef]
- Lee, J.-H.; Park, S.-J. Recent advances in preparations and applications of carbon aerogels: A review. Carbon 2020, 163, 1–18. [Google Scholar]
- Palansooriya, K.N.; Yang, Y.; Tsang, Y.F.; Sarkar, B.; Hou, D.; Cao, X.; Meers, E.; Rinklebe, J.; Kim, K.-H.; Ok, Y.S. Occurrence of contaminants in drinking water sources and the potential of biochar for water quality improvement: A review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 549–611. [Google Scholar] [CrossRef]
- Yahya, E.; Abdulsamad, M.A. In-vitro Antibacterial Activity of Carbopol-Essential Oils hydrogels. J. Appl. Sci. Process Eng. 2020, 7, 564–571. [Google Scholar] [CrossRef]
- Chkirida, S.; Zari, N.; Bouhfid, R. Insight into the bionanocomposite applications on wastewater decontamination. J. Water Process Eng. 2021, 43, 102198. [Google Scholar] [CrossRef]
- Mittal, J. Permissible synthetic food dyes in India. Resonance 2020, 25, 567–577. [Google Scholar] [CrossRef]
- Mota, I.G.C.; Neves, R.A.M.D.; Nascimento, S.S.D.C.; Maciel, B.L.L.; Morais, A.H.D.A.; Passos, T.S. Artificial dyes: Health risks and the need for revision of international regulations. Food Rev. Int. 2021, 1–16. [Google Scholar] [CrossRef]
- Wang, C.; Kim, J.; Tang, J.; Na, J.; Kang, Y.M.; Kim, M.; Lim, H.; Bando, Y.; Li, J.; Yamauchi, Y. Large-scale synthesis of MOF-derived superporous carbon aerogels with extraordinary adsorption capacity for organic solvents. Angew. Chem. 2020, 132, 2082–2086. [Google Scholar] [CrossRef]
- Li, K.; Zhou, M.; Liang, L.; Jiang, L.; Wang, W. Ultrahigh-surface-area activated carbon aerogels derived from glucose for high-performance organic pollutants adsorption. J. Colloid Interface Sci. 2019, 546, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Zhang, P.; Min, L.; Tang, J.; Sun, H. Synthesis of cellulose carbon aerogel via combined technology of wet ball-milling and TEMPO-mediated oxidation and its supersorption performance to ionic dyes. Bioresour. Technol. 2020, 315, 123815. [Google Scholar] [CrossRef]
- Lai, K.C.; Lee, L.Y.; Hiew, B.Y.Z.; Thangalazhy-Gopakumar, S.; Gan, S. Environmental application of three-dimensional graphene materials as adsorbents for dyes and heavy metals: Review on ice-templating method and adsorption mechanisms. J. Environ. Sci. 2019, 79, 174–199. [Google Scholar] [CrossRef]
- Ahmed, M.; Wiese, D.N. Short-term trends in Africa’s freshwater resources: Rates and drivers. Sci. Total Environ. 2019, 695, 133843. [Google Scholar] [CrossRef]
- Pradinaud, C.; Northey, S.; Amor, B.; Bare, J.; Benini, L.; Berger, M.; Boulay, A.-M.; Junqua, G.; Lathuillière, M.J.; Margni, M. Defining freshwater as a natural resource: A framework linking water use to the area of protection natural resources. Int. J. Life Cycle Assess. 2019, 24, 960–974. [Google Scholar]
- Elisadiki, J.; Kibona, T.E.; Machunda, R.L.; Saleem, M.W.; Kim, W.-S.; Jande, Y.A. Biomass-based carbon electrode materials for capacitive deionization: A review. Biomass Convers. Biorefinery 2020, 10, 1327–1356. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Wang, H.; Wu, X.; Shen, J. A positive-negative alternate adsorption effect for capacitive deionization in nano-porous carbon aerogel electrodes to enhance desalination capacity. Desalination 2019, 458, 45–53. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Gu, X.; Wu, N.; Zhang, R.; Shen, Y.; Zheng, B.; Wu, J.; Zhang, W.; Li, S. One-step turning leather wastes into heteroatom doped carbon aerogel for performance enhanced capacitive deionization. Microporous Mesoporous Mater. 2020, 303, 110303. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Yang, Z.; Yang, P.; Wang, J.; Shi, M.; Yu, F.; Ma, J. Industrially-prepared carbon aerogel for excellent fluoride removal by membrane capacitive deionization from brackish groundwaters. Sep. Purif. Technol. 2022, 297, 121510. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.; Li, J.; Wang, X. Cotton derived carbonaceous aerogels for the efficient removal of organic pollutants and heavy metal ions. J. Mater. Chem. A 2015, 3, 6073–6081. [Google Scholar] [CrossRef]
- Shen, G.; Xu, Y.; Liu, B. Preparation and adsorption properties of magnetic mesoporous Fe3C/carbon aerogel for arsenic removal from water. Desalination Water Treat. 2016, 57, 24467–24475. [Google Scholar] [CrossRef]
- Li, J.; Xia, C.; Cheng, R.; Lan, J.; Chen, F.; Li, X.; Li, S.; Chen, J.; Zeng, T.; Hou, H. Passivation of multiple heavy metals in lead–zinc tailings facilitated by straw biochar-loaded N-doped carbon aerogel nanoparticles: Mechanisms and microbial community evolution. Sci. Total Environ. 2022, 803, 149866. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, C.; Yang, Z.; Qin, Q.; Zhang, Z.; Wang, X.; Shen, J. Preparation of carbon aerogel electrode for electrosorption of copper ions in aqueous solution. Materials 2019, 12, 1864. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lu, K.-Q.; Zhang, F.; Tang, Z.-R.; Xu, Y.-J. 3D carbon quantum dots/graphene aerogel as a metal-free catalyst for enhanced photosensitization efficiency. Appl. Catal. B Environ. 2018, 233, 11–18. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, M.; Waterhouse, G.I.; Sun, J.; Shi, W.; Ai, S. Efficient removal of cadmium ions from water by adsorption on a magnetic carbon aerogel. Environ. Sci. Pollut. Res. 2021, 28, 5149–5157. [Google Scholar] [CrossRef]
- Alatalo, S.-M.; Pileidis, F.; Makila, E.; Sevilla, M.; Repo, E.; Salonen, J.; Sillanpaa, M.; Titirici, M.-M. Versatile cellulose-based carbon aerogel for the removal of both cationic and anionic metal contaminants from water. ACS Appl. Mater. Interfaces 2015, 7, 25875–25883. [Google Scholar] [CrossRef]
- Goel, J.; Kadirvelu, K.; Rajagopal, C.; Garg, V. Cadmium (II) uptake from aqueous solution by adsorption onto carbon aerogel using a response surface methodological approach. Ind. Eng. Chem. Res. 2006, 45, 6531–6537. [Google Scholar] [CrossRef]
- Song, Z.; Chen, X.; Gong, X.; Gao, X.; Dai, Q.; Nguyen, T.T.; Guo, M. Luminescent carbon quantum dots/nanofibrillated cellulose composite aerogel for monitoring adsorption of heavy metal ions in water. Opt. Mater. 2020, 100, 109642. [Google Scholar] [CrossRef]
- Kadirvelu, K.; Goel, J.; Rajagopal, C. Sorption of lead, mercury and cadmium ions in multi-component system using carbon aerogel as adsorbent. J. Hazard. Mater. 2008, 153, 502–507. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, C.; Hu, Y.; Cheng, H.; Shi, G.; Qu, L. A versatile, ultralight, nitrogen-doped graphene framework. Angew. Chem. Int. Ed. 2012, 51, 11371–11375. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Chen, J.; Hng, H.H.; Ma, J.; Chen, X. A leavening strategy to prepare reduced graphene oxide foams. Adv. Mater. 2012, 24, 4144–4150. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-Y.; Li, C.; Liang, H.-W.; Zhang, Y.-N.; Wang, X.; Chen, J.-F.; Yu, S.-H. Carbon nanofiber aerogels for emergent cleanup of oil spillage and chemical leakage under harsh conditions. Sci. Rep. 2014, 4, 4079. [Google Scholar] [CrossRef] [PubMed]
- Gui, X.; Wei, J.; Wang, K.; Cao, A.; Zhu, H.; Jia, Y.; Shu, Q.; Wu, D. Carbon nanotube sponges. Adv. Mater. 2010, 22, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Huang, X.; Wu, X.; Cao, X.; Tan, C.; Yin, Z.; Lu, X.; Sun, L.; Zhang, H. Carbon microbelt aerogel prepared by waste paper: An efficient and recyclable sorbent for oils and organic solvents. Small 2014, 10, 3544–3550. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H. Ultra-hydrophobic and mesoporous silica aerogel membranes for efficient separation of surfactant-stabilized water-in-oil emulsion separation. Sep. Purif. Technol. 2019, 212, 597–604. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, L.; Liu, D. Ultralight graphene/carbon nanotubes aerogels with compressibility and oil absorption properties. Materials 2018, 11, 641. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, S.; Ma, Q.; Jia, X.; Ma, P.-C. Preparation of carbon nanotubes/graphene hybrid aerogel and its application for the adsorption of organic compounds. Carbon 2017, 118, 765–771. [Google Scholar] [CrossRef]
- Cervin, N.T.; Aulin, C.; Larsson, P.T.; Wågberg, L. Ultra porous nanocellulose aerogels as separation medium for mixtures of oil/water liquids. Cellulose 2012, 19, 401–410. [Google Scholar] [CrossRef]
- Eom, S.; Kang, D.W.; Kang, M.; Choe, J.H.; Kim, H.; Kim, D.W.; Hong, C.S. Fine-tuning of wettability in a single metal–organic framework via postcoordination modification and its reduced graphene oxide aerogel for oil–water separation. Chem. Sci. 2019, 10, 2663–2669. [Google Scholar] [CrossRef]
- Gould, F.; Brown, Z.S.; Kuzma, J. Wicked evolution: Can we address the sociobiological dilemma of pesticide resistance? Science 2018, 360, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Gao, Y.; Xu, C.; Lian, Y. Determination of six organophosphorus pesticides in water samples by three-dimensional graphene aerogel-based solid-phase extraction combined with gas chromatography/mass spectrometry. RSC Adv. 2018, 8, 10277–10283. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Wang, B.; Cheng, J.; Xiao, D.; Xie, Z.; Zhao, J. 3D, eco-friendly metal-organic frameworks@ carbon nanotube aerogels composite materials for removal of pesticides in water. J. Hazard. Mater. 2021, 401, 123718. [Google Scholar] [CrossRef]
- Ahamad, T.; Naushad, M.; Alhabarah, A.N.; Alshehri, S.M. N/S doped highly porous magnetic carbon aerogel derived from sugarcane bagasse cellulose for the removal of bisphenol-A. Int. J. Biol. Macromol. 2019, 132, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Show, S.; Chakraborty, P.; Karmakar, B.; Halder, G. Sorptive and microbial riddance of micro-pollutant ibuprofen from contaminated water: A state of the art review. Sci. Total Environ. 2021, 786, 147327. [Google Scholar] [CrossRef]
- Dassanayake, R.S.; Gunathilake, C.; Abidi, N.; Jaroniec, M. Activated carbon derived from chitin aerogels: Preparation and CO2 adsorption. Cellulose 2018, 25, 1911–1920. [Google Scholar] [CrossRef]
- Aylaz, G.L.N.; Okan, M.; Duman, M.; Aydin, H.M. Study on cost-efficient carbon aerogel to remove antibiotics from water resources. ACS Omega 2020, 5, 16635–16644. [Google Scholar] [CrossRef]
- Mukherjee, S.; Halder, G. A review on the sorptive elimination of fluoride from contaminated wastewater. J. Environ. Chem. Eng. 2018, 6, 1257–1270. [Google Scholar] [CrossRef]
- Ling, Y.; Man, X.; Zhang, W.; Wang, D.; Wu, J.; Liu, Q.; Gu, M.; Lin, Y.; He, P.; Jia, T. Molybdenum trioxide impregnated carbon aerogel for gaseous elemental mercury removal. Korean J. Chem. Eng. 2020, 37, 641–651. [Google Scholar] [CrossRef]
- Zhang, X.; He, W.; Zhang, R.; Wang, Q.; Liang, P.; Huang, X.; Logan, B.E.; Fellinger, T.P. High-performance carbon aerogel air cathodes for microbial fuel cells. ChemSusChem 2016, 9, 2788–2795. [Google Scholar] [CrossRef]
- Sam, D.K.; Sam, E.K.; Durairaj, A.; Lv, X.; Zhou, Z.; Liu, J. Synthesis of biomass-based carbon aerogels in energy and sustainability. Carbohydr. Res. 2020, 491, 107986. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.N.; Minh, D.N.; Van Hung, N.; Minh, P.N.; Khoi, P.H. Carbon nanotube and graphene aerogels—The world’s 3D lightest materials for environment applications: A review. Int. J. Mater. Sci. Appl 2017, 6, 277. [Google Scholar]
- Xu, X.; Wang, R.; Nie, P.; Cheng, Y.; Lu, X.; Shi, L.; Sun, J. Copper nanowire-based aerogel with tunable pore structure and its application as flexible pressure sensor. ACS Appl. Mater. Interfaces 2017, 9, 14273–14280. [Google Scholar] [CrossRef] [PubMed]
- Mensing, J.P.; Lomas, T.; Tuantranont, A. 2D and 3D printing for graphene based supercapacitors and batteries: A review. Sustain. Mater. Technol. 2020, 25, e00190. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).