Abstract
Arthrospira platensis contains high protein content and quality, which makes it a promising feed source for livestock animals. However, this microalga presents a recalcitrant peptidoglycan cell wall, and its main proteins form protein-pigment complexes attached to the algal thylakoid membrane. The purpose of the present study was to evaluate the effect of mechanical/physical pretreatments (bead milling, extrusion, freeze-drying, heating, microwave and sonication) combined with commercial enzymes (trypsin or pancreatin) on the degradation of A. platensis proteins. Protein degradation was assessed through the quantification of total protein and protein fractions (18–26 kDa, 40–48 kDa and others) on SDS-PAGE gels and the evaluation of the coefficient of protein degradation (CPD). The results showed that no significant differences were found among treatments for CPD values, except for an increase related to 18–26 kDa protein (phycocyanin subunits) with extrusion combined with pancreatin. In addition, extrusion and microwave caused a decrease of total protein in algal supernatant probably due to a denaturation/aggregation and reduction of solubility. Overall, extrusion is a promising pretreatment for A. platensis cell wall disruption and protein bioaccessibility. Further studies could elucidate how the effect of extrusion on protein solubility affects the activity of peptidases on protein degradation.
1. Introduction
Microalgae have emerged as promising feed, food and industrial resources. Particularly, Arthrospira platensis, due to its high protein content (up to 76% dry matter), has been explored as a supplement or ingredient for livestock animals [1]. However, A. platensis has a recalcitrant peptidoglycan cell wall [2], and the main proteins with bioactive properties are covalently bound to pigments (i.e., phycocyanobilins), forming protein-pigment complexes. These complexes (phycocyanins), which are mainly composed of hexamers or trimers of c-phycocyanin and trimers of allophycocyanin, are linked with other phycobiliproteins to form the phycobilisomes, which are attached to the algal thylakoid membrane [3,4]. This intricate microalga structure reduces the bioaccessibility and digestibility of its high-quality proteins, and therefore, mechanical/physical and non-mechanical/physical pretreatments have been used to overcome these issues [5].
Mechanical/physical pretreatments, including bead milling [6], drying [7], high-pressure [8,9], high-temperature heat [3,4], manual grinding [8] and ultrasonication [6,8,10] have shown positive effects on protein extraction, particularly high-pressure [8,9]. Heating was also able to change the structural conformation of proteins, promoting reversible unfolding followed by irreversible protein unfolding, with the subsequent protein aggregation [3,4]. Non-mechanical/physical pretreatments, such as alkaline treatments [8] or the use of enzymes, have also proven to be effective in increasing the bioaccessibility of A. platensis nutrients. For instance, carbohydrases, such as lysozyme, were shown to effectively degrade the microalga cell wall [11] and release total protein from algal biomass. Moreover, trypsin and pancreatin, which simulate gastrointestinal digestion, could increase algal dry weight [12] and crude protein [13] digestibility up to 94% and 81%, respectively.
The synergistic effect of combining different mechanical/physical pretreatments, such as high-shear homogenization with pulsed electric fields [14] or ultrasonication with freezing and thawing [15], on increasing the extractability of soluble proteins and/or phycocyanins from A. platensis was previously reported. Similar results were obtained when associating mechanical/physical (e.g., ultrasonication) and enzymatic (e.g., lysozyme) pretreatments [16]. However, to the best of our knowledge, the latter combination has been scarcely explored in what concerns A. platensis biomass disruption and protein extraction and degradation and would be of upmost importance for enhancing the use of this microalga in animal feeding.
In the present study, we aimed to assess the effect of combining several mechanical/physical pretreatments (bead milling, freeze-drying, heating, microwave, sonication and extrusion) with trypsin (EC 3.4.21.4) or pancreatin (a mix of pancreatic peptidase, lipase and amylase) on the bioaccessibility of A. platensis proteins.
2. Materials and Methods
2.1. Microalga Mechanical/Physical Pretreatments
Arthrospira platensis dried powder (Allmicroalgae Natural Products SA, Pataias, Portugal) was submitted to six different pretreatments: bead milling, extrusion, freeze-drying, heating, microwave and sonication. For all treatments, the microalga was previously suspended at 20 g/L in PBS solution (1× PBS, BioWhittaker, Verviers, Belgium), except for freeze-drying and heating, where those procedures were carried out afterwards. The mechanical/physical pretreatments were chosen based on their previously reported efficiency for microalga cell wall disruption [6,8,17,18,19,20], following standardized conditions with some modifications optimized in previous preliminary assays (data not shown). For bead milling, 0.5 mm zirconium beads were used for a minimum of 50% filling instead of 65% [6], considering a time between 1 and 60 min [18]. The extrusion conditions were based on those already described [20], but with adaptations to A. platensis biomass and to scale it up to an industrial level. For the freeze-drying method, alga biomass was freeze-dried for 24 h instead of 3 h 10 min [7], to maximize the cell wall disruption time. The heating procedure was performed at a temperature of 70 °C instead of 90 °C [17] to avoid the excess denaturation and aggregation of algal proteins that occurs at 90 °C. Indeed, a stepwise transition in the spectroscopic properties of phycocyanin complexes was observed within the range of 50 °C ≤ T ≤ 70 °C [3]. The microwave technique was combined with sample boiling in order to associate the effects of microwave radiation and high temperature, which were proven to be effective in degrading microalga biomass [17,21]. The sonication procedure was conducted according to previous studies [18,21]. For a better comprehension of the pre-treatments applied, a brief description of the procedures follows. The bead milling was performed by adding 1 bead per mL of microalga suspension in a tube and placing it in a shaker (Multi Reax Heidolph Instruments, Schwabach, Germany) for 30 min at 2000 rpm. The freeze-drying consisted of disposing of 2 g of A. platensis sample in a freeze dryer (Labogene, CoolSafe, Frilabo, Milheirós, Portugal) incorporated with a vacuum pump (CRVpro2, model no. 3022-00, Welch, Ingersoll Rand, Swords, Ireland), for 24 h at −55 °C and 2.0 hPa, after freezing it overnight at −80 °C. The heating was carried out by exposing 0.2 g of microalga in a 16-mL glass tube to 70 °C for 30 min in a stove, for a final volume of 10 mL in PBS solution. The microwave procedure was performed by placing the A. plantesis suspension (0.3 g per 15 mL PBS in a 50 mL tube) in a microwave under the keep warm program until boiling. For microalga sonication, seven cycles at 70% potency were applied for 15 min (with manual agitation of the suspension in the middle time) in an ultrasound device (Bandelin ultrasonic homogenizer, Heinrichstraße, Berlin, Germany) with a maximum power setting of 200 W and incorporated with an immersion horn (20 Hz). The extrusion of A. platensis (94.1% of solids) followed specific conditions established by Sparos (Olhão, Algarve, Portugal): 340 mL of water addition/min, pressure at 34 bar and 118 °C for the last extrusion barrel. The screw rotation speed of the extruder was adjusted by a frequency converter. The time in which the microalga was subjected to this pressure and temperature varied between 3 and 7 s. Then, algal pellets were dried for 8 and 10 min by passing them through the area of a vibrating fluid bed dryer where the air was warmer (120 °C) for about 2 min, followed by gradual air cooling. In the end, pellets were ground to allow microalga suspension. The chemical composition of untreated A. platensis is presented in Table 1, which was conceded by Allmicroalgae. The chemical composition was determined using routine and widespread methods [22]. Briefly, dry matter (DM) was analyzed by drying a sample at 105 °C to a constant weight. The nitrogen content for crude protein determination was obtained using the Kjeldahl method. Ash content was determined after burning the sample at 525 °C. The crude fat value was obtained with Soxhlet extraction using petroleum. Gross energy and carbohydrates were determined by standardized calculations. Phycocyanin was extracted following the procedures described by Ritchie [23] and was analyzed by high-performance liquid chromatography (HPLC).

Table 1.
Chemical composition of Arthrospira platensis.
2.2. Incubation of Pretreated A. platensis with Enzymes
The incubation assay was performed in a 24-well microplate (n = 5), adding each enzyme at 20 µg/mL to the pretreated alga resuspension or the same volume of PBS, as previously reported [24,25]. The microplate was incubated at 37 °C and 160 rpm for 16 h, and then centrifuged for 15 min at 3210 g to recover 1 mL of supernatants. The enzymes used were pancreatin (350 FIP-U/g protease, 6000 FIP-U/g lipase, 7500 FIP-U/g amylase; Merck, Darmstadt, Germany) and trypsin (type II-S, 1000–2000 units/mg dry weight, Sigma-Aldrich, St Louis, MO, USA) dried powders from porcine pancreas.
2.3. Evaluation of Protein Fractions after Hydrolysis
Protein fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), as reported by Vizcaíno, et al. [26] with some modifications. Succinctly, supernatant samples were diluted (1:5) in sample buffer (prepared with 30% instead of 10% (v/v) β-mercaptoethanol) and boiled for 5 min. Afterwards, 12 µL (about 9.00 µg of protein for unhydrolyzed samples) of each replicate was loaded into 14% PAGE gels, together with a low molecular weight (LMW) protein marker (5 µL) containing seven proteins ranging from 18.5 to 96 KDa (Nzytech, Lisbon, Portugal). After scanning the gel image with the ChemiDoc MP Imaging System device (Bio-Rad Laboratories Lda, CA, USA), the relative densities of the two most prominent protein fractions (fraction 1, 18–26 kDa; fraction 2, 40–48 kDa; and other proteins) and total protein was determined using Image J software (NIH, Bethesda, MA, USA). The band of 40.0 kDa of the LMW marker (1.95 µg of protein) was used as external standard for this determination. The protein fraction of 18–26 kDa was the most predominant fraction in the gel and mainly corresponded to the α- and β-subunits of c-phycocyanin and allophycocyanin [3], whereas the fraction of 40–48 kDa contained unidentified but abundant proteins among the ones with a higher MW. The total protein extraction yield in the supernatant was determined as follows:
where
- PTRAT = protein obtained with pretreatments
- PCON = protein obtained with control
2.4. Calculation of the Coefficient of Protein Degradation
For an assessment of the extent of protein hydrolysis, the coefficient of protein degradation (CPD), as an adaptation to that previously reported by Alarcón, et al. [27] and Vizcaíno, et al. [26], was calculated for the quantified protein fractions. The CPD value was expressed as a percentage and established the relation between decreases in optical density for each protein band after the enzymatic hydrolysis relative to the optical density obtained without hydrolysis. It was calculated using the following expression:
where
- OD1 = optical density of the protein band before hydrolysis
- OD2 = optical density of the protein band after hydrolysis
2.5. Determination of Total Protein by the Bradford Method
The total protein present in the supernatant was quantified by spectrophotometry using the Bradford method [28]. Briefly, 1.5 mL of Bradford solution (PanReac, AppliChem, ITW Reagents, Darmstadt, Germany) was mixed with 30 μL PBS buffer: sample (1:3). The absorbance was read at 595 nm, after 5 min at room temperature. A standard curve was built using bovine serum albumin (BSA) at concentrations from 0.0125 to 1 mg/mL.
2.6. Determination of Total Peptides by O-Pthalaldehyde (OPA) Assay
The spectrophotometric OPA assay for measurement of free amino acids and peptides was performed as previously reported [26,29], but with some modifications. Succinctly, 200 μL of each sample was mixed with 100 μL of 20% trichloroacetic acid for protein precipitation, followed by a centrifugation at 12,000× g for 15 min. Then, 200 μL of supernatant, or distilled water for blank, was mixed with 1 mL of daily prepared OPA [29,30]. The absorbance was read at 340 nm after incubation at room temperature for 5 min. A standard curve was obtained with peptone at concentrations from 5 to 500 μg/mL.
2.7. Statistical Analysis
Data were analyzed using the general linear models of SAS to perform ANOVA and the Tukey–Kramer method (PDIFF option) for multiple comparisons of adjusted least square means. The homogeneity of variances was tested using Levene’s test. Values were considered significant when p < 0.05.
3. Results
3.1. Effect of Mechanical/Physical Pretreatments and Enzymatic Treatment on A. platensis Protein (Bradford Method) and Peptide (OPA Assay) Concentrations
Table 2 presents the influence of mechanical/physical pretreatments and enzymes on the amount of total protein and peptides in A. platensis. Total protein content determined by the Bradford method significantly decreased (p < 0.001) with extrusion and microwave pre-treatments. However, no significant effect (p > 0.050) of the enzymes was detected after performing these pre-treatments. In addition, an almost 2-fold decrease (p < 0.001) of total peptide concentration quantified by OPA assay was observed with extrusion when compared to no-pretreatment (control). The activity of pancreatin on extruded, freeze-dried and heated microalga caused an increase in total peptides (p < 0.010). A similar result (p < 0.050) was obtained when microwave-treated A. platensis was combined with trypsin.

Table 2.
Effect of mechanical/physical pretreatments combined or not with enzymes on the concentration of total protein (mg/mL) and peptides (µg/mL) in the Arthrospira platensis supernatant fraction determined by the Bradford method and the OPA assay, respectively (n = 5).
3.2. Effect of Mechanical/Physical Pretreatments and Enzymatic Treatment on A. platensis Protein Concentration Quantified in SDS-PAGE Gel
Table 3 shows the influence of mechanical/physical pretreatments and enzymes on the total protein and protein fraction concentrations of A. platensis. Extrusion and microwave significantly reduced (p < 0.001) the amount of protein fractions 1 (18–26 kDa) (F1) and 2 (40–48 kDa) (F2) and total proteins in the SDS-PAGE gel compared to no-pretreatment. Trypsin decreased (p < 0.050) the amount of F2 when no pretreatment or all pretreatments, except for microwave, were applied, but had no significant effect (p > 0.050) on F1. In addition, this enzyme reduced (p < 0.050) the concentration of other protein fractions and total protein in no pretreated or treated with bead milling algal biomass. Pancreatin decreased (p < 0.050) the amount of F1 in the supernatant after extrusion and heating, and a similar result occurred for F2 but also with no pretreatment and sonication. For other proteins and total protein, pancreatin led to protein degradation only in sonicated microalgae. Figure 1 represents the effect of mechanical/physical pretreatments, and of those most active (extrusion and microwave), on algal proteins combined or not with enzymes. The total protein extraction yield (n = 5) was 0.05 ± 0.196, −0.32 ± 0.207, 0.00 ± 0.206, 0.07 ± 0.206, −0.28 ± 0.135 and 0.12 ± 0.223 (means ± standard deviation), for bead milling, extrusion, freeze-drying, heating, microwave and sonication, respectively, with significant differences (p = 0.005) between extrusion and heating and sonication and also between microwave and sonication.

Table 3.
Effect of mechanical/physical pretreatments, combined or not with enzymes, on the concentration of total protein and protein fractions (mg/mL) of Arthrospira platensis supernatant fraction evaluated by SDS-PAGE gel (n = 5).
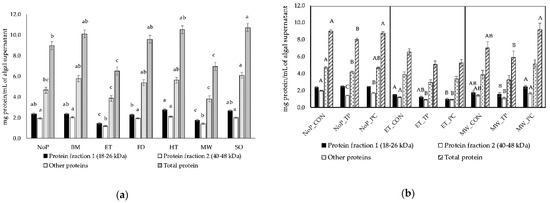
Figure 1.
Effect of mechanical/physical pretreatments (a) and enzymes (b) on the concentration of Arthrospira platensis protein fractions quantified in SDS-PAGE gels loaded with algal supernatant (n = 5): NoP, no pretreatment; BM, bead milling; ET, extrusion; FD, freeze-drying; HT, heating; MW, microwave; SO, sonication; CON, control; TP, trypsin; PC, pancreatin. a,b,c Significant differences among pretreatments for each fraction are indicated by different superscripts (p < 0.05). A,B,C Significant differences for each fraction obtained with no pretreatment, extrusion or microwave combined with or not with enzymes are indicated by different superscripts (p < 0.05).
3.3. Effect of Trypsin on the Degradation of Proteins of Pretreated A. platensis
Table 4 presents the effect of trypsin on the protein degradation coefficients of non- or pretreated A. platensis, and Figure 2 shows the most representative SDS-PAGE gels for the hydrolysis of A. platensis proteins by trypsin after alga pretreatment. The results show that no significant differences (p > 0.050) were found between treatments for CPD values, although the action of trypsin towards microalga proteins led to a decrease in total protein and protein fractions quantified in SDS-PAGE gels for all pretreatments and the control. Particularly, CPD values for total protein were about 2- and 1.6-fold numerically higher with bead milling or extrusion and microwave, respectively, than with the control. These results were mostly due to the effect of bead milling on F2 and other protein fractions, which predisposed it to trypsin hydrolysis, whereas extrusion and microwave acted on F1 and other fractions.

Table 4.
Effect of trypsin on the coefficient of protein degradation (CPD) of Arthrospira platensis biomass after several mechanical/physical pretreatments (n = 5).
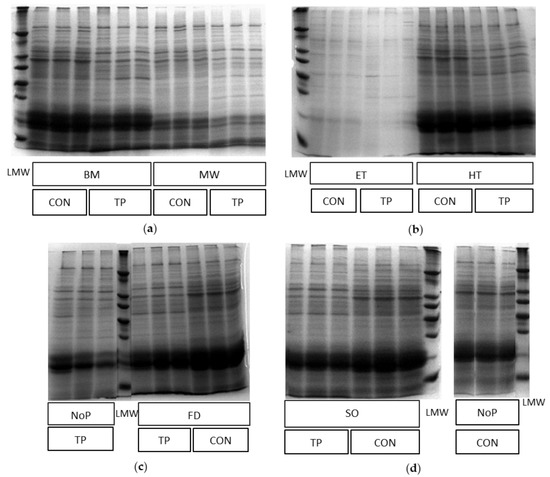
Figure 2.
Images of SDS-PAGE gels showing the hydrolysis effect of trypsin on Arthrospira platensis proteins after pretreatment (n = 3): TP, trypsin; CON, control; LMW, low molecular weight protein marker; (a) BM, bead milling; MW, microwave; (b) ET, extrusion; HT, heating; (c) NoP, no pretreatment; FD, freeze-drying; (d) SO, sonication; NoP, no pretreatment.
3.4. Effect of Pancreatin on the Degradation of Proteins of Pretreated A. platensis
The influence of pancreatin on the protein degradation coefficients of non- or pretreated A. platensis is shown in Table 5, whereas the most representative SDS-PAGE gels for protein hydrolysis are presented in Figure 3. A significant increase (p = 0.001) of F1 hydrolysis was found in microalga treated with extrusion, but no significant effects (p > 0.001) were observed between treatments in CPD values for F2, other proteins and total protein. However, all pretreatments numerically increased the hydrolysis of alga proteins by pancreatin, with a consequent decrease in total protein quantified in the SDS-PAGE gel. For instance, a non-significant 4- to 5-fold increase (p = 0.167) in CPD for total protein was found for extrusion, microwave and sonication compared to control. These results were mostly due to the effect of extrusion on F1, predisposing it to pancreatin hydrolysis, while microwave led to a CPD value for F1 between that obtained with control and extrusion, tended to increase F2 degradation (p = 0.079), and caused a non-significant (p = 0.657) 3-fold increase in CPD for other protein fractions. The effect of sonication on the degradation of F2 and other fractions was similar to that obtained with microwave.

Table 5.
Effect of pancreatin on the coefficient of protein degradation (CPD) of Arthrospira platensis biomass after several mechanical/physical pretreatments (n = 5).
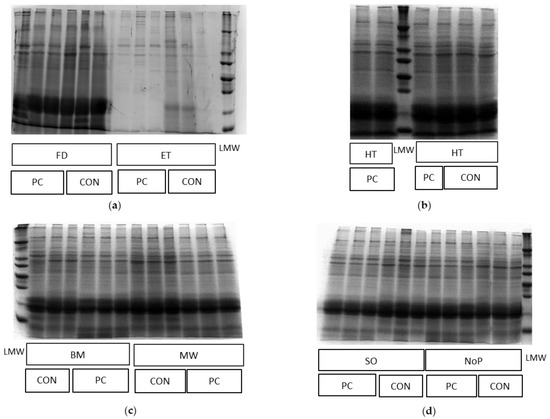
Figure 3.
Images of SDS-PAGE gels showing the hydrolysis effect of pancreatin on Arthrospira platensis proteins after pretreatment (n = 3): PC, pancreatin; CON, control; LMW, low molecular weight protein marker; (a) FD, freeze-drying; ET, extrusion; (b) HT, heating; (c) BM, bead milling; MW, microwave; (d) SO, sonication; NoP, no pretreatment.
4. Discussion
The pretreatment of A. platensis with extrusion and microwave significantly decreased the amount of the main protein fractions and total protein in the algal supernatant. A significant reduction of CPD for F1 (a protein fraction of 18 to 26 kDa, mainly composed of c-phycocyanin and allophycocyanin subunits) also occurred when extrusion was combined with pancreatin. However, trypsin was active toward F2 in non- or pretreated microalga, except for that treated with microwave. For total protein, this effect was only found with non-pretreated or bead milling-treated microalga. Moreover, pancreatin promoted F2 hydrolysis when no pretreatment or extrusion, heating and sonication was applied, but this enzyme mixture was only effective with sonication for total protein. The milder effect of trypsin on A. platensis proteins pretreated with extrusion and, particularly, with microwave, was probably due to a 1.4 and 1.3-fold reduction in total protein concentration, respectively, with these treatments, with less protein being available for hydrolysis. In addition, the higher variability between replicates for pre-treatments combined with trypsin than for those combined with pancreatin, which is reflected by a more than 2-fold increase in standard error of means (Table 3 and Table 4), can help explain these results.
The significant decrease of protein fractions and total protein with extrusion and microwave, as quantified in SDS-PAGE gels, relative to control, very likely indicates protein denaturation and aggregation with a decrease in its solubility. Although, to date, the effect of extrusion on protein extraction has not been reported for A. platensis, this treatment uses a combination of high-pressure and temperature conditions and has been proven to be effective for the extraction of polyunsaturated fatty acids and essential amino acids from Nannochloropsis oceanica biomass [20]. High-pressure was previously shown to disrupt the A. platensis cell wall [8,9] and denature algal proteins [9], whereas high-temperature can change the conformation and structure of proteins and phycocyanobilin chromophores [3,4], causing a reversible followed by a mostly irreversible unfolding and aggregation of proteins [4]. For instance, c-phycocyanin and allophycocyanin are heat-sensitive complexes, and, therefore, when submitted to high temperatures, particularly above 70 °C, a disruption of apoprotein secondary, tertiary and quaternary structures with a consequent relaxation of chromophores into cyclic conformation occurs [3]. Nevertheless, the fact that, even with protein denaturation, extrusion increased the availability of alga protein, mostly from phycocyanin subunits, for hydrolysis with pancreatin should be further explored. Possibly, the denaturation phenomenon enhanced the accessibility of protein-susceptible sites to proteolysis, which were not compromised by protein aggregation, as previously reported [31]. Moreover, the microwave-assisted extraction of proteins from algal biomass has been scarcely reported [19], although this method was described as an effective treatment to disrupt the microalga cell wall [19], leading, for instance, to a 94.9% disruption of Nannochloropsis oculata cells [17]. In addition, Esquivel-Hernández, et al. [32] showed that the use of microwaves could extract 2.28 and 4.11 µg/g of c-phycocyanin and a-phycocyanin, respectively, from A. platensis biomass. The mechanism of compound extraction using microwave radiation consists of electromagnetic waves dissipation in the medium, which causes vibration of water and other polar molecules, leading to an increase in temperature and evaporation of intracellular liquids with consequent high-pressure generation and disruption of cell walls [19]. Considering that, in the present study, the samples of A. platensis biomass were left in the microwave until boiling, it is understandable that, together with the microwave radiation effect, proteins denatured due to a high temperature within the algal structure [4].
The efficiency of bead milling and sonication in enhancing the activity of trypsin and pancreatin, respectively, towards A. platensis proteins was not previously reported, although these methods are widely used to disrupt microalga cell walls and extract algal proteins [33]. Bead milling was shown to increase the in vivo apparent digestibility coefficient of protein in Microchloropsis gaditana from 62 to 78% [34], and, when adapted as a stirred ball mill for disruption of A. platensis cell walls, this procedure led to an increase of up to 5% of in vitro protein digestibility [35]. A previous study using a similar mechanical procedure, i.e., manual grinding, described its effectiveness in disrupting A. platensis cell walls and extracting proteins from algal biomass, although it was less effective than high-pressure homogenization [8]. Ultrasonication was also reported as an efficient method for A. platensis protein extraction due to its effect on degrading algal cell walls, but without surpassing the results obtained with high-pressure technique [8]. Tavanandi, et al. [16] found that this procedure could extract allophycocyanin from A. platensis, particularly improving the yield obtained with enzyme (lysozyme)-associated extraction from 32.3 to 44.1 mg/g dry biomass. A high extraction efficiency for allophycocyanin (up to 93.11%) was also described when the pretreatment was associated with other mechanical/physical methods, such as freezing and thawing [15]. Sonication consists of ultrasound waves propagating in a liquid medium, which causes modifications in low- and high-pressure cycles with the formation of cavitation bubbles followed by their implosion, respectively, with the generation of shear stress that disrupts cell walls [36]. Thus, it was expected that, in the current study, sonication would disrupt the peptidoglycan-rich cell wall of A. platensis and allow pancreatin to hydrolyze total alga proteins, even though it had no effect on the hydrolysis of phycocyanin complexes.
5. Conclusions
Overall, extrusion was shown to be a promising mechanical/physical pretreatment for A. platensis cell wall disruption and enhancement of proteins, particularly phycocyanins, extraction and denaturation/aggregation. The hydrolysis of pigment-protein complexes was significant when the pretreatment was combined with pancreatin. However, extrusion seems to have a strong effect on protein denaturation and aggregation, leading to a significant reduction of total protein in the algal supernatant. This phenomenon requires, in future assays, an adjustment of enzyme dose to the amount of protein available in the supernatant instead of using a fixed enzyme concentration of 20 μg/mL for a better interpretation of the results.
Further studies are needed to understand if the decrease in algal protein solubility and modification of protein conformation would compromise the availability of enzyme active sites for hydrolysis. In addition, the use of other pretreatments, such as microwave, bead milling and sonication, to disrupt A. platensis cell walls and increase the activity of peptidases should not be discarded. In vivo studies are currently being performed by our team to evaluate the efficacy of extrusion associated with pancreatin on increasing the bioaccessibility of proteins in monogastric animals fed with high inclusion levels of A. platensis (up to 15–20% of the feed).
Author Contributions
Conceptualization, J.A.M.P.; data curation, M.P.S. and M.M.C.; formal analysis, M.P.S. and M.M.C.; investigation, M.P.S. and M.M.C.; writing—original draft preparation, M.M.C.; writing—review and editing, M.M.C. and J.A.M.P.; project administration, J.A.M.P.; funding acquisition, J.A.M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação para a Ciência e a Tecnologia grants (Lisbon, Portugal; UI/BD/153071/2022 to M.P.S., UIDB/00276/2020 to CIISA and LA/P/0059/2020 to AL4AnimalS), and by Portugal2020 project (Lisbon, Portugal; P2020/17/SI/70114/2019 and associated researcher contract to M.M.C.).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Martins, C.F.; Ribeiro, D.M.; Costa, M.; Coelho, D.; Alfaia, C.M.; Lordelo, M.; Almeida, A.M.; Freire, J.P.; Prates, J.A. Using microalgae as a sustainable feed resource to enhance quality and nutritional value of pork and poultry meat. Foods 2021, 10, 2933. [Google Scholar] [CrossRef] [PubMed]
- Van Eykelenburg, C. On the morphology and ultrastructure of the cell wall of Spirulina platensis. Antonie van Leeuwenhoek 1977, 43, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Böcker, L.; Hostettler, T.; Diener, M.; Eder, S.; Demuth, T.; Adamcik, J.; Reineke, K.; Leeb, E.; Nyström, L.; Mathys, A. Time-temperature-resolved functional and structural changes of phycocyanin extracted from Arthrospira platensis/Spirulina. Food Chem. 2020, 316, 126374. [Google Scholar] [CrossRef] [PubMed]
- Buecker, S.; Grossmann, L.; Loeffler, M.; Leeb, E.; Weiss, J. Thermal and acidic denaturation of phycocyanin from Arthrospira platensis: Effects of complexation with λ-carrageenan on blue color stability. Food Chem. 2022, 380, 132157. [Google Scholar] [CrossRef]
- Spínola, M.P.; Costa, M.M.; Prates, J.A.M. Digestive constraints of Arthrospira platensis in poultry and swine feeding. Foods 2022, 11, 2984. [Google Scholar] [CrossRef]
- Soto-Sierra, L.; Stoykova, P.; Nikolov, Z.L. Extraction and fractionation of microalgae-based protein products. Algal Res. 2018, 36, 175–192. [Google Scholar] [CrossRef]
- Stramarkou, M.; Papadaki, S.; Kyriakopoulou, K.; Tzovenis, I.; Chronis, M.; Krokida, M. Comparative analysis of different drying techniques based on the qualitative characteristics of Spirulina platensis biomass. J. Aquat. Food Prod. Technol. 2021, 30, 498–516. [Google Scholar] [CrossRef]
- Safi, C.; Ursu, A.V.; Laroche, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Aqueous extraction of proteins from microalgae: Effect of different cell disruption methods. Algal Res. 2014, 3, 61–65. [Google Scholar] [CrossRef]
- Ahmed, J.; Kumar, V. Effect of high-pressure treatment on oscillatory rheology, particle size distribution and microstructure of microalgae Chlorella vulgaris and Arthrospira platensis. Algal Res. 2022, 62, 102617. [Google Scholar] [CrossRef]
- Boukhari, N.; Doumandji, A.; Sabrine Ait chaouche, F.; Ferradji, A. Effect of ultrasound treatment on protein content and functional properties of Spirulina powder grown in Algeria. Med. J. Nutr. Metab. 2018, 11, 235–249. [Google Scholar] [CrossRef]
- Coelho, D.; Lopes, P.A.; Cardoso, V.; Ponte, P.; Bras, J.; Madeira, M.S.; Alfaia, C.M.; Bandarra, N.M.; Fontes, C.M.G.A.; Prates, J.A.M. A two-enzyme constituted mixture to improve the degradation of Arthrospira platensis microalga cell wall for monogastric diets. J. Anim. Physiol. Anim. Nutr. 2020, 104, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Mišurcová, L.; Kráčmar, S.; Klejdus, B.; Vacek, J. Nitrogen content, dietary fiber, and digestibility in algal food products. Czech J. Food Sci. 2010, 28, 27–35. [Google Scholar] [CrossRef]
- Niccolai, A.; Chini Zittelli, G.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of interest as food source: Biochemical composition and digestibility. Algal Res. 2019, 42, 101617. [Google Scholar] [CrossRef]
- Carullo, D.; Donsì, F.; Ferrari, G.; Pataro, G. Extraction improvement of water-soluble compounds from Arthrospira platensis through the combination of high-shear homogenization and pulsed electric fields. Algal Res. 2021, 57, 102341. [Google Scholar] [CrossRef]
- Tavanandi, H.A.; Chandralekha Devi, A.; Raghavarao, K. A newer approach for the primary extraction of allophycocyanin with high purity and yield from dry biomass of Arthrospira platensis. Sep. Purif. Technol. 2018, 204, 162–174. [Google Scholar] [CrossRef]
- Tavanandi, H.A.; Vanjari, P.; Raghavarao, K.S.M.S. Synergistic method for extraction of high purity allophycocyanin from dry biomass of Arthrospira platensis and utilization of spent biomass for recovery of carotenoids. Sep. Purif. Technol. 2019, 225, 97–111. [Google Scholar] [CrossRef]
- McMillan, J.R.; Watson, I.A.; Ali, M.; Jaafar, W. Evaluation and comparison of algal cell disruption methods: Microwave, waterbath, blender, ultrasonic and laser treatment. Appl. Energy 2013, 103, 128–134. [Google Scholar] [CrossRef]
- Safi, C.; Frances, C.; Ursu, A.V.; Laroche, C.; Pouzet, C.; Vaca-Garcia, C.; Pontalier, P.-Y. Understanding the effect of cell disruption methods on the diffusion of Chlorella vulgaris proteins and pigments in the aqueous phase. Algal Res. 2015, 8, 61–68. [Google Scholar] [CrossRef]
- Kapoore, R.V.; Butler, T.O.; Pandhal, J.; Vaidyanathan, S. Microwave-assisted extraction for microalgae: From biofuels to biorefinery. Biology 2018, 7, 18. [Google Scholar] [CrossRef]
- Wang, M.; Cheng, H.; Chen, S.; Wen, S.; Wu, X.; Zhang, D.; Yuan, Q.; Cong, W. Microalgal cell disruption via extrusion for the production of intracellular valuables. Energy 2018, 142, 339–345. [Google Scholar] [CrossRef]
- Bermúdez Menéndez, J.M.; Arenillas, A.; Menéndez Díaz, J.Á.; Boffa, L.; Mantegna, S.; Binello, A.; Cravotto, G. Optimization of microalgae oil extraction under ultrasound and microwave irradiation. J. Chem. Technol. Biotechnol. 2014, 89, 1779–1784. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Ritchie, R.J. Universal chlorophyll equations for estimating chlorophylls a, b, c, and d and total chlorophylls in natural assemblages of photosynthetic organisms using acetone, methanol, or ethanol solvents. Photosynthetica 2008, 46, 115–126. [Google Scholar] [CrossRef]
- Costa, M.; Pio, L.; Bule, P.; Cardoso, V.; Alfaia, C.M.; Coelho, D.; Brás, J.; Fontes, C.M.G.A.; Prates, J.A.M. An individual alginate lyase is effective in the disruption of Laminaria digitata recalcitrant cell wall. Sci. Rep. 2021, 11, 9706. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.M.; Pio, L.B.; Bule, P.; Cardoso, V.A.; Duarte, M.; Alfaia, C.M.; Coelho, D.F.; Brás, J.A.; Fontes, C.M.G.A.; Prates, J.A.M. Recalcitrant cell wall of Ulva lactuca seaweed is degraded by a single ulvan lyase from family 25 of polysaccharide lyases. Anim. Nutr. 2022, 9, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Vizcaíno, A.J.; Sáez, M.I.; Martínez, T.F.; Acién, F.G.; Alarcón, F.J. Differential hydrolysis of proteins of four microalgae by the digestive enzymes of gilthead sea bream and Senegalese sole. Algal Res. 2019, 37, 145–153. [Google Scholar] [CrossRef]
- Alarcón, F.J.; Moyano, F.J.; Díaz, M. Use of SDS-PAGE in the assessment of protein hydrolysis by fish digestive enzymes. Aquac. Int. 2001, 9, 255–267. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Sedighi, M.; Jalili, H.; Darvish, M.; Sadeghi, S.; Ranaei-Siadat, S.-O. Enzymatic hydrolysis of microalgae proteins using serine proteases: A study to characterize kinetic parameters. Food Chem. 2019, 284, 334–339. [Google Scholar] [CrossRef]
- Church, F.C.; Porter, D.H.; Catignani, G.L.; Swaisgood, H.E. An o-phthalaldehyde spectrophotometric assay for proteinases. Anal. Biochem. 1985, 146, 343–348. [Google Scholar] [CrossRef]
- Carbonaro, M.; Cappelloni, M.; Nicoli, S.F.; Lucarini, M.; Carnovale, E. Solubility-digestibility relationship of legume proteins. J. Agric. Food Chem. 1997, 45, 3387–3394. [Google Scholar] [CrossRef]
- Esquivel-Hernández, D.A.; Rodríguez-Rodríguez, J.; Rostro-Alanis, M.; Cuéllar-Bermúdez, S.P.; Mancera-Andrade, E.I.; Núñez-Echevarría, J.E.; García-Pérez, J.S.; Chandra, R.; Parra-Saldívar, R. Advancement of green process through microwave-assisted extraction of bioactive metabolites from Arthrospira platensis and bioactivity evaluation. Bioresour. Technol. 2017, 224, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Demarco, M.; Oliveira de Moraes, J.; Matos, Â.P.; Derner, R.B.; de Farias Neves, F.; Tribuzi, G. Digestibility, bioaccessibility and bioactivity of compounds from algae. Trends Food Sci. Technol. 2022, 121, 114–128. [Google Scholar] [CrossRef]
- Teuling, E.; Wierenga, P.A.; Agboola, J.O.; Gruppen, H.; Schrama, J.W. Cell wall disruption increases bioavailability of Nannochloropsis gaditana nutrients for juvenile Nile tilapia (Oreochromis niloticus). Aquaculture 2019, 499, 269–282. [Google Scholar] [CrossRef]
- Wild, K.J.; Steingaß, H.; Rodehutscord, M. Variability in nutrient composition and in vitro crude protein digestibility of 16 microalgae products. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1306–1319. [Google Scholar] [CrossRef]
- Suslick, K.S.; Price, G.J. Applications of ultrasound to materials chemistry. Annu. Rev. Mater. Sci. 1999, 29, 295–326. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).