Influence of Osmotic, Salt, and Combined Stress on Morphophysiological Parameters of Chenopodium quinoa Photosynthetic Organs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Growth Conditions
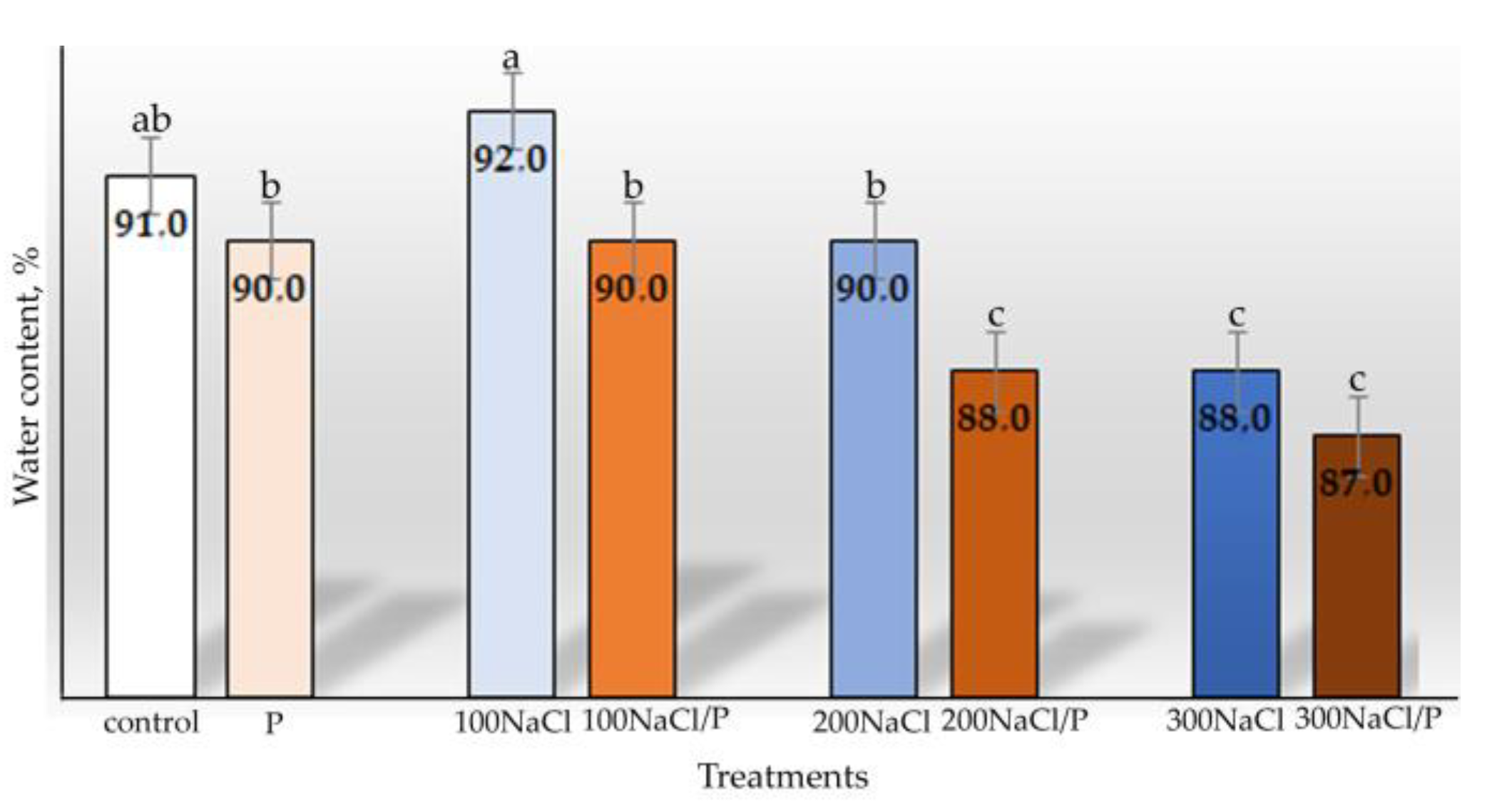
2.3. Water Content Determination
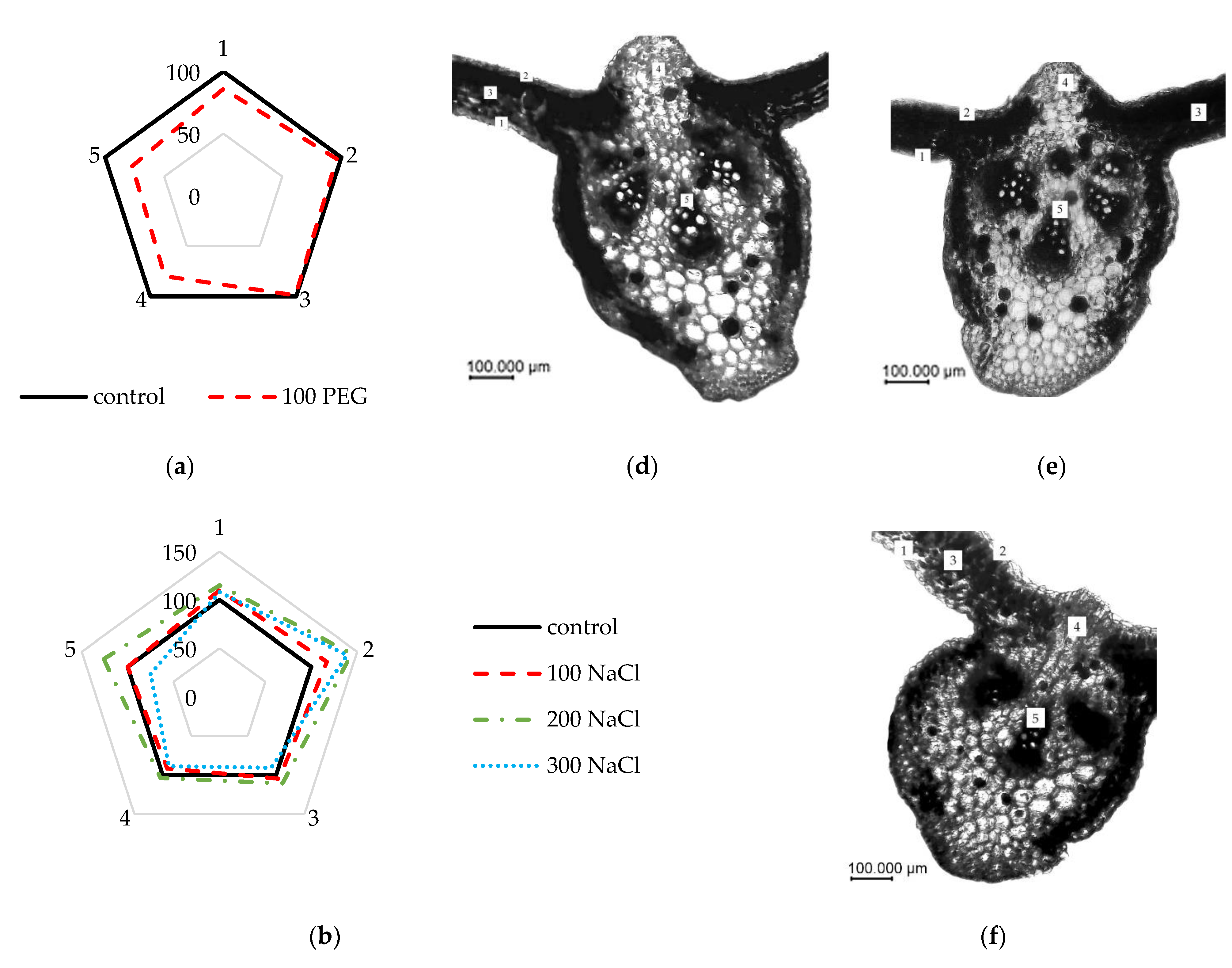
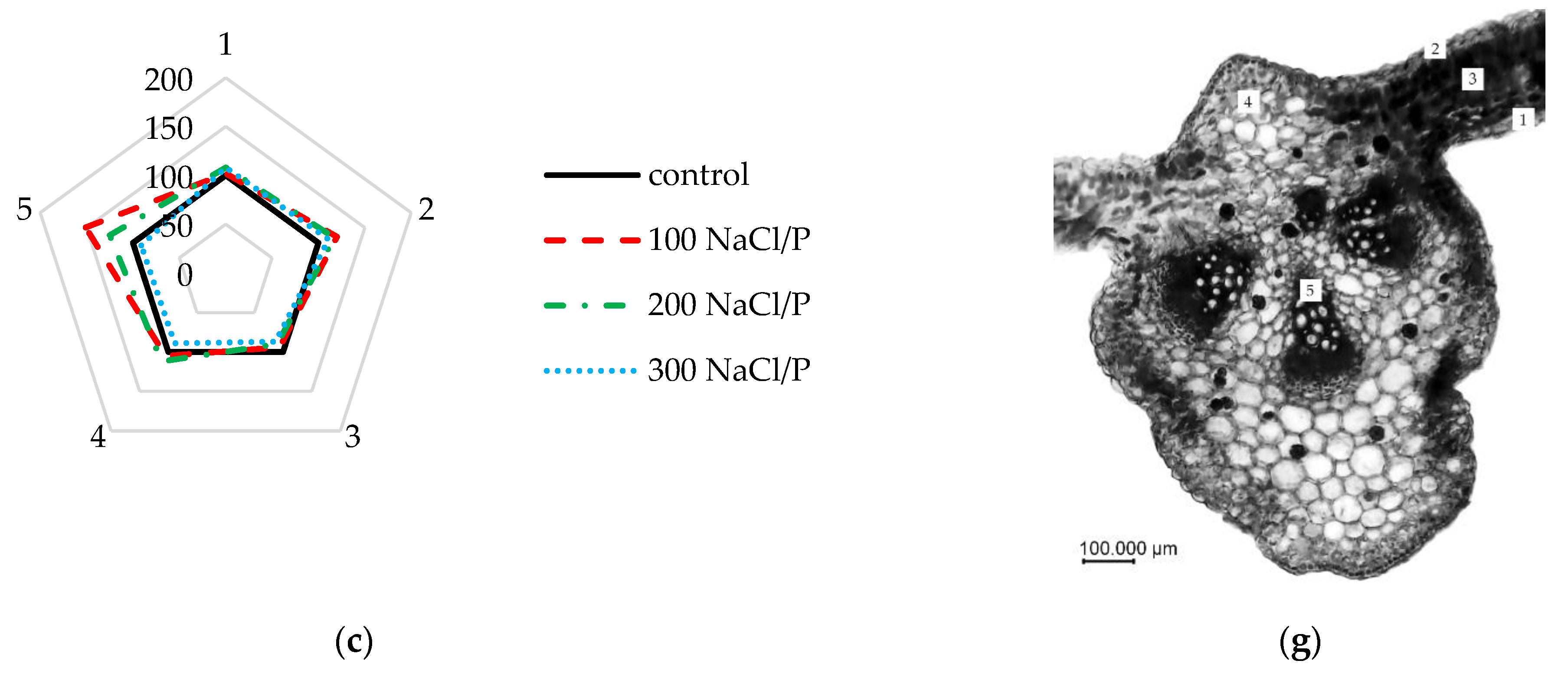
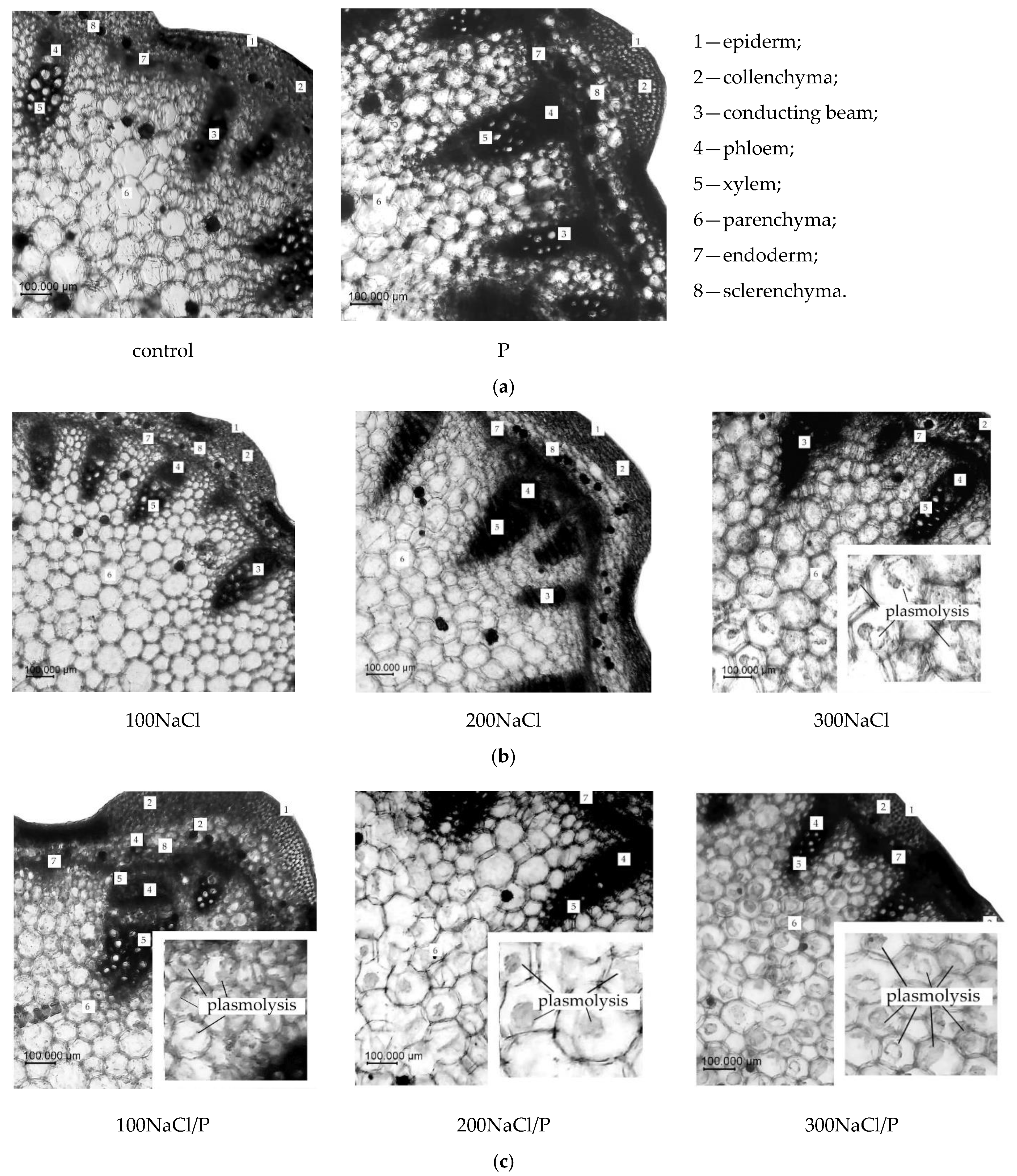
2.4. Analysis of Elements Changes in the Anatomical Structure
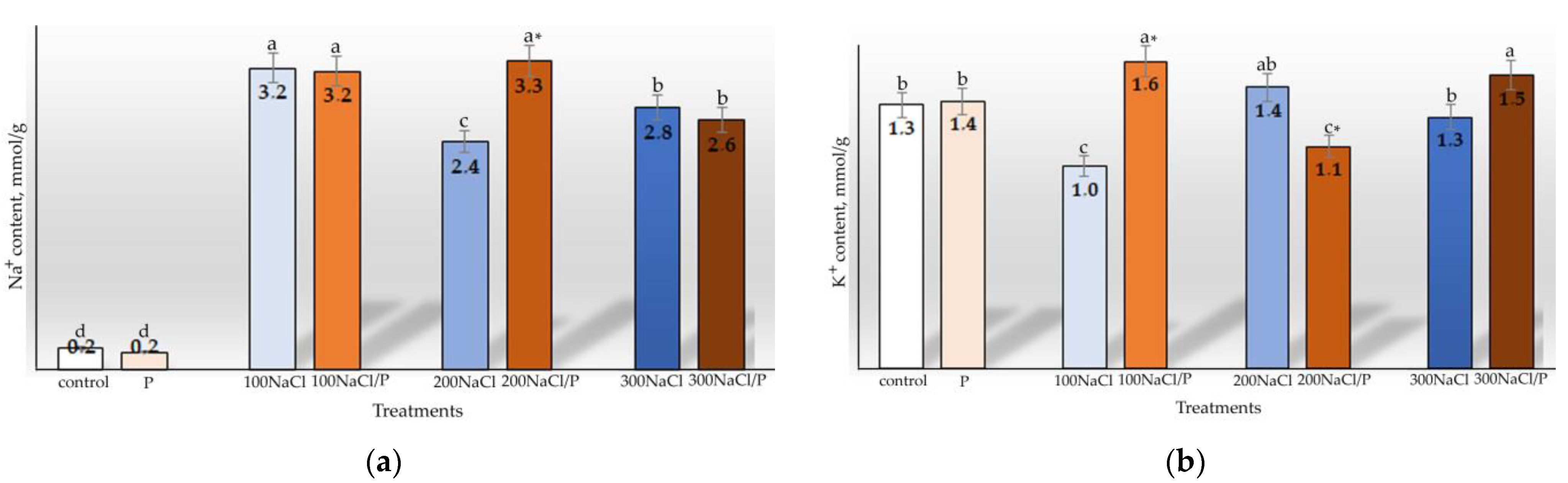
2.5. Determination of Ion Balance in Plant Tissues
2.6. Statistical Analysis
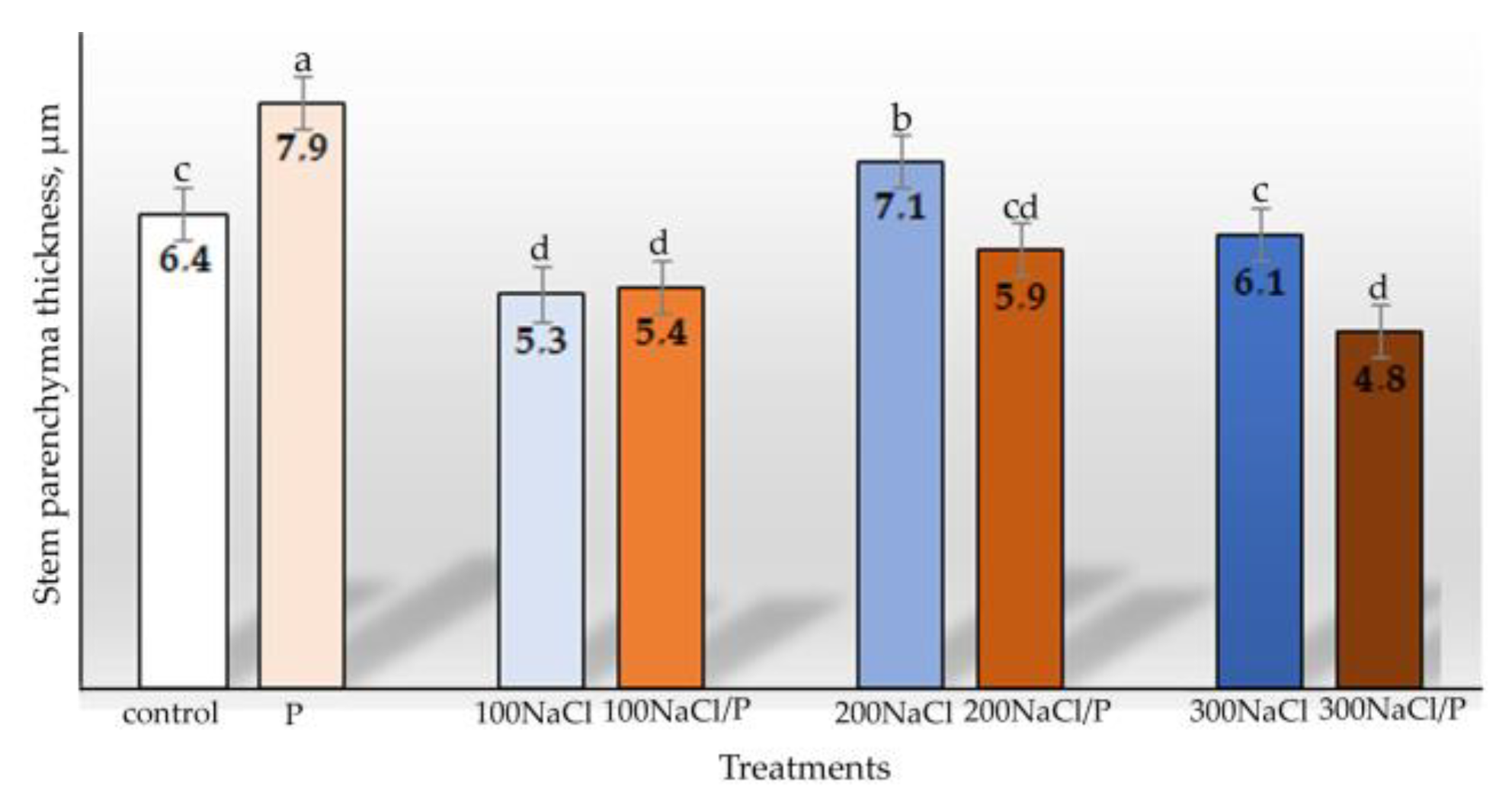
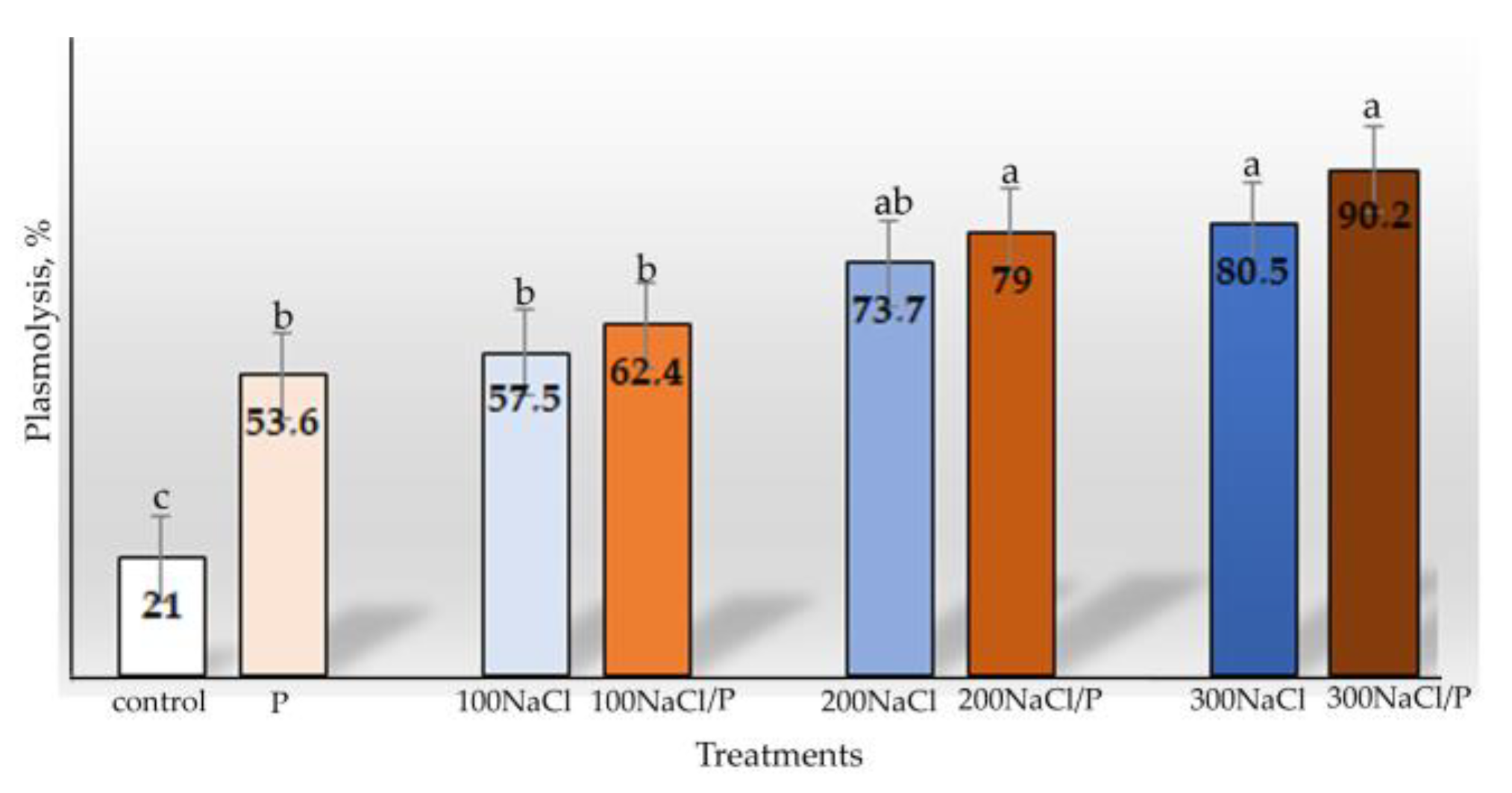
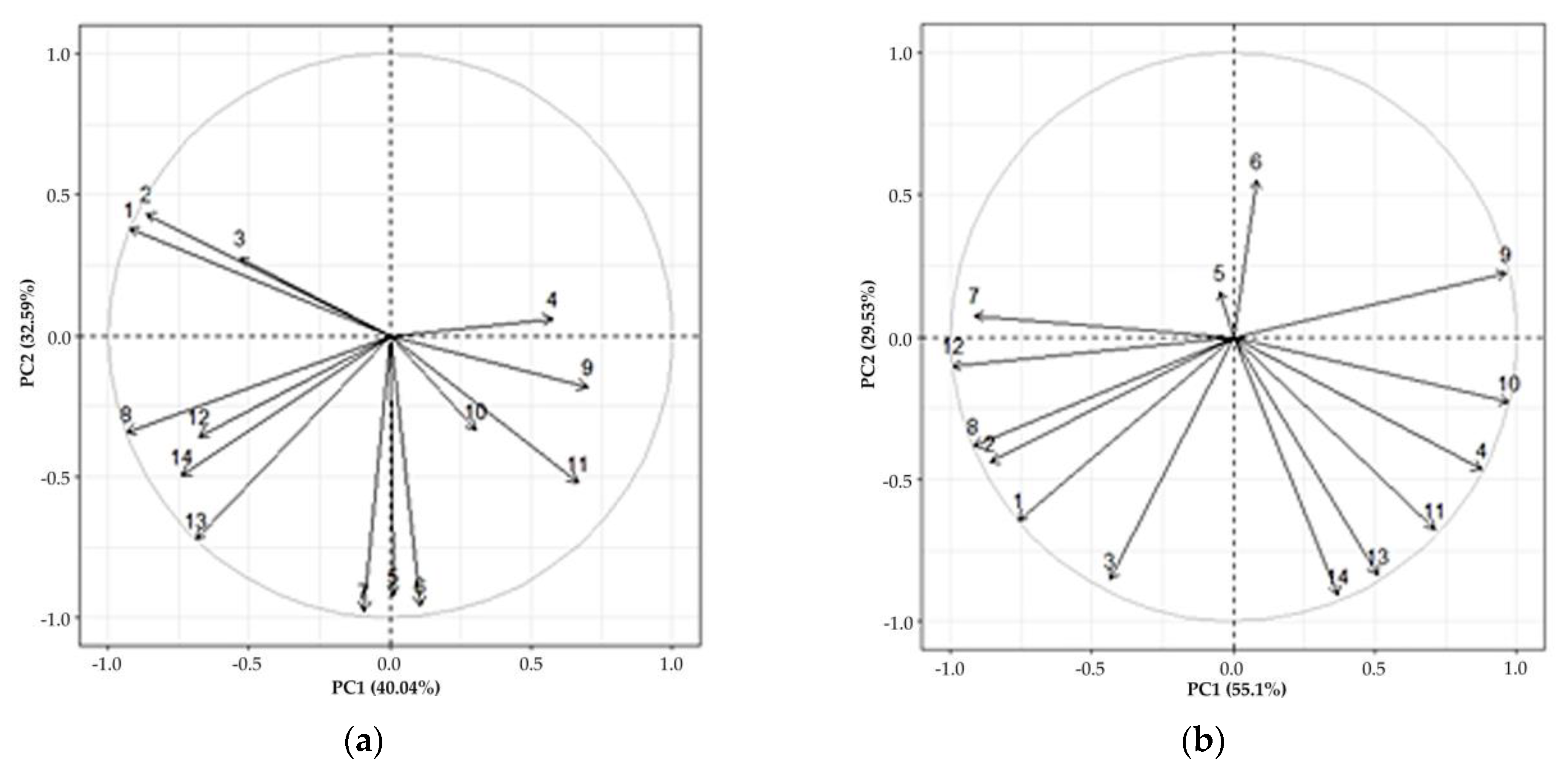
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hinojosa, L.; González, J.A.; Barrios-Masias, F.H.; Fuentes, F.; Murphy, K.M. Quinoa Abiotic Stress Responses: A Review. Plants 2018, 7, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razzaghi, F.; Ahmadi, S.H.; Jacobsen, S.-E.; Jensen, C.R.; Andersen, M.N. Effects of Salinity and Soil–Drying on Radiation Use Efficiency, Water Productivity and Yield of Quinoa (Chenopodium quinoa Willd.). J. Agron. Crop Sci. 2012, 198, 173–184. [Google Scholar] [CrossRef]
- Toderich, K.N.; Mamadrahimov, A.A.; Khaitov, B.B.; Karimov, A.A.; Soliev, A.A.; Nanduri, K.R.; Shuyskaya, E.V. Differential Impact of Salinity Stress on Seeds Minerals, Storage Proteins, Fatty Acids, and Squalene Composition of New Quinoa Genotype, Grown in Hyper-Arid Desert Environments. Front. Plant Sci. 2020, 11, 607102. [Google Scholar] [CrossRef] [PubMed]
- Rezzouk, F.Z.; Shahid, M.A.; Elouafi, I.A.; Zhou, B.; Araus, J.L.; Serret, M.D. Agronomic Performance of Irrigated Quinoa in Desert Areas: Comparing Different Approaches for Early Assessment of Salinity Stress. Agric. Water Manag. 2020, 240, 106205. [Google Scholar] [CrossRef]
- Iqbal, S.; Maqsood Ahmed Basra, S.; Afzal, I.; Wahid, A. Exploring Potential of Well Adapted Quinoa Lines for Salt Tolerance. Int. J. Agric. Biol. 2017, 19, 933–940. [Google Scholar] [CrossRef]
- Adolf, V.I.; Jacobsen, S.-E.; Shabala, S. Salt Tolerance Mechanisms in Quinoa (Chenopodium quinoa Willd.). Environ. Exp. Bot. 2013, 92, 43–54. [Google Scholar] [CrossRef]
- Prado, F.E.; Hilal, M.B.; Albornoz, P.L.; Gallardo, M.; Ruíz, V. Anatomical and Physiological Responses of Four Quinoa Cultivars to Salinity at Seedling Stage. Indian J. Sci. Technol. 2017, 10, 1–12. [Google Scholar] [CrossRef]
- Bhargava, A.; Srivastava, S. Response of Amaranthus sp. to Salinity Stress: A Review. In Emerging Research in Alternative Crops; Hirich, A., Choukr-Allah, R., Ragab, R., Eds.; Environment & Policy; Springer: Cham, Switzerland, 2020; pp. 245–263. ISBN 978-3-319-90472-6. [Google Scholar]
- Asher, A.; Galili, S.; Whitney, T.; Rubinovich, L. The Potential of Quinoa (Chenopodium quinoa) Cultivation in Israel as a Dual-Purpose Crop for Grain Production and Livestock Feed. Sci. Hortic. 2020, 272, 109534. [Google Scholar] [CrossRef]
- Nanduri, K.R.; Hirich, A.; Salehi, M.; Saadat, S.; Jacobsen, S.E. Quinoa: A New Crop for Harsh Environments. In Sabkha Ecosystems: Volume VI: Asia/Pacific; Gul, B., Böer, B., Khan, M.A., Clüsener-Godt, M., Hameed, A., Eds.; Tasks for Vegetation Science; Springer: Cham, Switzerland, 2019; pp. 301–333. ISBN 978-3-030-04417-6. [Google Scholar]
- Zurita-Silva, A.; Fuentes, F.; Zamora, P.; Jacobsen, S.-E.; Schwember, A.R. Breeding Quinoa (Chenopodium quinoa Willd.): Potential and Perspectives. Mol. Breed. 2014, 34, 13–30. [Google Scholar] [CrossRef]
- Choukr-Allah, R.; Rao, N.K.; Hirich, A.; Shahid, M.; Alshankiti, A.; Toderich, K.; Gill, S.; Butt, K.U.R. Quinoa for Marginal Environments: Toward Future Food and Nutritional Security in MENA and Central Asia Regions. Front. Plant Sci. 2016, 7, 346. [Google Scholar] [CrossRef]
- Jacobsen, S.-E.; Mujica, A.; Jensen, C.R. The Resistance of Quinoa (Chenopodium quinoa Willd.) to Adverse Abiotic Factors. Food Rev. Int. 2003, 19, 99–109. [Google Scholar] [CrossRef]
- Ruiz, K.B.; Biondi, S.; Oses, R.; Acuña-Rodríguez, I.S.; Antognoni, F.; Martinez-Mosqueira, E.A.; Coulibaly, A.; Canahua-Murillo, A.; Pinto, M.; Zurita-Silva, A.; et al. Quinoa Biodiversity and Sustainability for Food Security under Climate Change. A Review. Agron. Sustain. Dev. 2014, 34, 349–359. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, K.B.; Biondi, S.; Martínez, E.A.; Orsini, F.; Antognoni, F.; Jacobsen, S.-E. Quinoa—A Model Crop for Understanding Salt-Tolerance Mechanisms in Halophytes. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2016, 150, 357–371. [Google Scholar] [CrossRef]
- Cuevas, J.; Daliakopoulos, I.N.; del Moral, F.; Hueso, J.J.; Tsanis, I.K. A Review of Soil-Improving Cropping Systems for Soil Salinization. Agronomy 2019, 9, 295. [Google Scholar] [CrossRef] [Green Version]
- Tebini, M.; Luu, D.T.; Mguis, K.; Ben Ahmed, H.; Meddich, A.; Zribi, F.; Chalh, A. Physiological Exploration of Intra-Specific Variability in Salinity Tolerance of Amaranth. Russ. J. Plant Physiol. 2022, 69, 59. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahid, S.; Shahbaz, M.; Maqsood, M.F.; Farhat, F.; Zulfiqar, U.; Javed, T.; Fraz, A.M.; Alhomrani, M.; Alamri, A.S. Proline-Induced Modifications in Morpho-Physiological, Biochemical and Yield Attributes of Pea (Pisum sativum L.) Cultivars under Salt Stress. Sustainability 2022, 14, 13579. [Google Scholar] [CrossRef]
- Sánchez-Bermúdez, M.; del Pozo, J.C.; Pernas, M. Effects of Combined Abiotic Stresses Related to Climate Change on Root Growth in Crops. Front. Plant Sci. 2022, 13, 918537. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Tansley review abiotic and biotic stress combinations. N. Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
- Mahmood, U.; Hussain, S.; Hussain, S.; Ali, B.; Ashraf, U.; Zamir, S.; Al-Robai, S.A.; Alzahrani, F.O.; Hano, C.; El-Esawi, M.A. Morpho-physio-biochemical and Molecular Responses of Maize Hybrids to Salinity and Waterlogging during Stress and Recovery Phase. Plants 2021, 10, 1345. [Google Scholar] [CrossRef]
- Hussain, S.; Mehmood, U.; Ashraf, U.; Naseer, M.A. Combined Salinity and Waterlogging Stress in Plants: Limitations and Tolerance Mechanisms. In Climate Change and Crop Stress; Academic Press: Cambridge, MA, USA, 2022; pp. 95–112. [Google Scholar] [CrossRef]
- Reynolds-Henne, C.E.; Langenegger, A.; Mani, J.; Schenk, N.; Zumsteg, A.; Feller, U. Interactions between Temperature, Drought and Stomatal Opening in Legumes. Environ. Exp. Bot. 2010, 68, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, N.J.; Urwin, P.E. The interaction of plant biotic and abiotic stresses: From genes to the field. J. Exp. Bot. 2012, 63, 3523–3543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shabbir, R.; Singhal, R.K.; Mishra, U.N.; Chauhan, J.; Javed, T.; Hussain, S.; Kumar, S.; Anuragi, H.; Lal, D.; Chen, P. Combined Abiotic Stresses: Challenges and Potential for Crop Improvement. Agronomy 2022, 12, 2795. [Google Scholar] [CrossRef]
- Quan, R.; Lin, H.; Mendoza, I.; Zhang, Y.; Cao, W.; Yang, Y.; Shang, M.; Chen, S.; Pardo, J.M.; Guo, Y. SCABP8/CBL10, a Putative Calcium Sensor, Interacts with the Protein Kinase SOS2 to Protect Arabidopsis Shoots from Salt Stress. Plant Cell 2007, 19, 1415–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.J.; Kim, W.-Y.; Yun, D.-J. A Role for GIGANTEA. Plant Signal. Behav. 2013, 8, e24820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debez, A.; Ben Hamed, K.; Grignon, C.; Abdelly, C. Salinity Effects on Germination, Growth, and Seed Production of the Halophyte Cakile Maritima. Plant Soil 2004, 262, 179–189. [Google Scholar] [CrossRef]
- Gul, B.; Ansari, R.; Flowers, T.J.; Khan, M.A. Germination Strategies of Halophyte Seeds under Salinity. Environ. Exp. Bot. 2013, 92, 4–18. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin III, F.S.; Pons, T.L. Plant Physiological Ecology, 2nd ed.; Springer: New York, NY, USA, 2008; ISBN 978-0-387-78340-6. [Google Scholar]
- Mittler, R. Abiotic Stress, the Field Environment and Stress Combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Pandey, P.; Ramegowda, V.; Senthil-Kumar, M. Shared and Unique Responses of Plants to Multiple Individual Stresses and Stress Combinations: Physiological and Molecular Mechanisms. Front. Plant Sci. 2015, 6, 723. [Google Scholar] [CrossRef]
- Poljakoff-Mayber, A. Morphological and Anatomical Changes in Plants as a Response to Salinity Stress. In Plants in Saline Environments; Poljakoff-Mayber, A., Gale, J., Eds.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 1975; pp. 97–117. ISBN 978-3-642-80929-3. [Google Scholar]
- Munns, R.; James, R.A. Screening Methods for Salinity Tolerance: A Case Study with Tetraploid Wheat. Plant Soil 2003, 253, 201–218. [Google Scholar] [CrossRef]
- Hameed, M.; Ashraf, M.; Naz, N. Anatomical Adaptations to Salinity in Cogon Grass [Imperata cylindrica (L.) Raeuschel] from the Salt Range, Pakistan. Plant Soil 2009, 322, 229–238. [Google Scholar] [CrossRef]
- Ali, Q.; Ashraf, M. Induction of Drought Tolerance in Maize (Zea mays L.) due to Exogenous Application of Trehalose: Growth, Photosynthesis, Water Relations and Oxidative Defence Mechanism. J. Agron. Crop Sci. 2011, 97, 258–271. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under Stressful Environments: An Overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Mogazy, A.M.; Seleem, E.A.; Mohamed, G.F. Mitigating the Harmful Effects of Water Deficiency Stress on White Lupine (Lupinus albus L.) Plants using Algae Extract and Hydrogen Peroxide. J. Plant Prod. 2020, 11, 921–931. [Google Scholar] [CrossRef]
- Abobatta, W.F. Plant Responses and Tolerance to Combined Salt and Drought Stress. In Salt and Drought Stress Tolerance in Plants; Springer: Cham, Switzerland, 2020; pp. 17–52. [Google Scholar] [CrossRef]
- Barykina, R.; Veselova, T.; Devyatov, A.; Dzhalilova, K.K.; Ilyina, G.; Chubatova, N. Guide on Botanical Microtechique. In Basics Methods; MSU: Moscow, Russia, 2004; 312p. (In Russian) [Google Scholar]
- Hariadi, Y.; Marandon, K.; Tian, Y.; Jacobsen, S.-E.; Shabala, S. Ionic and Osmotic Relations in Quinoa (Chenopodium quinoa Willd.) Plants Grown at Various Salinity Levels. J. Exp. Bot. 2011, 62, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Shabala, S. Learning from Halophytes: Physiological Basis and Strategies to Improve Abiotic Stress Tolerance in Crops. Ann. Bot. 2013, 112, 1209–1221. [Google Scholar] [CrossRef]
- Shabala, S.; Bose, J.; Hedrich, R. Salt Bladders: Do They Matter? Trends Plant Sci. 2014, 19, 687–691. [Google Scholar] [CrossRef]
- Gonzales, J.A.; Prado, F.E. Germination in Relation to Salinity and Temperature in Chenopodium quinoa (Willd.). Agrochim. Italy 1992, 36, 101–107. [Google Scholar]
- Orsini, F.; Accorsi, M.; Gianquinto, G.; Dinelli, G.; Antognoni, F.; Carrasco, K.B.R.; Martinez, E.A.; Alnayef, M.; Marotti, I.; Bosi, S.; et al. Beyond the Ionic and Osmotic Response to Salinity in Chenopodium quinoa: Functional Elements of Successful Halophytism. Funct. Plant Biol. 2011, 38, 818–831. [Google Scholar] [CrossRef]
- Prado, F.E.; Boero, C.; Gallardo, M.R.A.; González, J.A. Effect of NaCl on Growth Germination and Soluble Sugars Content in Chenopodium quinoa Willd. Seeds 2000, 41, 27–34. [Google Scholar]
- Eisa, S.S.; Eid, M.A.; Abd, E.-S.E.H.; Hussin, S.A.; Abdel Ati, A.A.; El Bordeny, N.E.; Ali, S.H.; Al Sayed Hanan, M.A.; Lotfy, M.E.; Masoud, A.M.; et al. “Chenopodium quinoa” Willd. A New Cash Crop Halophyte for Saline Regions of Egypt. Aust. J. Crop Sci. 2017, 11, 343–351. [Google Scholar] [CrossRef]
- El-Afry, M.M. Anatomical Studies on Drought-Stressed Wheat Plants (Triticum aestivum L.) Treated with Some Bacterial Strains. Acta Biol. Szeged. 2012, 56, 165–174. [Google Scholar]
- Fghire, R.; Anaya, F.; Ali, O.I.; Benlhabib, O.; Ragab, R.; Wahbi, S. Physiological and Photosynthetic Response of Quinoa to Drought Stress. Chil. J. Agric. Res. 2015, 75, 174–183. [Google Scholar] [CrossRef] [Green Version]
- Terletskaya, N.V.; Lee, T.E.; Altayeva, N.A.; Kudrina, N.O.; Blavachinskaya, I.V.; Erezhetova, U. Some Mechanisms Modulating the Root Growth of Various Wheat Species under Osmotic-Stress Conditions. Plants 2020, 9, 1545. [Google Scholar] [CrossRef]
- Rivero, R.M.; Mestre, T.C.; Mittler, R.O.; Rubio, F.; Garcia-Sanchez, F.; Martinez, V. The Combined Effect of Salinity and Heat Reveals a Specific Physiological, Biochemical and Molecular Response in Tomato Plants. Plant Cell Environ. 2014, 37, 1059–1073. [Google Scholar] [CrossRef]
- Munns, R.; James, R.A.; Läuchli, A. Approaches to Increasing the Salt Tolerance of Wheat and Other Cereals. J. Exp. Bot. 2006, 57, 1025–1043. [Google Scholar] [CrossRef] [Green Version]
- Naz, N.; Rafique, T.; Hameed, M.; Ashraf, M.; Batool, R.; Fatima, S. Morpho-Anatomical and Physiological Attributes for Salt Tolerance in Sewan Grass (Lasiurus scindicus Henr.) from Cholistan Desert, Pakistan. Acta Physiol. Plant. 2014, 36, 2959–2974. [Google Scholar] [CrossRef]
- Hameed, M.; Ashraf, M.; Ahmad, M.S.A.; Naz, N. Structural and Functional Adaptations in Plants for Salinity Tolerance. In Plant Adaptation and Phytoremediation; Ashraf, M., Ozturk, M., Ahmad, M.S.A., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 151–170. ISBN 978-90-481-9370-7. [Google Scholar]
- Bonales-Alatorre, E.; Pottosin, I.; Shabala, L.; Chen, Z.-H.; Zeng, F.; Jacobsen, S.-E.; Shabala, S. Differential Activity of Plasma and Vacuolar Membrane Transporters Contributes to Genotypic Differences in Salinity Tolerance in a Halophyte Species, Chenopodium quinoa. Int. J. Mol. Sci. 2013, 14, 9267–9285. [Google Scholar] [CrossRef] [Green Version]
- Carden, D.E.; Walker, D.J.; Flowers, T.J.; Miller, A.J. Single-Cell Measurements of the Contributions of Cytosolic Na+ and K+ to Salt Tolerance. Plant Physiol. 2003, 131, 676–683. [Google Scholar] [CrossRef] [Green Version]
- Terletskaya, N.; Duisenbayeva, U.; Rysbekova, A.; Kurmanbayeva, M.; Blavachinskaya, I. Architectural Traits in Response to Salinity of Wheat Primary Roots. Acta Physiol. Plant. 2019, 41, 157. [Google Scholar] [CrossRef]
- Reinhardt, D.H.; Rost, T.L. Salinity Accelerates Endodermal Development and Induces an Exodermis in Cotton Seedling Roots. Environ. Exp. Bot. 1995, 35, 563–574. [Google Scholar] [CrossRef]
- Rashid, P.; Yasmin, F.; Karmoker, J. Effects of Salinity on Ion Transport and Anatomical Structure in Wheat (Triticum aestivum L. cv. Kanchan). Bangladesh J. Bot. 2001, 30, 65–69. [Google Scholar]
- Bahaji, A.; Mateu, I.; Sanz, A.; Cornejo, M.J. Common and Distinctive Responses of Rice Seedlings to Saline- and Osmotically-Generated Stress. Plant Growth Regul. 2002, 38, 83–94. [Google Scholar] [CrossRef]
- Terletskaya, N.; Kurmanbayeva, M. Change in Leaf Anatomical Parameters of Seedlings of Different Wheat Species under Conditions of Drought and Salt Stress. Pak. J. Bot. 2017, 49, 857–865. [Google Scholar]
- Al-Naggar, A.; Abd El-Salam, R.; Badran, A.; Boulos, S.; El-Moghazi Mai, M. Leaf Anatomical Characteristics and Its Heritability of Different Quinoa (Chenopodium auinoa Willd.) Genotypes as Influenced by Moderate and Severe Water Stress. Asian J. Biol. 2017, 4, 1–14. [Google Scholar] [CrossRef]
- Panfilova, O.V.; Golyaev, O.D. Adaptation of Currant to Action of a Drought and Abnormally High Temperatures (Review). Mod. Gard. 2015, 2, 88–98. [Google Scholar]
- Abd Elbar, O.H.; Farag, R.E.; Shehata, S.A. Effect of Putrescine Application on Some Growth, Biochemical and Anatomical Characteristics of Thymus vulgaris L. under Drought Stress. Ann. Agric. Sci. 2019, 64, 129–137. [Google Scholar] [CrossRef]
- Agami, R.A. Salicylic Acid Mitigates the Adverse Effect of Water Stress on Lettuce (Lactuca sativa L.). J. Appl. Sci. Res. 2013, 9, 5701–5711. [Google Scholar]
- Zhang, F.-J.; Zhang, K.-K.; Du, C.-Z.; Li, J.; Xing, Y.-X.; Yang, L.-T.; Li, Y.-R. Effect of Drought Stress on Anatomical Structure and Chloroplast Ultrastructure in Leaves of Sugarcane. Sugar Tech 2015, 17, 41–48. [Google Scholar] [CrossRef]
- Assem, N.; El Hafid, L.; Lamchouri, F. Impact of Water Stress on the Leaf Tissues of Two Moroccan Varieties of Durum Wheat (Triticum durum Desf). J. Mater. 2017, 8, 3583–3487. [Google Scholar]
- Ortega, L.; Fry, S.C.; Taleisnik, E. Why are Chloris gayana Leaves Shorter in Salt-Affected Plants? Analyses in the elongation zone. J. Exp. Bot. 2006, 57, 3945–3952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shepelov, V.V.; Malasai, V.M.; Penzev, A.F.; Kochmarsky, V.S.; Shelepov, A.V. Morphoobiology, Biology, Economic Value of Wheat; Mironovsky Institute of Wheat by V.N. Remeslo: Mironivka, Ukraine, 2004. [Google Scholar]
- Azmi, A.R.; Alam, S.M. Effect of Salt Stress on Germination, Growth, Leaf Anatomy and Mineral Element Composition of Wheat Cultivars. Acta Physiol. Plant. 1990, 12, 215–224. [Google Scholar]
- Munns, R. Comparative Physiology of Salt and Water Stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, A.J.; Murphy, K.M. Quinoa Cultivation for Temperate North America: Considerations and Areas for Investigation. In Quinoa: Improvement and Sustainable Production; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 173–192. ISBN 978-1-118-62804-1. [Google Scholar]
- Yolcu, S. Determination of Sowing and Harvest Times on Hay Yield and Quality of Quinoa (Chenopodium quinoa Willd.) Plant Grown under Irrigated Conditions in Igdir. Ph.D. Thesis, Igdir University, Igdir, Turkey, 2018. [Google Scholar]
- Jurin, J., II. An Account of Some Experiments Shown before the Royal Society; with an Enquiry into the Cause of the Ascent and Suspension of Water in Capillary Tubes. Philos. Trans. R. Soc. Lond. 1718, 30, 739–747. [Google Scholar] [CrossRef]
- Munns, R.; Gilliham, M. Salinity Tolerance of Crops—What Is the Cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef]


| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|
| Control | P | 100NaCl | 200NaCl | 300NaCl | 100 NaCl/P | 200NaCl/P | 300NaCl/P |
| 14 days, solution 50% Hoagland | 10 days per solution 50% Hoagland + 4 days PEG-6000 at a concentration of 12.5% (m/v) | 14 days, solution 50% Hoagland + 100 mM NaCl | 14 days, solution 50% Hoagland + 200 mM NaCl | 14 days, solution 50% Hoagland + 300 mM NaCl | 10 days per solution 50% Hoagland + 100 mM NaCl + 4 days PEG-6000 | 10 days per solution 50% Hoagland + 200 mM NaCl + 4 days PEG-6000 | 10 days per solution 50% Hoagland + 300 mM NaCl + 4 days PEG-6000 |
| Variable and Source of Variation | df | F | p | Variable and Source of Variation | df | F | p |
|---|---|---|---|---|---|---|---|
| Plantgrowth | Area of stem xylem | ||||||
| Osmotic stress | 1 | 3.044 | 0.100 | Osmotic stress | 1 | 1.560 | 0.230 |
| Salt stress | 3 | 14.720 | <0.001 | Salt stress | 3 | 12.930 | <0.001 |
| Combined stress | 3 | 0.381 | 0.768 | Combined stress | 3 | 3.968 | 0.027 |
| Water content | Percentage of plasmolysis in cells | ||||||
| Osmotic stress | 1 | 7.149 | 0.017 | Osmotic stress | 1 | 6.723 | 0.020 |
| Salt stress | 3 | 7.624 | 0.002 | Salt stress | 3 | 17.373 | <0.001 |
| Combined stress | 3 | 0.337 | 0.799 | Combined stress | 3 | 1.691 | 0.209 |
| Leaf biomass | The thickness of the adaxial leaf epidermis | ||||||
| Osmotic stress | 1 | 123.183 | <0.001 | Osmotic stress | 1 | 2.909 | 0.107 |
| Salt stress | 3 | 86.311 | <0.001 | Salt stress | 3 | 4.048 | 0.026 |
| Combined stress | 3 | 3.472 | 0.041 | Combined stress | 3 | 0.465 | 0.711 |
| Na+ ion content | The thickness of the abaxial leaf epidermis | ||||||
| Osmotic stress | 1 | 3.120 | 0.096 | Osmotic stress | 1 | 3.207 | 0.092 |
| Salt stress | 3 | 192.443 | <0.001 | Salt stress | 3 | 3.257 | 0.049 |
| Combined stress | 3 | 6.176 | 0.005 | Combined stress | 3 | 1.106 | 0.376 |
| K+ ion content | Thickness of mesophyll | ||||||
| Osmotic stress | 1 | 4.289 | 0.055 | Osmotic stress | 1 | 2.362 | 0.144 |
| Salt stress | 3 | 0.889 | 0.468 | Salt stress | 3 | 0.668 | 0.584 |
| Combined stress | 3 | 11.302 | <0.001 | Combined stress | 3 | 0.524 | 0.672 |
| K+/Na+ ratio | The thickness of the central leaf vein | ||||||
| Osmotic stress | 1 | 1.742 | 0.205 | Osmotic stress | 1 | 0.010 | 0.923 |
| Salt stress | 3 | 6.517 | 0.004 | Salt stress | 3 | 5.546 | 0.008 |
| Combined stress | 3 | 21.531 | <0.001 | Combined stress | 3 | 3.650 | 0.035 |
| The thickness of stem parenchyma | Diameter of the leaf central vascular bundle | ||||||
| Osmotic stress | 1 | 0.158 | 0.696 | Osmotic stress | 1 | 2.071 | 0.169 |
| Salt stress | 3 | 2.337 | 0.112 | Salt stress | 3 | 10.271 | 0.001 |
| Combined stress | 3 | 1.349 | 0.294 | Combined stress | 3 | 4.735 | 0.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terletskaya, N.V.; Erbay, M.; Zorbekova, A.N.; Prokofieva, M.Y.; Saidova, L.T.; Mamirova, A. Influence of Osmotic, Salt, and Combined Stress on Morphophysiological Parameters of Chenopodium quinoa Photosynthetic Organs. Agriculture 2023, 13, 1. https://doi.org/10.3390/agriculture13010001
Terletskaya NV, Erbay M, Zorbekova AN, Prokofieva MY, Saidova LT, Mamirova A. Influence of Osmotic, Salt, and Combined Stress on Morphophysiological Parameters of Chenopodium quinoa Photosynthetic Organs. Agriculture. 2023; 13(1):1. https://doi.org/10.3390/agriculture13010001
Chicago/Turabian StyleTerletskaya, Nina V., Malika Erbay, Aigerim N. Zorbekova, Maria Yu Prokofieva, Luizat T. Saidova, and Aigerim Mamirova. 2023. "Influence of Osmotic, Salt, and Combined Stress on Morphophysiological Parameters of Chenopodium quinoa Photosynthetic Organs" Agriculture 13, no. 1: 1. https://doi.org/10.3390/agriculture13010001
APA StyleTerletskaya, N. V., Erbay, M., Zorbekova, A. N., Prokofieva, M. Y., Saidova, L. T., & Mamirova, A. (2023). Influence of Osmotic, Salt, and Combined Stress on Morphophysiological Parameters of Chenopodium quinoa Photosynthetic Organs. Agriculture, 13(1), 1. https://doi.org/10.3390/agriculture13010001







