Effects of Nutrients and Growth Regulators on Seed Germination and Development of Juvenile Rhizome Proliferation of Gastrodia elata In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Floral Induction and Artificial Pollination of G. elata
Fruit Capsule Collection after Pollination
2.2. Medium for Seed Germination In Vitro
2.2.1. Test of Carbohydrate, Protein, and Other Additives on Seed Germination (Table S1)
2.2.2. Plant Growth Regulators Tested for Seed Germination
2.3. Protocorms/Juvenile Rhizomes (Jrhs) Proliferation Test
2.3.1. Test of Different Cytokinins on Jrhs Proliferation (Table S6)
2.3.2. Test of BA and IAA Combination on Jrhs Proliferation
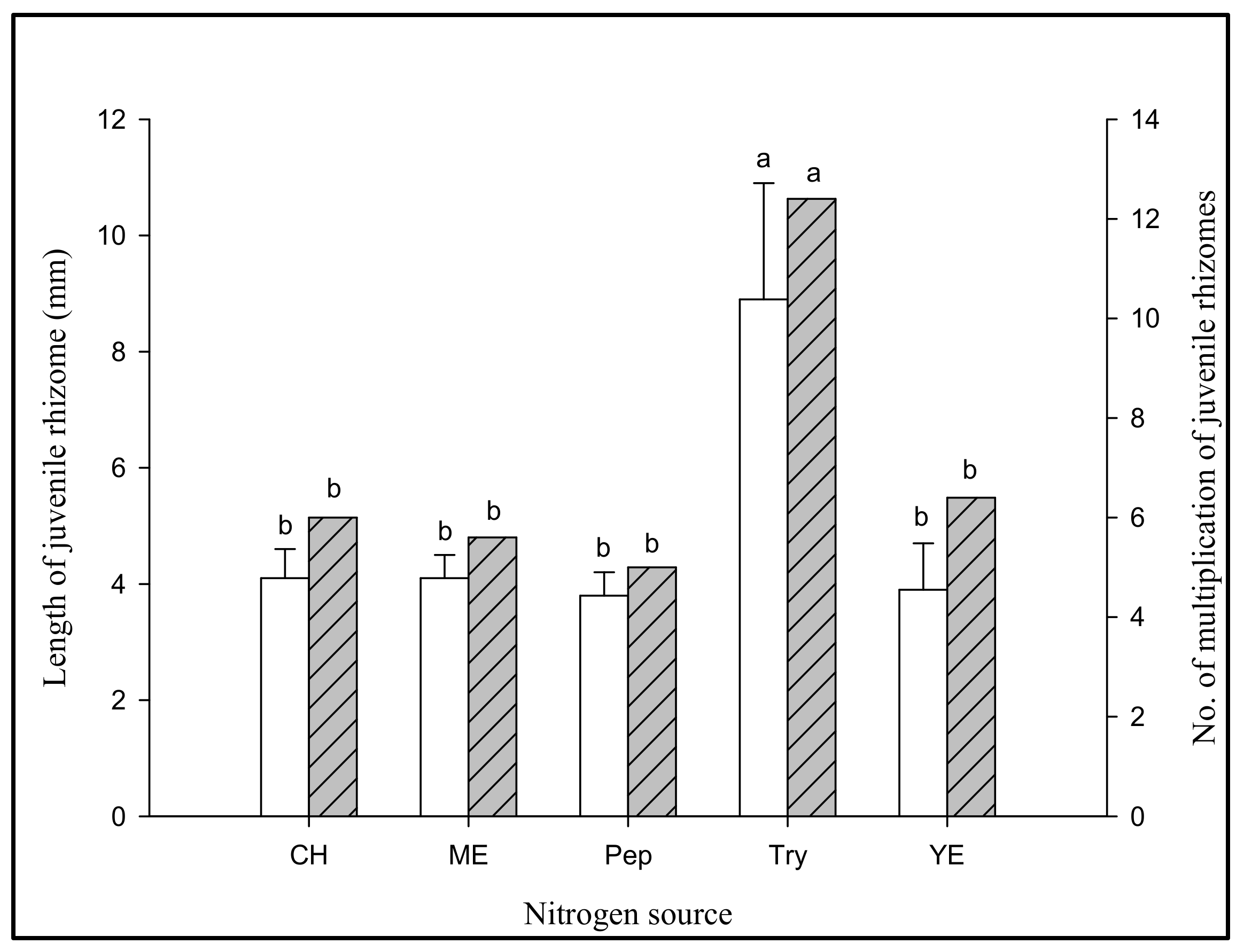
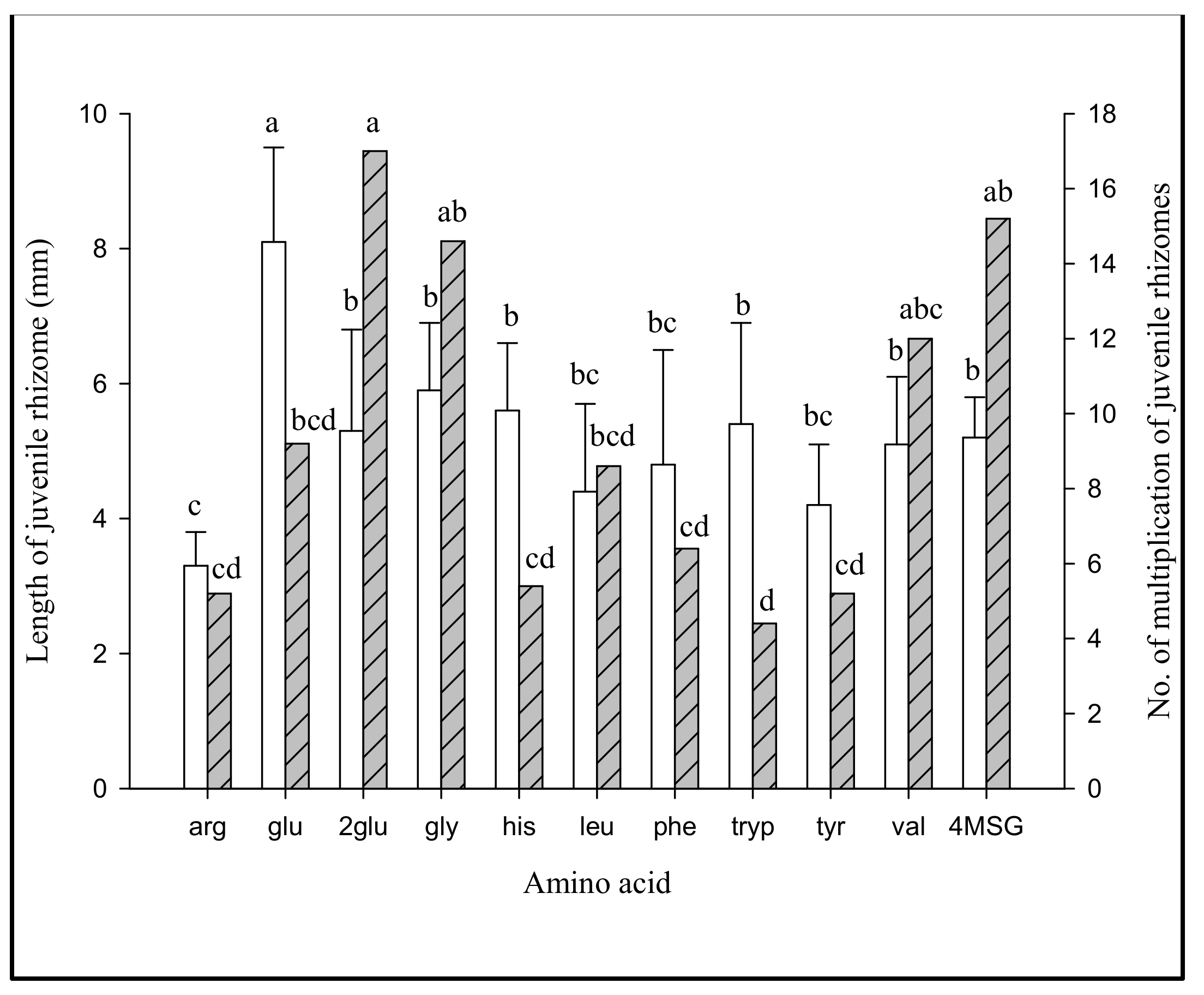
2.3.3. Assessment of Protein (Nitrogen Source), Amino Acids and B Vitamins for Jrhs Proliferation (Table S7)
2.3.4. Test of Chitosan on Jrhs Proliferation (Table S8)
2.3.5. Combination of BA with IAA or NAA on Jrhs Proliferation
2.3.6. Test of Various Basal Salts on Proliferation of Jrhs (Table S9)
2.3.7. Test of Different pH in Culture Medium on Proliferation of Jrhs
2.4. Statistical Analysis
3. Results and Discussion
3.1. Aseptic Seeding of G. elata on the Culture Medium
3.2. Protocorm/Juvenile Rhizomes Proliferation Test
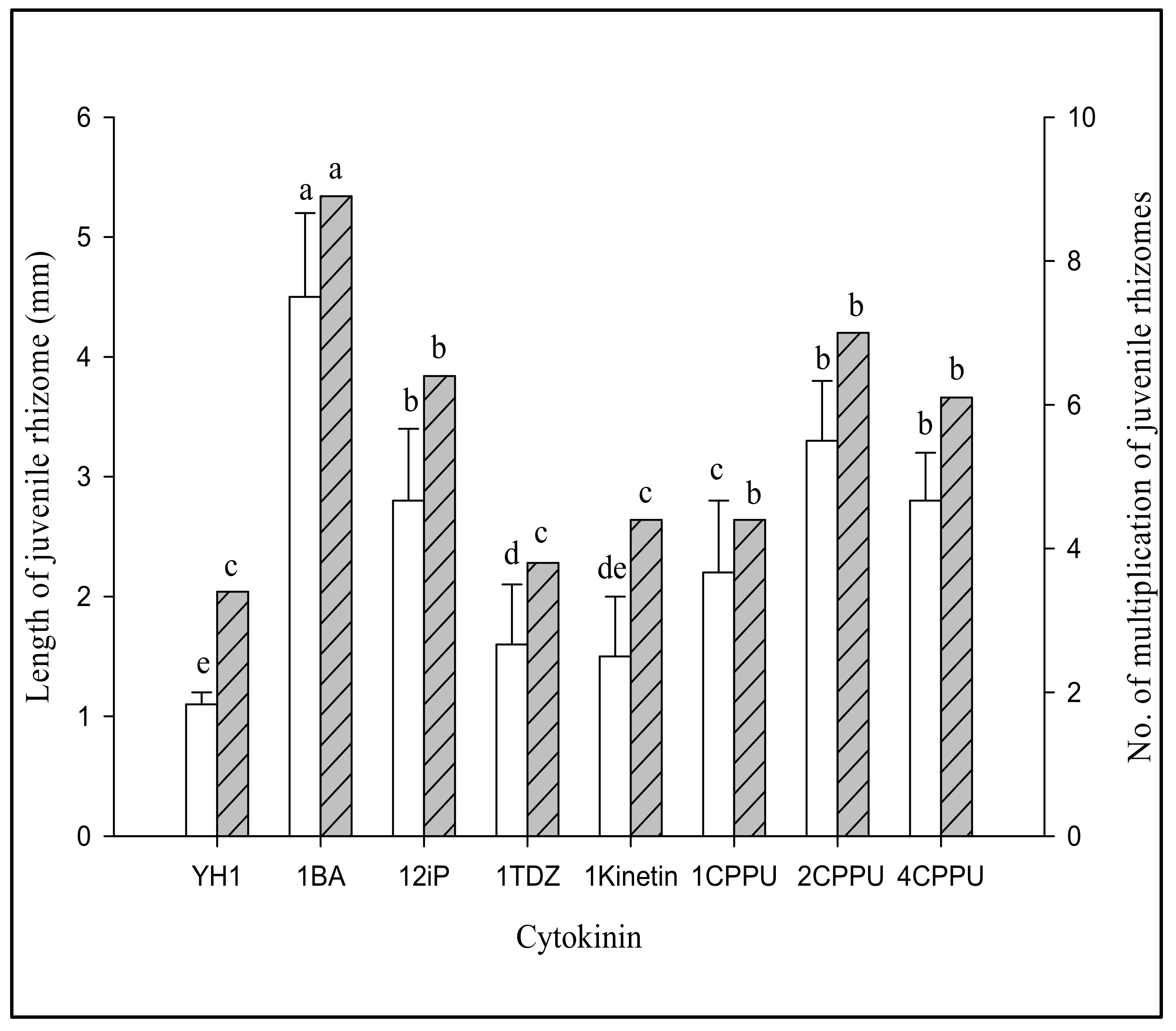
3.2.1. Effect of Different Cytokinins on Proliferation of G. elata Jrhs
3.2.2. Effect of BA and IAA Concentrations on the Proliferation of G. elata Jrhs
3.2.3. Effect of Jrhs Proliferation on Media Containing Proteins, Amino Acids, B Vitamins, and Plant Growth Regulators
3.2.4. Effect of Chitosan Concentrations on the Proliferation of G. elata Jrhs
3.2.5. Effect of IAA or NAA Concentrations on the Proliferation of G. elata Jrhs
3.2.6. The Effect of Medium Containing Various Basal Salts and pH on the Proliferation of G. elata Jrhs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Li, M.; Kang, R.X.; Shi, J.G.; Liu, G.T.; Zhang, J.J. NHBA isolated from Gastrodia elata exerts sedative and hypnotic effects in sodium pentobarbital-treated mice. Pharmacol. Biochem. Behav. 2012, 102, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Lai, Y.S.; Lin, S.H.; Lu, K.H.; Lin, Y.E.; Panyod, S.; Ho, C.T.; Sheen, L.Y. Anti-depressant effects of Gastrodia elata Blume and its compounds gastrodin and 4-hydroxybenzyl alcohol, via the monoaminergic system and neuronal cytoskeletal remodeling. J. Ethnopharmacol. 2016, 182, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Matias, M.; Silvestre, S.; Falcão, A.; Alves, G. Gastrodia elata and epilepsy: Rationale and therapeutic potential. Phytomedicine 2016, 23, 1511–1526. [Google Scholar] [CrossRef]
- Duan, X.; Wang, W.; Liu, X.; Yan, H.; Dai, R.; Lin, Q. Neuroprotective effect of ethyl acetate extract from Gastrodia elata against transient focal cerebral ischemia in rats induced by middle cerebral artery occlusion. J. Tradit. Chin. Med. 2015, 35, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.W.; Liu, Z.Y.; Zhang, F.L.; Zhang, L.; Li, F.; Liu, S.Y.; He, J.Y.; Xiao, Z.C. Post-stroke gastrodin treatment ameliorates ischemic injury and increases neurogenesis and restores the Wnt/β-Catenin signaling in focal cerebral ischemia in mice. Brain Res. 2019, 1712, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.S.; Zhou, S.F.; Wang, Q.; Guo, J.N.; Liang, H.M.; Deng, J.B.; He, W.Y. Gastrodin suppresses BACE1 expression under oxidative stress condition via inhibition of the PKR/eIF2α pathway in Alzheimer’s disease. Neuroscience 2016, 325, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, N. Floral morphology and pollination in Gastrodia elata, a mycoheterotrophic orchid. Plant Species Biol. 2017, 32, 173–178. [Google Scholar] [CrossRef]
- Zhou, Y.; Liang, Z.S.; Zhang, Y.J.; Mu, X.Q. Study of reproductive biology and artificial pollination of G. elata Bl. J. Northwest Sci.-Tech. Univ. Agric. For. 2005, 33, 33–36. [Google Scholar]
- Xu, J.T.; Fan, L. Cytodifferentiation of the seeds (protocorms) and vegetative propagation corms colonized by mycorrhizal fungi. Acta Bot. Sin. 2001, 43, 1003–1010. [Google Scholar]
- Ogura-Tsujita, Y.; Gebauer, G.; Hashimoto, T.; Umata, H.; Yukawa, T. Evidence for novel and specialized mycorrhizal parasitism, the orchid Gastrodia confusa gains carbon from saprotrophic Mycena. Proc. R. Soc. B Biol. Sci. 2009, 276, 761–767. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.C.; Qin, L.Y.; He, H.Y.; Yu, X.L.; Yang, M.Z.; Zhang, H.B. Dynamics of fungal communities during Gastrodia elata growth. BMC Microbiol. 2019, 19, 158. [Google Scholar] [CrossRef]
- Xie, X.Q. Effect of Armillariella mellea on sexual reproduction of Gastrodia elata f. glauca in Ganzi prefecture. Hubei Agric. Sci. 2011, 50, 3562–3565. [Google Scholar]
- Ren, L.Y.; Zhao, H.; Liu, X.L.; Zong, T.K.; Qiao, M.; Liu, S.Y.; Liu, X.Y. Transcriptome reveals roles of lignin-modifying enzymes and abscisic acid in the symbiosis of Mycena and Gastrodia elata. Int. J. Mol. Sci. 2021, 22, 6557. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Zhou, T.; Jiang, W.K.; Guo, P.L. Analysis of ecological recycling modes for Gastrodia elata cultivation. China J. Chin. Mater. Med. 2020, 45, 2036. (In Chinese) [Google Scholar] [CrossRef]
- Xu, J.; Ou, X.H.; Jiang, W.K. Effect of Gastrodia elata-Phallus impudicus sequential planting pattern on soil microbial community structure. China J. Chin. Mater. Med. 2020, 45, 463–471. [Google Scholar] [CrossRef]
- Arditti, J. Fundamentals of Orchid Biology; John Wiley & Sons: New York, NY, USA, 1992; p. 691. [Google Scholar]
- Vacin, E.F.; Went, F.W. Some pH changes in nutrient solutions. Bot. Gaz. 1949, 110, 605–613. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Dian, J.Y.; Liang, H.X. On the relation between the percentage of germination and the degree of ripeness of the seeds of Gastrodia elata. Acta Bot. Yunnan 1982, 4, 303–306. [Google Scholar]
- Arditti, J.; Clements, M.; Fast, G.; Hadley, G.; Nishimura, G.; Ernst, R. Orchid seed germination and seedling culture—A manual. In Orchid Biology—Reviews and Perspectives; Arditti, J., Ed.; Cornell University Press: New York, NY, USA, 1982; Volume II, pp. 243–370. [Google Scholar]
- Lindén, B. Two new methods for pretreatment of seeds of Northern orchids to improve germination in axenic culture. Ann Botanici Fennici 1992, 29, 305–313. [Google Scholar]
- De Pauw, M.A.; Remphrey, W.R. In vitro germination of three Cypripedium species in relation to time of seed collection, media, and cold treatment. Can. J. Bot. 1993, 71, 879–885. [Google Scholar] [CrossRef]
- Van Waes, J.M.; Debergh, P.C. In vitro germination of some Western European orchids. Physiol. Plant 1986, 67, 253–261. [Google Scholar] [CrossRef]
- Van der Kinderen, G. Abscisic acid in terrestrial orchid seeds: A possible impact on their germination. Lindleyana 1987, 2, 84–87. [Google Scholar]
- Yamazaki, J.; Miyoshi, K. In vitro asymbiotic germination of immature seed and formation of protocorm by Cephalanthera falcata (Orchidaceae). Ann. Bot. 2006, 98, 1197–1206. [Google Scholar] [CrossRef]
- Lu, I.L.; Lee, C.J.; Lee, N. Effect of medium composition on seed germination in vitro of Cymbidium ensifolium var. misericors. J. Chinese Soc. Hort. Sci. 1992, 38, 161–169. [Google Scholar]
- Yeh, C.H.; Chen, B.L.; Lin, H.J.; Liao, F.S. Studies on seed development and in vitro germination of Gastrodia elata. Bull. Taoyuan Dist. Agric. Res. Ext. Stat. 2013, 74, 1–14. [Google Scholar]
- Juang, J.H.; Lee, N. Effect of niacin, coconut milk and banana homogenate on seed germination and seedling growth of Pleione formosana. J. Chin. Soc. Hort. Sci. 1986, 32, 132–138. [Google Scholar]
- Lee, Y.I.; Lee, N. Effect of capsual maturity, medium composition and suspension culture on in vitro germination of Paphiopedilum primulinum seed. J. Chin. Soc. Hort. Sci. 2001, 47, 147–156. [Google Scholar]
- Arditti, J. Factors affecting the germination of orchid seed. Bot. Rev. 1967, 33, 1–97. [Google Scholar] [CrossRef]
- Chang, C.; Chen, Y.C.; Yen, H.F. Protocorm or rhizome? The morphology of seed germination in Cymbidium dayanum Reichb. Bot. Bull. Acad. Sin. 2005, 46, 71–74. [Google Scholar]
- Chen, Y.; Aviad, T. Effects of humic substances on plant growth. In Humic Substances in Soil and Crop Sciences: Selected Readings; MacCarthy, P., Clapp, C.E., Malcolm, R.L., Bloom, P.R., Eds.; Soil Science Society of America, Inc.: Madison, WI, USA, 1990; pp. 161–186. [Google Scholar]
- Kadota, M.; Niimi, Y. Effects of cytokinin types and their concentrations on shoot proliferation and hyperhydricity in in vitro pear cultivar shoots. Plant Cell Tissue Organ Cult. 2003, 72, 261–265. [Google Scholar] [CrossRef]
- Mok, D.W.; Mok, M.C. Cytokinin metabolism and action. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 89–118. [Google Scholar] [CrossRef] [PubMed]
- Subotić, A.; Jevremović, S.; Grubišić, D. Influence of cytokinins on in vitro morphogenesis in root cultures of Centaurium erythraea-Valuable medicinal plant. Sci. Hortic. 2009, 120, 386–390. [Google Scholar] [CrossRef]
- Huang, C.H.; Chung, J.P. Efficient indirect induction of protocorm-like bodies and shoot proliferation using field-grown axillary buds of a Lycaste hybrid. Plant Cell Tissue Organ Cult. 2010, 106, 31–38. [Google Scholar] [CrossRef]
- Hiregoudar, L.V.; Murthy, H.N.; Bhat, J.G.; Nayeem, A.; Hema, B.P.; Hahn, E.J.; Paek, K.Y. Rapid clonal propagation of Vitex trifolia. Biol. Plant 2006, 50, 291–294. [Google Scholar] [CrossRef]
- Branca, C.; Bucci, G.; Domiano, P.; Ricci, A.; Torelli, A.; Bassi, M. Auxin structure and activity on tomato morphogenesis in vitro and pea stem elongation. Plant Cell Tissue Organ Cult. 1991, 24, 105–114. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Hüner, N.P.A. Introduction to Plant Physiology, 4th ed.; John Wiley & Sons, Inc.: Danvers, MA, USA, 2008. [Google Scholar]
- Schaller, G.E.; Bishopp, A.; Kieber, J.J. The yin-yang of hormones, cytokinin and auxin interactions in plant development. Plant Cell 2015, 27, 44–63. [Google Scholar] [CrossRef]
- Cai, Y.P.; Yu, L.W.; Zhang, H.Y.; Chen, B.Q. Tissue culture and clonal propagation of Gastrodia elata. Chin. Trad. Herb. Drug 2001, 32, 445–446. [Google Scholar]
- Arditti, J. Niacin biosynthesis in germinating x Laeliocattleya orchid embryos and young seedlings. Am. J. Bot. 1967, 54, 291–298. [Google Scholar] [CrossRef]
- Cooper, J.L.; Hilton, B.L.; Arditti, J.; Tarr, J.B. Niacin biosynthesis in leaf discs and seedlings of Cattleya skinneri (Orchidaceae). New Phytol. 1982, 91, 621–628. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; De Klerk, G.J. The components of plant tissue culture media II, Organic addition, osmotic and pH effects, and support systems. In Plant Propagation by Tissue Culture; George, E.F., Hall, M.A., De Klerk, G.J., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 115–173. [Google Scholar]
- Wu, S.C.; Lee, N. In vitro seed germination of Phaius tankervilliae. J. Chin. Soc. Hort. Sci. 1991, 37, 183–198. [Google Scholar]
- Kazemiani, S.; Motallebi-Azar, A.R.; Panahandeh, J.; Mokhtarzadeh, S.; Ozdemir, F.A. Shoot proliferation from potato (Solanum tuberosum cv. agria) under different concentration of MS include vitamins and BAP medium. Prog. Nutr. 2018, 20, 160–166. [Google Scholar] [CrossRef]
- Burguieres, E.; McCue, P.; Kwon, Y.I.; Shetty, K. Effect of vitamin C and folic acid on seed vigour response and phenolic-linked antioxidant activity. Bioresour. Technol. 2007, 98, 1393–1404. [Google Scholar] [CrossRef]
- Nge, K.L.; New, N.; Chandrkrachang, S.; Stevens, W.F. Chitosan as a growth stimulator in orchid tissue culture. Plant Sci. 2006, 170, 1185–1190. [Google Scholar] [CrossRef]
- Guan, Y.J.; Hu, J.; Wang, X.J.; Shao, C.X. Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. J. Zhejiang Univ. Sci. B 2009, 10, 427–433. [Google Scholar] [CrossRef]
- No, H.K.; Lee, K.S.; Kim, I.D.; Park, M.J.; Kim, S.D.; Meyers, S.P. Chitosan treatment affects yield, ascorbic acid content, and hardness of soybean sprouts. J. Food Sci. 2003, 68, 680–685. [Google Scholar] [CrossRef]
- Zhou, Y.G.; Yang, Y.D.; Qi, Y.G.; Zhang, Z.M.; Wang, X.J.; Hu, X.J. Effects of chitosan on some physiological activity in germinating seed of peanut. J. Pean Sci. 2002, 31, 22–25. [Google Scholar]
- Chang, Y.C.; Lee, N. Tissue culture of Pleione formosana Hayata. J. Chin. Soc. Hort. Sci. 1992, 38, 80–90. [Google Scholar]
- Filho, A.R.; Vesco, L.L.D.; Nodari, R.O.; Lischka, R.W.; Müller, C.V.; Guerra, M.P. Tissue culture for the conservation and mass propagation of Vriesea reitzii Leme and Costa, a bromeliad threatened of extinction from the Brazilian Atlantic Forest. Biodivers Conserv. 2005, 14, 1799–1808. [Google Scholar] [CrossRef]
- Juang, J.H.; Lee, N. Effect of activated charcoal, sucrose and mineral concentration on seed germination and seedling growth of Pleione formosana. J. Chin. Soc. Hort. Sci. 1986, 32, 61–69. [Google Scholar]
- George, E.F.; Hall, M.A.; De Klerk, G.J. The components of plant tissue culture media I, macro- and micro-nutrients. In Plant Propagation by Tissue Culture; George, E.F., Hall, M.A., De Klerk, G.J., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 65–113. [Google Scholar]
- Hadwiger, L.A.; Beckman, J.M. Chitosan as a component of pea-Fusarium solani interactions. Plant Physiol. 1980, 66, 205–211. [Google Scholar] [CrossRef]

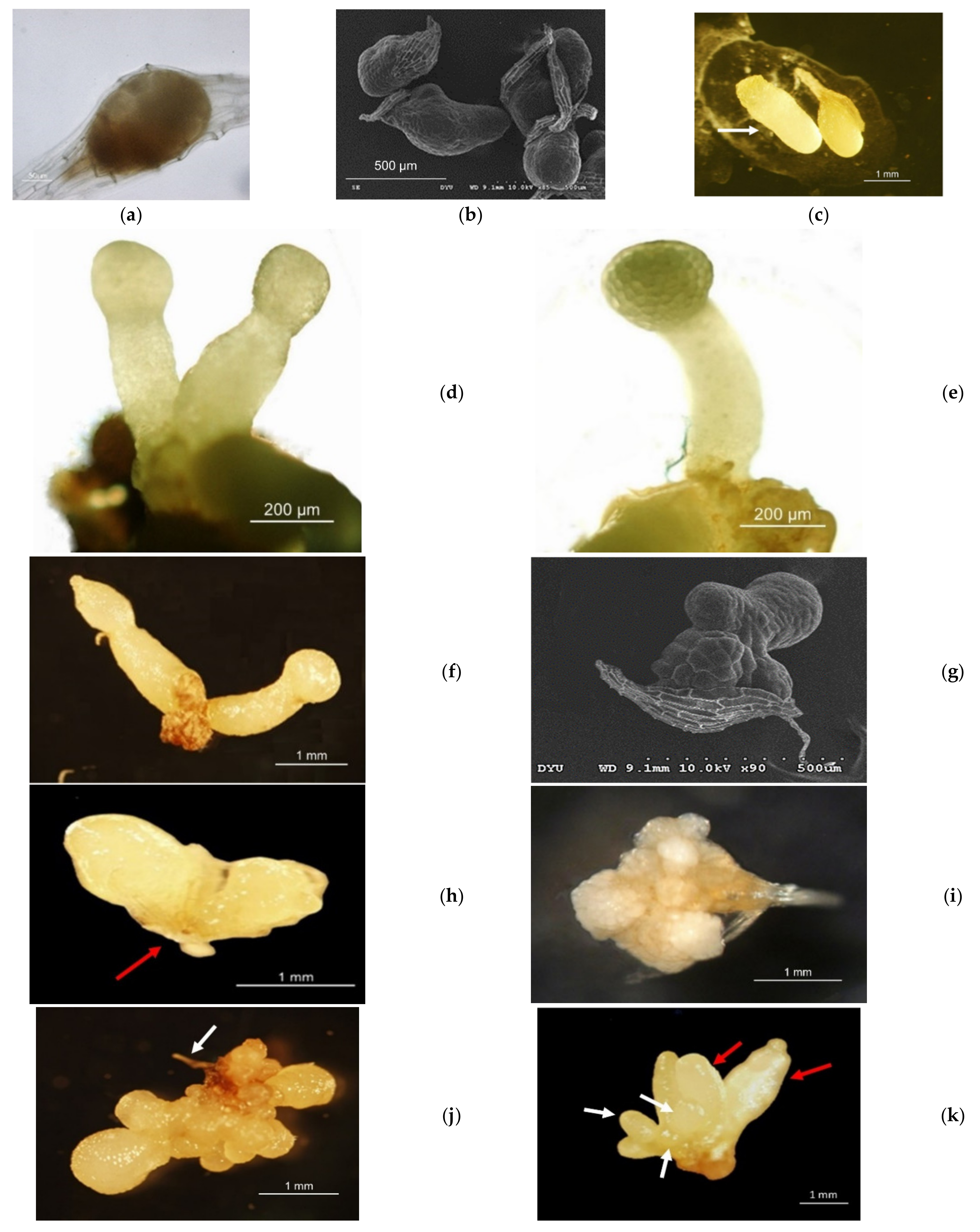
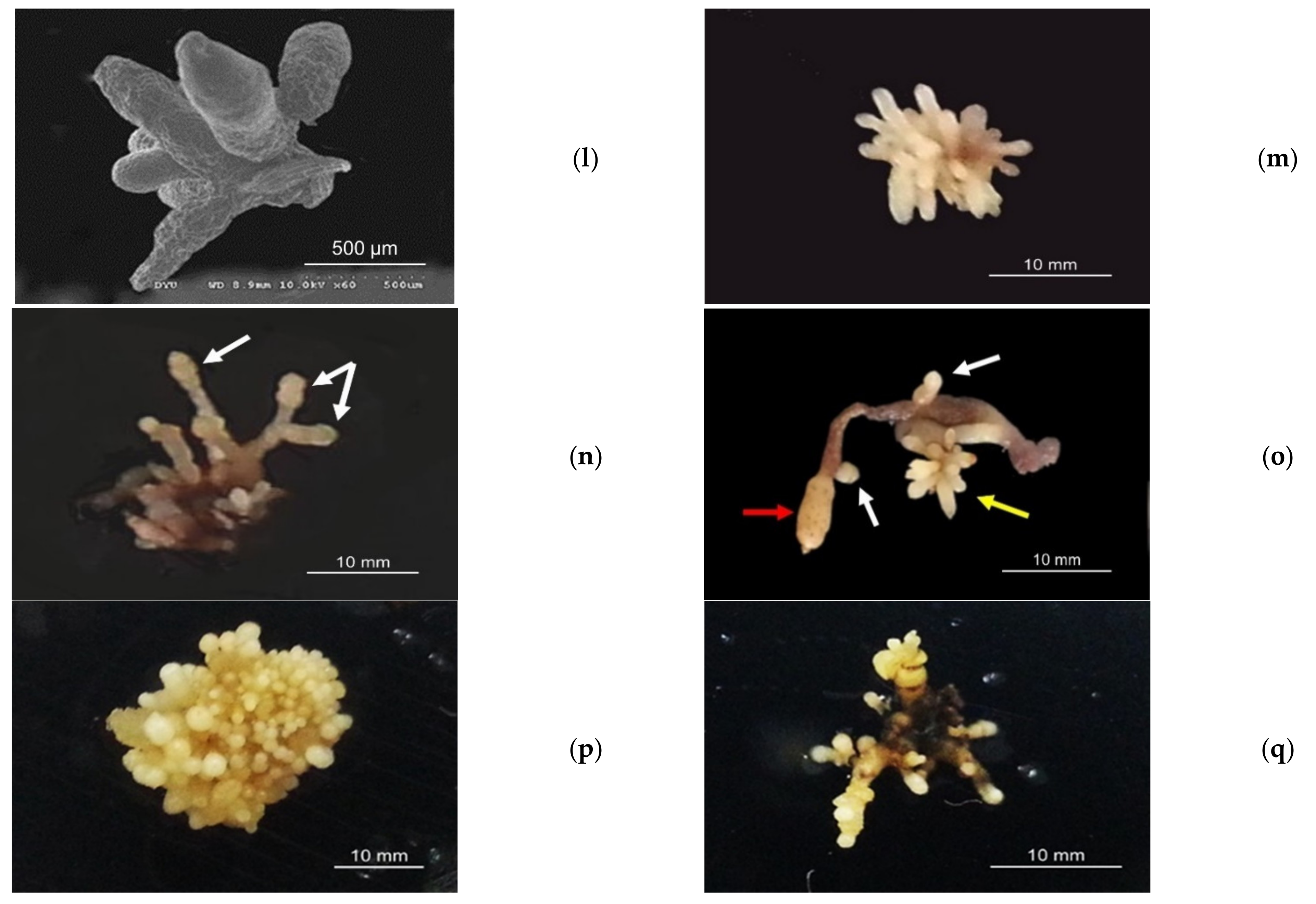
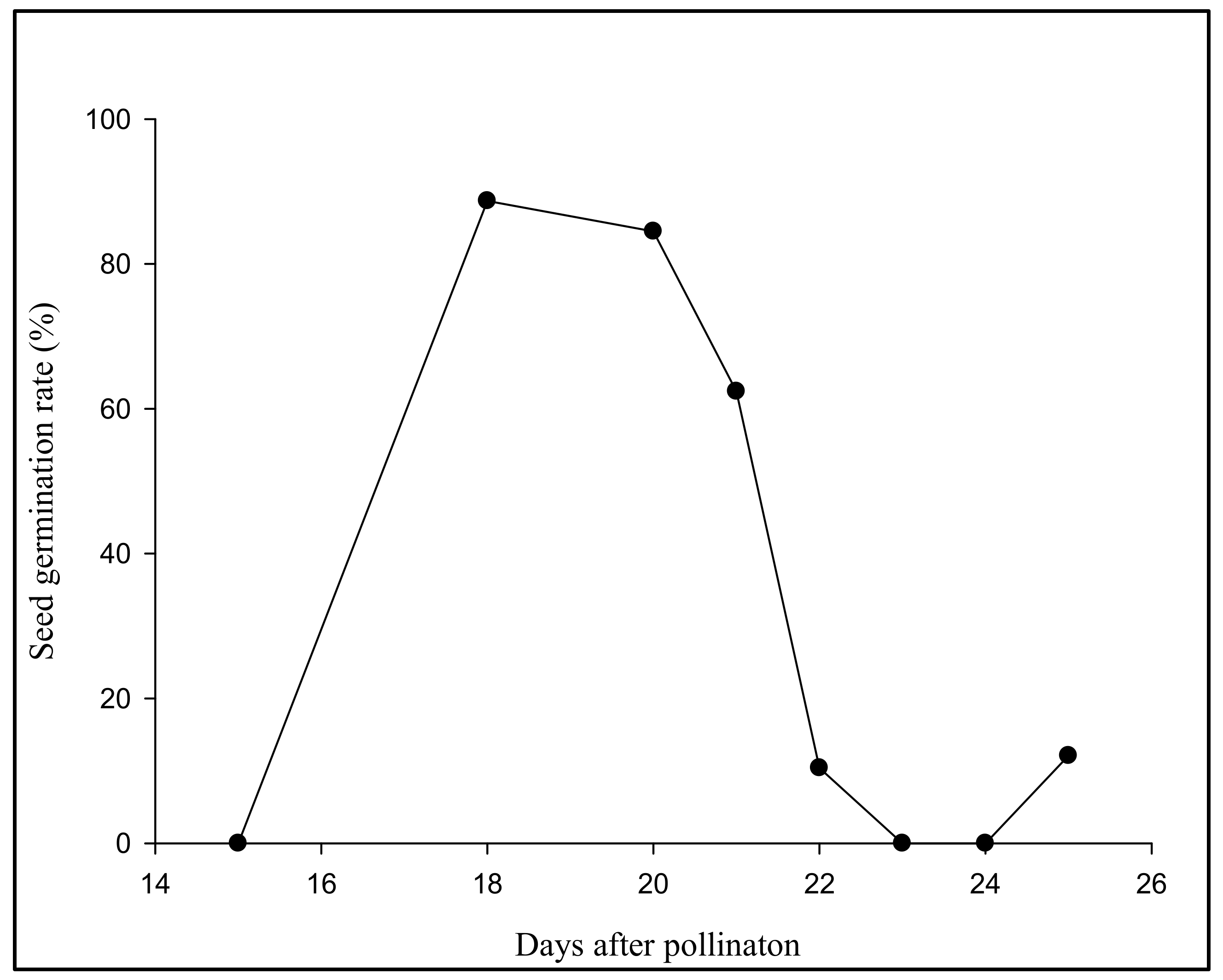

| Harvest DAP | Fruit Pod Code | Medium Code | Fruit Pod Code/Medium Germination Occurred | Medium/Average Protocorm Length (mm) |
|---|---|---|---|---|
| 7 March | SA1-SA8 | Va, VGc20, VGc30, VS20, VS30, VGcS10, VCW150, VCW200 | - | - |
| (15 DAP) | ||||
| 10 March | SB1,SB2, SB3, SB4, SB6, SB7, SC1-SC9, SD1-SD7 | Va, VGc20, VGc30, VS20, VS30, VGcS10, VCW100, VCW150, VCW200, VHGc20 | SC2/Y2A, Y2L, Y2Pp; | Y2/1 mm |
| (18 DAP) | VHGc30, Y2, Y2A, Y2CW150, Y2H, Y2L, Y2MSG,Y2Pp, Y2Pt, Y3, Y3A, Y3CW150, Y3H, Y3L, Y3MSG, Y3Pp, Y3Pt, VP0.2, VP0.4, VIPc,VIPd, VIPe,VIPf, VIPg, VIPh | SC3/Y2, Y2H, Y2MSG, Y2Pp, Y2Pt; | Y2A/1 mm | |
| SC4/Y2A, Y2L, Y2MSG; | Y2H/2 mm | |||
| SC5/Y2L, Y2Pt; | Y2L/1 mm | |||
| SC8/Y2A, Y2H, Y2L, Y2MSG; | Y2MSG/1 mm | |||
| SD1/Y3A, Y3MSG, Y3Pp, Y3Pt; | Y2Pp/2 mm | |||
| SD4/Y3, Y3A, Y3H, Y3L, Y3MSG, Y3Pt | Y2Pt/2 mm | |||
| Y3/0.5 mm | ||||
| Y3A/1 mm | ||||
| Y3H/1 mm | ||||
| Y3L/0.5 mm | ||||
| Y3MSG/1 mm | ||||
| Y3Pp/1.5 mm | ||||
| Y3Pt/1.5 mm | ||||
| 12 March | SD1-SD12, SD14-SD25, SD28 | Va, VS20, VS30, VGcS10, VCW100, VCW150, VCW200, Y3, Y3A, Y3MSG, Y3H, Y3L, Y3Pp, Y3Pt, Y3CW150, YH, YHG0.5, YHG1, YHG2, YHI0.2, YHI0.5, YHI1, YHI2, YP0.1, YP0.2, | SD1/Y3, Y3MSG; | VS30/0.5 mm |
| (20 DAP) | YIPa, YIPb, YCW, YCWa | SD2/VS30, Y3, Y3A, Y3Pp, VCW200; | VCW200/0.5 mm | |
| SD3/Y3MSG, Y3H, Y3L; | Y3/0.5 mm | |||
| SD24/YHI0.5, YHI1, YHI2, YIPb | Y3A/1 mm | |||
| Y3MSG/1 mm | ||||
| Y3H/1 mm | ||||
| Y3L/1 mm | ||||
| Y3Pp/1 mm | ||||
| YHI0.5/0.5 mm | ||||
| YHI1/1 mm | ||||
| YHI2/1 mm | ||||
| YIPb/1 mm | ||||
| 13 March | SE1-SE19 | VH, VI0.5, VI1, VI2, VP0.2, VP0.4, VIPc,VIPd, VIPe, VIPf, VIPg, VIPh, VCW100, Y2, Y2A,Y2CW150, Y2G, Y2H, Y2L, Y2Pp, Y2Pt | SE2/VI0.5, VI1, VI2; | VI0.5/1 mm |
| (21 DAP) | SE3/VI0.5, VI1, VI2 | VI1/1 mm | ||
| VI2/1 mm | ||||
| 14 March | SE26, SE27, SG1-SG10, SG14-SG34 | YH, YIG1-YIG6 | SG2/YIG2, YIG3, YIG4, YIG5, YIG6; | YIG1/0.5 mm |
| (22 DAP) | VI0.5, VI1, VI2, VP0.2, VP0.4, VIPc,VIPd, VIPe, VIPf, VIPg, VIPh, | SG5/YIG1 | YIG2/0.5 mm | |
| VCW100 | SG21/VI1; | YIG3/0.5 mm | ||
| SG15, SG17, SG18, SG19, SG23, | YIG4/1 mm | |||
| SG22/VI0.5, VI1 | YIG5/1 mm | |||
| YIG6/1 mm | ||||
| VI0.5/0.5 mm | ||||
| VI1/0.5 mm | ||||
| 15 March | SH1-SH14 | VGP1-6, Va | - | - |
| (23 DAP) | ||||
| 16 March | SJ1-SJ13, | VGP1-6, Va | - | - |
| (24 DAP) | SK1-SK28 | |||
| 17 March | SL1, | VCW100, | SM8, SM17, SM18, SM19, SM20, SM21, SN1, SN2/CW100 | VCW100/0.5 mm |
| (25 DAP) | SL3-SL20, SM1-SM21, | VGcS10 | ||
| SN1, SN2, |
| Medium Code | Growth Regulator (mg/L) | Length (mm) | No. of Multiplication of Juvenile Rhizomes | |
|---|---|---|---|---|
| BA | IAA | |||
| YBI 1 | 0 | 0 | 1.1 ± 0.1 e | 1.3 f |
| YBI 2 | 0 | 1 | 2.1 ± 0.2 d | 1.5 ef |
| YBI 3 | 0 | 2 | 2.1 ± 0.2 d | 1.9 ef |
| YBI 4 | 0 | 4 | 2.2 ± 0.3 d | 1.9 ef |
| YBI 5 | 1 | 0 | 1.2 ± 0.2 e | 2.1 e |
| YBI 6 | 1 | 1 | 3.7 ± 0.4 b | 2.9 d |
| YBI 7 | 1 | 2 | 4.0 ± 0.3 b | 4.3 b |
| YBI 8 | 1 | 4 | 2.3 ± 0.2 d | 3.4 cd |
| YBI 9 | 2 | 0 | 3.9 ± 0.5 b | 4.5 b |
| YBI 10 | 2 | 1 | 5.1 ± 0.4 a | 5.4 a |
| YBI 11 | 2 | 2 | 3.1 ± 0.2 c | 3.6 c |
| YBI 12 | 2 | 4 | 3.0 ± 0.3 c | 3.9 bc |
| Medium Code | Chitosan (g/L) | Length (mm) | No. of Multiplication of Juvenile Rhizomes |
|---|---|---|---|
| YH3(control) | 0 | 5.4 ± 0.4 c | 19.0 c |
| TM * | 1 | 9.0 ± 1.0 b | 27.8 b |
| CHT1 | 1 | 12.8 ± 1.5 a | 34.8 a |
| CHT2 | 2 | 10.0 ± 1.4 b | 24.6 bc |
| Medium Code | Growth Regulator (mg/L) | Length (mm) | No. of Multiplication of Juvenile Rhizomes | ||
|---|---|---|---|---|---|
| BA | IAA | NAA | |||
| YBI1 | 2 | 1 | 0 | 12.8 ± 1.5 b | 34.8 a |
| YBI2 | 2 | 2 | 0 | 13.0 ± 1.1 b | 34.6 a |
| YBN1 | 2 | 0 | 1 | 13.3 ± 0.8 ab | 34.2 a |
| YBN2 | 2 | 0 | 2 | 14.7 ± 0.7 a | 37.6 a |
| Medium Code | Length (mm) | No. of Multiplication of Juvenile Rhizomes |
|---|---|---|
| Y1 | 12.4 ± 1.1 bc | 37.4 c |
| Y1+1/2AB | 13.4 ± 1.1 b | 45.0 bc |
| 1/2AB | 9.6 ± 1.7 d | 37.4 c |
| YS 5.6 | 13.4 ± 1.2 b | 51.4 b |
| YS 6.0 | 17.2 ± 0.8 a | 76.6 a |
| YS 6.5 | 11.6 ± 1.1 c | 37.0 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, C.-H.; Liang, Z.-C.; Shieh, W.-J.; Chang, S.-L.; Ho, W.-J. Effects of Nutrients and Growth Regulators on Seed Germination and Development of Juvenile Rhizome Proliferation of Gastrodia elata In Vitro. Agriculture 2022, 12, 1210. https://doi.org/10.3390/agriculture12081210
Hsieh C-H, Liang Z-C, Shieh W-J, Chang S-L, Ho W-J. Effects of Nutrients and Growth Regulators on Seed Germination and Development of Juvenile Rhizome Proliferation of Gastrodia elata In Vitro. Agriculture. 2022; 12(8):1210. https://doi.org/10.3390/agriculture12081210
Chicago/Turabian StyleHsieh, Chi-Hung, Zeng-Chin Liang, Wen-Jang Shieh, Shin-Liang Chang, and Wai-Jane Ho. 2022. "Effects of Nutrients and Growth Regulators on Seed Germination and Development of Juvenile Rhizome Proliferation of Gastrodia elata In Vitro" Agriculture 12, no. 8: 1210. https://doi.org/10.3390/agriculture12081210
APA StyleHsieh, C.-H., Liang, Z.-C., Shieh, W.-J., Chang, S.-L., & Ho, W.-J. (2022). Effects of Nutrients and Growth Regulators on Seed Germination and Development of Juvenile Rhizome Proliferation of Gastrodia elata In Vitro. Agriculture, 12(8), 1210. https://doi.org/10.3390/agriculture12081210






