Effects of Nitrogen and Phosphorus Fertilization on Photosynthetic Properties of Leaves and Agronomic Characters of Alfalfa over Three Consecutive Years
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site Description and Experimental Design
2.2. Determination of Physical and Chemical Properties of Soil
2.3. Plant Material and Growth Measurements
2.4. Determination of Nitrogen and Phosphorus Contents
2.5. Determination of Stomatal Aperture
2.6. Determination of Photosynthetic Pigment Content
2.7. Determination of Antioxidant Indices
2.8. Statistical Analysis
3. Results
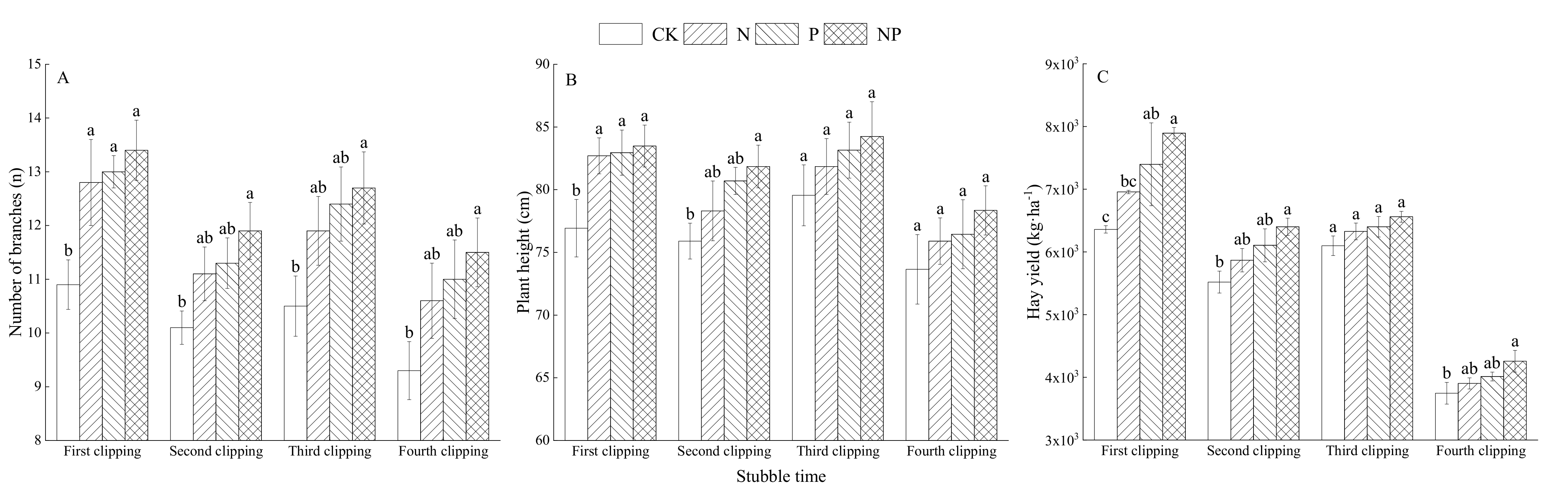
3.1. Effects of N and P Fertilization on Agronomic Parameters and Hay Yield of Alfalfa
3.2. Effects of N and P Fertilization on Nitrogen and Phosphorus Content of Alfalfa Leaves
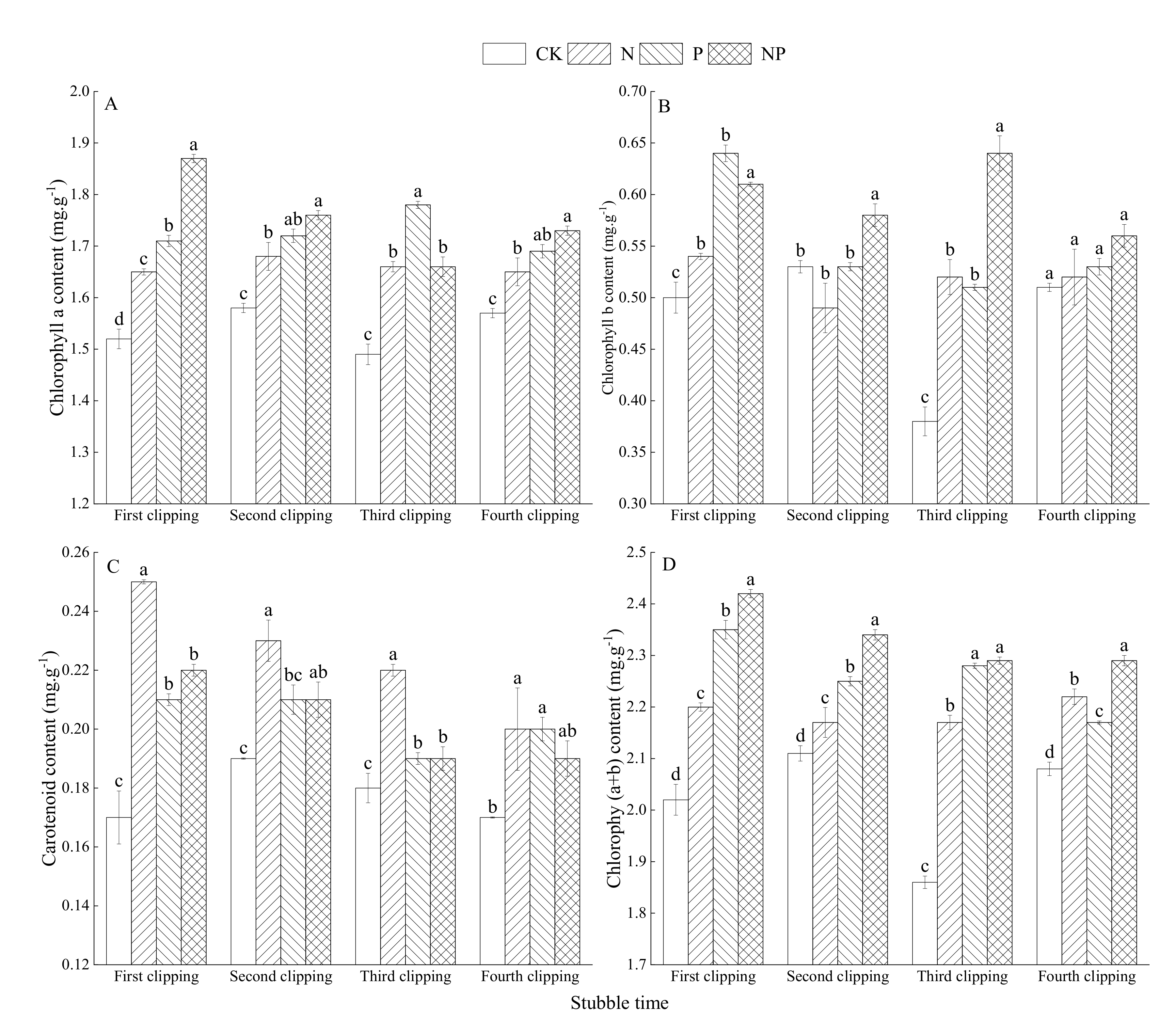
3.3. Effects of N and P Fertilization on Photosynthetic Pigment Content of Alfalfa Leaves
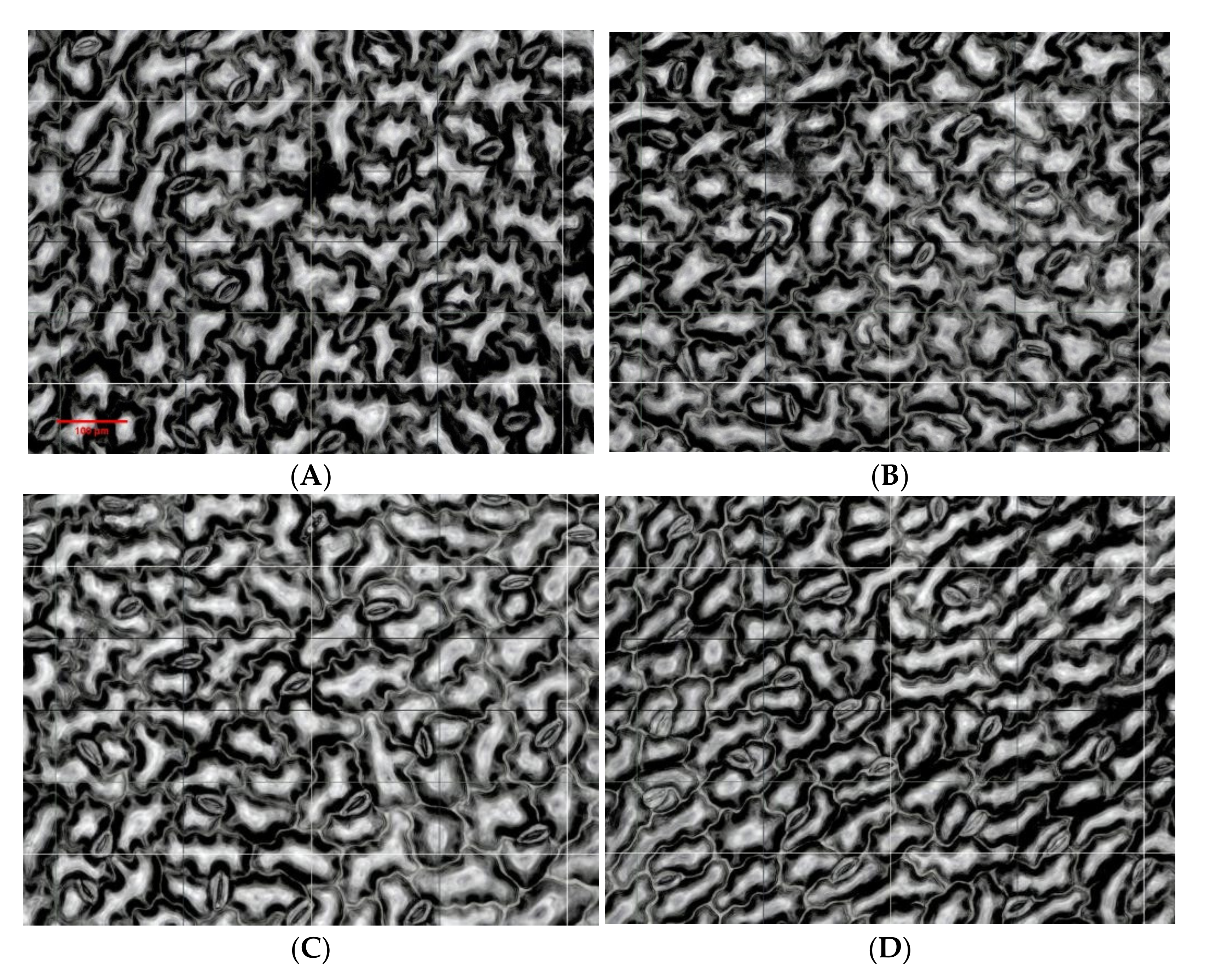
3.4. Effects of N and P Fertilization on Stomatal Aperture of Alfalfa Leaves
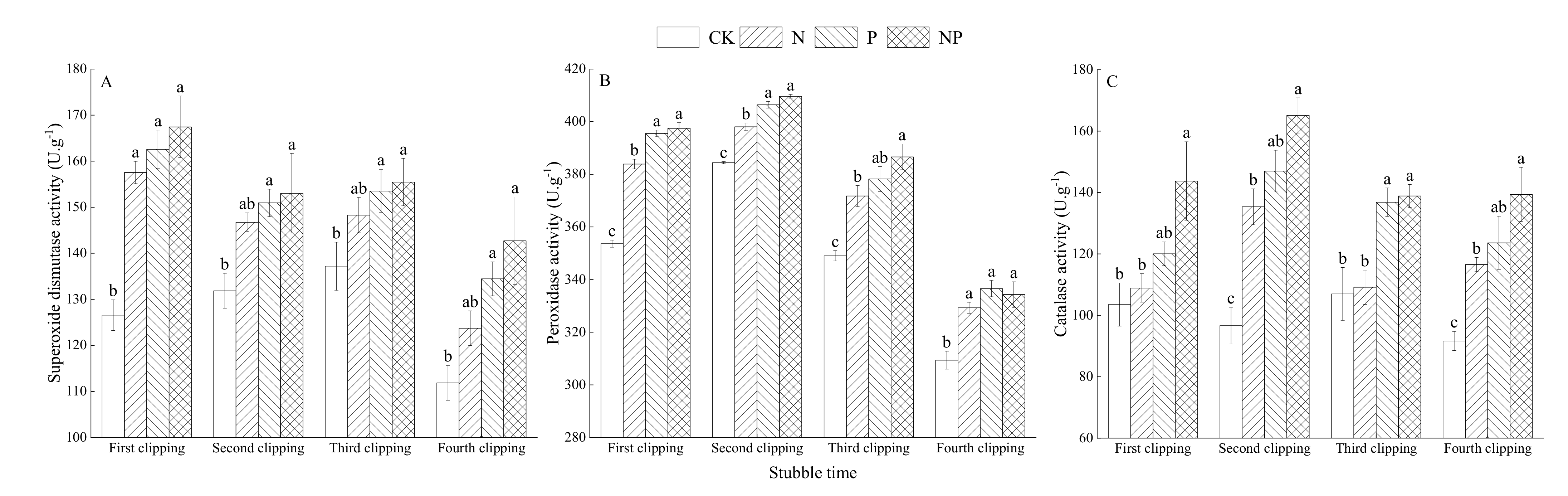
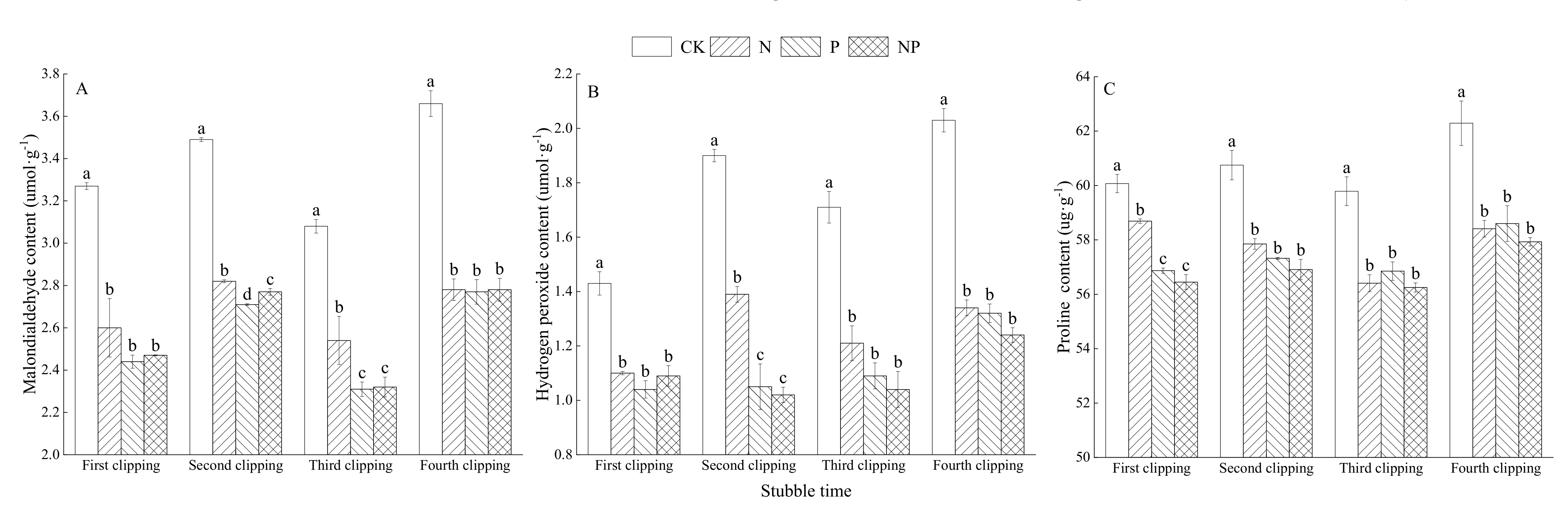
3.5. Effects of N and P Fertilization on Antioxidant Indices of Alfalfa Leaves
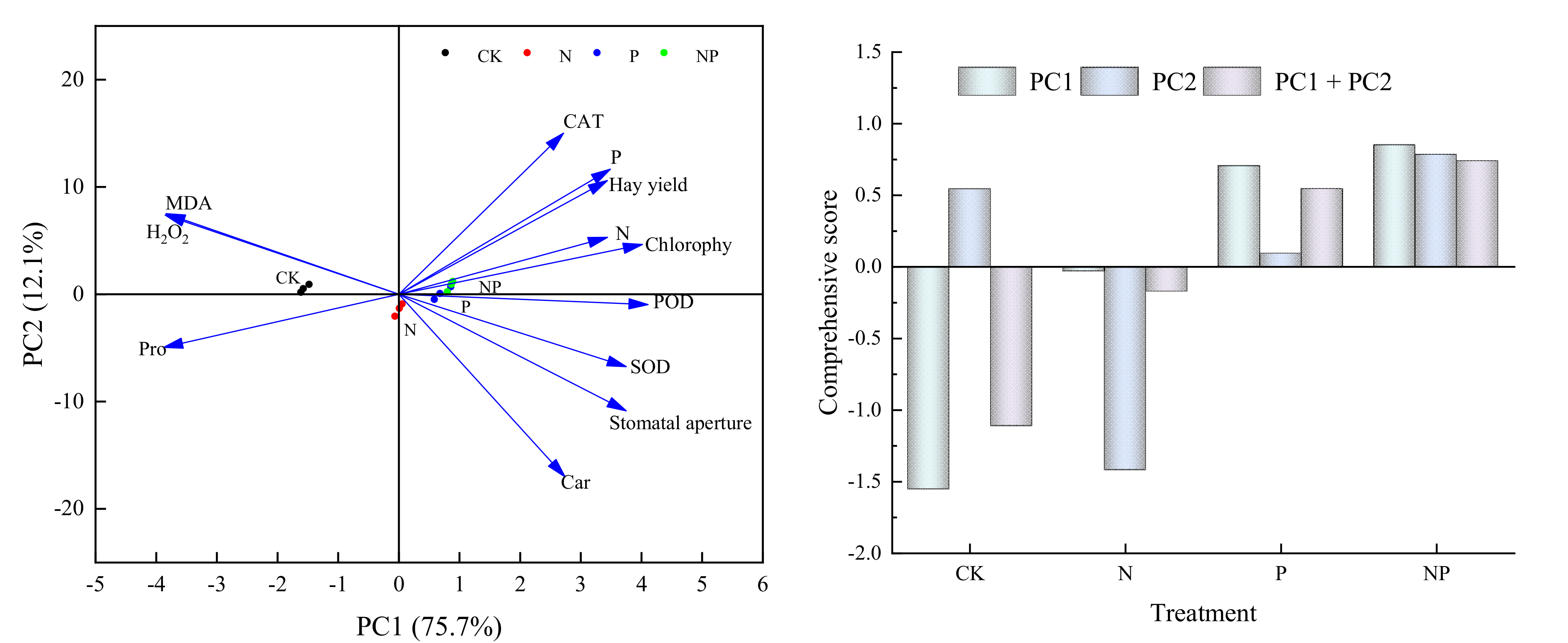
3.6. Effect of Nitrogen and Phosphorus on the Relationship between Leaf Physiological Factors and Alfalfa Hay Yield and Comprehensive Score
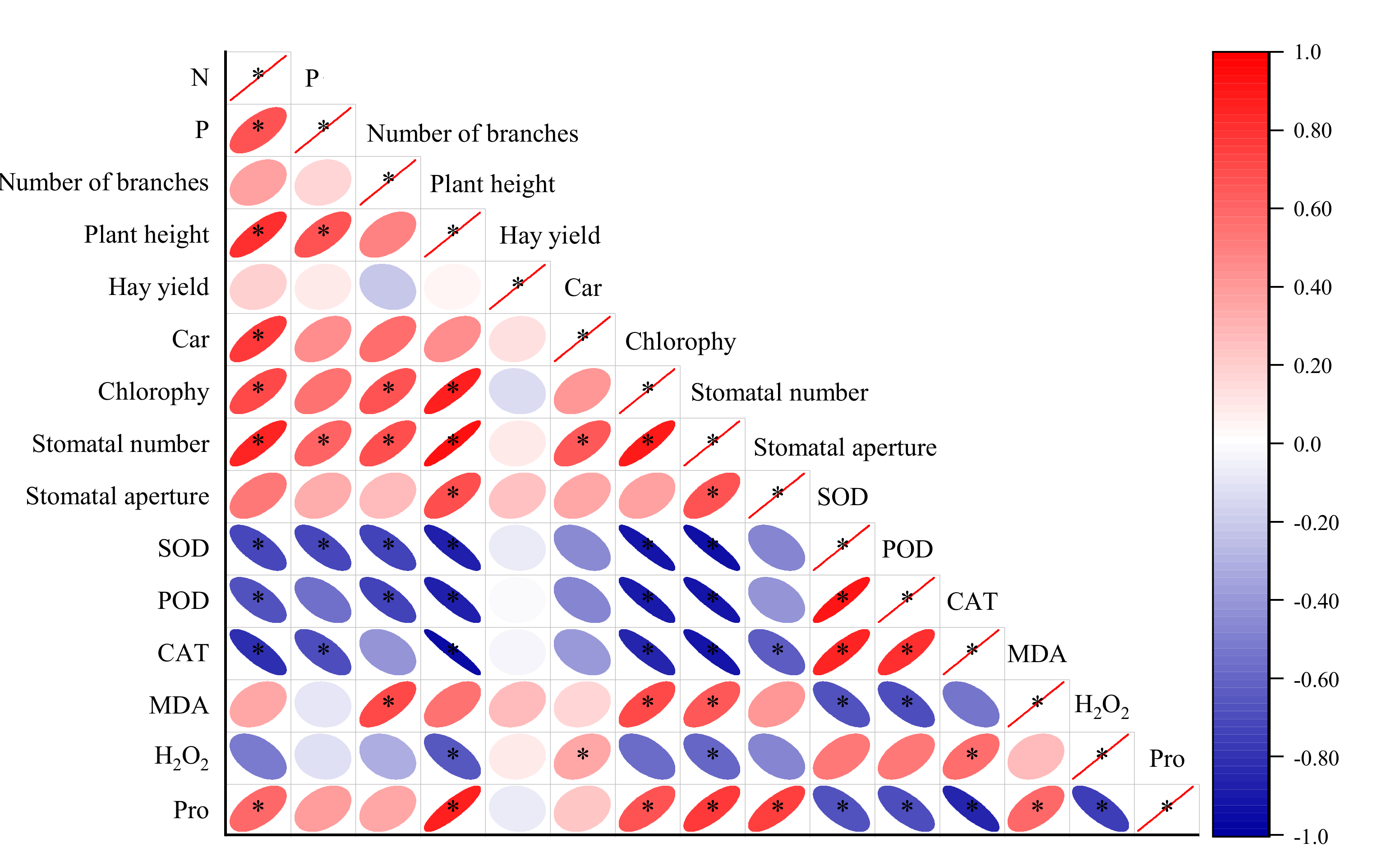
3.7. Relationship between Leaf Nutrition and Growth Performance, Photosynthetic Physiological Properties and Antioxidant Capacity
4. Discussion
4.1. Agronomic Traits and Hay Yield of Alfalfa under NP Fertilization
4.2. Nitrogen, Phosphorus and Photosynthetic Pigments Content of Alfalfa Leaves under NP Fertilization
4.3. Stomata Aperture of Alfalfa Leaves under NP Fertilization
4.4. Antioxidant Enzymes, Oxidizing Substance and Proline of Alfalfa Leaves under NP Fertilization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, G.R.; Jia, Y.L.; He, N.P.; Zhu, J.X.; Chen, Z.; Wang, Q.W.; Piao, S.L.; Liu, X.J.; He, H.; Guo, X.B.; et al. Stabilization of Atmospheric Nitrogen Deposition in China over the Past Decades. Nat. Geosci. 2019, 6, 424–429. [Google Scholar] [CrossRef]
- Lu, X.K.; Mao, Q.G.; Gilliam, F.S.; Luo, Y.Q.; Mo, J.M. Nitrogen Deposition Contributes to Soil Acidification in Tropical Ecosystems. Glob. Chang. Biol. 2014, 12, 3790–3801. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.F.; Wu, J.G.; Clark, C.M.; Naeem, S.; Pan, Q.M.; Huang, J.H.; Zhang, L.X.; Han, X.G. Tradeoffs and Thresholds in the Effects of Nitrogen Addition on Biodiversity and Ecosystem Functioning: Evidence from Inner Mongolia Grasslands. Glob. Chang. Biol. 2010, 16, 358–372. [Google Scholar] [CrossRef]
- Lu, X.T.; Reed, S.; Yu, Q.; He, N.P.; Wang, Z.W. Convergent Responses of Nitrogen and Phosphorus Resorption to Nitrogen Inputs in a Semiarid Grassland. Glob. Chang. Biol. 2013, 19, 2775–2784. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gao, X.P.; Li, X.Y.; Lin, L.S.; Zeng, F.J.; Gui, D.W.; Lu, Y. Nitrogen (N) and Phosphorus (P) Resorption of Two Dominant Alpine Perennial Grass Species in Response to Contrasting N and P Availability. Environ. Exp. Bot. 2016, 127, 37–44. [Google Scholar] [CrossRef]
- Li, Y.; Niu, S.L.; Yu, G.R. Aggravated Phosphorus Limitation on Biomass Production under Increasing Nitrogen Loading: A Meta-Analysis. Glob. Chang. Biol. 2016, 22, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Chen, Q.S.; Elser, J.J.; He, N.P.; Wu, H.H.; Zhang, G.M.; Wu, J.G.; Bai, Y.F.; Han, X.G. Linking Stoichiometric Homoeostasis with Ecosystem Structure, Functioning and Stability. Ecol. Lett. 2010, 13, 1390–1399. [Google Scholar] [CrossRef]
- Qiao, J.; Yang, L.Z.; Yan, T.M.; Xue, F.; Zhao, D. Nitrogen Fertilizer Reduction in Rice Production for Two Consecutive Years in the Taihu Lake Area. Agric. Ecosyst. Environ. 2012, 146, 103–112. [Google Scholar] [CrossRef]
- Lkhagvasuren, B.; Schoenau, J.J.; Anderson, D.W.; Malhi, S.S. Plant and soil responses to nitrogen and phosphorus fertilization of bromegrass-dominated haylands in Saskatchewan, Canada. Grass Forage Sci. 2011, 66, 351–360. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, J.; Gao, F.; Liu, P.; Zhao, B.; Zhang, J.W. Photosynthetic Characteristics and Chloroplast Ultrastructure of Summer Maize Response to Different Nitrogen Supplies. Front. Plant Sci. 2018, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Gao, P.C.; Tong, Y.A.; Norse, D.; Lu, Y.L.; Powlson, D. Overcoming Nitrogen Fertilizer Over-Use through Technical and Advisory Approaches: A Case Study from Shaanxi Province, Northwest China. Agric. Ecosyst. Environ. 2015, 209, 89–99. [Google Scholar] [CrossRef]
- Zhao, S.C.; Qiu, S.J.; Cao, C.Y.; Zheng, C.L.; Zhou, W.; He, P. Responses of Soil Properties, Microbial Community and Crop Yields to Various Rates of Nitrogen Fertilization in a Wheat-Maize Cropping System in North-Central China. Agric. Ecosyst. Environ. 2014, 194, 29–37. [Google Scholar] [CrossRef]
- Boyce, R.L.; Larson, J.R.; Sanford, R.L. Phosphorus and Nitrogen Limitations to Photosynthesis in Rocky Mountain Bristlecone Pine (Pinas Aristata) in Colorado. Tree Physiol. 2006, 26, 1477–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kant, S.; Bi, Y.M.; Rothstein, S.J. Understanding Plant Response to Nitrogen Limitation for the Improvement of Crop Nitrogen Use Efficiency. J. Exp. Bot. 2011, 62, 1499–1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Jiang, H.; Zhao, H.X.; Korpelainen, H.; Li, C.Y. Sexually Different Physiological Responses of Populus Cathayana to Nitrogen and Phosphorus Deficiencies. Tree Physiol. 2014, 34, 343–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Qin, J.J.; He, F.F.; Li, H.; Liu, T.X.; Polle, A.; Peng, C.H.; Luo, Z.B. Net Fluxes of Ammonium and Nitrate in Association with H+ Fluxes in Fine Roots of Populus Popularis. Planta 2013, 237, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Gusewell, S. N:P Ratios in Terrestrial Plants: Variation and Functional Significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Takashima, T.; Hikosaka, K.; Hirose, T. Photosynthesis or Persistence: Nitrogen Allocation in Leaves of Evergreen and Deciduous Quercus Species. Plant Cell Environ. 2004, 27, 1047–1054. [Google Scholar] [CrossRef]
- Lu, Y.J.; Duursma, R.A.; Farrior, C.E.; Medlyn, B.E.; Feng, X. Optimal Stomatal Drought Response Shaped by Competition for Water and Hydraulic Risk Can Explain Plant Trait Covariation. New Phytol. 2019, 225, 1206–1217. [Google Scholar] [CrossRef]
- Sack, L.; Scoffoni, C.; John, G.P.; Poorter, H.; Mason, C.M.; Mendez-Alonzo, R.; Donovan, L.A. Leaf Mass per Area Is Independent of Vein Length per Area: Avoiding Pitfalls When Modelling Phenotypic Integration (reply to Blonder et al. 2014). J. Exp. Bot. 2014, 65, 5115–5123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, T.X.; Song, L.; Goulding, K.; Zhang, F.S.; Liu, X.J. Cumulative and Partially Recoverable Impacts of Nitrogen Addition on a Temperate Steppe. Ecol. Appl. 2018, 28, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Lue, X.T.; Cui, Q.; Wang, Q.B.; Han, X.G. Nutrient Resorption Response to Fire and Nitrogen Addition in a Semi-Arid Grassland. Ecol. Eng. 2011, 37, 534–538. [Google Scholar] [CrossRef]
- Gan, H.H.; Jiao, Y.; Jia, J.B.; Wang, X.L.; Li, H.; Shi, W.G.; Peng, C.H.; Polle, A.; Luo, Z.B. Phosphorus and Nitrogen Physiology of Two Contrasting Poplar Genotypes When Exposed to Phosphorus and/or Nitrogen Starvation. Tree Physiol. 2016, 36, 22–38. [Google Scholar] [CrossRef] [Green Version]
- Lu, R.K. Soil Agricultural Chemistry Analysis Method; Agricultural Scientech Press: Beijing, China, 1999; pp. 150–152. [Google Scholar]
- Zhou, X.Q.; Li, H.W.; He, J.; Wang, Q.J.; Zhang, X.R. Design of multi-segment in situ soil sampler testing bulk density. Trans. Chin. Soc. Agric. Eng. 2008, 24, 127–130. [Google Scholar]
- Xiong, H.; Ma, C.E.; Li, L.; Zeng, H.; Guo, D.I. Stomatal Characteristics of Ferns and Angiosperms and Their Responses to Changing Light Intensity at Different Habitats. Chin. J. Plant Ecol. 2014, 38, 868–877. [Google Scholar] [CrossRef]
- Zhao, X.; Feng, B.H.; Chen, T.T.; Zhang, C.X.; Tao, L.X.; Fu, G.F. Transcriptome Analysis of Pale-Green Leaf Rice Reveals Photosynthetic Regulatory Pathways. Acta Physiol. Plant. 2017, 39, 274. [Google Scholar] [CrossRef]
- Li, J.Y.; Guo, Q.X.; Zhang, J.X.; Korpelainen, H.; Li, C.Y. Effects of Nitrogen and Phosphorus Supply on Growth and Physiological Traits of Two Larix Species. Environ. Exp. Bot. 2016, 130, 206–215. [Google Scholar] [CrossRef]
- Rasse, D.P. Nitrogen Deposition and Atmospheric CO2 Interactions on Fine Root Dynamics in Temperate Forests: A Theoretical Model Analysis. Glob. Chang. Biol. 2002, 8, 486–503. [Google Scholar] [CrossRef]
- Liu, J.Y.; Hui, J.F.; Sun, M.Y.; Liu, X.S.; Lu, W.H.; Ma, C.H.; Zhang, Q.B. Effects of Phosphorus Application and Inoculation Arbuscular Mycorrhizae Fungi (AMF) and Phosphate Solubilizing Bacteria on Dry Matter Yield and Phosphorus Use Efficiency of Alfalfa. Trans. Chin. Soc. Agric. Eng. 2020, 36, 142–149. [Google Scholar] [CrossRef]
- Marklein, A.R.; Houlton, B.Z. Nitrogen Inputs Accelerate Phosphorus Cycling Rates across a Wide Variety of Terrestrial Ecosystems. New Phytol. 2012, 193, 696–704. [Google Scholar] [CrossRef]
- Xu, H.S.; Zhu, L.; Mei, Y. Effects of High Levels of Nitrogen and Phosphorus on Perennial Ryegrass (Lolium Perenne L.) and Its Potential in Bioremediation of Highly Eutrophic Water. Environ. Sci. Pollut. Res. 2020, 28, 9475–9483. [Google Scholar] [CrossRef]
- Thompson, J.B.; Slot, M.; Dalling, J.W.; Winter, K.; Turner, B.L.; Zalamea, P.C. Species-Specific Effects of Phosphorus Addition on Tropical Tree Seedling Response to Elevated CO2. Funct. Ecol. 2019, 33, 1871–1881. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Cornelissen, J.H.C.; Falster, D.S.; Garnier, E.; Hikosaka, K.; Lamont, B.B.; Lee, W.; Oleksyn, J.; Osada, N.; et al. Assessing the Generality of Global Leaf Trait Relationships. New Phytol. 2005, 166, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Aerts, R.; Chapin, F.S.; Fitter, A.H.; Raffaelli, D.G. The Mineral Nutrition of Wild Plants Revisited: A Re-Evaluation of Processes and Patterns. Adv. Ecol. Res. 2000, 30, 1–67. [Google Scholar] [CrossRef]
- Sun, J.G.; Liu, C.C.; Hou, J.H.; He, N.P. Spatial Variation of Stomatal Morphological Traits in Grassland Plants of the Loess Plateau. Ecol. Indic. 2021, 128, 107857. [Google Scholar] [CrossRef]
- Chaerle, L.; Saibo, N.; Straeten, D. Tuning the Pores: Towards Engineering Plants for Improved Water Use Efficiency. Trends Biotechnol. 2005, 23, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.Y.; Fang, K.; Bian, H.W.; Zhu, M.Y. Advances in Stomatal Development and Its Regulation Factors. Plant Physiol. Commun. 2015, 52, 782–788. [Google Scholar] [CrossRef]
- Chen, T.T.; Fu, W.M.; Yu, J.; Feng, B.H.; Li, G.Y.; Fu, G.F.; Tao, L.X. The Photosynthesis Characteristics of Colored Rice Leaves and Its Relation with Antioxidant Capacity and Anthocyanin Content. Sci. Agric. Sin. 2022, 55, 467–478. [Google Scholar] [CrossRef]
- Sanita, d.T.L.; Pawlik-Skowronska, B.; Vurro, E.; Vattuone, Z.; Kalinowska, R.; Restivo, F.M.; Musetti, R.; Skowronski, T. First and Second Line Mechanisms of Cadmium Detoxification in the Lichen Photobiont Trebouxia Impressa (Chlorophyta). Environ. Pollut. 2008, 151, 280–286. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Tong, T.; Tian, B.; Fang, Y.X.; Pan, J.J.; Zheng, J.J.; Xue, D. Physiological, Biochemical and Molecular Responses of Barley Seedlings to Aluminum Stress. Phyton-Int. J. Exp. Bot. 2019, 88, 253–260. [Google Scholar] [CrossRef]
- Ahmad, H.; Hayat, S.; Ali, M.; Liu, T.; Cheng, Z.H. The Combination of Arbuscular Mycorrhizal Fungi Inoculation (Glomus Versiforme) and 28-Homobrassinolide Spraying Intervals Improves Growth by Enhancing Photosynthesis, Nutrient Absorption, and Antioxidant System in Cucumber (Cucumis Sativus L.) under Salinity. Ecol. Evol. 2018, 8, 5724–5740. [Google Scholar] [CrossRef] [PubMed]
- Hajiboland, R.; Aliasgharzadeh, N.; Laiegh, S.F.; Poschenrieder, C. Colonization with Arbuscular Mycorrhizal Fungi Improves Salinity Tolerance of Tomato (Solanum Lycopersicum L.) Plants. Plant Soil 2010, 331, 313–327. [Google Scholar] [CrossRef]
- Xue, J.R.; Pang, T.; Liu, J.G. Changes in the Temperature Tolerance Profile of Gracilariopsis Lemaneiformis from the Perspective of Photosynthesis, Respiration, and Biochemical Markers after Many Years of Vegetative Propagation. J. Appl. Phycol. 2022, 34, 1045–1058. [Google Scholar] [CrossRef]
- Lei, P.; Xu, Z.Q.; Liang, J.F.; Luo, X.H.; Zhang, Y.X.; Feng, X.H.; Xu, H. Poly (Gamma-Glutamic Acid) Enhanced Tolerance to Salt Stress by Promoting Proline Accumulation in Brassica Napus L. Plant Growth Regul 2016, 78, 233–241. [Google Scholar] [CrossRef]

| Total Nitrogen g kg−1 | Alkaline Nitrogen mg·kg−1 | Total Phosphorus g·kg−1 | Available Phosphorus mg·kg−1 | Available Potassium mg·kg−1 | Bulk Density g·cm−3 | Organic Matter g·kg−1 |
|---|---|---|---|---|---|---|
| 1.18 | 145.47 | 0.53 | 19.30 | 119.8 | 1.54 | 39.5 |
| Treatment | Urea (N) | Monoammonium Phosphate (P) | Monoammonium Phosphate (P2O5 52 %) kg·ha−1 | Monoammonium Phosphate (N 12.2 %) kg·ha−1 | Urea (N 46 %) kg·ha−1 |
|---|---|---|---|---|---|
| CK | 0 | 0 | 0 | 0 | 0 |
| N (120 kg·ha−1) | 120 | 0 | 0 | 0 | 260.9 + 51 |
| P with low N (100 + 24 kg·ha−1) | 0 | 100 | 192.3 | 23.5 | 0 |
| N and P (120 + 100 kg·ha−1) | 120 | 100 | 192.3 | 23.5 | 260.9 |
| Stubble Time | Treatment | Stomatal Number (n) | Stomatal Longitu-dinal Diameter (μm) | Stomatal Trans-verse Diameter (μm) | Stomatal Aper-ture (μm2) |
|---|---|---|---|---|---|
| First clipping | CK | 17.5 ± 0.77 a | 29.43 ± 0.76 b | 10.00 ± 0.44 c | 230.97 ± 11.31 b |
| N | 19.4 ± 1.01 a | 30.37 ± 0.89 ab | 12.11 ± 0.30 a | 288.16 ± 8.78 a | |
| P | 19.4 ± 1.25 a | 32.80 ± 0.79 a | 11.29 ± 0.30 ab | 290.58 ± 9.81 a | |
| NP | 20.3 ± 1.13 a | 32.29 ± 0.86 a | 11.10 ± 0.27 b | 282.05 ± 11.69 a | |
| Second clipping | CK | 15.3 ± 0.49 b | 28.38 ± 0.50 b | 6.98 ± 0.18 b | 155.87 ± 5.59 b |
| N | 18.2 ± 1.35 a | 29.79 ± 0.35 a | 8.55 ± 0.33 a | 199.76 ± 7.59 a | |
| P | 19.8 ± 0.79 a | 30.49 ± 0.26 a | 8.54 ± 0.14 a | 204.46 ± 3.75 a | |
| NP | 19.5 ± 0.56 a | 29.44 ± 0.57 ab | 8.80 ± 0.27 a | 203.62 ± 7.62 a | |
| Third clipping | CK | 16.9 ± 0.77 a | 30.93 ± 0.69 b | 10.14 ± 0.35 a | 246.23 ± 11.80 a |
| N | 19.5 ± 1.11 a | 31.87 ± 0.78 ab | 10.25 ± 0.30 a | 255.95 ± 8.20 a | |
| P | 19.6 ± 1.25 a | 33.30 ± 0.78 ab | 10.43 ± 0.23 a | 272.60 ± 9.58 a | |
| NP | 19.3 ± 0.57 a | 33.79 ± 0.76 a | 10.24 ± 0.28 a | 272.38 ± 10.47 a | |
| Fourth clipping | CK | 19.7 ± 2.04 a | 28.83 ± 1.15 a | 6.27 ± 0.34 b | 141.08 ± 7.72 b |
| N | 20.3 ± 1.30 a | 30.86 ± 1.59 a | 7.54 ± 0.37 a | 185.17 ± 16.49 a | |
| P | 22.7 ± 1.18 a | 30.52 ± 0.67 a | 8.33 ± 0.40 a | 201.03 ± 13.86 a | |
| NP | 20.0 ± 1.23 a | 29.80 ± 0.66 a | 7.67 ± 0.47 a | 180.99 ± 14.34 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Huang, R.; Yang, K.; Ma, C.; Zhang, Q. Effects of Nitrogen and Phosphorus Fertilization on Photosynthetic Properties of Leaves and Agronomic Characters of Alfalfa over Three Consecutive Years. Agriculture 2022, 12, 1187. https://doi.org/10.3390/agriculture12081187
Zhao J, Huang R, Yang K, Ma C, Zhang Q. Effects of Nitrogen and Phosphorus Fertilization on Photosynthetic Properties of Leaves and Agronomic Characters of Alfalfa over Three Consecutive Years. Agriculture. 2022; 12(8):1187. https://doi.org/10.3390/agriculture12081187
Chicago/Turabian StyleZhao, Jiantao, Rongzheng Huang, Kaixin Yang, Chunhui Ma, and Qianbing Zhang. 2022. "Effects of Nitrogen and Phosphorus Fertilization on Photosynthetic Properties of Leaves and Agronomic Characters of Alfalfa over Three Consecutive Years" Agriculture 12, no. 8: 1187. https://doi.org/10.3390/agriculture12081187
APA StyleZhao, J., Huang, R., Yang, K., Ma, C., & Zhang, Q. (2022). Effects of Nitrogen and Phosphorus Fertilization on Photosynthetic Properties of Leaves and Agronomic Characters of Alfalfa over Three Consecutive Years. Agriculture, 12(8), 1187. https://doi.org/10.3390/agriculture12081187






