Multigenerational Effects of Short-Term High Temperature on the Development and Reproduction of the Zeugodacus cucurbitae (Coquillett, 1899)
Abstract
1. Introduction
2. Materials and Methods
2.1. Pest Source for Testing and Pest Management
2.2. Test Reagents and Materials
2.3. The Setting of Short-Term High-Temperature Treatment
2.4. Observations on the Effects of Oviposition Ability and Egg Development
2.5. Observations on the Effects of Survival and Development
2.6. Statistical Analysis of Data
3. Results
3.1. F1 Only of Z. cucurbitae Was Exposed to Short-Term High Temperature
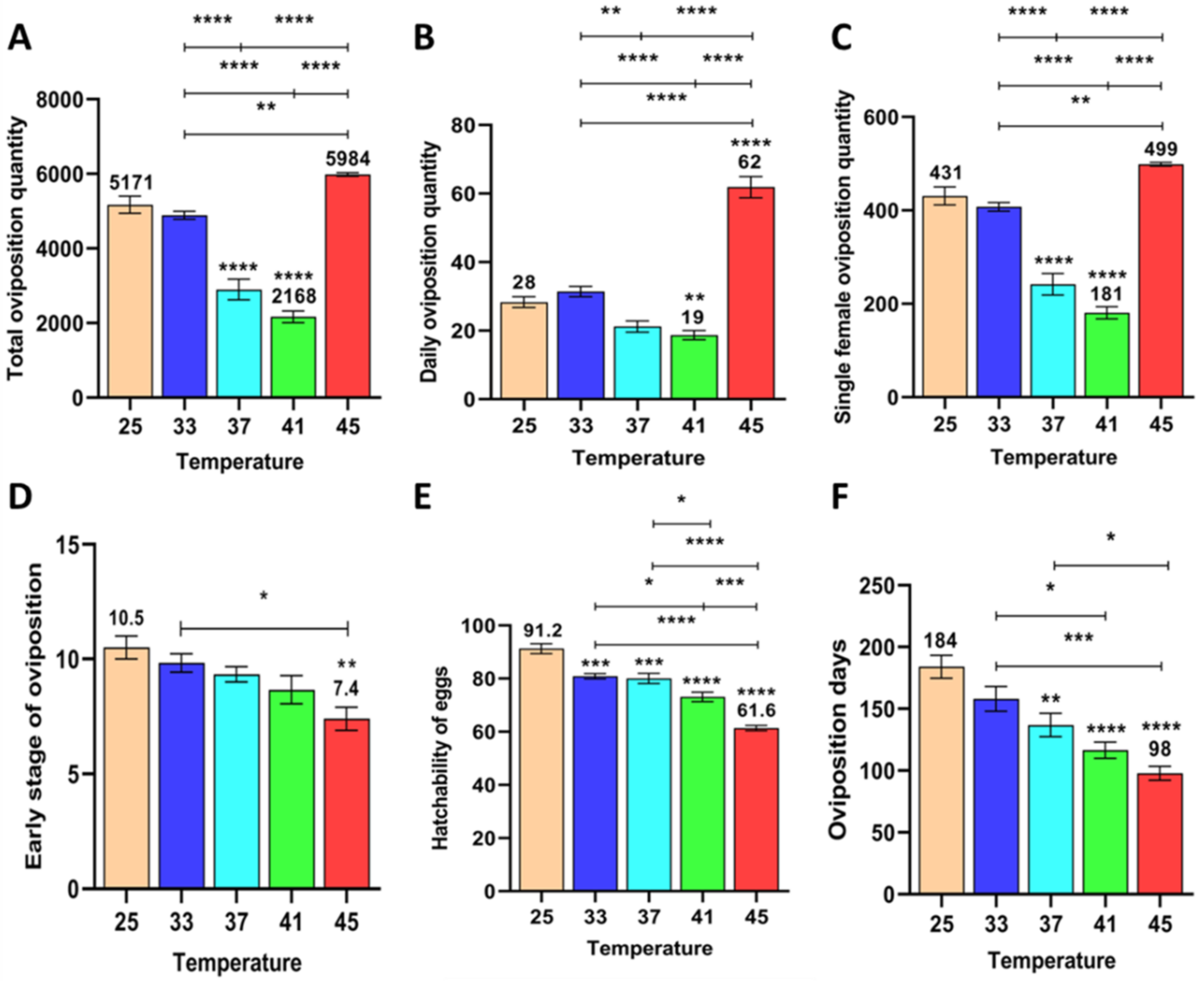
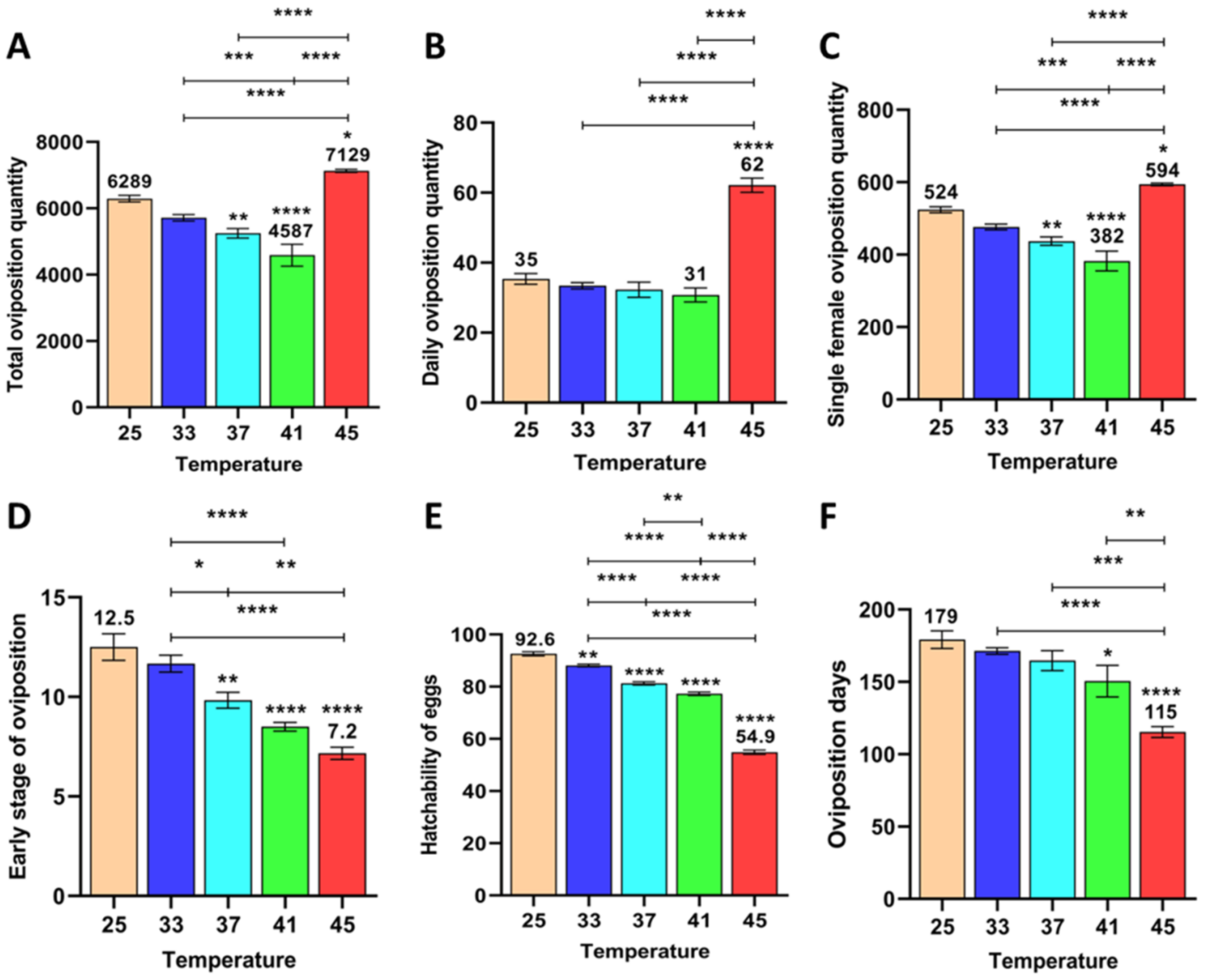
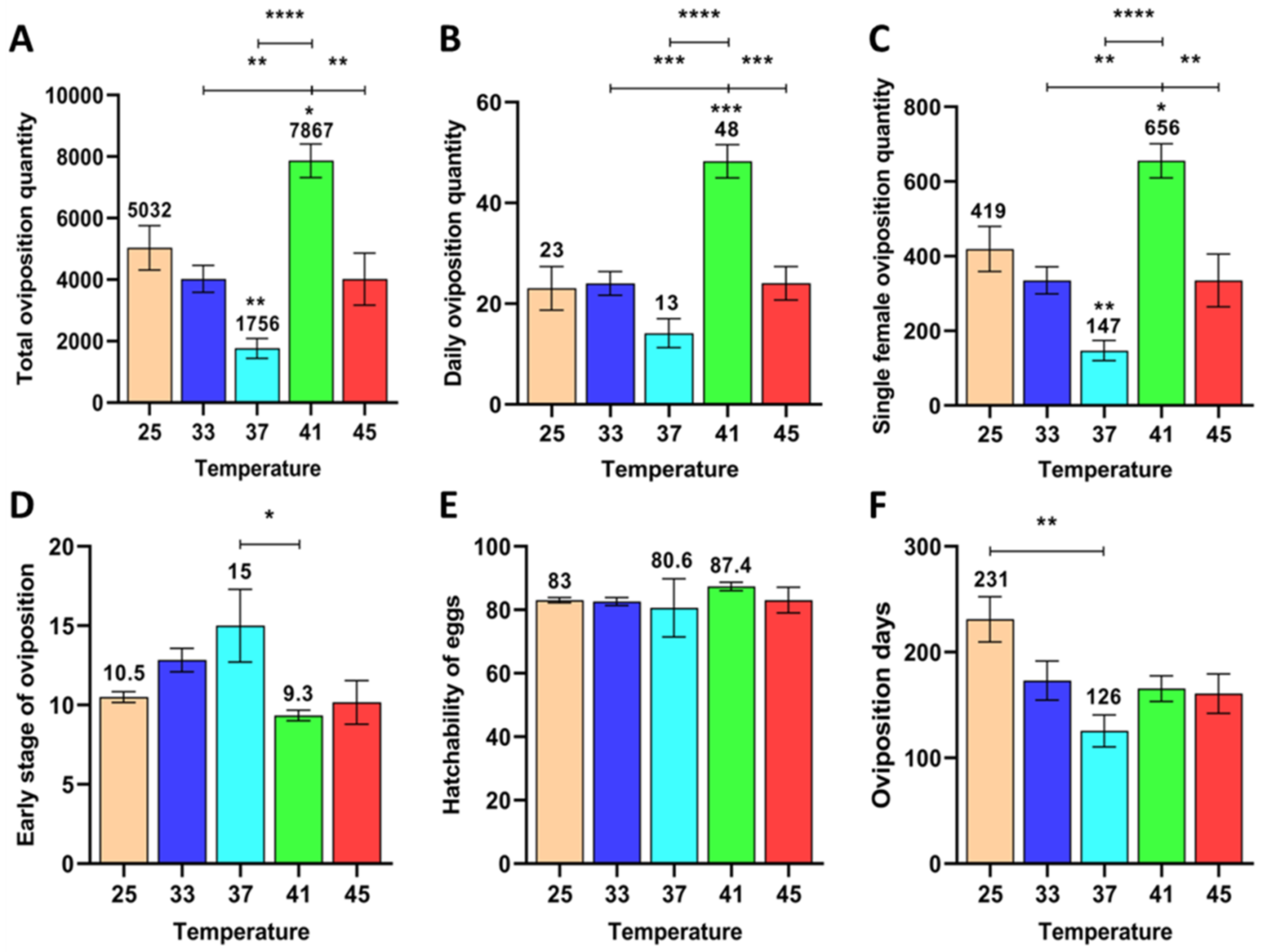
3.1.1. Effects of Short-Term High Temperature on Oviposition and Egg Development of Z. cucurbitae (F1)
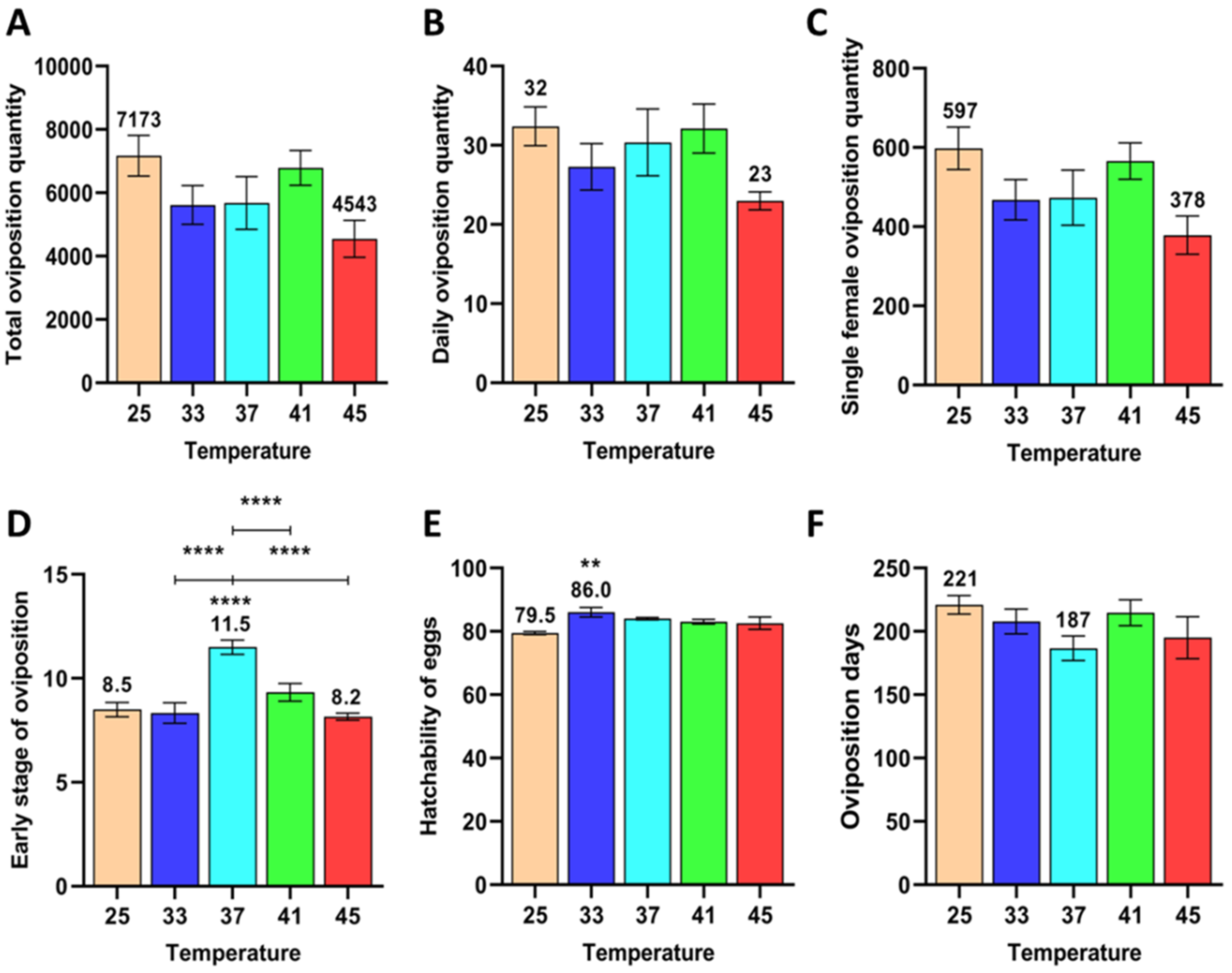
3.1.2. Effect of Short-Term High Temperature on Oviposition and Egg Development of Z. cucurbitae (F2)
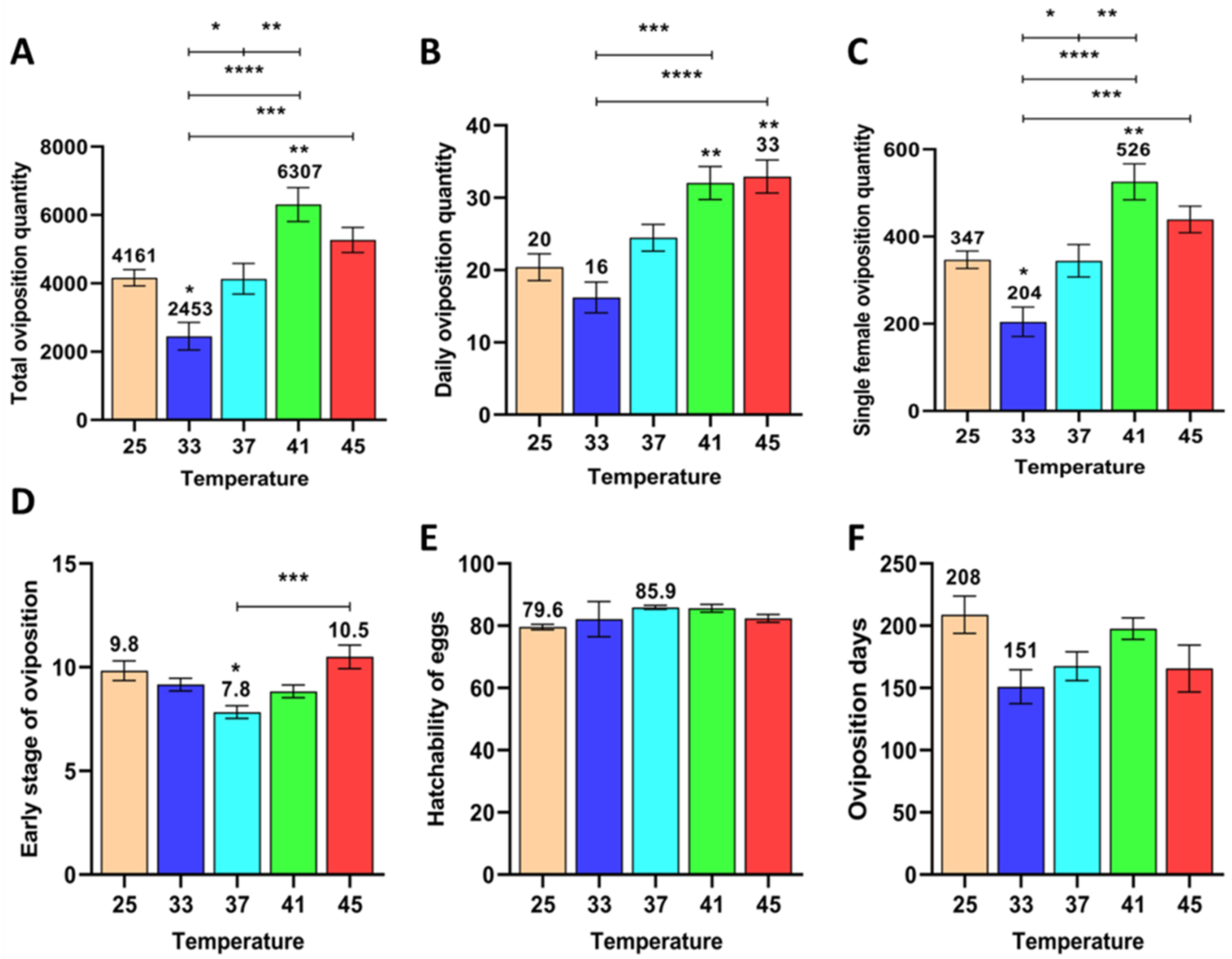
3.1.3. Effect of Short-Term High Temperature on Oviposition and Egg Development of Z. cucurbitae (F3)
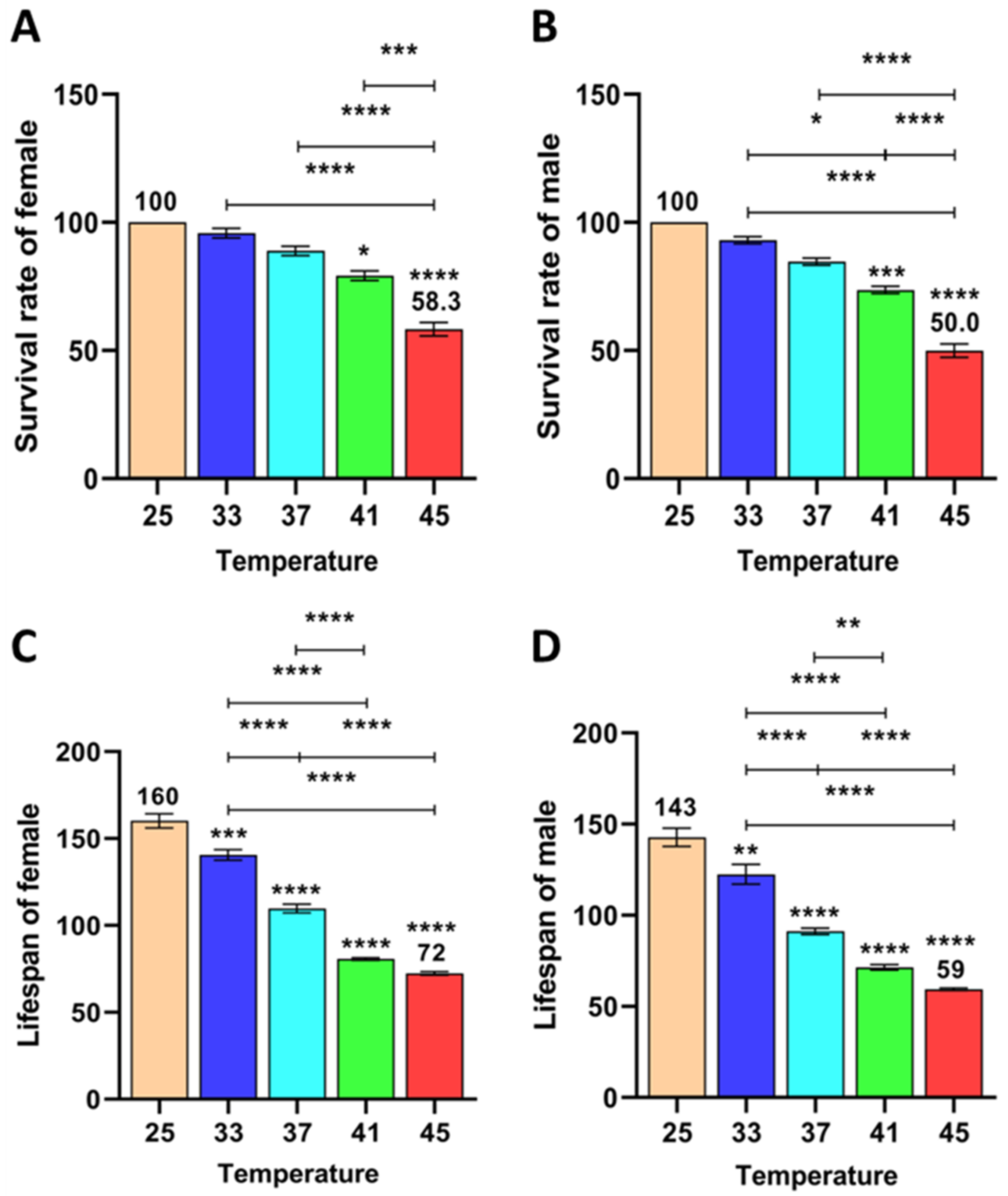
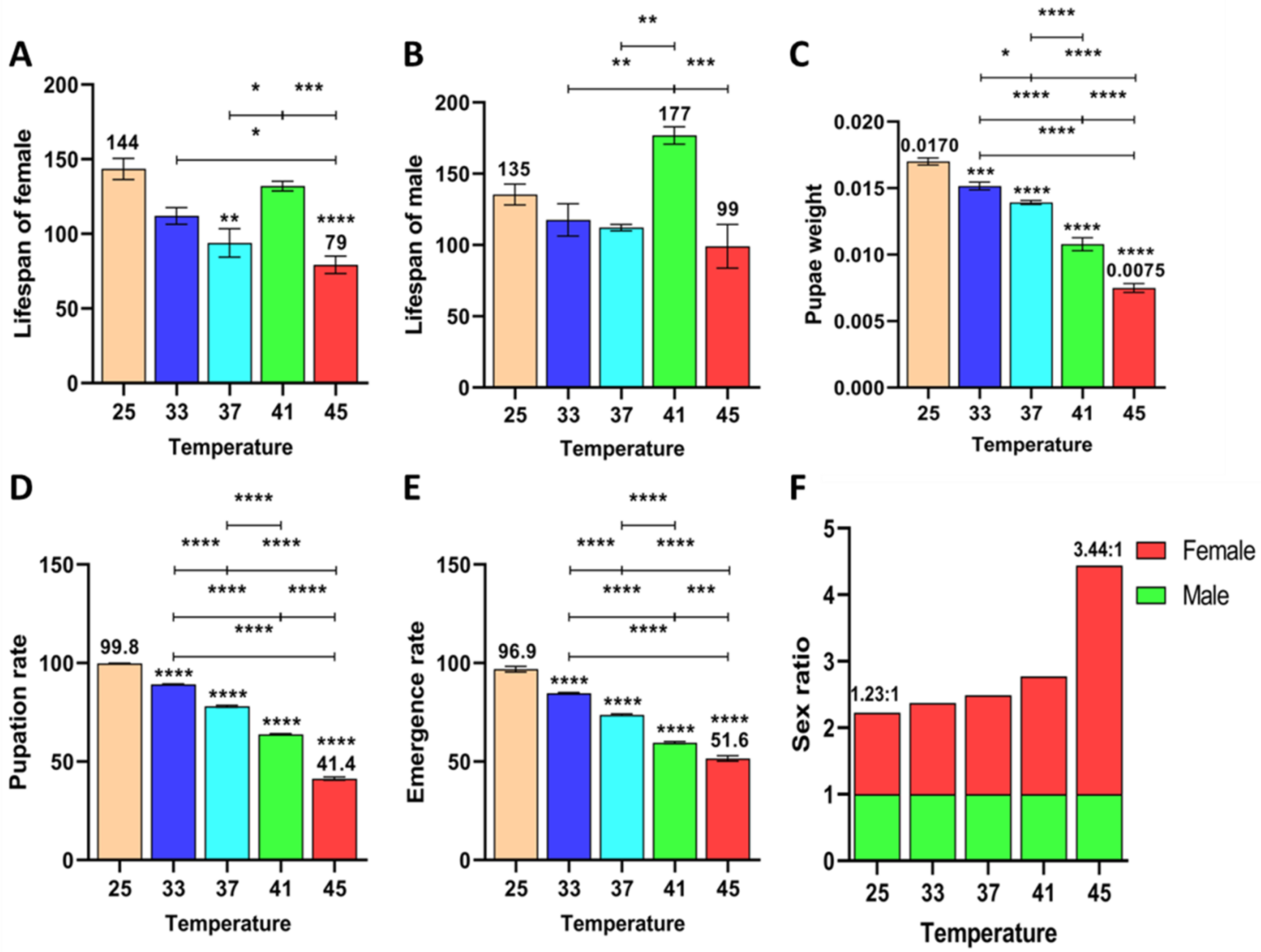
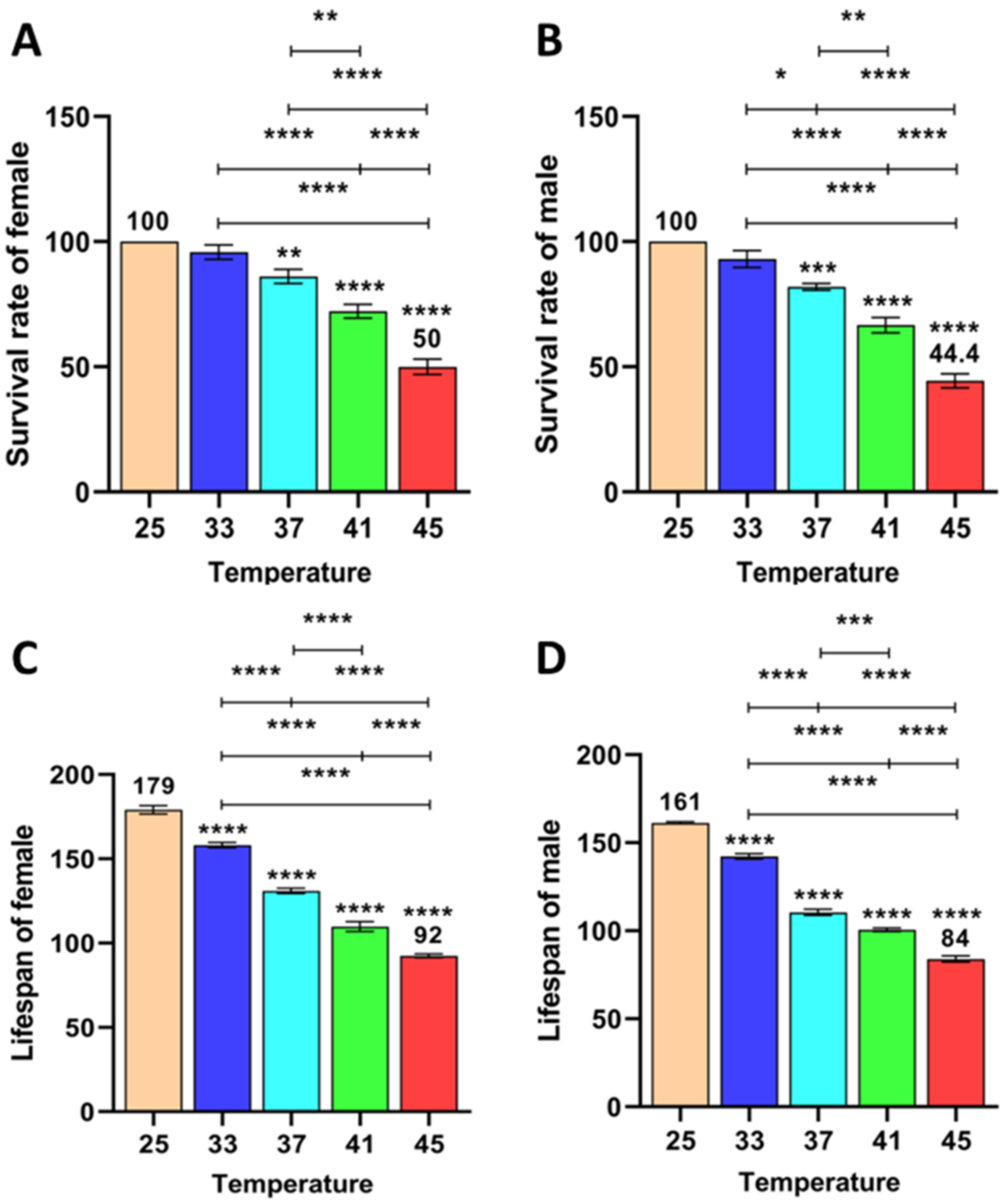
3.1.4. Effect of Short-Term High Temperature on Survival and Development of Z. cucurbitae (F1)
3.1.5. Effect of Short-Term High Temperature on Survival and Development of Z. cucurbitae (F2)
3.1.6. Effect of Short-Term High Temperature on Survival and Development of Z. cucurbitae (F3)
3.2. Both the F1 and F2 of Z. cucurbitae Were Exposed to Short-Term High Temperature
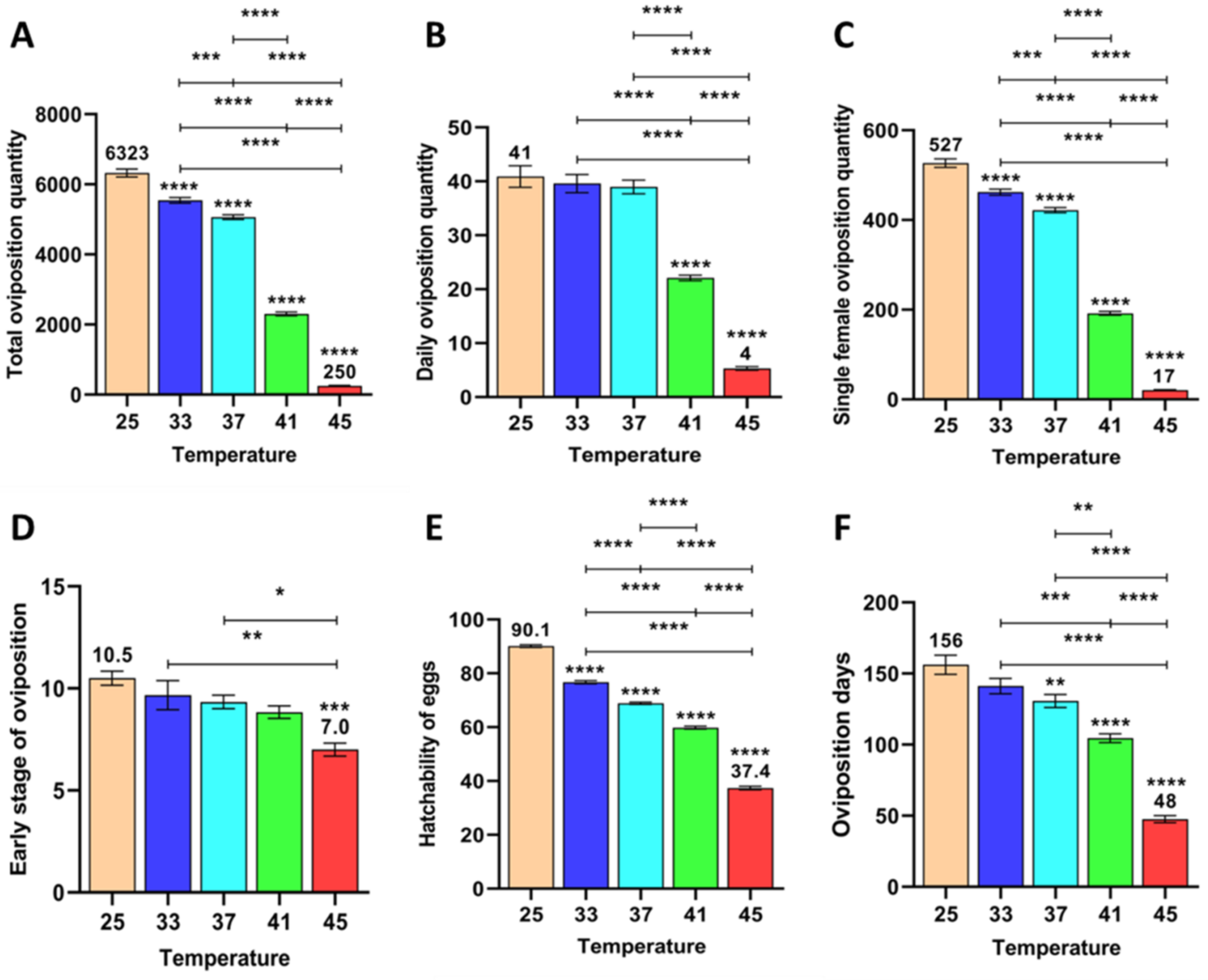
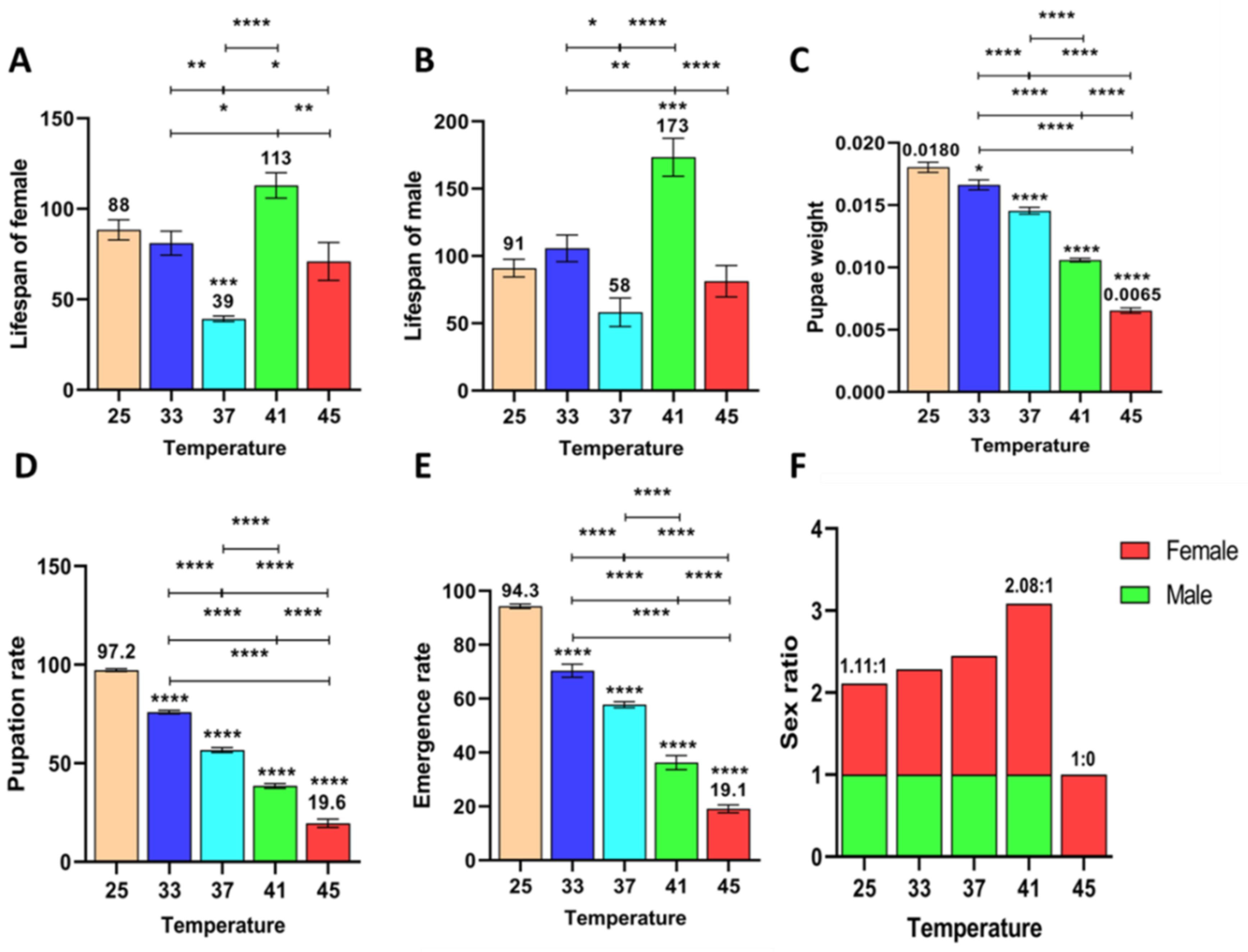
3.2.1. Effect of Short-Term High Temperature on Oviposition and Egg Development of Z. cucurbitae (F1)
3.2.2. Effect of Short-Term High Temperature on Oviposition and Egg Development of Z. cucurbitae (F2)
3.2.3. Effect of Short-Term High Temperature on Oviposition and Egg Development of Z. cucurbitae (F3)
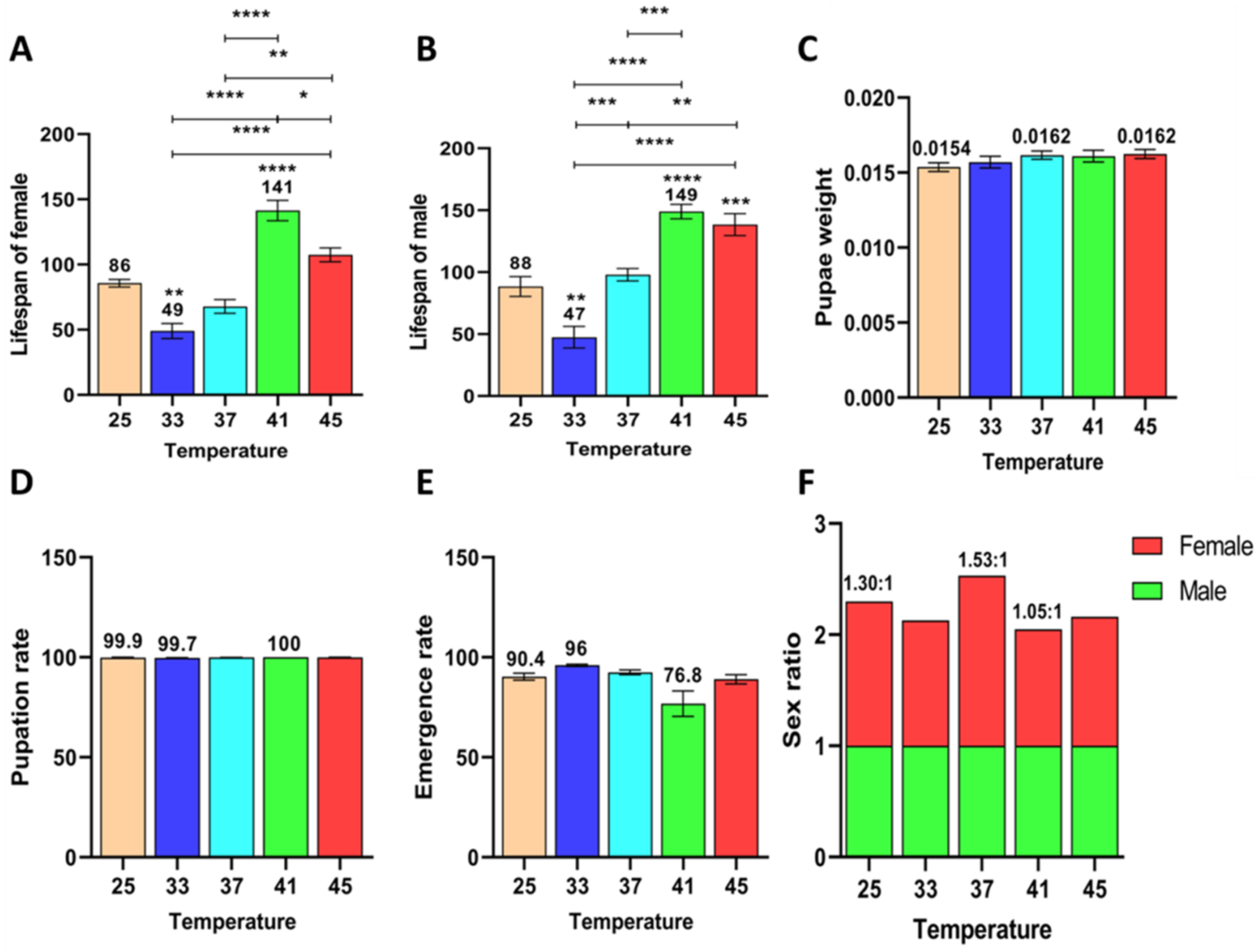
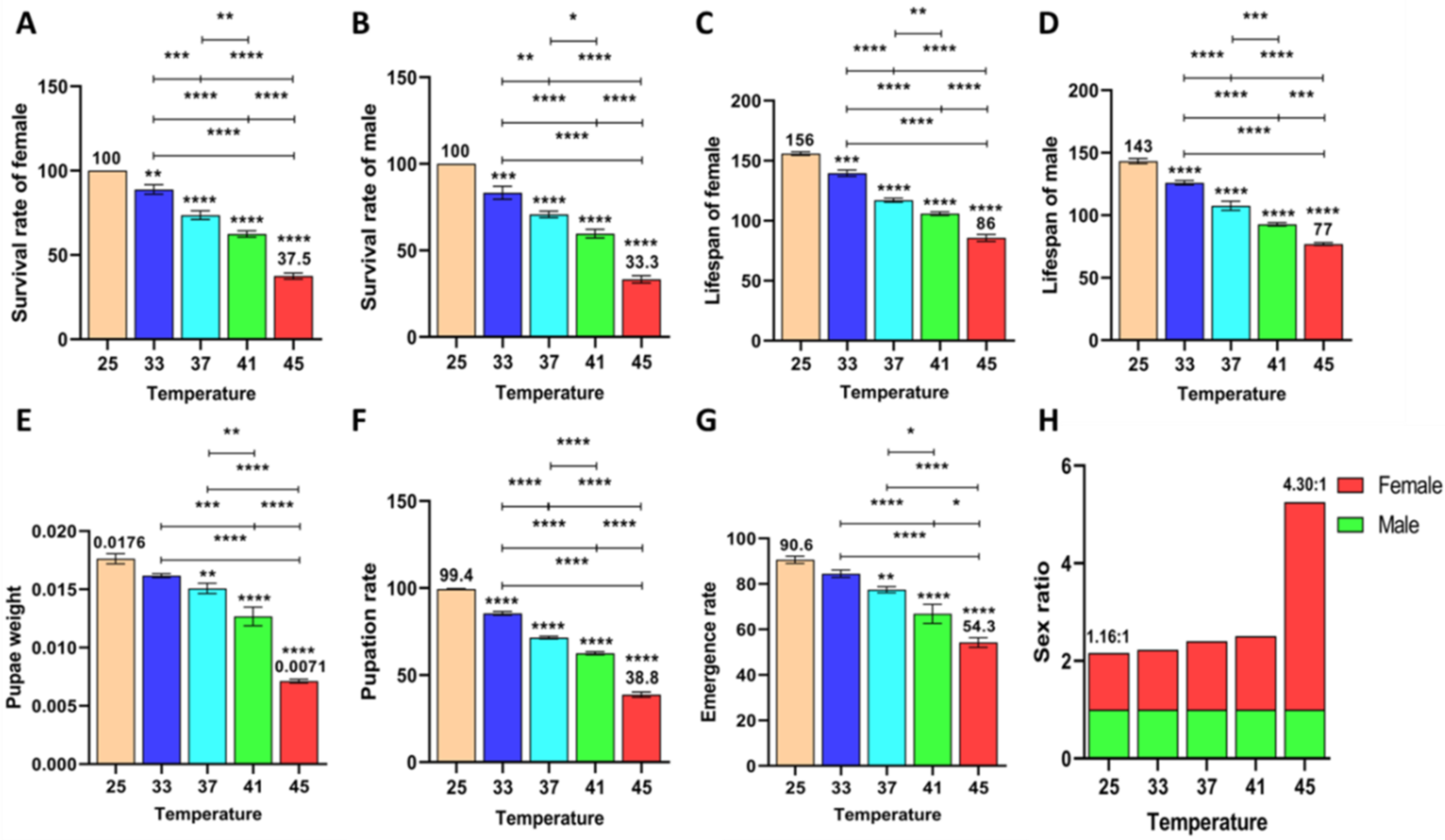
3.2.4. Effect of Short-Term High Temperature on Survival and Development of Z. cucurbitae (F1)
3.2.5. Effect of Short-Term High Temperature on Survival and Development of Z. cucurbitae (F2)
3.2.6. Effect of Short-Term High Temperature on Survival and Development of Z. cucurbitae (F3)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.; Bairlein, F. Ecological Responses to Recent Climate Change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Easterling, D.R.; Meehl, G.A.; Parmesan, C.; Changnon, S.A.; Karl, T.R.; Mearns, L.O. Climate Extremes: Observations, Modeling, and Impacts. Science 2000, 289, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Ma, C.S. The Impacts of Extreme High Temperature on Insect Populations under Climate Change: A Review. Sci Sin. Vitae 2016, 46, 556–564. [Google Scholar] [CrossRef][Green Version]
- Battisti, A.; Stastny, M.; Buffo, E.; Larsson, S. A Rapid Altitudinal Range Expansion in the Pine Processionary Moth Produced by the 2003 Climatic Anomaly. Glob. Change Biol. 2006, 12, 662–671. [Google Scholar] [CrossRef]
- Yamamura, K.; Yokozawa, M.; Nishimori, M.; Ueda, Y.; Yokosuka, T. How to Analyze Long-Term Insect Population Dynamics under Climate Change: 50-Year Data of Three Insect Pests in Paddy Fields. Popul. Ecol. 2006, 48, 31–48. [Google Scholar] [CrossRef]
- Clarke, A. Costs and Consequences of Evolutionary Temperature Adaptation. Trends Ecol. Evol. 2003, 18, 573–581. [Google Scholar] [CrossRef]
- Luo, S.; Ahola, V.; Shu, C.; Xu, C.; Wang, R. Heat Shock Protein 70 Gene Family in the Glanville Fritillary Butterfly and Their Response to Thermal Stress. Gene 2015, 556, 132–141. [Google Scholar] [CrossRef]
- Li, D.W.; Wu, J.H.; Qin, Z.Q. Study on the Heat Resistance and Survival Effect of Dysmicoccus neobrevipes Beardsley (Hemiptera: Pseudococcidae) to Short-Time High Temperature Stress. Chin. J. Trop. Agric. 2021, 41, 74–80. [Google Scholar]
- Li, H.B. Effects of High Temperature on Survival Rate and Reproduction of Frankliniella occidentalis Larvae. Guizhou Agric. Sci. 2021, 49, 52–59. [Google Scholar]
- Eggert, H.; Diddens, D.; Buhr, M.F.; Kurtz, J. A Temperature Shock can Lead to Trans-Generational Immune Priming in the Red Flour Beetle, Tribolium Castaneum. Ecol. Evol. 2015, 5, 1318–1326. [Google Scholar] [CrossRef]
- Gilchrist, G.W.; Huey, R.B. Parental and Developmental Temperature Effects on the Thermal Dependence of Fitness in Drosophila melanogaster. Evolution 2001, 55, 209–214. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Kong, Q.; Yuan, S.Y.; Shen, D.R.; Xie, K.; Wang, C.M. Effects of Temperature and Relative Humidity Stress on the Survival and Fecundity of the Melon Fly, Bactrocera cucurbitae (Diptera: Tephritidae). North. Hortic. 2019, 15, 36–43. [Google Scholar]
- Ahn, J.J.; Choi, K.S.; Huang, Y.B. Thermal effects on the development of Zeugodacus cucurbitae (Coquillett)(Diptera: Tephritidae) and model validation. Phytoparasitica 2022, 50, 601–616. [Google Scholar] [CrossRef]
- Back, E.A.; Pemberton, C.E. Life history of the melon fly. J. Agric. Res. 1914, 3, 269–274. [Google Scholar]
- Raza, M.M.; Khan, M.A.; Arshad, M.; Sagheer, M.; Sattar, Z.; Shafi, J.; Sattar, A. Impact of global warming on insects. Arch. Phytopathol. Plant Prot. 2015, 48, 84–94. [Google Scholar] [CrossRef]
- Zhou, S.H. Studies on the Responses of Bactrocera Cucurbitae to High Temperature Stress and its Molecular Basis. PhD Thesis, Hainan University, Haikou, China, 2016. [Google Scholar]
- Wei, S.D.; Huang, S.S.; Wang, Y.Q.; Zeng, D.Q.; Ling, Y. Effect of temperature on the development and reproduction of Bactrocera cucurbitae (Coquillett) population. J. South Agric. 2011, 42, 744–747. [Google Scholar]
- Liang, L.N.; Zhang, W.; Ma, G.; Hoffmann, A.A.; Ma, C.S. A single hot event stimulates adult performance but reduces egg survival in the oriental fruit moth, Grapholitha molesta. PLoS ONE 2014, 9, e116339. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Sgrò, C.M. Climate change and evolutionary adaptation. Nature 2011, 470, 479–485. [Google Scholar] [CrossRef]
- Tseng, M.; Kaur, K.M.; Soleimani, P.S.; Sarai, K.; Chan, D.; Yao, C.H.; Fograscher, K. Decreases in beetle body size linked to climate change and warming temperatures. J. Anim. Ecol. 2018, 87, 647–659. [Google Scholar] [CrossRef]
- Zhou, S.; Li, L.; Zeng, B.; Fu, Y. Effects of short-term high-temperature conditions on oviposition and differential gene expression of Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae). Int. J. Pest Manag. 2020, 66, 332–340. [Google Scholar] [CrossRef]
- Zeng, B.; Zhu, W.; Fu, Y.; Zhou, S. Response mechanism of oviposition and relevant protein expression of Bactrocera cucurbitae (Coquillet) to short-term high-temperature conditions. Neotrop. Entomol. 2019, 48, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Ma, C.S.; Zhao, Q.H.; Ma, G.; Yang, H.P. Effects of heat stress on physiological and biochem icalmechanism s of insects: A literature review. Acta Ecol. Sin. 2007, 4, 1565–1572. [Google Scholar]
- Ghazanfar, M.U.; Hagenbucher, S.; Romeis, J.; Grabenweger, G.; Meissle, M. Fluctuating temperatures influence the susceptibility of pest insects to biological control agents. J. Pest Sci. 2020, 93, 1007–1018. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Tada, A.; Musolin, D.L.; Hari, N.; Hosokawa, T.; Fujisaki, K.; Fukatsu, T. Collapse of insect gut symbiosis under simulated climate change. mBio 2016, 7, e01578-16. [Google Scholar] [CrossRef] [PubMed]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Chang. Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- Gu, X.P.; Huang, Y.Y.; Zhang, J.Y.; Zhang, X.M.; Chen, G.H. Effects of short-term high-temperature stress on growth, development and reproduction of melon fly. J. Environ. Entomol. 2020, 42, 391–399. [Google Scholar] [CrossRef]
- Mousseau, T.A.; Fox, C.W. The adaptive significance of maternal effects. Trends Ecol. Evol. 1998, 13, 403–407. [Google Scholar] [CrossRef]
- Hopper, K.R. Risk-spreading and bet-hedging in insect population biology. Annu. Rev. Entomol. 1999, 44, 535–560. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, B.; Lian, Y.; Jia, J.; Liu, Y.; Wang, A.; Yang, H.; Li, J.; Yang, S.; Peng, S.; Zhou, S. Multigenerational Effects of Short-Term High Temperature on the Development and Reproduction of the Zeugodacus cucurbitae (Coquillett, 1899). Agriculture 2022, 12, 954. https://doi.org/10.3390/agriculture12070954
Zeng B, Lian Y, Jia J, Liu Y, Wang A, Yang H, Li J, Yang S, Peng S, Zhou S. Multigenerational Effects of Short-Term High Temperature on the Development and Reproduction of the Zeugodacus cucurbitae (Coquillett, 1899). Agriculture. 2022; 12(7):954. https://doi.org/10.3390/agriculture12070954
Chicago/Turabian StyleZeng, Bei, Yuyang Lian, Jingjing Jia, Yang Liu, Aqiang Wang, Heming Yang, Jinlei Li, Shuyan Yang, Sihua Peng, and Shihao Zhou. 2022. "Multigenerational Effects of Short-Term High Temperature on the Development and Reproduction of the Zeugodacus cucurbitae (Coquillett, 1899)" Agriculture 12, no. 7: 954. https://doi.org/10.3390/agriculture12070954
APA StyleZeng, B., Lian, Y., Jia, J., Liu, Y., Wang, A., Yang, H., Li, J., Yang, S., Peng, S., & Zhou, S. (2022). Multigenerational Effects of Short-Term High Temperature on the Development and Reproduction of the Zeugodacus cucurbitae (Coquillett, 1899). Agriculture, 12(7), 954. https://doi.org/10.3390/agriculture12070954






