Effect of Watermelon (Citrullus lanatus) Extract on Carbohydrates-Hydrolyzing Enzymes In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Watermelon Extract Preparation
2.2. Measurement of Total Phenolic Compound (TPC)
2.3. Identification and Quantification of Watermelon Bioactive Compounds
2.3.1. Carotenoid and Chlorophyll Analysis
2.3.2. Citrulline Analysis
2.4. Rat α-Glucosidase and α-Amylase Inhibition Assays
2.5. Statistical Analysis
3. Results
3.1. TPC and Bioactive Compounds of Watermelon Extracts
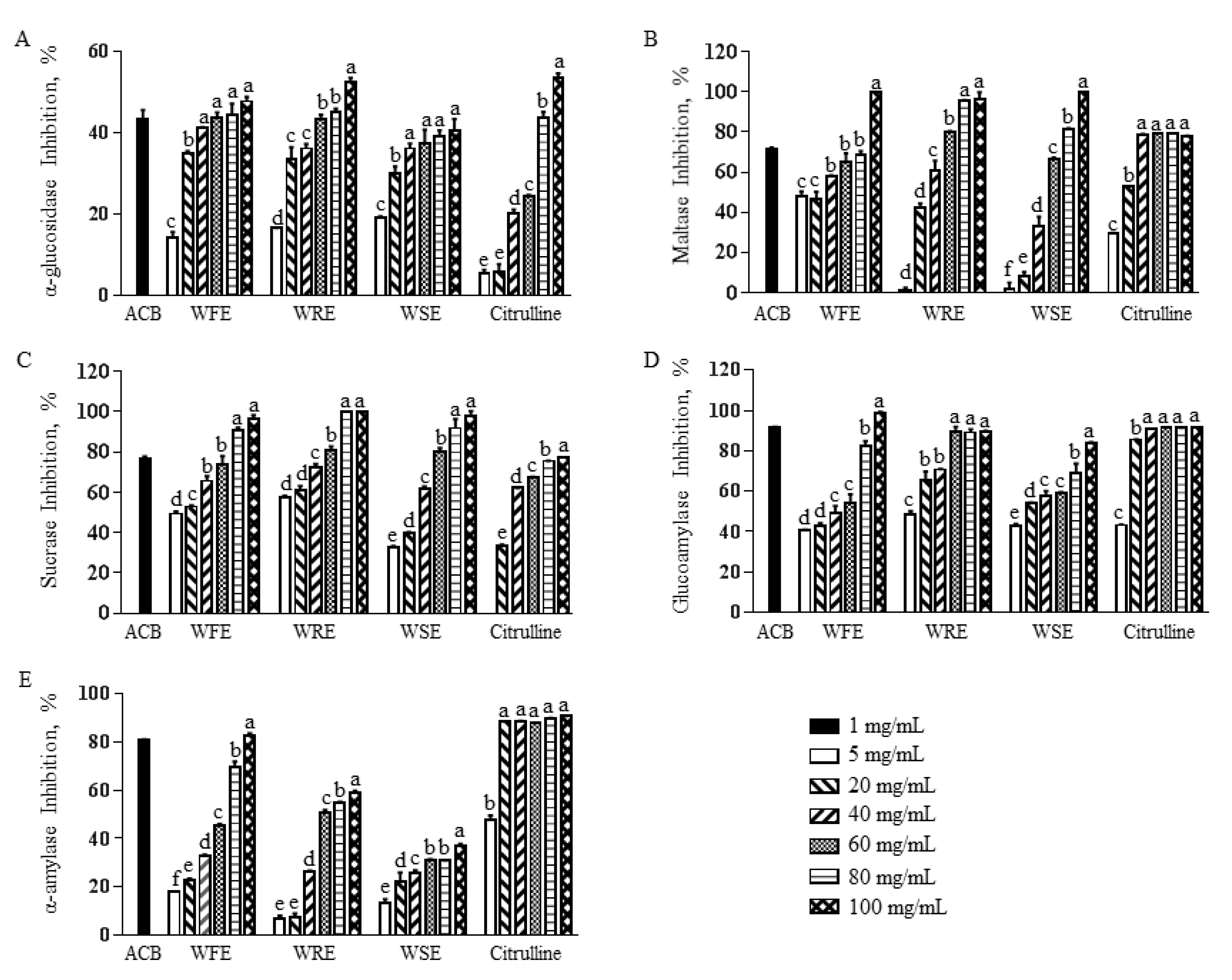
3.2. Inhibitory Effect of Watermelon Extract and Citrulline on α-Glucosidase Activity
3.3. Inhibitory Effect of Watermelon Extract and Citrulline on Pancreatic α-Amylase Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ceriello, A. Postprandial hyperglycemia and diabetes complications: Is it time to treat? Diabetes 2005, 54, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ajiboye, B.O.; Shonibare, M.T.; Oyinloye, B.E. Antidiabetic activity of watermelon (Citrullus lanatus) juice in alloxan-induced diabetic rats. J. Diabetes Metab. Disord. 2020, 19, 343–352. [Google Scholar] [CrossRef]
- Kast, R. Acarbose related diarrhea: Increased butyrate upregulates prostaglandin E. Inflamm. Res. 2002, 51, 117–118. [Google Scholar] [CrossRef]
- Kumar, S.; Narwal, S.; Kumar, V.; Prakash, O. α-glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharmacogn. Rev. 2011, 5, 19. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Zhang, G.; Liao, Y.; Gong, D. Inhibitory kinetics and mechanism of kaempferol on α-glucosidase. Food Chem. 2016, 190, 207–215. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, G.; Pan, J.; Wang, Y. α-Glucosidase inhibition by luteolin: Kinetics, interaction and molecular docking. Int. J. Biol. Macromol. 2014, 64, 213–223. [Google Scholar] [CrossRef]
- Zamuz, S.; Munekata, P.E.S.; Gullón, B.; Rocchetti, G.; Montesano, D.; Lorenzo, J.M. Citrullus lanatus as source of bioactive components: An up-to-date review. Trends Food Sci. Technol. 2021, 111, 208–222. [Google Scholar] [CrossRef]
- Lum, T.; Connolly, M.; Marx, A.; Beidler, J.; Hooshmand, S.; Kern, M.; Liu, C.; Hong, M.Y. Effects of fresh watermelon consumption on the acute satiety response and cardiometabolic risk factors in overweight and obese adults. Nutrients 2019, 11, 595. [Google Scholar] [CrossRef] [Green Version]
- Oseni, O.; Odesanmi, O.; Oladele, F. Antioxidative and antidiabetic activities of watermelon (Citrullus lanatus) juice on oxidative stress in alloxan-induced diabetic male Wistar albino rats. Niger. Med. J. J. Niger. Med. Assoc. 2015, 56, 272. [Google Scholar] [CrossRef] [Green Version]
- Elkahoui, S.; Bartley, G.E.; Yokoyama, W.H.; Friedman, M. Dietary supplementation of potato peel powders prepared from conventional and organic russet and non-organic gold and red potatoes reduces weight gain in mice on a high-fat diet. J. Agric. Food Chem. 2018, 66, 6064–6072. [Google Scholar] [CrossRef]
- Aderiye, B.; David, O.; Fagbohun, E.; Faleye, J.; Olajide, O. Immunomodulatory and phytomedicinal properties of watermelon juice and pulp (Citrullus lanatus Linn): A review. GSC Biol. Pharm. Sci. 2020, 11, 153–165. [Google Scholar] [CrossRef]
- Bantle, J.P. Dietary fructose and metabolic syndrome and diabetes. J. Nutr. 2009, 139, 1263s–1268s. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.; Choi, W.; Kim, S.; Ha, T. Anti-diabetic effect of watermelon (Citrullus vulgaris Schrad) on Streptozotocin-induced diabetic mice. Food Sci. Biotechnol. 2011, 20, 251–254. [Google Scholar] [CrossRef]
- Becraft, A.R.; Sturm, M.L.; Mendez, R.L.; Park, S.H.; Lee, S.I.; Shay, N.F. Intake of watermelon or its byproducts alters glucose metabolism, the microbiome, and hepatic proinflammatory metabolites in high-fat–fed male C57BL/6 J mice. J. Nutr. 2020, 150, 434–442. [Google Scholar] [CrossRef]
- Wong, S.Y.; Wu, L.; Lu, P.; Ojo, B.; Tang, M.; Clarke, S.; Keirns, B.; Lucas, E.A.; Smith, B.; Chowanadisai, W. Drinking watermelon juice shift the gut microbiome in diabetic mice (P20-025-19). Curr. Dev. Nutr. 2019, 3, nzz040.P020-025-019. [Google Scholar] [CrossRef]
- Angeline, C.; Krishnakumari, S. Qualitative phtochemistry profile of watermelon (Citrullus vulgaris schrad) rind extracts with different solvent. Asian J. Pharm. Clin. Res. 2015, 8, 62–65. [Google Scholar]
- Edori, O.; Marcus, A. Phytochemical screening and physiologic functions of metals in seed and peel of Citrullus lanatus (Watermelon). Int. J. Green Herb. Chem. B 2017, 6, 35–46. [Google Scholar]
- Otieno, D.; Lee, E.J.; Lee, S.G.; Richard, C.; Kang, H.W. Optimizing process of brewing onion peel tea using a response surface methodology. NFS J. 2020, 20, 22–27. [Google Scholar] [CrossRef]
- Van Hung, P.; Hatcher, D.W.; Barker, W. Phenolic acid composition of sprouted wheats by ultra-performance liquid chromatography (UPLC) and their antioxidant activities. Food Chem. 2011, 126, 1896–1901. [Google Scholar] [CrossRef]
- Wu, G.; Meininger, C.J. Analysis of citrulline, arginine, and methylarginines using high-performance liquid chromatography. Methods Enzymol. 2008, 440, 177–189. [Google Scholar]
- Malunga, L.N.; Eck, P. Inhibition of intestinal α-glucosidase and glucose absorption by feruloylated arabinoxylan mono-and oligosaccharides from corn bran and wheat aleurone. J. Nutr. Metab. 2016, 2016, 1932532. [Google Scholar] [CrossRef] [Green Version]
- Kwon, Y.-I.I.; Vattem, D.A.; Shetty, K. Evaluation of clonal herbs of Lamiaceae species for management of diabetes and hypertension. Asia Pac. J. Clin. Nutr. 2006, 15, 107. [Google Scholar]
- Qi, J.; Kim, S.M. α-Glucosidase inhibitory activities of lutein and zeaxanthin purified from green alga Chlorella ellipsoidea. J. Ocean Univ. China 2018, 17, 983–989. [Google Scholar] [CrossRef]
- Jibril, M.M.; Abdul-Hamid, A.; Ghazali, H.M.; Dek, M.S.P.; Ramli, N.S.; Jaafar, A.H.; Karrupan, J.; Mohammed, A.S. Antidiabetic antioxidant and phytochemical profile of yellow-fleshed seeded watermelon (Citrullus lanatus) extracts. J. Food Nutr. Res. 2019, 7, 82–95. [Google Scholar]
- Lee, B.-H.; Hamaker, B.R. Maltase has most versatile α-hydrolytic activity among the mucosal α-glucosidases of the small intestine. J. Pediatr. Gastroenterol. Nutr. 2018, 66, S7–S10. [Google Scholar] [CrossRef]
- Simsek, M.; Quezada-Calvillo, R.; Nichols, B.L.; Hamaker, B.R. Phenolic compounds increase the transcription of mouse intestinal maltase-glucoamylase and sucrase-isomaltase. Food Funct. 2017, 8, 1915–1924. [Google Scholar] [CrossRef]
- Zheng, Y.-G.; Shentu, X.-P.; Shen, Y.-C. Inhibition of porcine small intestinal sucrase by valienamine. J. Enzym. Inhib. Med. Chem. 2005, 20, 49–53. [Google Scholar] [CrossRef]
- Lo Piparo, E.; Scheib, H.; Frei, N.; Williamson, G.; Grigorov, M.; Chou, C.J. Flavonoids for controlling starch digestion: Structural requirements for inhibiting human α-amylase. J. Med. Chem. 2008, 51, 3555–3561. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, C.; Zhang, B.; Huang, Q. The inhibitory effects of flavonoids on α-amylase and α-glucosidase. Crit. Rev. Food Sci. Nutr. 2020, 60, 695–708. [Google Scholar] [CrossRef]
- Mushtaq, M.; Sultana, B.; Bhatti, H.N.; Asghar, M. RSM based optimized enzyme-assisted extraction of antioxidant phenolics from underutilized watermelon (Citrullus lanatus Thunb.) rind. J. Food Sci. Technol. 2015, 52, 5048–5056. [Google Scholar] [CrossRef] [Green Version]
- Simsek, M.; Quezada-Calvillo, R.; Ferruzzi, M.G.; Nichols, B.L.; Hamaker, B.R. Dietary phenolic compounds selectively inhibit the individual subunits of maltase-glucoamylase and sucrase-isomaltase with the potential of modulating glucose release. J. Agric. Food Chem. 2015, 63, 3873–3879. [Google Scholar] [CrossRef] [PubMed]
- Abu-Reidah, I.M.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Profiling of phenolic and other polar constituents from hydro-methanolic extract of watermelon (Citrullus lanatus) by means of accurate-mass spectrometry (HPLC–ESI–QTOF–MS). Food Res. Int. 2013, 51, 354–362. [Google Scholar] [CrossRef]
- Yang, J.-P.; He, H.; Lu, Y.-H. Four flavonoid compounds from Phyllostachys edulis leaf extract retard the digestion of starch and its working mechanisms. J. Agric. Food Chem. 2014, 62, 7760–7770. [Google Scholar] [CrossRef] [PubMed]
- Maoto, M.M.; Beswa, D.; Jideani, A.I. Watermelon as a potential fruit snack. Int. J. Food Prop. 2019, 22, 355–370. [Google Scholar] [CrossRef] [Green Version]
| WFE | WRE | WSE | |
|---|---|---|---|
| Chlorophyll | 83.7 ± 1.6 b | 26.4 ± 0.3 b | 7880.4 ± 132.2 a |
| Lutein | 5.3 ± 0.1 b | 11.2 ± 0.1 b | 1273.0 ± 11.1 a |
| β-carotene | 1.2 ± 0.1 b | 2.3 ± 0.1 b | 20.7 ± 0.8 a |
| Citrulline | 684.4 ± 2.8 c | 1017.1 ± 64.3 b | 3101.0 ± 5.5 a |
| Acarbose | WFE | WRE | WSE | Citrulline | |
|---|---|---|---|---|---|
| α-glucosidase | 0.03 ± 0.00 c | 8.12 ± 0.95 b | 11.95 ± 2.80 b | 5.68 ± 0.94 b | 43.49 ± 2.57 a |
| Maltase | 0.02 ± 0.00 d | 26.58 ± 0.46 b | 0.02 ± 0.00 d | 44.30 ± 1.17 a | 6.48 ± 0.32 c |
| Sucrase | 0.02 ± 0.00 d | 10.19 ± 0.13 c | 13.00 ± 1.00 b | 16.00 ± 0.55 a | 0.01 ± 0.00 d |
| Glucoamylase | 0.01 ± 0.00 d | 23.00 ± 0.70 a | 5.03 ± 0.21 c | 10.11 ± 0.59 b | 0.01 ± 0.00 d |
| α-amylase | 0.07 ± 0.00 d | 32.82 ± 0.79 a | 0.03 ± 0.01 d | 12.15 ±1.75 b | 3.20 ± 0.76 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balogun, O.; Otieno, D.; Brownmiller, C.R.; Lee, S.-O.; Kang, H.W. Effect of Watermelon (Citrullus lanatus) Extract on Carbohydrates-Hydrolyzing Enzymes In Vitro. Agriculture 2022, 12, 772. https://doi.org/10.3390/agriculture12060772
Balogun O, Otieno D, Brownmiller CR, Lee S-O, Kang HW. Effect of Watermelon (Citrullus lanatus) Extract on Carbohydrates-Hydrolyzing Enzymes In Vitro. Agriculture. 2022; 12(6):772. https://doi.org/10.3390/agriculture12060772
Chicago/Turabian StyleBalogun, Olugbenga, Dammah Otieno, Cindi R. Brownmiller, Sun-Ok Lee, and Hye Won Kang. 2022. "Effect of Watermelon (Citrullus lanatus) Extract on Carbohydrates-Hydrolyzing Enzymes In Vitro" Agriculture 12, no. 6: 772. https://doi.org/10.3390/agriculture12060772
APA StyleBalogun, O., Otieno, D., Brownmiller, C. R., Lee, S.-O., & Kang, H. W. (2022). Effect of Watermelon (Citrullus lanatus) Extract on Carbohydrates-Hydrolyzing Enzymes In Vitro. Agriculture, 12(6), 772. https://doi.org/10.3390/agriculture12060772






