Physiological and Biochemical Parameters of Leaves for Evaluation of the Potato Yield
Abstract
:1. Introduction
2. Materials and Methods
2.1. Objects of Study and Growing Conditions
2.2. Research Methodology
2.2.1. Growth and Yield Parameters
2.2.2. Plant Material
2.2.3. Physiological Parameters of Leaves
2.2.4. Lipid Analysis
2.2.5. Protein Analysis
2.2.6. Proline Analysis
2.3. Statistical Analysis of the Data
3. Results
3.1. Environmental Conditions
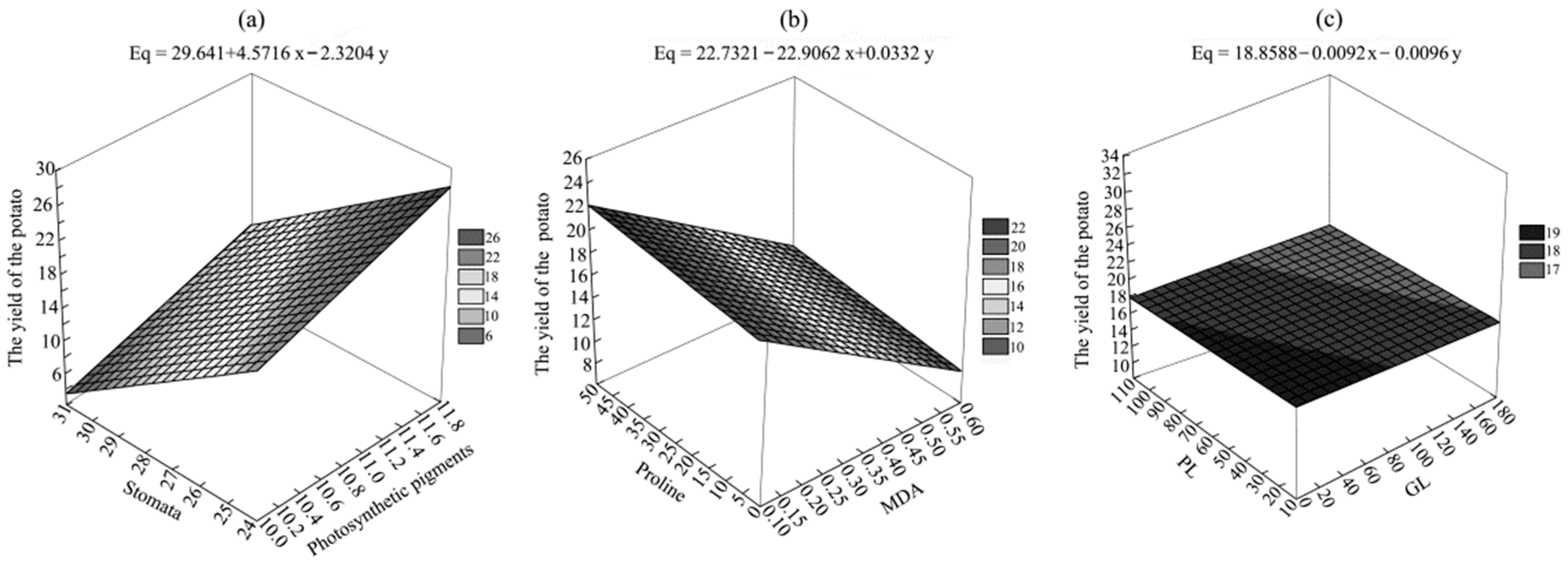
3.2. Potato Yield and Its Components
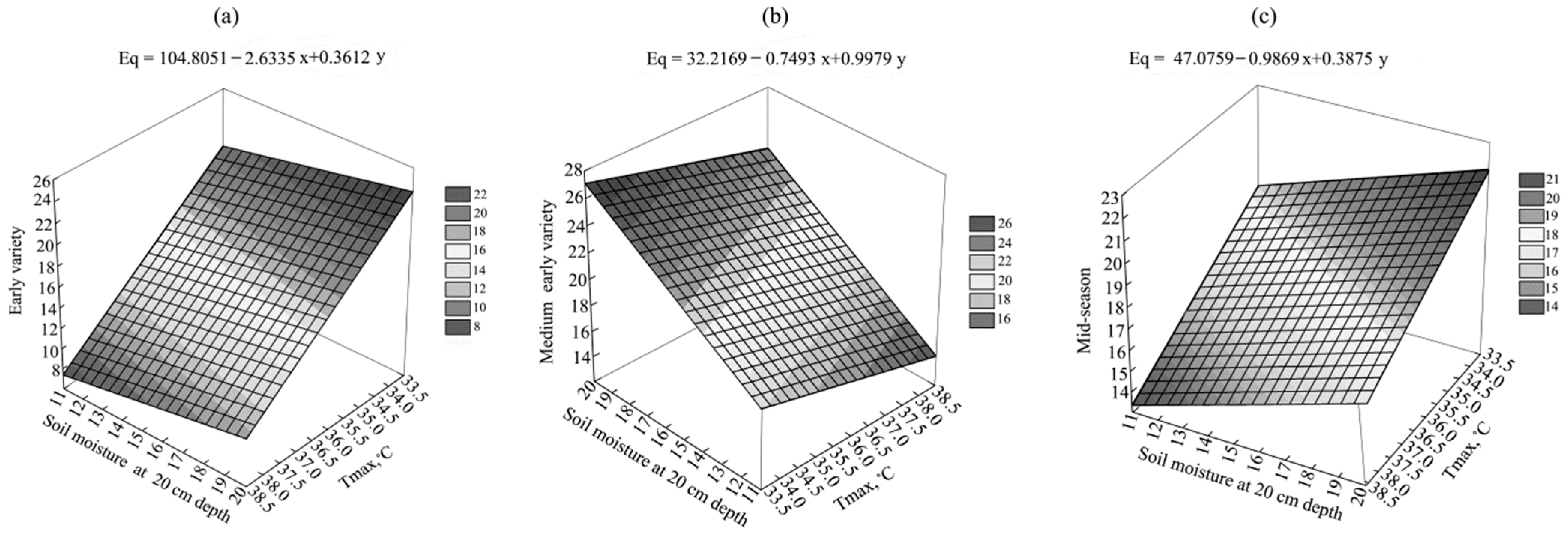
3.3. Potato Plant Growth
3.4. Functional Parameters of Leaves
3.5. Elements of the Antioxidant System
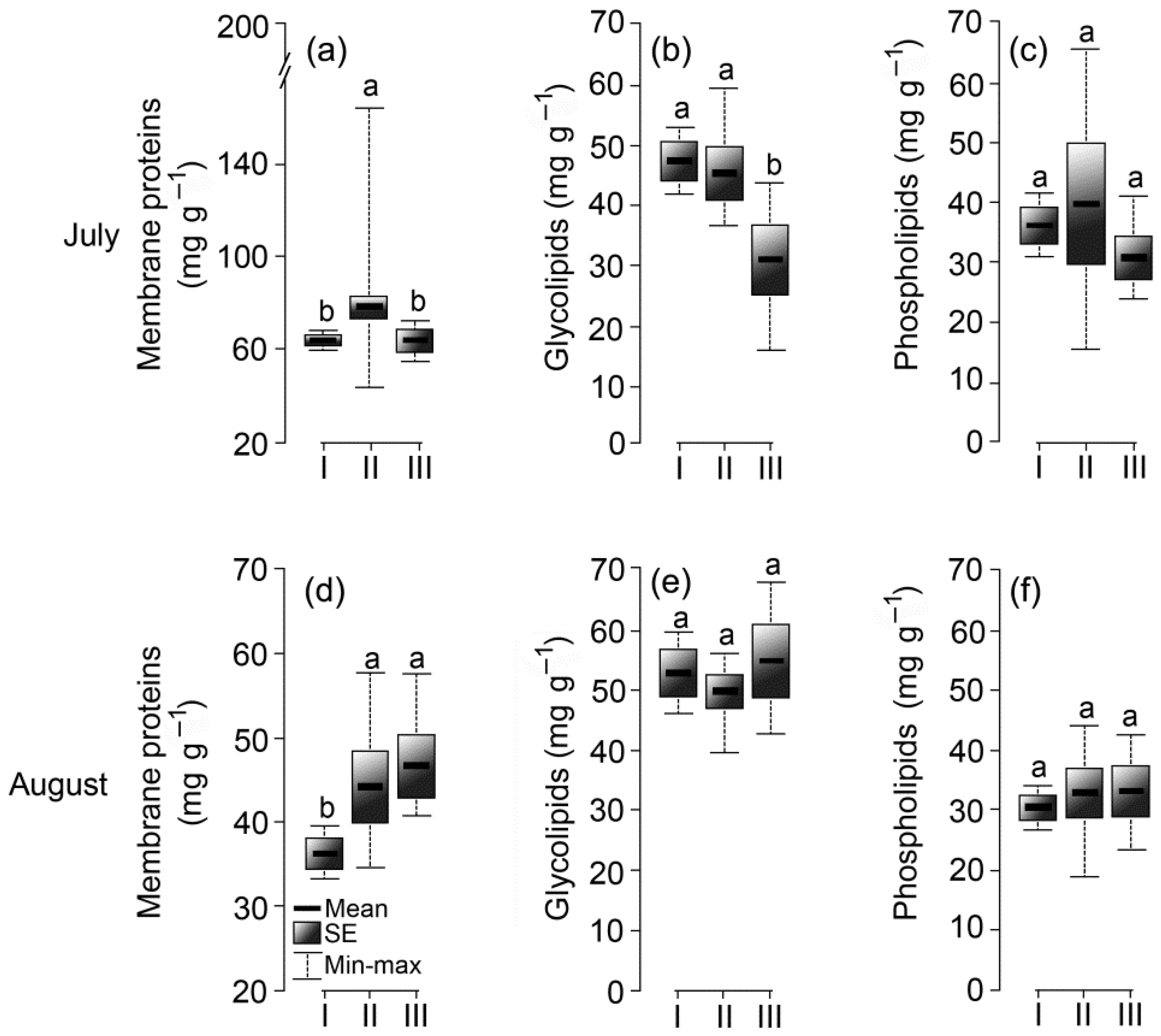
3.6. Membrane Complex
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- George, T.S.; Taylor, M.A.; Dodd, I.C.; White, P.J. Climate Change and Consequences for Potato Production: A Review of Tolerance to Emerging Abiotic Stress. Potato Res. 2018, 60, 239–268. [Google Scholar] [CrossRef]
- Cabello, R.; Monneveux, P.; De Mendiburu, F.; Bonierbale, M. Comparison of yield based drought tolerance indices in improved varieties, genetic stocks and landraces of potato (Solanum tuberosum L.). Euphytica 2013, 193, 147–156. [Google Scholar] [CrossRef]
- Demirel, U.; Çalişkan, S.; Yavuz, C.; Tindaş, I.; Polgar, Z.; Vaszily, Z.; Cernák, I.; Çalişkan, M.E. Assessment of morphophysiological traits for selection of heat-tolerant potato genotypes. Turk. J. Agric. For. 2017, 41, 218–232. [Google Scholar] [CrossRef] [Green Version]
- Jeffery, R.A. Physiology of Crop Response to Drought. In Potato Ecology and Modeling of Crops Under Conditions Limiting Growth; Haverkortand, A.J., MacKerron, D.K.L., Eds.; Academic Publishers: Wageningen, The Netherlands, 1995; pp. 61–74. [Google Scholar] [CrossRef]
- Schafleitner, R.; Gutierrez, R.; Espino, R.; Gaudin, A.; Pérez, J.; Martínez, M.; Domínguez, A.; Tincopa, L.; Alvarado, C.; Numberto, G.; et al. Field screening for variation of drought tolerance in Solanum tuberosum L. by agronomical, physiological and genetic analysis. Potato Res. 2007, 50, 71–85. [Google Scholar] [CrossRef]
- Burton, W.G. Challenges for stress physiology in potato. Am. Potato J. 1981, 58, 3–14. [Google Scholar] [CrossRef]
- Van Der Zaag, D.E. Reliability and significance of a simple method of estimating the potential yield of the potato crop. Potato Res. 1984, 27, 51–73. [Google Scholar] [CrossRef]
- Ahn, Y.J.; Claussen, K.; Zimmerman, J.L. Genotypic differences in the heat-shock response and thermotolerance in four potato cultivars. Plant Sci. 2004, 166, 901–911. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Tang, R.; Niu, S.; Zhang, G.; Chen, G.; Haroon, M.; Yang, Q.; Rajora, O.P.; Li, X.-Q. Physiological and growth responses of potato cultivars to heat stress. Botany 2018, 96, 897–912. [Google Scholar] [CrossRef]
- Monneveux, P.; Ramírez, D.A.; Pino, M.-T. Drought tolerance in potato (S. tuberosum L.): Can we learn from drought tolerance research in cereals? Plant Sci. 2013, 205–206, 76–86. [Google Scholar] [CrossRef]
- Krystyna, Z.; Dominika, B.-M.; Artur, N. Differences in size and architecture of the potato cultivars root system and their tolerance to drought stress. Plant Soil Environ. 2017, 63, 159–164. [Google Scholar] [CrossRef] [Green Version]
- Deblonde, P.; Ledent, J. Effects of moderate drought conditions on green leaf number, stem height, leaf length and tuber yield of potato cultivars. Eur. J. Agron. 2001, 14, 31–41. [Google Scholar] [CrossRef]
- Kumar, S.; Asrey, A.; Mandal, G. Effect of differential irrigation regimes on potato (Solanum tuberosum) yield and post-harvest attributes. Indian J. Agric. Sci. 2007, 77, 366–368. [Google Scholar]
- Eiasu, B.K.; Soundy, P.; Hammes, P.S. Response of potato (Solarium tuberosum) tuber yield components to gel-polymer soil amendments and irrigation regimes. N. Z. J. Crop Hortic. Sci. 2007, 35, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Aliche, E.B.; Oortwijn, M.; Theeuwen, T.P.J.M.; Bachem, C.W.B.; van Eck, H.J.; Visser, R.G.F.; van der Linden, C.G. Genetic mapping of tuber size distribution and marketable tuber yield under drought stress in potatoes. Euphytica 2019, 215, 186. [Google Scholar] [CrossRef] [Green Version]
- Rykaczewska, K.; Zarzyńska, K.; Boguszewska-Mańkowska, D. Architecture of the root system of potato cultivars grown in aeroponics. Electron. J. Pol. Agric. Univ. 2018, 21, 02. [Google Scholar] [CrossRef]
- Tabalenkova, G.N.; Golovko, T.K. Production the Process of Cultivated Plants in Cold Conditions the Climate; Nauka: St. Petersburg, Russia, 2010. (In Russian) [Google Scholar]
- Struik, P.C.; Geertsema, J.; Custers, C.H.M.G. Effects of shoot, root and stolon temperature on the development of the potato (Solanum tuberosum L.) plant. Potato Res. 1989, 32, 151–158. [Google Scholar] [CrossRef]
- Hancock, R.D.; Morris, W.L.; Ducreux, L.J.M.; Morris, J.A.; Usman, M.; Verrall, S.R.; Fuller, J.; Simpson, C.G.; Zhang, R.; Hedley, P.E.; et al. Physiological, biochemical and molecular responses of the potato (Solanum tuberosum L.) plant to moderately elevated temperature. Plant Cell Environ. 2014, 37, 439–450. [Google Scholar] [CrossRef]
- Mahgoub, H.; Eisa, G.; Youssef, M. Molecular, biochemical and anatomical analysis of some potato (Solanum tuberosum L.) cultivars growing in Egypt. J. Genet. Eng. Biotechnol. 2015, 13, 39–49. [Google Scholar] [CrossRef]
- Germ, M. The response of two potato cultivars on combined effects of selenium and drought. Acta Agric. Slov. 2008, 9, 121–137. [Google Scholar] [CrossRef]
- Gervais, T.; Creelman, A.; Li, X.-Q.; Dahal, K. Potato response to drought stress: Physiological and growth basis. Front. Plant Sci. 2021, 12, 698060. [Google Scholar] [CrossRef]
- Ivanov, L.A.; Ronzhina, D.A.; Yudina, P.K.; Zolotareva, N.V.; Kalashnikova, I.V.; Ivanova, L.A. Seasonal dynamics of the chlorophyll and carotenoid content in the leaves of steppe and forest plants on species and community level. Rus. J. Plant Physiol. 2020, 67, 453–462. [Google Scholar] [CrossRef]
- Boudière, L.; Michaud, M.; Petroutsos, D.; Rébeillé, F.; Falconet, D.; Bastien, O.; Roy, S.; Finazzi, G.; Rolland, N.; Jouhet, J.; et al. Glycerolipids in photosynthesis: Composition, synthesis and trafficking. Biochim. Biophys. Acta 2014, 1837, 470–480. [Google Scholar] [CrossRef]
- Rozentsvet, O.A.; Bogdanova, E.S.; Nesterov, V.N.; Shevchenko, S.N.; Bakunov, A.L.; Milekhin, A.V.; Rubtsov, S.L. Productivity and dynamics of morphological, physiological and biochemical parameters of potatoes in arid climate. Doklady Biol. Sci. 2021, 497, 65–68. [Google Scholar] [CrossRef]
- Impa, S.M.; Nadaradjan, S.; Jagadish, S.V.K. Drought Stress Induced Reactiveoxygen Species and Anti-Oxidants in Plants. In Abiotic Stress Responses in Plants: Metabolism, Productivity and Sustainability; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 131–148. [Google Scholar] [CrossRef]
- Lorenzen, J.; Lafta, A.M. Effect of heat stress on enzymes that affect sucrose levels in potato shoots. J. Am. Soc. Hort. Sci. 1996, 121, 1152–1156. [Google Scholar] [CrossRef] [Green Version]
- Hossain, A.M.; Hoque, M.A.; Burritt, D.J.; Fujita, M. Proline Protects Plants against Abiotic Oxidative Stress. In Oxidative Damage to Plants Antioxidant Networks and Signaling; Ahmad, P., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 477–522. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, D.; Kumar, P.; Malik, P.S.; Kumar, M. Proline accumulation in the leaves of four potato cultivars in response to water stress. Plant Arch. 2020, 20, 3510–3514. [Google Scholar]
- Raymundo, R.; Asseng, S.; Cammarano, D.; Quiroz, R. Potato, sweet potato, and yam models for climate change: A review. Field Crops Res. 2014, 166, 173–185. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Douse, R., Packer, L., Eds.; Academic Press Inc.: New York, NY, USA, 1987; pp. 350–382. [Google Scholar] [CrossRef]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Kates, M. Techniques of Lipidology. In Laboratory Techniques in Biochemistry and Molecular Biology; Work, T.S., Work, E., Eds.; Elsevier: Amsterdam, The Netherlands, 1975; pp. 269–610. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Hijmans, R.J. The effect of climate change on global potato production. Am. J. Potato Res. 2003, 80, 271–279. [Google Scholar] [CrossRef]
- Wolf, S.; Olesinski, A.A.; Rudich, J.; Marani, A. Effect of high temperature on photosynthesis in potatoes. Ann. Bot. 1990, 65, 179–185. [Google Scholar] [CrossRef]
- Hastilestari, B.R.; Lorenz, J.; Reid, S.; Hofmann, J.; Pscheidt, D.; Sonnewald, U.; Sonnewald, S. Deciphering source and sink responses of potato plants (Solanum tuberosum L.) to elevated temperatures. Plant Cell Environ. 2018, 41, 2600–2616. [Google Scholar] [CrossRef]
- Sprenger, H.; Kurowsky, C.; Horn, R.; Erban, A.; Seddig, S.; Rudack, K.; Fischer, A.; Walther, D.; Zuther, E.; Köhl, K.; et al. The drought response of potato reference cultivars with contrasting tolerance. Plant Cell Environ. 2016, 39, 2370–2389. [Google Scholar] [CrossRef]
- Obidiegwu, J.E.; Bryan, G.J.; Jones, H.G.; Prashar, A. Coping with drought: Stress and adaptive responses in potato and perspectives for improvement. Front. Plant Sci. 2015, 6, 542. [Google Scholar] [CrossRef] [Green Version]
- Petukhov, A.S.; Khritokhin, N.; Petukhova, G. Lipid peroxidation in plants cells under conditions of the urban environment. RUDN J. Ecol. Life Saf. 2018, 26, 82–90. [Google Scholar] [CrossRef]
- Mansour, M.M.F.; Ali, E.F. Evaluation of proline functions in saline conditions. Phytochemistry 2017, 140, 52–68. [Google Scholar] [CrossRef]
- Farhad, M.-S.; Babak, A.M.; Reza, Z.M.; Hassan, R.-S.M.; Afshin, T. Response of proline, soluble sugars, photosynthetic pigments and antioxidant enzymes in potato (Solanum tuberosum L.) to different irrigation regimes in greenhouse condition. Aust. J. Crop Sci. 2011, 5, 55–60. [Google Scholar]
- Matysik, J.; Bhalu, B.A.; Mohanty, P. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci. 2002, 82, 525–532. [Google Scholar]




| Variety | Number of Tubers Per 1 Plant | Average Weight of Tubers, g | Yield, t/ha | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2021 | 2019 | 2020 | 2021 | 2019 | 2020 | 2021 | |
| Early | 5.0 ± 0.8 | 6.3 ± 1.1 | 7.0 ± 0.8 | 108.9 ± 16.1 | 74.2 ± 15.3 | 54.8 ± 12.0 | 21.2 ± 2.7 | 21.8 ± 3.7 | 18.4 ± 4.8 |
| Medium-early | 6.8 ± 2.0 | 6.5 ± 0.5 | 6.7 ± 2.0 | 96.8 ± 13.6 | 70.3 ± 9.3 | 48.2 ± 9.5 | 25.7 ± 6.1 | 20.8 ± 3.2 | 14.6 ± 4.4 |
| Mid-season | 5.7 ± 1.9 | 6.7 ± 1.5 | 7.1 ± 0.9 | 89.4 ± 6.1 | 59.8 ± 7.0 | 43.7 ± 11.5 | 20.9 ± 7.9 | 19.0 ± 3.7 | 14.2 ± 4.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozentsvet, O.; Bogdanova, E.; Nesterov, V.; Bakunov, A.; Milekhin, A.; Rubtsov, S.; Dmitrieva, N. Physiological and Biochemical Parameters of Leaves for Evaluation of the Potato Yield. Agriculture 2022, 12, 757. https://doi.org/10.3390/agriculture12060757
Rozentsvet O, Bogdanova E, Nesterov V, Bakunov A, Milekhin A, Rubtsov S, Dmitrieva N. Physiological and Biochemical Parameters of Leaves for Evaluation of the Potato Yield. Agriculture. 2022; 12(6):757. https://doi.org/10.3390/agriculture12060757
Chicago/Turabian StyleRozentsvet, Olga, Elena Bogdanova, Viktor Nesterov, Alexey Bakunov, Alexey Milekhin, Sergei Rubtsov, and Nadezhda Dmitrieva. 2022. "Physiological and Biochemical Parameters of Leaves for Evaluation of the Potato Yield" Agriculture 12, no. 6: 757. https://doi.org/10.3390/agriculture12060757
APA StyleRozentsvet, O., Bogdanova, E., Nesterov, V., Bakunov, A., Milekhin, A., Rubtsov, S., & Dmitrieva, N. (2022). Physiological and Biochemical Parameters of Leaves for Evaluation of the Potato Yield. Agriculture, 12(6), 757. https://doi.org/10.3390/agriculture12060757






