Integrated Organic-Inorganic Nitrogen Fertilization Mitigates Nitrous Oxide Emissions by Regulating Ammonia-Oxidizing Bacteria in Purple Caitai Fields
Abstract
:1. Introduction
2. Material and Methods
2.1. Experimental Site
2.2. Experimental Design
2.3. Gas Sampling
2.4. Soil Sampling and Measurement
2.5. Measurement of Yield and Calculation of Yield-Scaled N2O Emissions
2.6. Absolute Real-Time Quantitative Polymerase Chain Reaction (PCR) Analysis
2.7. Statistical Analysis
3. Results
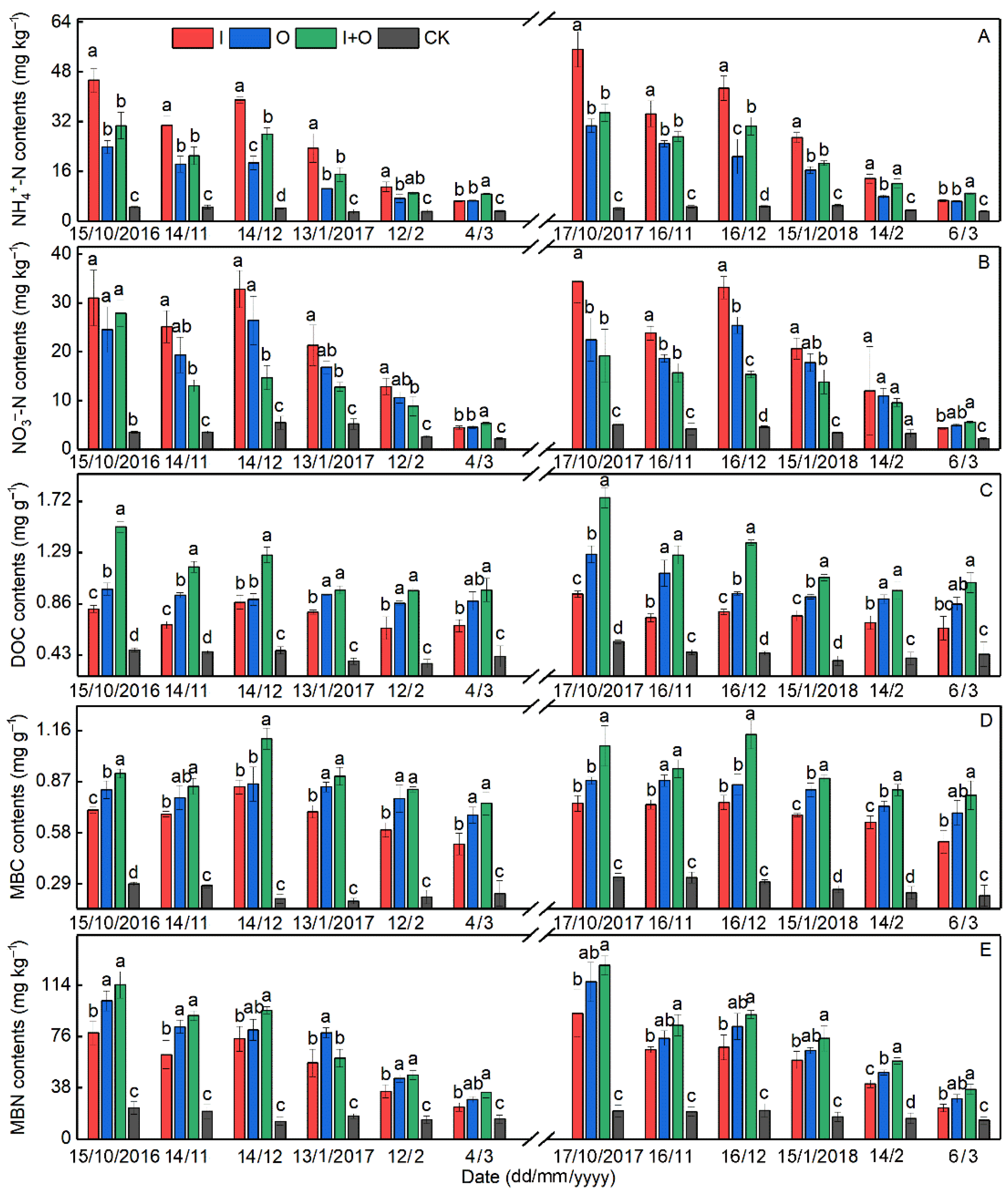
3.1. Soil Chemical Properties
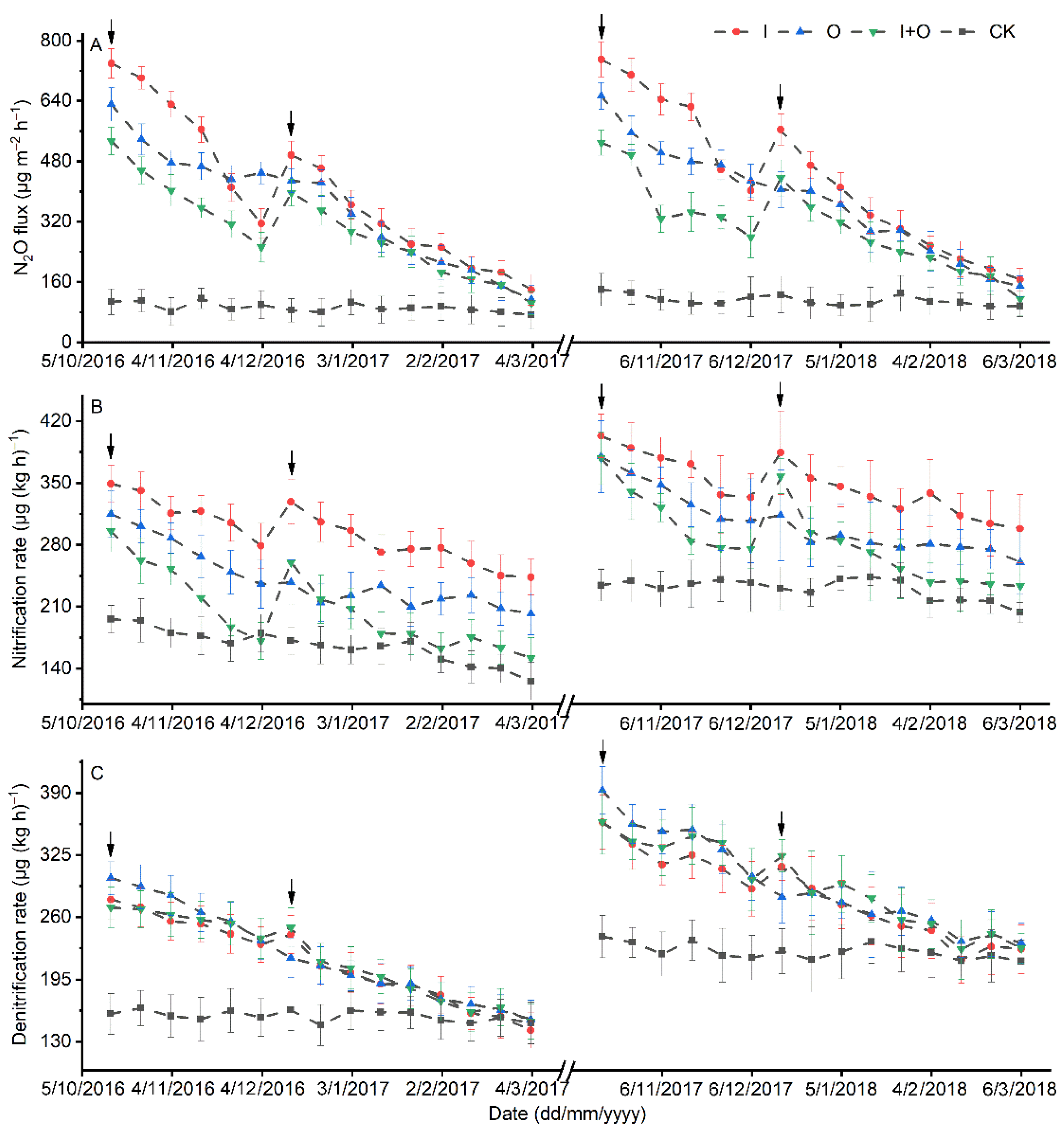
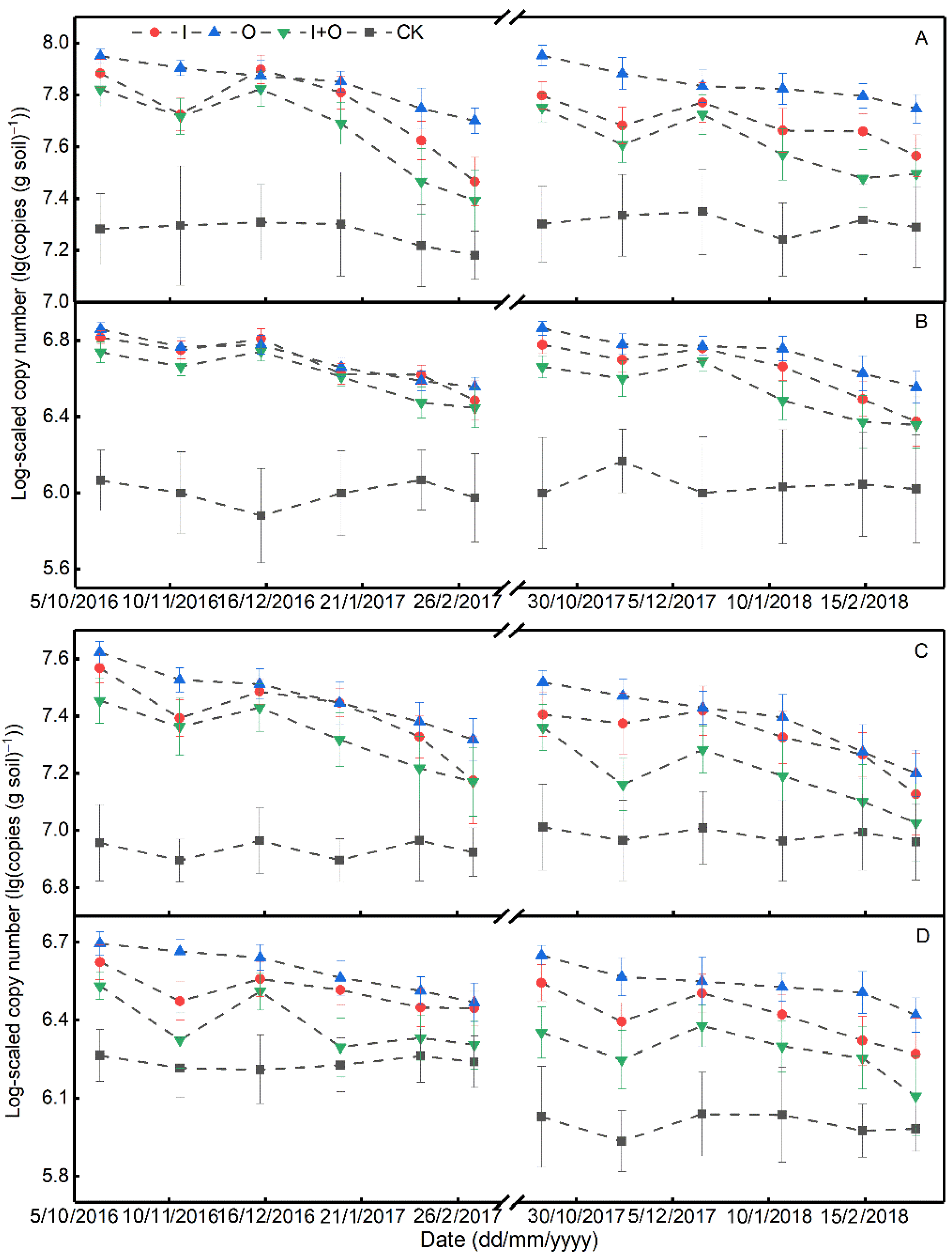
3.2. Soil Nitrification and Denitrification Rates, and Abundances of Nitrifier and Denitrifier Genes
3.3. N2O Emissions
3.4. Correlation among Soil Chemical Properties, Nitrifier/Denitrifier Abundance, Nitrification/Denitrification Rate and N2O Flux
3.5. Yield and Yield-Scaled N2O Emission
4. Discussion
4.1. Effect of N Fertilizer Type on Soil Properties
4.2. Effect of N Fertilizer Type on the Abundance and Activity of Nitrifiers/Denitrifiers
4.3. Effect of N Fertilizer Type on N2O Emissions
4.4. Effect of N Fertilizer Type on Yield and Yield-Scaled N2O Emission
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IPCC. 2013 Climate Change—The Physical Science Basis, Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous oxide N2O. The dominant ozone-depleting substance emitted in the 21st century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [Green Version]
- Mosier, A.; Kroeze, C.; Nevison, C.; Oenema, O.; Seitzinger, S.; van Cleemput, O. Closing the global N2O budget, nitrous oxide emissions through the agricultural nitrogen cycle. Nutr. Cycl. Agroecosyst. 1998, 52, 225–248. [Google Scholar] [CrossRef]
- Schindler, D.W.; Hecky, R.E. Eutrophication, More Nitrogen Data Needed. Science 2009, 324, 721–722. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Xiao, J.; Liu, F.; Goodman, B.A.; Li, W.; Jia, Z.; Ran, W.; Zhang, R.; Shen, Q.; Yu, G. Contrasting effects of inorganic and organic fertilisation regimes on shifts in Fe redox bacterial communities in red soils. Soil Biol. Biochem. 2018, 117, 56–67. [Google Scholar] [CrossRef]
- Urzedo, D.I.; Franco, M.P.; Pitombo, L.M.; Carmo, J.B. Effects of organic and inorganic fertilizers on greenhouse gas GHG emissions in tropical forestry. For. Ecol. Manag. 2013, 310, 37–44. [Google Scholar] [CrossRef]
- Aguilera, E.; Lassaletta, L.; Sanz-Cobena, A.; Garnier, J.; Vallejo, A. The potential of organic fertilizers and water management to reduce N2O emissions in Mediterranean climate cropping systems. A review. Agric. Ecosyst. Environ. 2013, 164, 32–52. [Google Scholar] [CrossRef] [Green Version]
- Verdi, L.; Kuikman, P.J.; Orlandini, S.; Mancini, M.; Napoli, M.; Marta, A.D. Does the use of digestate to replace mineral fertilizers have less emissions of N2O and NH3? Agric. For. Meteorol. 2019, 269–270, 112–118. [Google Scholar] [CrossRef]
- Ding, W.; Luo, J.; Li, J.; Yu, H.; Fan, J.; Liu, D. Effect of long-term compost and inorganic fertilizer application on background N2O and fertilizer-induced N2O emissions from an intensively cultivated soil. Sci. Total Environ. 2013, 465, 115–124. [Google Scholar] [CrossRef]
- Agegnehu, G.; Bass, A.M.; Nelson, P.N.; Bird, M.I. Benefits of biochar, compost and biochar-compost for soil quality, maize yield and greenhouse gas emissions in a tropical agricultural soil. Sci. Total Environ. 2016, 543, 295–306. [Google Scholar] [CrossRef]
- Case, S.D.C.; McNamara, N.P.; Reay, D.S.; Stott, A.W.; Grant, H.K.; Whitaker, J. Biochar suppresses N2O emissions while maintaining N availability in a sandy loam soil. Soil Biol. Biochem. 2015, 81, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Sandrin, T.R.; Herman, D.C.; Maier, R.M. Physiological Methods. In Environmental Microbiology; Maier, R.M., Pepper, I.L., Gerba, C.P., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 191–223. [Google Scholar]
- Richarson, D.J. Bacterial respiration, a flexible process for a changing environment. Microbiology 2000, 146, 551–571. [Google Scholar]
- Norton, J.M.; Alzerreca, J.J.; Suwa, Y.; Klotz, M.G. Diversity of ammonia monooxygenase operon in autotrophic ammonia-oxidizing bacteria. Arch. Microbiol. 2002, 177, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; Stres, B.; Rosenquist, M.; Hallin, S. Phylogenetic analysis of nitrite, nitric oxide, and nitrous oxide respiratory enzymes reveal a complex evolutionary history for denitrification. Mol. Biol. Evol. 2008, 25, 1955–1966. [Google Scholar] [CrossRef] [PubMed]
- Levy-Booth, D.J.; Prescott, C.E.; Grayston, S.J. Microbial functional genes involved in nitrogen fixation, nitrification and denitrification in forest ecosystems. Soil Biol. Biochem. 2014, 75, 11–25. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, X.; Ju, X. Linkage between N2O emission and functional gene abundance in an intensively managed calcareous fluvo-aquic soil. Sci. Rep. 2017, 7, 43283. [Google Scholar] [CrossRef]
- Wang, C.; Li, H.; Li, Y.; Meng, Q.; Xie, F.; Xu, Y.; Wan, Z. Genetic characterization and fine mapping BrCER4 in involved cuticular wax formation in purple cai-tai Brassica rapa L. var purpurea. Mol. Breed. 2019, 39, 12. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Z.; Guo, L.; Cai, M.; Cao, C. Emissions of CH4 and CO2 from double rice cropping systems under varying tillage and seeding methods. Atmos. Environ. 2013, 80, 438–444. [Google Scholar] [CrossRef]
- Yao, K.Y. Determination of soil microbial activities and character. In Soil Microbial Ecology and Experimental Technology; Yao, K.Y., Huang, C.Y., Eds.; Science Press: Beijing, China, 2006; pp. 187–192. (In Chinese) [Google Scholar]
- Witt, C.; Gaunt, J.L.; Galicia, C.C.; Ottow, J.C.G.; Neue, H.U. A rapid chloroform-fumigation extraction method for measuring soil microbial biomass carbon and nitrogen in flooded rice soils. Biol. Fertil. Soils 2000, 30, 510–519. [Google Scholar] [CrossRef]
- Allison, L.E. Organic carbon. In Methods of Soil Analysis Part II; Black, C.A., Ed.; American Society of Agronomy Publications: Madison, WI, USA, 1965; pp. 1367–1376. [Google Scholar]
- Zaman, M.; Saggar, S.; Blennerhassett, J.D.; Singh, J. Effect of urease and nitrification inhibitors on N transformation, gaseous emissions of ammonia and nitrous oxide, pasture yield and N uptake in grazed pasture system. Soil Biol. Biochem. 2009, 41, 1270–1280. [Google Scholar] [CrossRef]
- Evans, J.D. Straight Forward Statistics for the Behavioral Sciences; Brooks/Cole Publishing: Pacific Grove, CA, USA, 1996. [Google Scholar]
- Pan, G.; Zhou, P.; Li, Z.; Smith, P.; Chen, X. Combined inorganic/organic fertilization enhances N efficiency and increases rice productivity through organic carbon accumulation in a rice paddy from the Tai Lake region, China. Agric. Ecosyst. Environ. 2009, 131, 274–280. [Google Scholar] [CrossRef]
- Zhang, J.; Zhuang, M.; Shan, N.; Zhao, Q.; Li, H.; Wang, L. Substituting organic manure for compound fertilizer increases yield and decreases NH3 and N2O emissions in an intensive vegetable production systems. Sci. Total. Environ. 2019, 670, 1184–1189. [Google Scholar] [CrossRef] [PubMed]
- Neumann, G. Root Exudates and Nutrient Cycling. In Nutrient Cycling in Terrestrial Ecosystems. Soil Biology; Marschner, P., Rengel, Z., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 123–157. [Google Scholar]
- Ali, M.A.; Sattar, M.A.; Islam, M.N.; Inubushi, K. Integrated effects of organic, inorganic and biological amendments on methane emission, soil quality and rice productivity in irrigated paddy ecosystem of Bangladesh, field study of two consecutive rice growing seasons. Plant Soi. 2014, 378, 239–252. [Google Scholar] [CrossRef]
- Wang, F.; Chen, S.; Wang, Y.; Zhang, Y.; Hu, C.; Liu, B. Long-term nitrogen fertilization elevates the activity and abundance of nitrifying and denitrifying microbial communities in an upland soil, implications for nitrogen loss from intensive agricultural systems. Front. Microbiol. 2018, 23, 02424. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Hu, H.; Ding, Q.; Song, M.; Xu, X.; Zheng, Y.; Guo, L. Influence of nitrogen fertilization on soil ammonia oxidizer and denitrifier abundance, microbial biomass, and enzyme activities in an alpine meadow. Biol. Fertil. Soils 2013, 50, 703–713. [Google Scholar] [CrossRef]
- Han, J.; Shi, J.; Zeng, L.; Xu, J.; Wu, L. Impacts of continuous excessive fertilization on soil potential nitrification activity and nitrifying microbial community dynamics in greenhouse system. J. Soil Sediments 2017, 17, 471–480. [Google Scholar] [CrossRef]
- Grießmeier, V.; Leberecht, K.; Gescher, J. NO3− removal efficiency in field denitrification beds, key controlling factors and main implications. Environ. Microb. Rep. 2019, 11, 316–329. [Google Scholar] [CrossRef]
- Kindaichi, T.; Ito, T.; Okabe, S. Ecophysiological interaction between nitrifying bacteria and heterotrophic bacteria in autotrophic nitrifying biofilms as determined by microautoradiography-fluorescence in situ hybridization. Appl. Environ. Biol. 2004, 70, 1641–1650. [Google Scholar] [CrossRef] [Green Version]
- Martens, D.A. Denitrification. In Encyclopedia of Soils in the Environment; Hillel, D., Ed.; Academic Press: Cambridge, MA, USA, 2005; pp. 378–382. [Google Scholar]
- Inglett, P.W.; Reddy, K.R.; Corstanje, R. Anaerobic soils. In Encyclopedia of Soils in the Environment; Hillel, D., Ed.; Academic Press: Cambridge, MA, USA, 2005; pp. 72–78. [Google Scholar]
- Zhang, J.; Zhang, F.; Yang, J.; Wang, J.; Cai, M.; Li, C.; Cao, C. Emissions of N2O and NH3, and nitrogen leaching from direct seeded rice under different tillage practices in central China. Agric. Ecosyst. Environ. 2011, 140, 164–173. [Google Scholar]
- Xu, Y.; Guo, L.; Xie, L.; Yun, A.; Li, Y.; Zhang, X.; Zhao, X.; Diao, T. Characteristics of background emissions and emission factors of N2O from major upland fields in China. Sci. Agric. Sin. 2016, 49, 1729–1743. [Google Scholar]
- Bateman, E.J.; Baggs, E.M. Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol. Fertil. Soils. 2005, 41, 379–388. [Google Scholar] [CrossRef]
- Ding, W.; Cai, Y.; Cai, Z.; Yagi, K.; Zheng, X. Nitrous oxide emissions from an intensively cultivated maize–wheat rotation soil in the north china plain. Sci. Total. Environ. 2007, 373, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Dobbie, K.; Smith, K. Nitrous oxide emission factors for agricultural soils in Great Britain, the impact of soil water-filled pore space and other controlling variables. Glob. Chang. Biol. 2003, 9, 204–218. [Google Scholar] [CrossRef]
- Shcherbak, I.; Millar, N.; Robertson, G.P. Global metaanalysis of the nonlinear response of soil nitrous oxide N2O emissions to fertilizer nitrogen. Proc. Natl. Acad. Sci. USA 2014, 111, 9199–9204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyamadzawo, G.; Wuta, M.; Nyamangara, J.; Smith, J.L.; Rees, R.M. Nitrous oxide and methane emissions from cultivated seasonal wetland dambo soils with inorganic, organic and integrated nutrient management. Nutr. Cycl. Agroecosyst. 2014, 100, 161–175. [Google Scholar] [CrossRef]

| Treatment | Basal Fertilizer (15 October 2016 and 17 October 2017) | Topdressing Fertilizer (14 December 2016 and 16 December 2017) |
|---|---|---|
| I | 750 kg compound fertilizer ha−1 | 245 kg urea ha−1 |
| O | 2250 kg organic fertilizer ha−1 + 112.5 kg KCl ha−1 + 264.5 kg calcium superphosphate ha−1 | − |
| I+O | 1125 kg organic fertilizer ha−1 + 375 kg compound fertilizer ha−1 + 56 kg KCl ha−1 + 133 kg calcium superphosphate ha−1 | 122.3 kg urea ha−1 |
| CK | − | − |
| Year | Treatment | CE (kg ha−1) | EFN2O (%) | Yield (t ha−1) | Yield-Scaled N2O Emission (t CO2-eq. t−1 Yield) |
|---|---|---|---|---|---|
| 2016 | I | 8.54 ± 0.05 a | 2.92 ± 0.07 a | 17.09 ± 0.65 b | 0.13 ± 0.00 b |
| O | 7.63 ± 0.09 b | 2.51 ± 0.10 b | 13.08 ± 0.84 c | 0.16 ± 0.01 a | |
| I+O | 6.33 ± 0.13 c | 1.94 ± 0.12 c | 18.65 ± 1.05 a | 0.09 ± 0.00 c | |
| CK | 1.98 ± 0.13 d | – | 6.76 ± 0.59 d | 0.08 ± 0.00 d | |
| 2017 | I | 9.24 ± 0.16 a | 3.05 ± 0.08 a | 15.76 ± 1.29 b | 0.16 ± 0.01 b |
| O | 7.96 ± 0.16 b | 2.48 ± 0.08 b | 11.69 ± 0.73 c | 0.18 ± 0.01 a | |
| I+O | 6.58 ± 0.21 c | 1.86 ± 0.10 c | 17.74 ± 0.76 a | 0.10 ± 0.00 c | |
| CK | 2.38 ± 0.02 d | – | 6.42 ± 0.14 d | 0.10 ± 0.00 c |
| Growing Season | Treatments | AOB-amoA | AOA-amoA | nirK | nirS |
|---|---|---|---|---|---|
| 2016 | I vs. O | 38.9 | 14.0 | 25.8 | 21.3 |
| I vs. I+O | 39.9 | 12.9 | 24.0 | 23.2 | |
| O vs. I+O | 38.2 | 10.1 | 23.3 | 28.4 | |
| 2017 | I vs. O | 36.7 | 15.3 | 23.5 | 24.5 |
| I vs. I+O | 37.8 | 10.6 | 26.6 | 25.0 | |
| O vs. I+O | 35.0 | 13.7 | 23.5 | 27.8 |
| Growing Season | Variable | AOB-amoA | AOA-amoA | nirK | nirS | NR | DNR | N2O Flux |
|---|---|---|---|---|---|---|---|---|
| 2016 | NH4+–N | ns | ns | ns | ns | 0.42 ** | ns | 0.39 ** |
| NO3––N | 0.29 * | 0.47 ** | 0.30 * | 0.32 * | 0.25 ** | ns | 0.47 ** | |
| DOC | ns | –0.21 * | ns | ns | –0.52 ** | ns | –0.34 * | |
| MBC | –0.52 * | ns | ns | ns | –0.46 ** | ns | –0.58 ** | |
| MBN | –0.65 * | ns | ns | ns | –0.40 ** | ns | –0.45 ** | |
| AOB-amoA | 1 | 0.61 ** | 0.77 ** | 0.60 ** | 0.72 ** | ns | 0.46 ** | |
| AOA-amoA | - | 1 | 0.52 ** | 0.62 ** | 0.22 * | ns | ns | |
| nirK | - | - | 1 | 0.62 ** | 0.33 * | ns | 0.40 ** | |
| nirS | - | - | - | 1 | 0.36 ** | 0.28 * | 0.35 ** | |
| NR | - | - | - | - | 1 | ns | 0.56 ** | |
| DNR | - | - | - | - | - | 1 | ns | |
| 2017 | NH4+–N | ns | ns | ns | ns | 0.46 ** | ns | 0.34 ** |
| NO3––N | ns | 0.38 ** | 0.42 ** | 0.35 ** | 0.31 * | ns | 0.35 ** | |
| DOC | ns | –0.31 * | ns | ns | –0.47 * | ns | –0.39 ** | |
| MBC | –0.48 * | ns | ns | ns | –0.49 * | ns | –0.47 ** | |
| MBN | –0.72 ** | ns | ns | ns | –0.54 * | ns | –0.34 * | |
| AOB-amoA | 1 | 0.68 ** | 0.68 ** | 0.72 ** | 0.74 ** | ns | 0.58 * | |
| AOA-amoA | - | 1 | 0.49 ** | 0.70 ** | ns | ns | ns | |
| nirK | - | - | 1 | 0.69 ** | 0.35 * | ns | 0.36 ** | |
| nirS | - | - | - | 1 | 0.31 ** | ns | 0.27 * | |
| NR | - | - | - | - | 1 | ns | 0.49 ** | |
| DNR | - | - | - | - | - | 1 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, D.; Cao, C.; Li, C. Integrated Organic-Inorganic Nitrogen Fertilization Mitigates Nitrous Oxide Emissions by Regulating Ammonia-Oxidizing Bacteria in Purple Caitai Fields. Agriculture 2022, 12, 723. https://doi.org/10.3390/agriculture12050723
Fan D, Cao C, Li C. Integrated Organic-Inorganic Nitrogen Fertilization Mitigates Nitrous Oxide Emissions by Regulating Ammonia-Oxidizing Bacteria in Purple Caitai Fields. Agriculture. 2022; 12(5):723. https://doi.org/10.3390/agriculture12050723
Chicago/Turabian StyleFan, Daijia, Cougui Cao, and Chengfang Li. 2022. "Integrated Organic-Inorganic Nitrogen Fertilization Mitigates Nitrous Oxide Emissions by Regulating Ammonia-Oxidizing Bacteria in Purple Caitai Fields" Agriculture 12, no. 5: 723. https://doi.org/10.3390/agriculture12050723
APA StyleFan, D., Cao, C., & Li, C. (2022). Integrated Organic-Inorganic Nitrogen Fertilization Mitigates Nitrous Oxide Emissions by Regulating Ammonia-Oxidizing Bacteria in Purple Caitai Fields. Agriculture, 12(5), 723. https://doi.org/10.3390/agriculture12050723






