Long-Term Monocultures of American Ginseng Change the Rhizosphere Microbiome by Reducing Phenolic Acids in Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Samples Collection
2.2. Soil Analysis
2.3. American Ginseng Pot Experiment
2.4. Effects of Exogenous Phenolic Acid Concentration and Extracts of Soils with Different American Ginseng Growing History on Pathogen Suppression
2.5. Bioinformatics
2.6. Statistical Analysis
3. Results
3.1. Physical and Chemical Properties, and Phenolic Acid Content of Soils with Different American Ginseng Cropping Histories
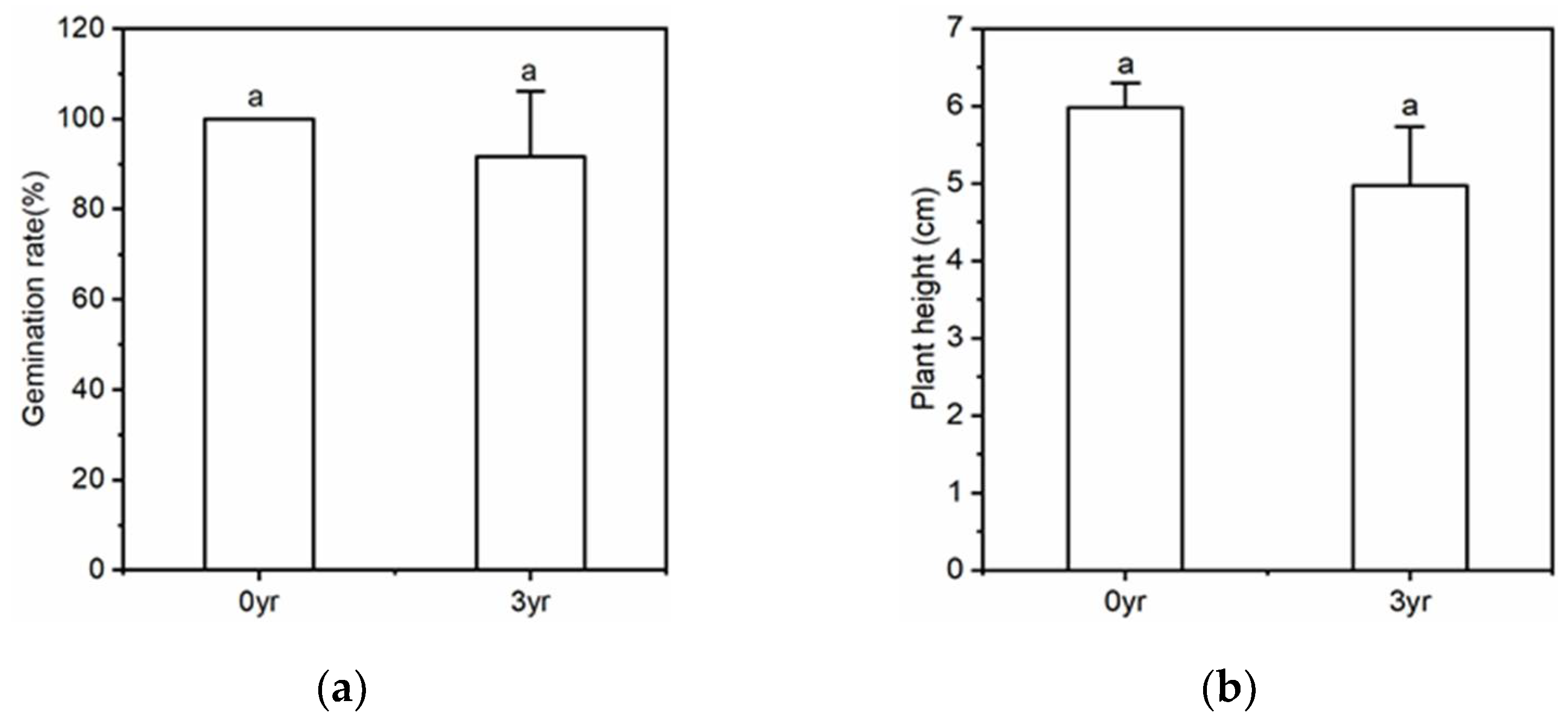
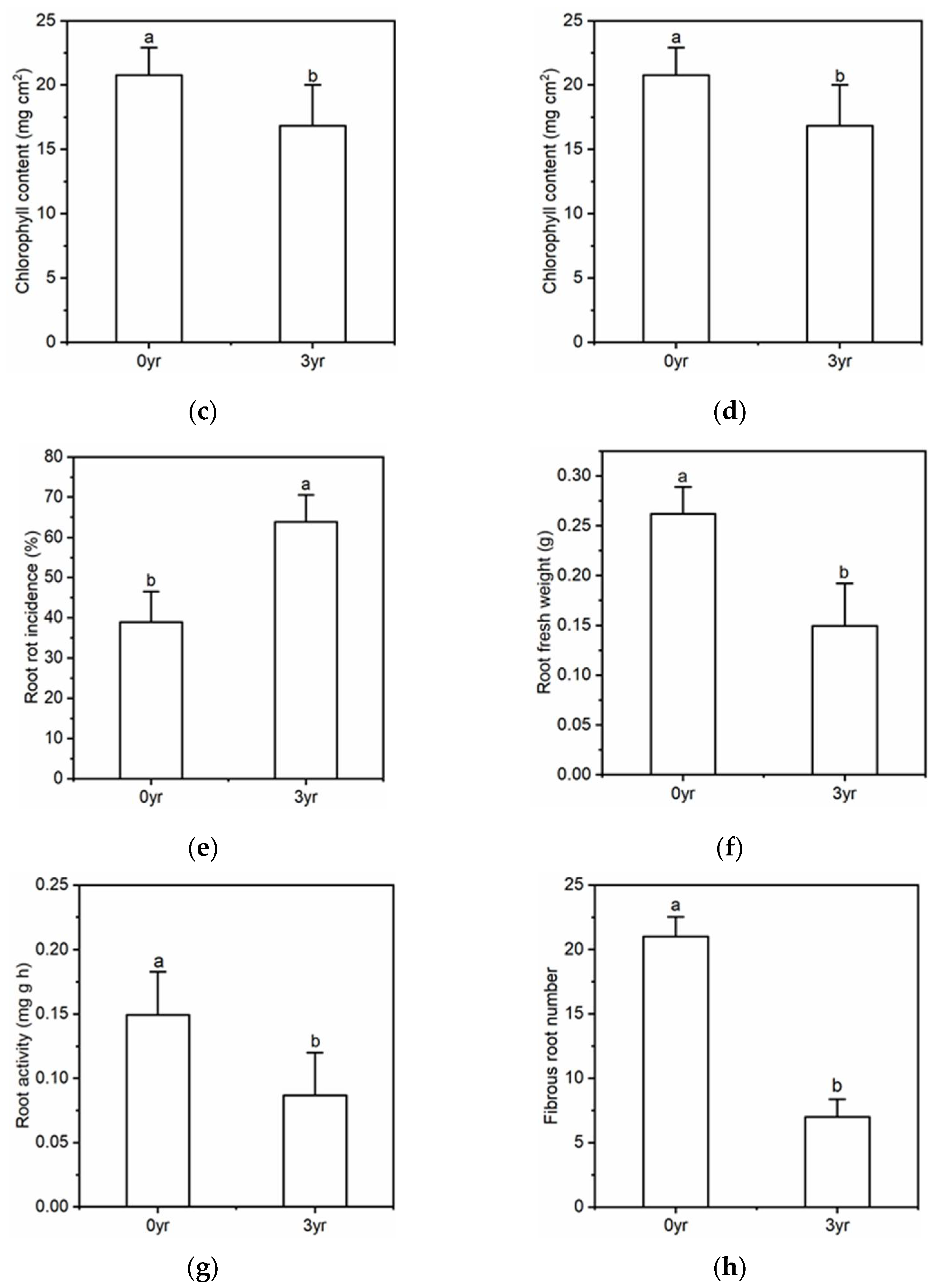
3.2. Effects of Soils with Different Monoculture Histories on the Growth of American Ginseng
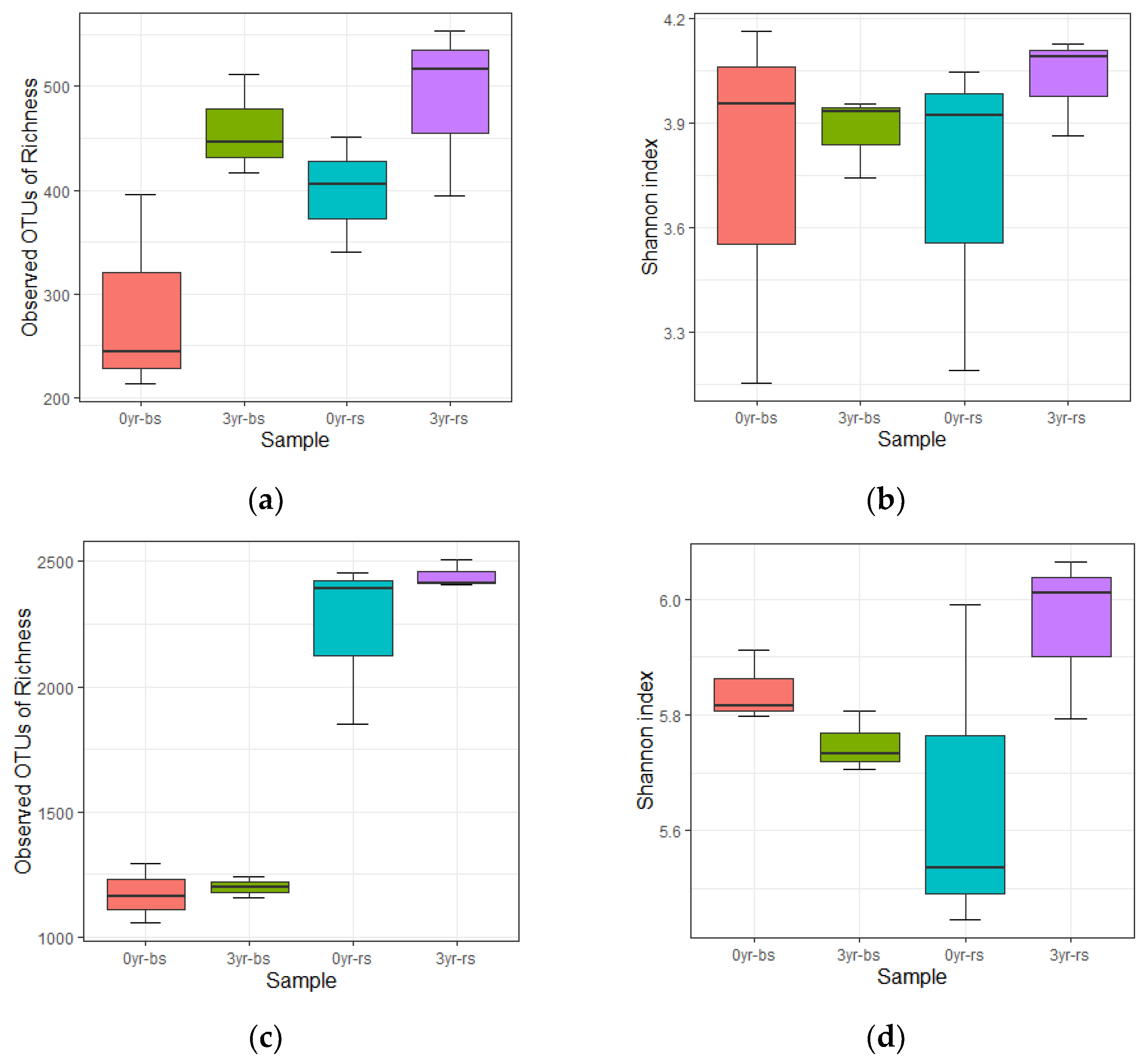
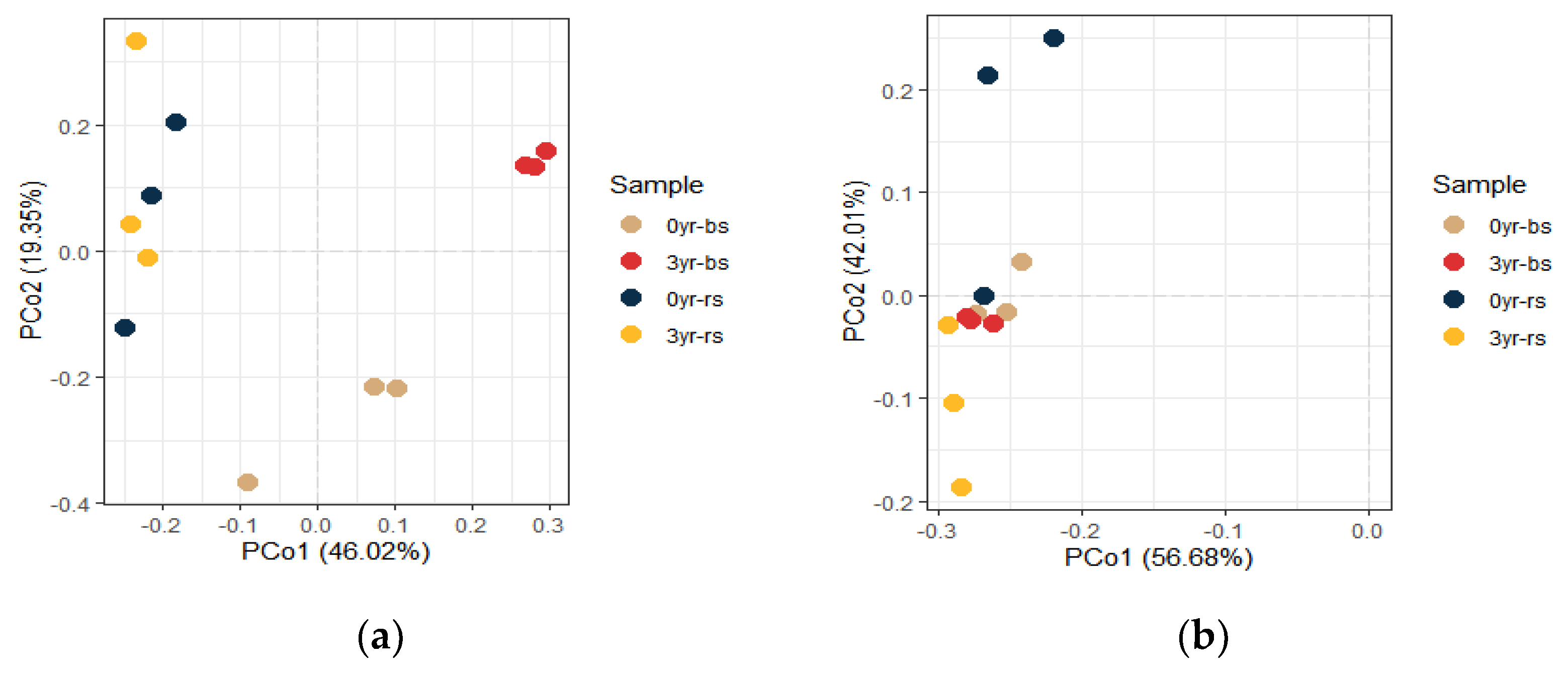
3.3. Rhizosphere Microbial Community of American Ginseng Influenced by Soils with Different Monoculture Histories
3.4. Effects of Exogenous Phenolic Acids and Soil Extracts on Suppression of American Ginseng Root Pathogens
4. Discussion
4.1. Monoculture Changed the Rhizosphere Soil Microbial Community of American Ginseng
4.2. Phenolic Acids Promote the Succession of the Rhizosphere Microbial Community of American Ginseng
4.3. Phenolic Acids Hinder the Growth of American Ginseng
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| DF | F | p-Value | |
|---|---|---|---|
| Table 1. The physical and chemical properties of the soils with different monoculture history of American ginseng. | |||
| OM | 11 | 2.458 | 0.137 |
| TN | 11 | 3.536 | 0.068 |
| AN | 11 | 38.741 | <0.01 |
| TP | 11 | 5.285 | 0.027 |
| AP | 11 | 109.014 | <0.01 |
| TK | 11 | 2.768 | 0.111 |
| AK | 11 | 33.944 | <0.01 |
| Table 2. The effect of phenolic acid concentrations on the growth of American ginseng pathogens. | |||
| Fusarium solani | 11 | 12.634 | <0.01 |
| Cylindrocarpon destructans | 11 | 31.704 | <0.01 |
| Rhizoctonia solani | 11 | 15.271 | <0.01 |
| Fusarium oxysporum | 11 | 66 | <0.01 |
| Table 3. The effect of soil extracts on the growth of American ginseng pathogens. | |||
| Fusarium solani | 5 | 3.245 | 0.015 |
| Cylindrocarpon destructans | 5 | 7.539 | <0.01 |
| Rhizoctonia solani | 5 | 2.498 | 0.189 |
| Fusarium oxysporum | 5 | 0.001 | 0.98 |
| Figure 1. The effect of monoculture history of American ginseng on soil phenolic acid. | |||
| Total phenolic acid | 11 | 80.527 | <0.01 |
| Figure 2. The effect of soil with different monoculture history on the growth of American ginseng. | |||
| Germination rate | 5 | 1 | 0.374 |
| Plant height | 5 | 4.568 | 0.099 |
| Chlorophyll content | 5 | 3.185 | 0.015 |
| Root rot incidence | 5 | 1.373 | <0.01 |
| Root fresh weight | 5 | 14.608 | 0.019 |
| Root activity | 5 | 5.064 | <0.01 |
| Fibrous root numbers | 5 | 54.226 | 0.018 |
| Figure 3. Microbiome diversity in bulk soil and American ginseng rhizosphere when grown in soils with different monoculture history. | |||
| Fungal OTUs | 11 | 4.441 | 0.041 |
| Fungal Shannon | 11 | 0.432 | 0.736 |
| Bacterial OTUs | 11 | 42.157 | <0.01 |
| Bacterial Shannon | 11 | 5.75 | 0.023 |
| Sample ID | Raw | After Quality Control | Annotated as Fungi |
|---|---|---|---|
| 0 yr-bs1 | 61,233 | 61,141 | 55,637 |
| 0 yr-bs2 | 23,963 | 23,707 | 21,277 |
| 0 yr-bs3 | 45,562 | 45,421 | 38,851 |
| 3 yr-bs1 | 47,819 | 47,753 | 43,188 |
| 3 yr-bs2 | 63,154 | 63,097 | 56,345 |
| 3 yr-bs3 | 50,591 | 50,499 | 44,698 |
| 0 yr-rs1 | 61,843 | 61,835 | 28,026 |
| 0 yr-rs2 | 42,746 | 42,741 | 21,609 |
| 0 yr-rs3 | 57,472 | 57,471 | 9697 |
| 3 yr-rs1 | 61,940 | 61,926 | 41,707 |
| 3 yr-rs2 | 57,768 | 57,754 | 38,278 |
| 3 yr-rs3 | 47,378 | 47,371 | 14,066 |
References
- Qi, L.W.; Wang, C.Z.; Yuan, C.S. Ginsenosides from American ginsen: Chemical and pharmacological diversity. Phytochemistry 2011, 72, 689–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Wang, S.; Ge, X.; Zhang, M. Authentication of American ginsen (Panax quinquefolius L.) from different origins by linear discriminant analysis of multi-elements. Eur. Food Res. Technol. 2021, 247, 2657–2666. [Google Scholar] [CrossRef]
- Manici, L.M.; Kelderer, M.; Franke-Whittle, I.H.; Rühmer, T.; Baab, G.; Nicoletti, F.; Caputo, F.; Topp, A.; Insam, H.; Naef, A. Relationship between root-endophytic microbial communities and replant disease in specialized apple growing areas in Europe. Appl. Soil Ecol. 2013, 72, 207–214. [Google Scholar] [CrossRef]
- Tan, Y.; Cui, Y.; Li, H.; Kuang, A.; Li, X.; Wei, Y.; Ji, X. Rhizospheric soil and root endogenous fungal diversity and composition in response to continuous Panax notoginseng cropping practices. Microbiol. Res. 2017, 194, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Wolińska, A.; Kruczyńska, A.; Podlewski, J.; Słomczewski, A.; Grządziel, J.; Gałązka, A.; Kuźniar, A. Does the use of an intercropping mixture really improve the biology of monocultural soils & mdash; a search for bacterial indicators of sensitivity and resistance to long-term maize monoculture. Agronomy 2022, 12, 613. [Google Scholar]
- Cui, J.; Zhang, E.; Zhang, X.; Wang, Q.; Liu, Q. Effects of 2,4-di-tert-butylphenol at different concentrations on soil functionality and microbial community structure in the Lanzhou lily rhizosphere. Appl. Soil Ecol. 2022, 172, 104367. [Google Scholar] [CrossRef]
- Huang, L.F.; Song, L.X.; Xia, X.J.; Mao, W.H.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Plant-Soil feedbacks and soil sickness: From mechanisms to application in agriculture. J. Chem. Ecol. 2013, 39, 232–242. [Google Scholar] [CrossRef]
- Chen, S.; Yu, H.; Zhou, X.; Wu, F. Cucumber (Cucumis sativus L.) seedling rhizosphere Trichoderma and Fusarium spp. communities altered by vanillic acid. Front. Microbiol. 2018, 9, 2195. [Google Scholar] [CrossRef] [Green Version]
- Tian, G.L.; Bi, Y.M.; Cheng, J.D.; Zhang, F.F.; Zhou, T.H.; Sun, Z.J.; Zhang, L.S. High concentration of ferulic acid in rhizosphere soil accounts for the occurrence of Fusarium wilt during the seedling stages of strawberry plants. Physiol. Mol. Plant Pathol. 2019, 108, 101435. [Google Scholar] [CrossRef]
- Xin, A.; Li, X.; Jin, H.; Yang, X.; Zhao, R.; Liu, J.; Qin, B. The accumulation of reactive oxygen species in root tips caused by autotoxic allelochemicals—A significant factor for replant problem of Angelica sinensis (Oliv.) Diels. Ind. Crops Prod. 2019, 138, 111432. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, G.; Wu, F. Responses of soil microbial communities in the rhizosphere of cucumber (Cucumis sativus L.) to exogenously applied p-hydroxybenzoic acid. J. Chem. Ecol. 2012, 38, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.S.; Shen, S.H.; Han, J.M.; Liu, Y.D.; Liu, S.D. The effect in vitro of exogenously applied p-hydroxybenzoic acid on Fusarium oxysporum f. sp. niveum. Phytopathol. Mediterr. 2009, 48, 439–446. [Google Scholar]
- Ray, S.; Mishra, S.; Bisen, K.; Singh, S.; Sarma, B.K.; Singh, H.B. Modulation in phenolic root exudate profile of Abelmoschus esculentus expressing activation of defense pathway. Microbiol. Res. 2018, 207, 100. [Google Scholar] [CrossRef] [PubMed]
- Badri, D.V.; Chaparro, J.M.; Zhang, R.; Shen, Q.; Vivanco, J.M. Application of natural blends of phytochemicals derived from the root exudates of Arabidopsisto the soil reveal that phenolic-related compounds predominantly modulate the soil microbiome. J. Biol. Chem. 2013, 288, 4502–4512. [Google Scholar] [CrossRef] [Green Version]
- Zwetsloot, M.J.; Kessler, A.; Bauerle, T.L. Phenolic root exudate and tissue compounds vary widely among temperate forest tree species and have contrasting effects on soil microbial respiration. New Phytol. 2018, 218, 530–541. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Gong, J.; Xu, M. Implications of continuous and rotational cropping practices on soil bacterial communities in pineapple cultivation. Eur. J. Soil Biol. 2020, 97, 103172. [Google Scholar] [CrossRef]
- Li, L.; Yao, Y.F.; Qin, F.C. Spatial variability of soil total nitrogen, available phosphorus and available potassium in huanghuadianzi watershed. Chin. J. Ecol. 2015, 34, 373–379. [Google Scholar]
- Bilias, F.; Barbayiannis, N. Potassium availability: An approach using thermodynamic parameters derived from quantity-intensity relationships. Geoderma 2019, 338, 355–364. [Google Scholar] [CrossRef]
- Roberts, T.; Ross, W.; Norman, R.; Slaton, N.; Wilson, C. Predicting nitrogen fertilizer needs for rice in arkansas using Alkaline Hydrolyzable-Nitrogen. Soil Sci. Soc. Am. J. 2011, 75, 1161. [Google Scholar] [CrossRef]
- Swift, R.S. Organic Matter Characterization, Methods of Soil Analysis; Part 3; Chemical Methods; Soil Science Society of America: Madison, WI, USA, 1996; pp. 1011–1070. [Google Scholar] [CrossRef]
- Wu, L.; Jie, L.; Wang, W.; Dai, D.; Cheng, X.; Zhang, Z.; Yang, Y. Identification and content determination of phenolic acids of rhizosphere soil of Panax Notoginseng. Mod. Tradit. Chin. Med. Mater. Med.-World Sci. Technol. 2014, 12, 825–829. [Google Scholar] [CrossRef]
- Chen, M.; Xie, L.; FU, Z.; Zou, J. Comparison of two methods for measurement of phytoplanktonic chlorophyll-a. Environ. Monit. Manag. Technol. 2016, 28, 46–48. [Google Scholar]
- Zhu, X.; Liang, M.; Ma, Y. A review report on the experiments for the determination of root activity by TTC Method. Guangdong Chem. Ind. 2020, 47, 211–212. [Google Scholar]
- Li, J.; Philp, J.; Li, J.; Wei, Y.; Li, H.; Yang, K.; Ryder, M.; Toh, R.; Zhou, Y.; Denton, M.; et al. Trichoderma harzianum inoculation reduces the incidence of clubroot disease in Chinese cabbage by regulating the rhizosphere microbial community. Microorganisms 2020, 8, 1325. [Google Scholar] [CrossRef] [PubMed]
- Durairaj, K.; Velmurugan, P.; Park, J.H.; Chang, W.S.; Park, Y.J.; Senthilkumar, P.; Choi, K.M.; Lee, J.H.; Oh, B.T. An investigation of biocontrol activity pseudomonas and bacillus strains against Panax ginseng root rot fungal phytopathogens. Biol. Control 2018, 125, 138–146. [Google Scholar] [CrossRef]
- Yao, C.Z.; Jiang, Y.T.; Yang, Y.T.; Ma, Y.Y.; Pan, L.I.; Li, Y.I.; Sun, Y.Q.; Chen, J.; Zhao, J. Allelopathic effect of extracts from Panax notoginseng mono-cropped soil on its root rot pathogens. Chin. J. Appl. Ecol. 2020, 31, 2227–2235. [Google Scholar] [CrossRef]
- Bruce Williamson, G.; Richardson, D. Bioassays for allelopathy: Measuring treatment responses with independent controls. J. Chem. Ecol. 1988, 14, 181–187. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [Green Version]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef] [Green Version]
- Benitez, M.S.; Ewing, P.M.; Osborne, S.L.; Lehman, R.M. Rhizosphere microbial communities explain positive effects of diverse crop rotations on maize and soybean performance. Soil Biol. Biochem. 2021, 159, 108309. [Google Scholar] [CrossRef]
- Dong, L.; Xu, J.; Zhang, L.; Yang, J.; Liao, B.; Li, X.; Chen, S. High-throughput sequencing technology reveals that continuous cropping of American ginseng results in changes in the microbial community in arable soil. Chin. Med. 2017, 12, 18. [Google Scholar] [CrossRef] [Green Version]
- Ji, L.; Tian, L.; Nasir, F.; Chang, J.; Chang, C.; Zhang, J.; Li, X.; Tian, C. Impacts of replanting American ginseng on fungal assembly and abundance in response to disease outbreaks. Arch. Microbiol. 2021, 203, 2157–2170. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Ding, C.F.; Hua, K.; Zhang, T.I.; Zhang, Y.N.; Zhao, L.; Yang, Y.R.; Liu, J.G.; Wang, X.X. Soil sickness of peanuts is attributable to modifications in soil microbes induced by peanut root exudates rather than to direct allelopathy. Soil Biol. Biochem. 2014, 78, 149–159. [Google Scholar] [CrossRef]
- Nicol, R.W.; Yousef, L.; Traquair, J.A.; Bernards, M.A. Ginsenosides stimulate the growth of soilborne pathogens of American ginseng. Phytochemistry 2003, 64, 257–264. [Google Scholar] [CrossRef]
- Inderjit; Streibig, J.C.; Olofsdotter, M. Joint action of phenolic acid mixtures and its significance in allelopathy research. Physiol. Plant 2002, 114, 422–428. [Google Scholar] [CrossRef]
- Cecile, B.; Yang, X.H.; Weston, L.A. The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 2003, 256, 67–83. [Google Scholar] [CrossRef]
- Aparicio, V.; Costa, J.L. Soil quality indicators under continuous cropping systems in the Argentinean Pampas. Soil Tillage Res. 2007, 96, 155–165. [Google Scholar] [CrossRef]
- Zhang, X.; Lang, D.; Zhang, E.; Wang, Z. Effect of autotoxicity and soil microbes in continuous cropping soil on Angelica sinensis seedling growth and rhizosphere soil microbial population. Chin. Herb. Med. 2015, 7, 88–93. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Mei, X.; Li, Y.; Wu, J.; Li, Y.; Wang, H.; Huang, H.; Yang, M.; He, X.; et al. Phenolic acids released in maize rhizosphere during maize-soybean intercropping inhibit Phytophthora blight of soybean. Front. Plant Sci. 2020, 11, 886. [Google Scholar] [CrossRef]
- Chung, I.-M.; Lim, J.-J.; Ahn, M.-S.; Jeong, H.-N.; An, T.-J.; Kim, S.-H. Comparative phenolic compound profiles and antioxidative activity of the fruit, leaves, and roots of Korean ginseng (Panax ginseng Meyer) according to cultivation years. J. Ginseng Res. 2016, 40, 68–75. [Google Scholar] [CrossRef] [Green Version]



| Characteristics | 0 yr | 1 yr | 2 yr | 3 yr |
|---|---|---|---|---|
| OM (g kg−1) | 22.77 ± 2.88 a | 21.50 ± 0.92 a | 21.60 ± 0 a | 19.33 ± 0.95 a |
| TN (g kg−1) | 1.63 ± 0.08 a | 1.57 ± 0.08 a | 1.48 ± 0.05 a | 1.49 ± 0.07 a |
| AN (mg kg−1) | 189.33 ± 0.58 b | 177.67 ± 2.52 ba | 184.00 ± 1.73 ba | 191.67 ± 1.53 a |
| TP (g kg−1) | 0.60 ± 0.07 a | 0.51 ± 0.34 a | 0.49 ± 0.01 b | 0.57 ± 0.03 a |
| AP (mg kg−1) | 115.67 ± 1.15 a | 96.40 ± 0.82 b | 115.33 ± 1.54 a | 114.33 ± 2.52 a |
| TK (g kg−1) | 19.20 ± 0.79 a | 19.63 ± 0.76 a | 19.03 ± 0.29 a | 20.50 ± 0.75 a |
| AK (g kg−1) | 0.14 ± 0.01 a | 0.16 ± 0.01 a | 0.11 ± 0.01 b | 0.15 ± 0.01 a |
| Concentration (mg L−1) | Fusarium Solani | Cylindrocarpon Destructans | Rhizoctonia Solani | Fusarium Oxysporum |
|---|---|---|---|---|
| RI% | RI% | RI% | RI% | |
| 2.5 | 9.75 ± 0.06 a | 5.23 ± 0.02 a | 4.79 ± 0.02 a | −3.45 ± 0 a |
| 5.0 | 8.58 ± 0.04 a | 2.67 ± 0.02 a | 5.51 ± 0.05 a | −3.45 ± 0 a |
| 10 | 7.31 ± 0.02 a | 3.90 ± 0.04 a | 10.68 ± 0.04 a | −6.90 ± 0.03 a |
| 50 | −12.70 ± 0.07 b | −13.89 ± 0.02 b | −11.11 ± 0.05 b | −22.99 ± 0.02 b |
| Ratio (Methanol:Soil) | Fusarium Solani | Cylindrocarpon Destructans | Rhizoctonia Solani | Fusarium Oxysporum |
|---|---|---|---|---|
| RI% | RI% | RI% | RI% | |
| 2:1 | 11.94 ± 0.04 a | 13.72 ± 0.03 b | 28.97 ± 0.02 a | 5.35 ± 0.03 a |
| 5:1 | 7.30 ± 0.04 b | 22.58 ± 0.05 a | 26.17 ± 0.02 a | 5.42 ± 0.03 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, S.; Wei, Y.; Li, H.; Zhao, Z.; Hu, J.; Philp, J.; Ryder, M.; Toh, R.; Li, J.; Zhou, Y.; et al. Long-Term Monocultures of American Ginseng Change the Rhizosphere Microbiome by Reducing Phenolic Acids in Soil. Agriculture 2022, 12, 640. https://doi.org/10.3390/agriculture12050640
An S, Wei Y, Li H, Zhao Z, Hu J, Philp J, Ryder M, Toh R, Li J, Zhou Y, et al. Long-Term Monocultures of American Ginseng Change the Rhizosphere Microbiome by Reducing Phenolic Acids in Soil. Agriculture. 2022; 12(5):640. https://doi.org/10.3390/agriculture12050640
Chicago/Turabian StyleAn, Shuhui, Yanli Wei, Hongmei Li, Zhongjuan Zhao, Jindong Hu, Joshua Philp, Maarten Ryder, Ruey Toh, Jishun Li, Yi Zhou, and et al. 2022. "Long-Term Monocultures of American Ginseng Change the Rhizosphere Microbiome by Reducing Phenolic Acids in Soil" Agriculture 12, no. 5: 640. https://doi.org/10.3390/agriculture12050640
APA StyleAn, S., Wei, Y., Li, H., Zhao, Z., Hu, J., Philp, J., Ryder, M., Toh, R., Li, J., Zhou, Y., & Denton, M. D. (2022). Long-Term Monocultures of American Ginseng Change the Rhizosphere Microbiome by Reducing Phenolic Acids in Soil. Agriculture, 12(5), 640. https://doi.org/10.3390/agriculture12050640






