Effect of a Ridge-Furrow Mulching System and Limited Supplementary Irrigation on N2O Emission Characteristics and Grain Yield of Winter Wheat (Triticum aestivum L.) Fields under Dryland Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site Description
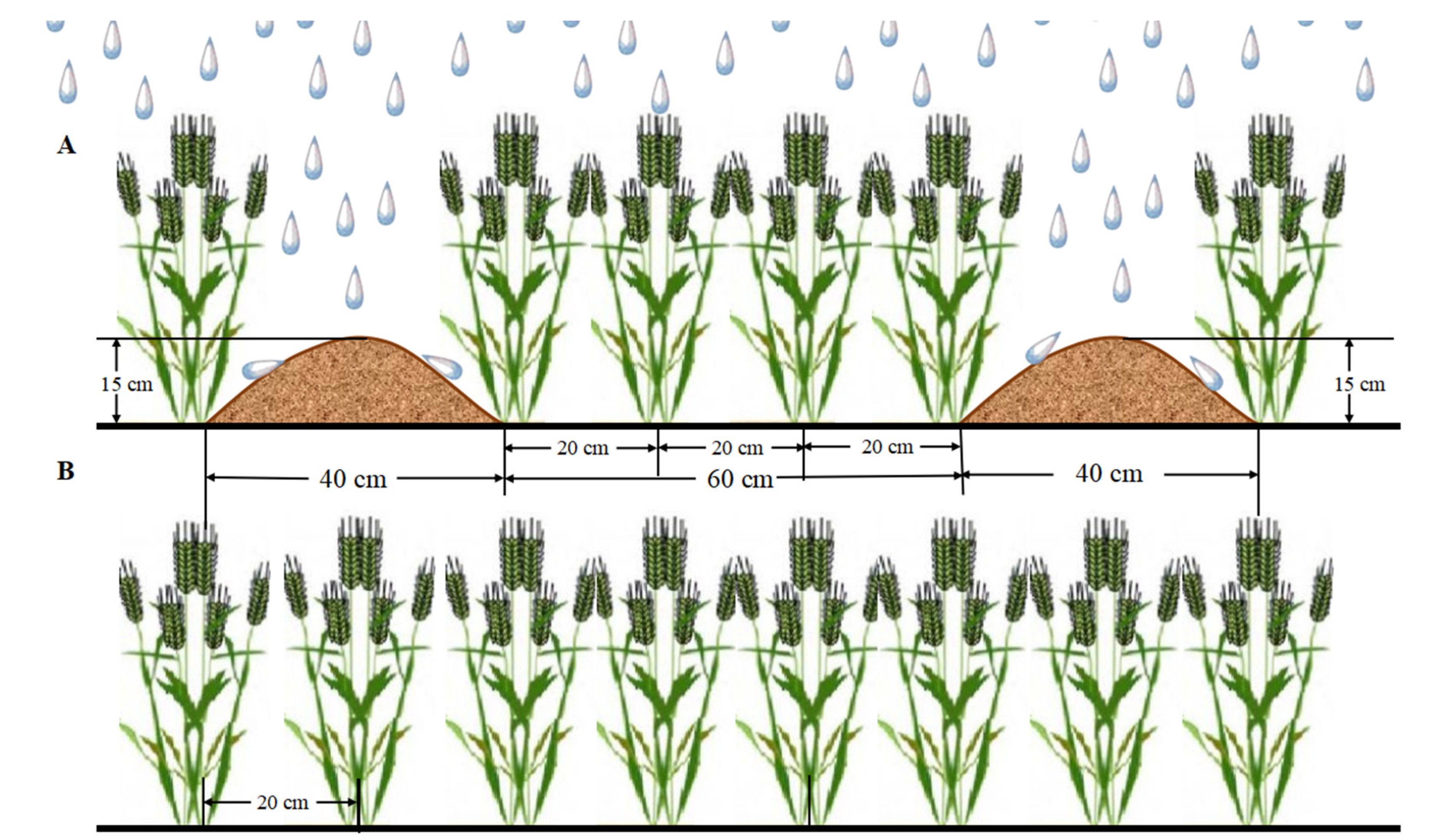
2.2. Experimental Design and Treatments
2.3. Sample Collection and Analysis
2.3.1. N2O Emission Fluxes
2.3.2. Soil Nitrate Nitrogen Content and Denitrifying Enzyme Activities
2.3.3. Grain Yield
2.4. Statistical Analysis
3. Results
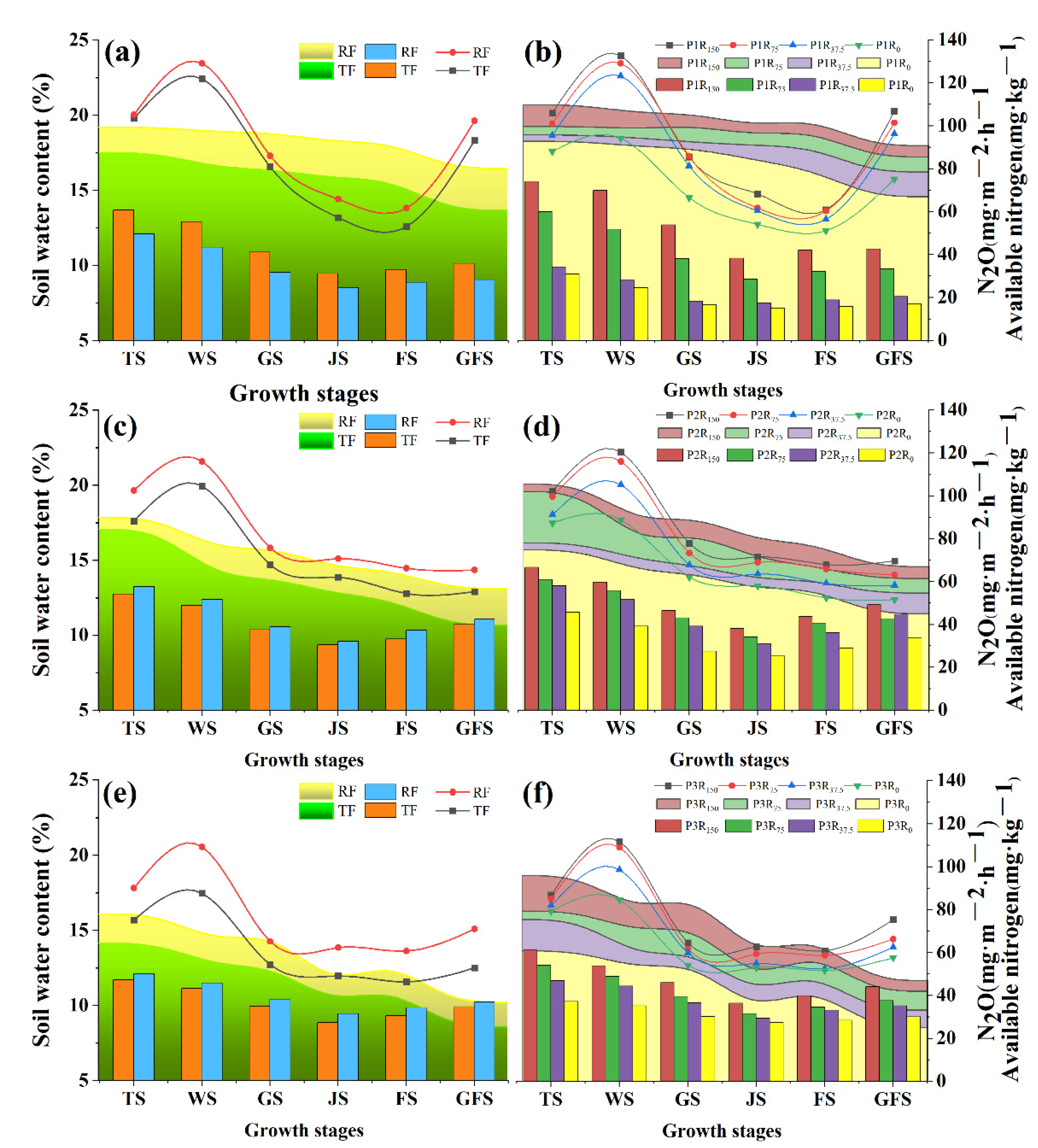
3.1. N2O Emissions, Soil Water Contents, and Soil Available Nitrogen Contents under RF with Supplementary Irrigation
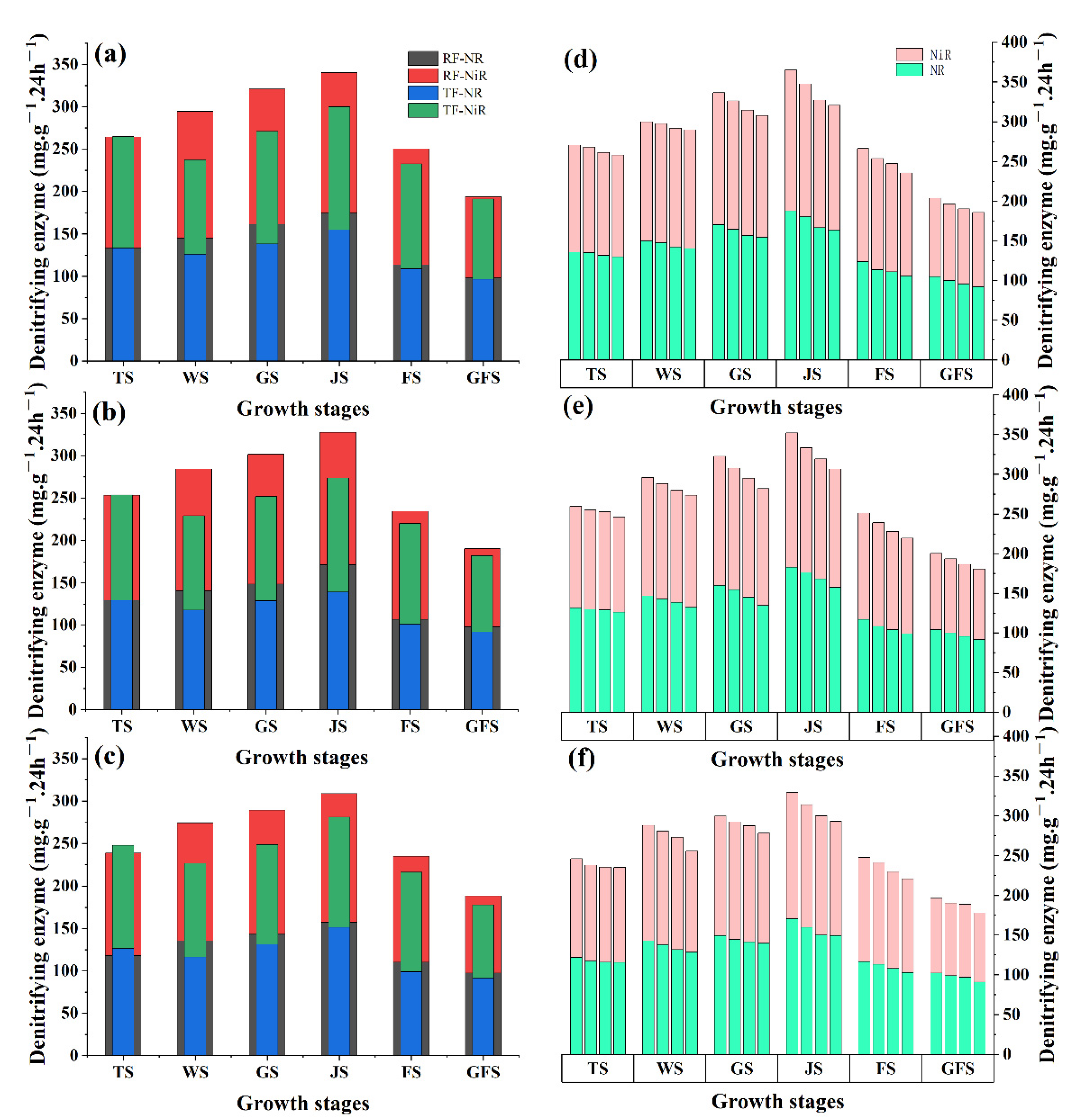
3.2. Denitrifying Enzyme (Nitrate Reductase and Nitrite Reductase) Activities under RF and Supplementary Irrigation
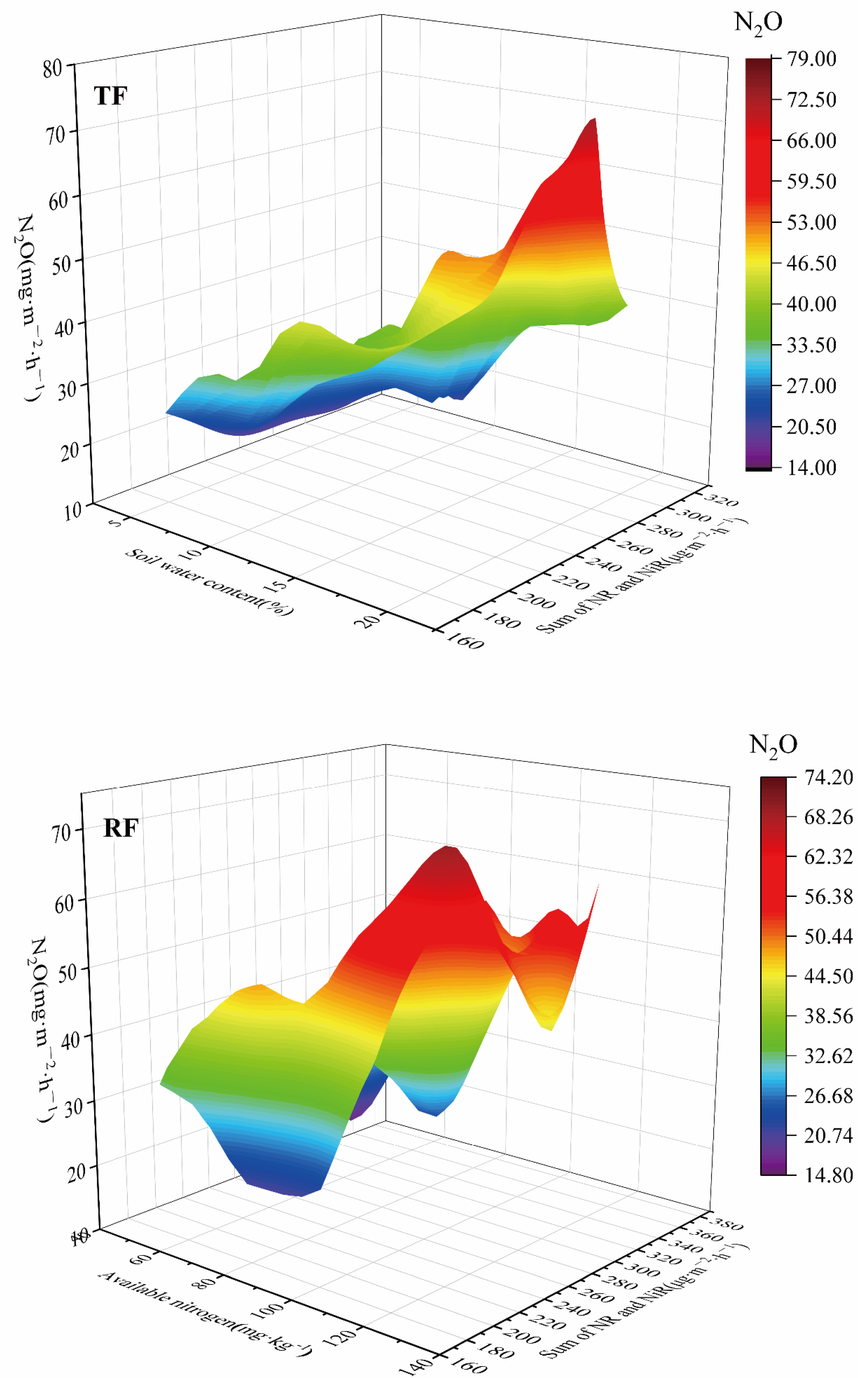
3.3. Relationships among N2O Emissions, Soil Water Contents, Soil Available Nitrogen Contents, and Denitrifying Enzyme Activities under RF and TF
3.4. Effect of the Ridge-Furrow Mulching System on Grain Yield
4. Discussion
4.1. N2O Emissions and Grain Yield from Winter Wheat Fields under RF with Different Amounts of Rainfall and Supplementary Irrigation
4.2. Relationships among Soil N2O Emissions Fluxes, Soil Water Contents, Soil Available Nitrogen Contents, and Denitrifying Enzyme Activities under RF and TF
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meyer, L. Climate Change (2007): Mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Nakicenovic, N.; Swart, R. IPCC Special Report on Emissions Scenarios: A Special Report of Working Group III of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revi, A.; Satterthwaite, D.E.; Aragón-Durand, F.; Corfee-Morlot, J.; Solecki, W. Urban Areas in Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. In Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Liu, X.; Shi, L.; Engel, B.A.; Sun, S.; Zhao, X.; Wu, P.; Wang, Y. New challenges of food security in Northwest China: Water footprint and virtual water perspective. J. Clean. Prod. 2019, 245, 118939. [Google Scholar] [CrossRef]
- Werner, C.; Butterbach-Bahl, K.; Haas, E.; Hickler, T.; Kiese, R. A global inventory of N2O emissions from tropical rainforest soils using a detailed biogeochemical model. Glob. Biogeochem. Cycles 2007, 21. [Google Scholar] [CrossRef]
- Sánchez-Martín, L.; Arce, A.; Benito, A.; Garcia-Torres, L.; Vallejo, A. Influence of drip and furrow irrigation systems on nitrogen oxide emissions from a horticultural crop. Soil Biol. Biochem. 2008, 40, 1698–1706. [Google Scholar] [CrossRef]
- Cusack, D.F.; Silver, W.L.; Torn, M.S.; Burton, S.D.; Firestone, M.K. Changes in microbial community characteristics and soil organic matter with nitrogen additions in two tropical forests. Ecology 2011, 92, 621–632. [Google Scholar] [CrossRef] [Green Version]
- Enwall, K.; Philippot, L.; Hallin, S. Activity and Composition of the Denitrifying Bacterial Community Respond Differently to Long-Term Fertilization. Appl. Environ. Microbiol. 2005, 71, 8335–8343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, F.; Liu, Z.; Mou, H.; Li, J.; Zhang, P.; Jia, Z. Impact of farmland mulching practices on the soil bacterial community structure in the semiarid area of the loess plateau in China. Eur. J. Soil Biol. 2019, 92, 8–15. [Google Scholar] [CrossRef]
- Mancinelli, R.; Marinari, S.; Brunetti, P.; Radicetti, E.; Campiglia, E. Organic mulching, irrigation and fertilization affect soil CO2 emission and C storage in tomato crop in the Mediterranean environment. Soil Tillage Res. 2015, 152, 39–51. [Google Scholar] [CrossRef]
- Ju, X.; Lu, X.; Gao, Z.; Chen, X.; Su, F.; Kogge, M.; Römheld, V.; Christie, P.; Zhang, F. Processes and factors controlling N2O production in an intensively managed low carbon calcareous soil under sub-humid monsoon conditions. Environ. Pollut. 2011, 159, 1007–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, T.L.; Suddick, E.C.; Six, J. Reduced nitrous oxide emissions and increased yields in California tomato cropping systems under drip irrigation and fertigation. Agric. Ecosyst. Environ. 2013, 170, 16–27. [Google Scholar] [CrossRef]
- Huang, G.B.; Qiang, C.H.A.I.; Feng, F.X.; Yu, A.Z. Effects of Different Tillage Systems on Soil Properties, Root Growth, Grain Yield, and Water Use Efficiency of Winter Wheat (Triticum aestivum L.) in Arid Northwest China. J. Integr. Agric. 2012, 11, 74–84. [Google Scholar] [CrossRef]
- Thornton, K.P.; Boone, R.B.; Ramirezvillegas, J. Climate Change Impacts on Livestock. Ann. Chir. 2015, 125, 18–25. [Google Scholar]
- Zhang, Y.; Niu, H. Influences of two irrigation systems on soil NO emissions from vineyards in Ningxia, China. J. Univ. Chin. Acad. Sci. 2016, 33, 178–186. [Google Scholar]
- Ran, Y.; Bai, X.; Long, Y.; Ai, P. Yield and Quality of Rice under the Effects of Digestate Application. Agriculture 2022, 12, 514. [Google Scholar] [CrossRef]
- Lei, H.; Ke, L.I.; Feng, K.; Pan, H.; Yang, H. Effects of Post-Irrigation Aeration on Pakchoi Water Use Efficiency and Nutrient Uptake Characteristics in Three Kinds of Soil; Journal of Drainage & Irrigation Machinery Engineering: Zhenjiang, China, 2018. [Google Scholar]
- Dong, Z.; Zhang, X.; Li, J.; Zhang, C.; Wei, T.; Yang, Z.; Cai, T.; Zhang, P.; Ding, R.; Jia, Z. Photosynthetic characteristics and grain yield of winter wheat (Triticum aestivum L.) in response to fertilizer, precipitation, and soil water storage before sowing under the ridge and furrow system: A path analysis. Agric. For. Meteorol. 2019, 272–273, 12–19. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Ma, X.; Liu, X.; Zhang, P.; Cai, T.; Jia, Z. Ridge-furrow mulching system and supplementary irrigation can reduce the greenhouse gas emission intensity. Sci. Total Environ. 2020, 717, 137262. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Wang, L.Q. Relationship between soil composition and water evaporation in guanzhong Plain. Soil Bull. 1986, 2, 49–52. [Google Scholar]
- Hou, H.; Chen, H.; Cai, H.; Yang, F.; Li, D.; Wang, F. CO2 and N2O emissions from Lou soils of greenhouse tomato fields under aerated irrigation. Atmos. Environ. 2016, 132, 69–76. [Google Scholar] [CrossRef]
- Rolston, D.E. Gas Flux. In Methods of Soil Analysis, Part 1, 2nd ed.; Klute, A., Ed.; ASA; SSSA: Madison, WI, USA, 1989. [Google Scholar]
- He, W.X.; Wei, Y.Y.; Cai, S.H. Study on determination methods and influencing factors of soil denitrification enzyme activity. J. Northwest AF Univ. (Nat. Sci. Ed.) 2006, 1, 127–130. [Google Scholar]
- Bao, S.D. Soil Agrochemical Analysis, 3rd ed.; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Baggs, E.M. A review of stable isotope techniques for N2O source partitioning in soils: Recent progress, remaining challenges and future considerations. Rapid Commun. Mass Spectrom. 2008, 22, 1664–1672. [Google Scholar] [CrossRef]
- Castro-Barros, C.M.; Jia, M.; van Loosdrecht, M.C.; Volcke, E.I.; Winkler, M.K. Evaluating the potential for dissimilatory nitrate reduction by anammox bacteria for municipal wastewater treatment. Bioresour. Technol. 2017, 233, 363–372. [Google Scholar] [CrossRef]
- Maris, S.; Teira-Esmatges, M.; Arbonés, A.; Rufat, J. Effect of irrigation, nitrogen application, and a nitrification inhibitor on nitrous oxide, carbon dioxide and methane emissions from an olive (Olea europaea L.) orchard. Sci. Total Environ. 2015, 538, 966–978. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Wang, J.; Zhang, Q.; Fu, X.; Li, Z. Effect of cover cropping on soil greenhouse gas emissions during summer fallow under manipulated rainfall. Acta Prataculturae Sin. 2018, 27, 27–38. [Google Scholar]
- Xu, Y.; Ma, X.; Wang, Y.; Ali, S.; Cai, T.; Jia, Z. Effects of ridge-furrow mulching system with supplementary irrigation on soil respiration in winter wheat fields under different rainfall conditions. Agric. Water Manag. 2020, 239, 106237. [Google Scholar] [CrossRef]
- Ali, S.; Xu, Y.; Jia, Q.; Ahmad, I.; Wei, T.; Ren, X.; Zhang, P.; Din, R.; Cai, T.; Jia, Z. Cultivation techniques combined with deficit irrigation improves winter wheat photosynthetic characteristics, dry matter translocation and water use efficiency under simulated rainfall conditions. Agric. Water Manag. 2018, 201, 207–218. [Google Scholar] [CrossRef]
- Wang, J.; Sainju, U.M.; Barsotti, J.L. Residue Placement and Rate, Crop Species, and Nitrogen Fertilization Effects on Soil Greenhouse Gas Emissions. J. Environ. Prot. 2012, 3, 1238–1250. [Google Scholar] [CrossRef] [Green Version]
- Amha, Y.; Bohne, H. Denitrification from the horticultural peats: Effects of pH, nitrogen, carbon, and moisture contents. Biol. Fertil. Soils 2011, 47, 293–302. [Google Scholar] [CrossRef]
- Bateman, J.E.; Baggs, E.M. Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol. Fertil. Soils 2005, 41, 379–388. [Google Scholar] [CrossRef]
- Yang, P.; Lai, D.Y.; Huang, J.F.; Tong, C. Effect of drainage on CO2, CH4, and N2O fluxes from aquaculture ponds during winter in a subtropical estuary of China. J. Environ. Sci. 2017, 65, 72–82. [Google Scholar] [CrossRef]
- Allende-Montalbán, R.; Martín-Lammerding, D.; del Mar Delgado, M.; Porcel, M.A.; Gabriel, J.L. Nitrate Leaching in Maize (Zea mays L.) and Wheat (Triticum aestivum L.) Irrigated Cropping Systems under Nitrification Inhibitor and/or Intercropping Effects. Agriculture 2022, 12, 478. [Google Scholar] [CrossRef]
- Pang, J.W.; Wang, Y.H.; Liu, C.; Liu, D.H.; Zhang, Y.; Yang, B.P.; Jia, Z.K.; Zhang, P. Effect of different fertilization amount on soil moisture and corn yield in dry ridges and ridges. J. Plant Nutr. Fertil. 2021, 27, 826–836. [Google Scholar]
- Wu, C.H.; Pu, X.K.; Zhou, Y.J.; Mian, Y.M.; Miao, F.F.; Li, R. Effect of rainwater cover on soil hydrothermal fertilizer and potato yield. Crop J. 2021, 47, 2208–2219. [Google Scholar]
- Li, R.; Wang, Y.L.; Wu, P.N.; Sun, R.P.; Qiu, J.X.; Su, M.; Hou, X.F. The ridge mulching in Ningnan dry area improves the potato yield. J. Agric. Eng. 2017, 33, 168–175. [Google Scholar]
- Zhang, Y.; Han, H.; Zhang, D. Effects of ridging and mulching combined practices on proso millet growth and yield in semi-arid regions of China. Field Crops Res. 2017, 213, 65–74. [Google Scholar] [CrossRef]
- Allen, K.S.; Plattner, G.K.; Nauels, A.; Xia, Y.; Stocker, T.F. Climate Change 2013: The Physical Science Basis. An overview of the Working Group 1 contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC). In EGU General Assembly Conference Abstracts; EGU General Assembly: Vienna, Austria, 2007; Volume 18, pp. 95–123. [Google Scholar]
- Wang, L.; Hao, D.C.; Fan, S.; Xie, H.; Bao, X.; Jia, Z.; Wang, L. N2O Emission and Nitrification/Denitrification Bacterial Communities in Upland Black Soil under Combined Effects of Early and Immediate Moisture. Agriculture 2022, 12, 330. [Google Scholar] [CrossRef]
- Barnard, R.; Leadley, P.W.; Hungate, B.A. Global change, nitrification, and denitrification: A review. Glob. Biogeochem. Cycles 2005, 19. [Google Scholar] [CrossRef]
- Kool, M.D.; Dolfing, J.; Wrage, N.; Groenigen, J.W.V. Nitrifier denitrification as a distinct and significant source of nitrous oxide from soil. Soil Biol. Biochem. 2011, 43, 174–178. [Google Scholar] [CrossRef]
- Xiong, Z.; Xie, Y.; Xing, G.; Zhu, Z.; Butenhoff, C. Measurements of nitrous oxide emissions from vegetable production in China. Atmos. Environ. 2006, 40, 2225–2234. [Google Scholar]
- Ali, S.; Xu, Y.; Jia, Q.; Ahmad, I.; Ma, X.; Yan, Z.; Cai, T.; Ren, X.; Zhang, P.; Jia, Z. Interactive effects of planting models with limited irrigation on soil water, temperature, respiration and winter wheat production under simulated rainfall conditions. Agric. Water Manag. 2018, 204, 198–211. [Google Scholar] [CrossRef]
- Hu, W.H.; Chen, D.; He, J.Z. Microbial regulation of terrestrial nitrous oxide formation: Understanding the biological pathways for prediction of emission rates. FEMS Microbiol. Rev. 2015, 5, 5. [Google Scholar] [CrossRef]
- Huang, T.; Gao, B.; Hu, X.-K.; Lu, X.; Well, R.; Christie, P.; Bakken, L.R.; Ju, X.-T. Ammonia-oxidation as an engine to generate nitrous oxide in an intensively managed calcareous Fluvo-aquic soil. Sci. Rep. 2014, 4, 3950. [Google Scholar] [CrossRef] [PubMed]
- Braker, G.; Conrad, R. Chapter 2—Diversity, Structure, and Size of N2O-Producing Microbial Communities in Soils—What Matters for Their Functioning? Elsevier Science & Technology: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Sander, B.O.; Schneider, P.; Romasanta, R.; Samoy-Pascual, K.; Sibayan, E.B.; Asis, C.A.; Wassmann, R. Potential of Alternate Wetting and Drying Irrigation Practices for the Mitigation of GHG Emissions from Rice Fields: Two Cases in Central Luzon (Philippines). Agriculture 2020, 10, 350. [Google Scholar] [CrossRef]
- He, F.; Jiang, R.; Chen, Q.; Zhang, F.; Su, F. Nitrous oxide emissions from an intensively managed greenhouse vegetable cropping system in Northern China. Environ. Pollut. 2009, 157, 1666–1672. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Zhang, Z.; Ye, X.; Kao, C.M.; Chen, S. Long-term effect of temperature on N2O emission from the denitrifying activated sludge. J. Biosci. Bioeng. 2014, 117, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Ding, J.; Li, Y.; Lin, W.; Mao, L. The effects of soil water content on N2O emissions and isotopic signature of nitrification and denitrification. Sci. Agric. Sin. 2017, 50, 4747–4758. [Google Scholar]
- Kim, S.-W.; Fushinobu, S.; Zhou, S.; Wakagi, T.; Shoun, H. The Possible Involvement of Copper-Containing Nitrite Reductase (NirK) and Flavohemoglobin in Denitrification by the FungusCylindrocarpon tonkinense. Biosci. Biotechnol. Biochem. 2010, 74, 1403–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Huang, G.H. The relationship between denitrification enzyme activity in soil and N2O emissions. Chin. J. Appl. Ecol. 1999, 3, 329–331. [Google Scholar]
- Zheng, J.-Q.; Han, S.-J.; Ren, F.-R.; Zhou, Y.-M.; Zhang, Y. Effects of long-term elevated CO2 on N2-fixing, denitrifying and nitrifying enzyme activities in forest soils under Pinus sylvestriformis in Changbai Mountain. J. For. Res. 2008, 19, 283–287. [Google Scholar] [CrossRef]
- Cardon, Z.G.; Hungate, B.A.; Cambardella, C.A.; Chapin Iii, F.S.; Field, C.B.; Holland, E.A.; Mooney, H.A. Contrasting effects of elevated CO2 on old and new soil carbon pools. Soil Biol. Biochem. 2001, 33, 365–373. [Google Scholar] [CrossRef]
- Rehman, I.; Riaz, M.; Ali, S.; Arif, M.; Ali, S.; Alyemeni, M.; Alsahli, A. Evaluating the Effects of Biochar with Farmyard Manure under Optimal Mineral Fertilizing on Tomato Growth, Soil Organic C and Biochemical Quality in a Low Fertility Soil. Sustainability 2021, 13, 2652. [Google Scholar] [CrossRef]
- Zhu, L.X. Effects of nitrogen application and plastic film mulching on soil microbial biomass and soil enzyme activity in spring maize farmland under dry farming. Agric. Res. Arid. Areas 2019, 37, 7. [Google Scholar]
- Li, S.; Liu, X.; Zhang, W.; Ge, Z.; Xie, N.; Sun, L.; An, T.; Wang, J. Effects of long-term fertilization and film mulching on the abundance of soil bacteria, Archaea and ammonia oxidizing microorganisms. Chin. Sci. Bull. 2019, 50, 8. [Google Scholar]
- Almaraz, J.J.; Mabood, F.; Zhou, X.; Madramootoo, C.; Rochette, P.; Ma, B.-L.; Smith, D.L. Carbon Dioxide and Nitrous Oxide Fluxes in Corn Grown under Two Tillage Systems in Southwestern Quebec. Soil Sci. Soc. Am. J. 2009, 73, 113–119. [Google Scholar] [CrossRef]
- Jia, Q.; Xu, Y.; Ali, S.; Sun, L.; Ding, R.; Ren, X.; Zhang, P.; Jia, Z. Strategies of supplemental irrigation and modified planting densities to improve the root growth and lodging resistance of maize (Zea mays L.) under the ridge-furrow rainfall harvesting system. Field Crops Res. 2018, 224, 48–59. [Google Scholar] [CrossRef]
- Xiang, S.R.; Doyle, A.; Holden, P.A.; Schimel, J.P. Drying and rewetting effects on C and N mineralization and microbial activity in surface and subsurface California grassland soils. Soil Biol. Biochem. 2008, 40, 2281–2289. [Google Scholar] [CrossRef]
- Treseder, K.K. Nitrogen Additions and Microbial Biomass: A Global Meta-analysis. In AGU Fall Meeting Abstracts; American Geophysical Union: Washington, DC, USA, 2008; Volume 2008, p. B11D-0400. [Google Scholar]
- Jia, Q.; Sun, L.; Ali, S.; Zhang, Y.; Liu, D.; Kamran, M.; Zhang, P.; Jia, Z.; Ren, X. Effect of planting density and pattern on maize yield and rainwater use efficiency in the Loess Plateau in China. Agric. Water Manag. 2018, 202, 19–32. [Google Scholar] [CrossRef]


| Treatments | Grain Yield (t·ha−1) | Treatments | Grain Yield (t·ha−1) | Treatments | Grain Yield (t·ha−1) |
|---|---|---|---|---|---|
| P1R150 | 7.73 ± 0.52a | P2R150 | 7.30 ± 0.30a | P3R150 | 4.42 ± 0.83de |
| P1R75 | 7.38 ± 0.84a | P2R75 | 6.89 ± 1.15b | P3R75 | 3.70 ± 0.27ef |
| P1R37.5 | 6.82 ± 0.39ab | P2R37.5 | 6.02 ± 0.65c | P3R37.5 | 3.20 ± 1.83f |
| P1R0 | 5.33 ± 0.39cd | P2R0 | 4.49 ± 1.69de | P3R0 | 2.91 ± 0.11fg |
| P1T150 | 7.04 ± 0.70ab | P2T150 | 6.65 ± 0.28b | P3T150 | 4.17 ± 1.03e |
| P1T75 | 6.15 ± 0.52bc | P2T75 | 4.58 ± 1.18de | P3T75 | 3.14 ± 0.56f |
| P1T37.5 | 5.29 ± 1.06cd | P2T37.5 | 4.21 ± 0.85e | P3T37.5 | 3.11 ± 1.68f |
| P1T0 | 4.75 ± 0.78de | P2T0 | 3.17 ± 1.12f | P3T0 | 2.07 ± 2.16g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Wang, Y.; Ma, X.; Cai, T.; Jia, Z. Effect of a Ridge-Furrow Mulching System and Limited Supplementary Irrigation on N2O Emission Characteristics and Grain Yield of Winter Wheat (Triticum aestivum L.) Fields under Dryland Conditions. Agriculture 2022, 12, 621. https://doi.org/10.3390/agriculture12050621
Xu Y, Wang Y, Ma X, Cai T, Jia Z. Effect of a Ridge-Furrow Mulching System and Limited Supplementary Irrigation on N2O Emission Characteristics and Grain Yield of Winter Wheat (Triticum aestivum L.) Fields under Dryland Conditions. Agriculture. 2022; 12(5):621. https://doi.org/10.3390/agriculture12050621
Chicago/Turabian StyleXu, Yueyue, Yingxin Wang, Xiangcheng Ma, Tie Cai, and Zhikuan Jia. 2022. "Effect of a Ridge-Furrow Mulching System and Limited Supplementary Irrigation on N2O Emission Characteristics and Grain Yield of Winter Wheat (Triticum aestivum L.) Fields under Dryland Conditions" Agriculture 12, no. 5: 621. https://doi.org/10.3390/agriculture12050621
APA StyleXu, Y., Wang, Y., Ma, X., Cai, T., & Jia, Z. (2022). Effect of a Ridge-Furrow Mulching System and Limited Supplementary Irrigation on N2O Emission Characteristics and Grain Yield of Winter Wheat (Triticum aestivum L.) Fields under Dryland Conditions. Agriculture, 12(5), 621. https://doi.org/10.3390/agriculture12050621






