Complex Mixture of Arvensic Acids Isolated from Convolvulus arvensis Roots Identified as Inhibitors of Radicle Growth of Broomrape Weeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. General Experimental Procedures
2.3. Preparation of Convolvulus Arvensis Extracts
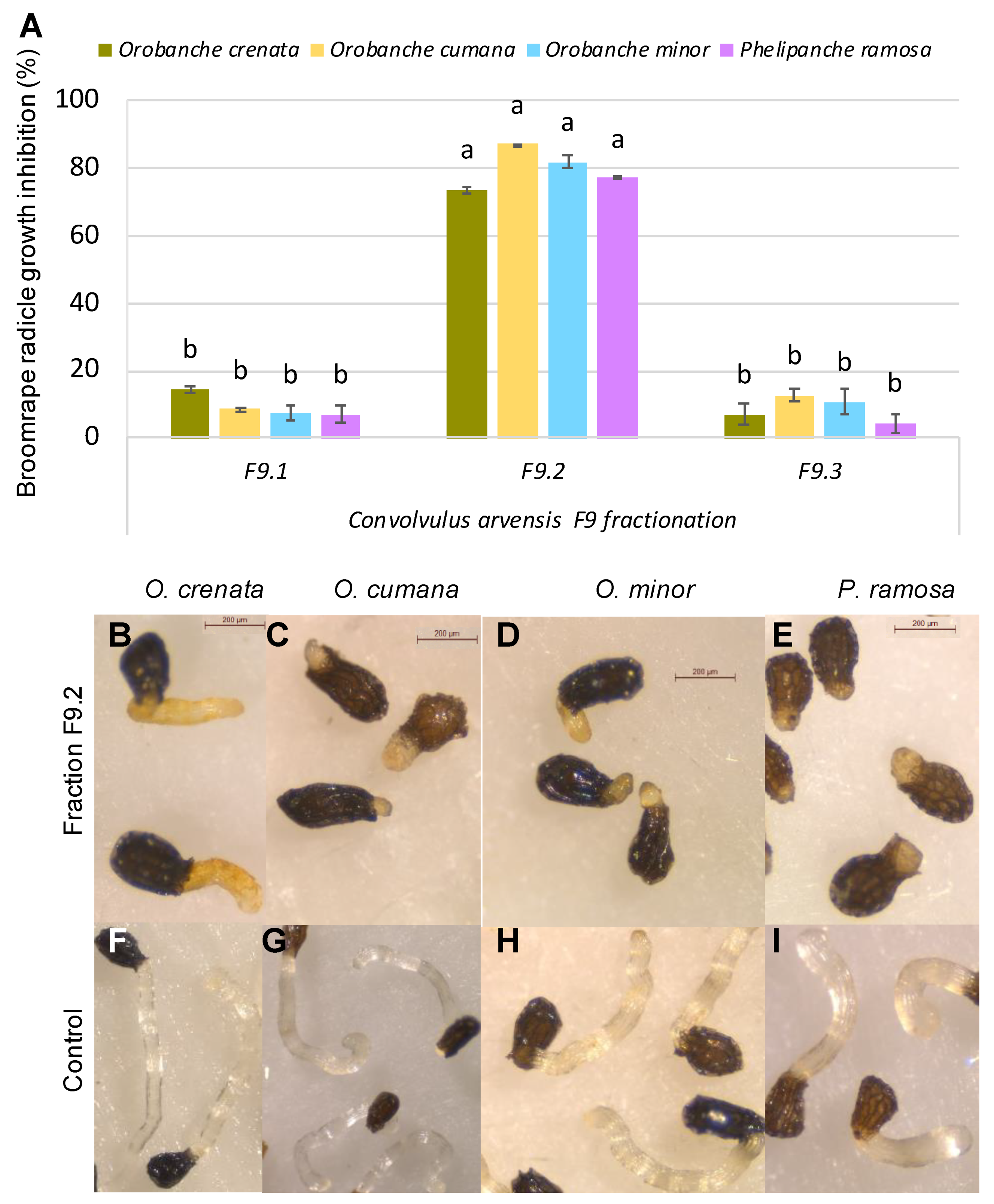
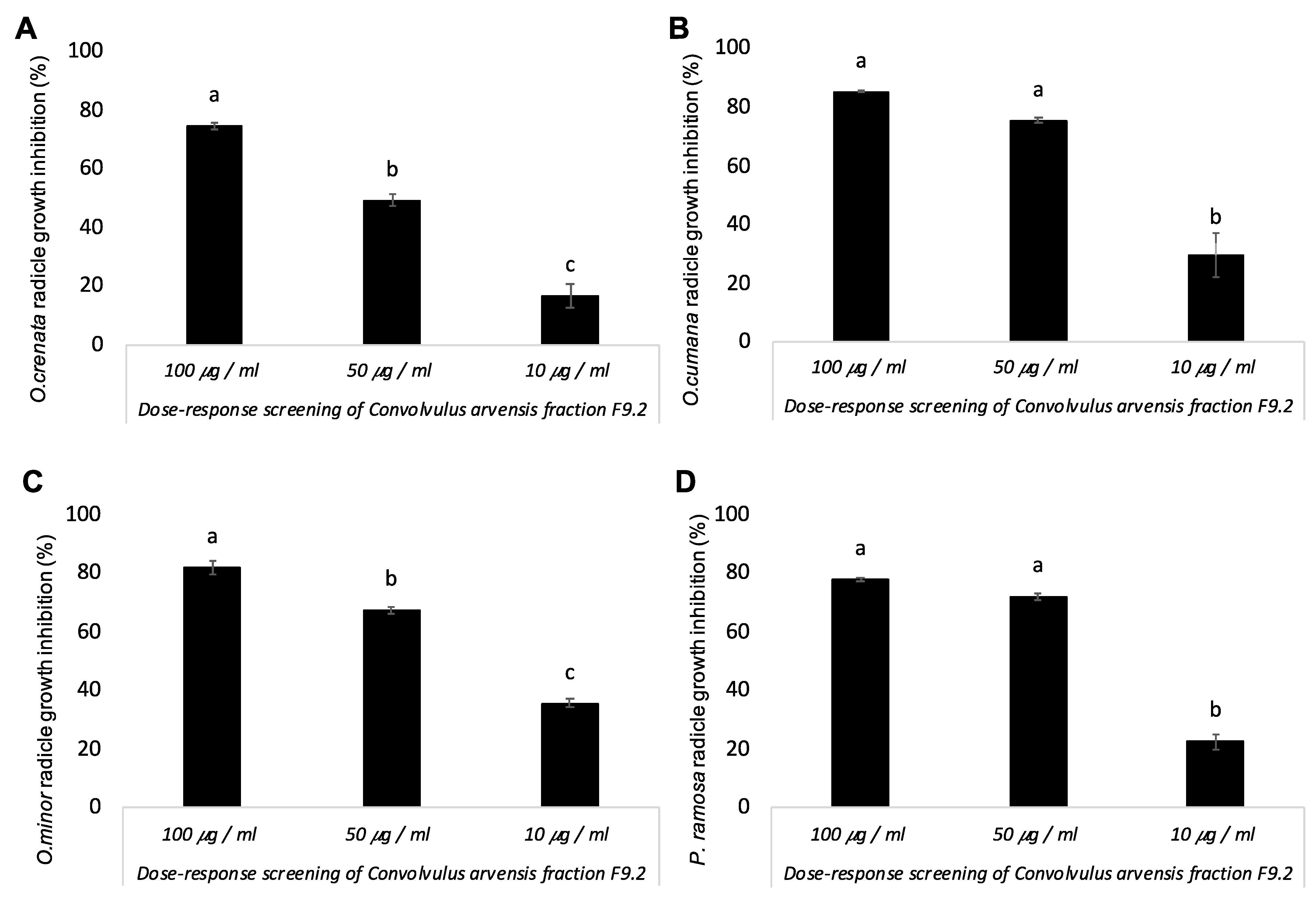
2.4. Radicle Growth Bioassays
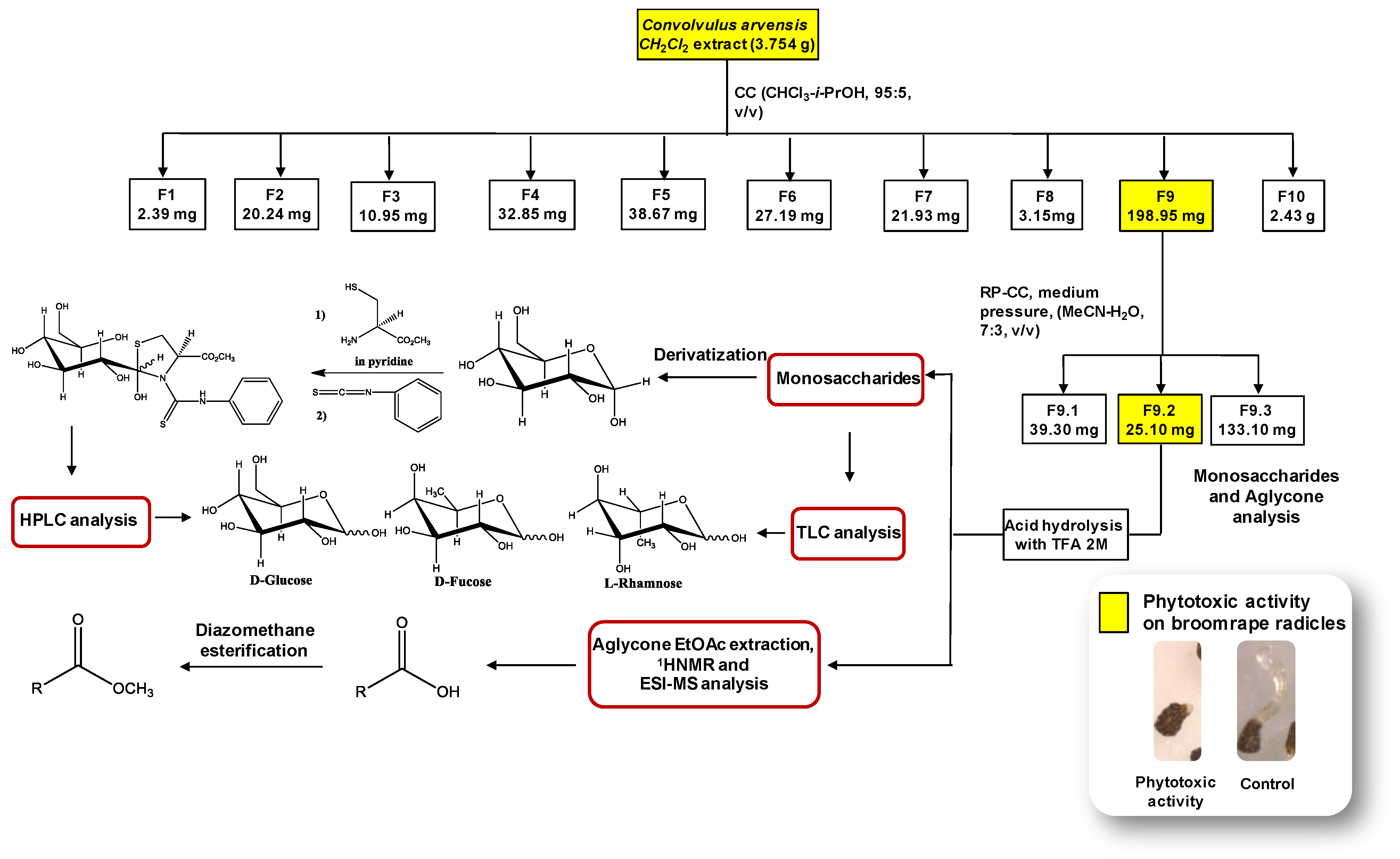
2.5. Activity-Guided Fractionation of Convolvulus Dichloromethane Extract
2.6. Acid Hydrolysis of Glycosides Fractions, Extraction of Aglicones and TLC Analysis of Monosaccharides
2.7. Derivatization of Monosaccharides and the HPLC Analysis of the Aldose Enantiomers Derivative
2.8. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Parker, C.; Riches, C.R. Parasitic Weeds of the World: Biology and Control; CAB International: Wallingford, UK, 1993. [Google Scholar]
- Fernández-Aparicio, M.; Flores, F.; Rubiales, D. The effect of Orobanche crenata infection severity in faba bean, field pea and grass pea productivity. Front. Plant Sci. 2016, 7, 1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, C. Observations on the current status of Orobanche and Striga problems worldwide. Pest Manag. Sci. 2009, 65, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Parker, C. The parasitic weeds of the Orobanchaceae. In Parasitic Orobanchaceae; Joel, D.M., Gressel, J., Musselman, L.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 313–344. [Google Scholar]
- Joel, D.M. The long-term approach to parasitic weeds control: Manipulation of specific developmental mechanisms of the parasite. Crop Prot. 2000, 19, 753–758. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Sillero, J.C.; Rubiales, D. Resistance to broomrape in wild lentils (Lens spp.). Plant Breed. 2009, 128, 266–270. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Sillero, J.C.; Rubiales, D. Resistance to broomrape species (Orobanche spp.) in common vetch (Vicia sativa L.). Crop Prot. 2009, 28, 7–12. [Google Scholar] [CrossRef]
- Eizenberg, H.; Hershenhorn, J.; Ephrath, J.H.; Kanampiu, F. Chemical control. In Parasitic Orobanchaceae; Joel, D.M., Gressel, J., Musselman, L.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 415–432. [Google Scholar]
- Cimmino, A.; Fernández-Aparicio, M.; Andolfi, A.; Basso, S.; Rubiales, D.; Evidente, A. Effect of fungal and plant metabolites on broomrapes (Orobanche and Phelipanche spp.) seed germination and radicle growth. J. Agric. Food Chem. 2014, 62, 10485–10492. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Cimmino, A.; Evidente, A.; Rubiales, D. Inhibition of Orobanche crenata seed germination and radicle growth by allelochemicals identified in cereals. J. Agric. Food Chem. 2013, 61, 9797–9803. [Google Scholar] [CrossRef] [Green Version]
- Dayan, F.E.; Duke, S.O. Natural compounds as next-generation herbicides. Plant Physiol. 2014, 166, 1090–1105. [Google Scholar] [CrossRef] [Green Version]
- Macías, F.A.; Molinillo, J.M.; Varela, R.M.; Galindo, J.C. Allelopathy—A natural alternative for weed control. Pest Manag. Sci. 2007, 63, 327–348. [Google Scholar] [CrossRef]
- Westwood, J.H.; Charudattan, R.; Duke, S.O.; Fennimore, S.A.; Marrone, P.; Slaughter, D.C.; Swanton, C.; Zollinger, R. Weed management in 2050: Perspectives on the future of weed science. Weed Sci. 2018, 66, 275–285. [Google Scholar] [CrossRef] [Green Version]
- Preston, R.E. Convolvulus. Jepson Flora Project. 2012. Available online: http://ucjeps.berkeley.edu/eflora/eflora_display.php?tid=11474 (accessed on 10 February 2022).
- Balicevic, R.; Ravlić, M.; Kneževic, M.; Serezlija, I. Allelopathic effect of field bindweed (Convolvulus arvensis L.) water extracts on germination and initial growth of maize. J. Anim. Plant Sci. 2014, 24, 1844–1848. [Google Scholar]
- Kazinczi, G.; Takacs, A.; Horvath, J. Crop-Weed Competition between sunflower (Helianthus annuus L.) and Convolvulus arvensis L. in substitutive experiments. Commun. Agric. Appl. Biol. Sci. 2006, 71, 781–786. [Google Scholar] [PubMed]
- Rahimzadeh, F.; Tobeh, A.; Jamaati-e-Somarin, S. Study of allelopathic effects of aqueous extracts of roots and seeds of goosefoot, red-root amaranth and field bindweed on germination and growth of lentil seedlings. Intl. J. Agron. Plant Prod. 2012, 3, 318–326. [Google Scholar]
- Shahrokhi, S.; Kheradmand, B.; Mehrpouyan, M.; Farboodi, M.; Akbarzaded, M. Effect of different concentrations of aqueous extract of bindweed, Convolvulus arvensis L. on initial growth of Abidar barley (Hordeum vulgare) cultivar in greenhouse. Int. Proc. Chem. Biol. Environ. Eng. 2011, 24, 474–478. [Google Scholar]
- Yarnia, M. Comparison of field bindweed (Convolvulus arvensis L.) and bermuda grass (Cynodon dactylon L.) organs residues on yield and yield components of bread wheat (Triticum aestivum L.). Adv. Environ. Biol. 2010, 4, 414–421. [Google Scholar]
- Om, H.; Dhiman, S.D.; Kumar, S.; Kumar, H. Allelopathic response of Phalaris minor to crop and weed plants in rice–wheat system. Crop Prot. 2002, 21, 699–705. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Cimmino, A.; Soriano, G.; Masi, M.; Vilariño, S.; Evidente, A. Assessment of weed root extracts for allelopathic activity against Orobanche and Phelipanche species. Phytopathol. Mediterr. 2021, 60, 455–466. [Google Scholar] [CrossRef]
- Tanaka, T.; Nakashima, T.; Ueda, T.; Tomii, K.; Kouno, I. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef] [Green Version]
- Fan, B.Y.; Lu, Y.; Yin, H.; He, Y.; Li, J.L.; Chen, G.T. Arvensic Acids A-D, novel heptasaccharide glycosidic acids as the alkaline hydrolysis products of crude resin glycosides from Convolvulus arvensis. Fitoterapia 2018, 131, 209–214. [Google Scholar] [CrossRef]
- Fan, B.Y.; He, Y.; Lu, Y.; Yang, M.; Zhu, Q.; Chen, G.T.; Li, J.L. Glycosidic acids with unusual aglycone units from Convolvulus arvensis. J. Nat. Prod. 2019, 82, 1593–1598. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; He, Y.; Yang, M.; Fan, B.Y. Arvensic Acids K and L, components of resin glycoside fraction from Convolvulus arvensis. Nat. Prod. Res. 2021, 35, 2303–2307. [Google Scholar] [CrossRef] [PubMed]
- Hegab, M.M.; Ghareib, H.R. Methanol extract potential of field bindweed (Convolvulus arvensis L.) for wheat growth enhancement. Int. J. Bot. 2010, 6, 334–342. [Google Scholar] [CrossRef] [Green Version]
- Lechat, M.M.; Pouvreau, J.B.; Péron, T.; Gauthier, M.; Montiel, G.; Véronési, C.; Todoroki, Y.; Le Bizec, B.; Monteau, F.; Macherel, D.; et al. PrCYP707A1, an ABA catabolic gene, is a key component of Phelipanche ramosa seed germination in response to the strigolactone analogue GR24. J. Exp. Bot. 2012, 63, 5311–5322. [Google Scholar] [CrossRef] [PubMed]
- Westwood, J.H.; Foy, C.L. Influence of nitrogen on germination and early development of broomrape (Orobanche spp.). Weed Sci. 1999, 47, 2–7. [Google Scholar] [CrossRef]
- Boulet, C.; Labrousse, P.; Arnaud, M.C.; Zehhar, N.; Fer, A. Weed species present various responses to Orobanche ramosa. In Proceedings of the Seventh International Parasitic Weed Symposium, Faculté des Sciences de Nantes, Nantes, France, 5–8 June 2001; pp. 228–231. [Google Scholar]
- Boulet, C.; Pineault, D.; Benharrat, H.; Simier, P.; Delavault, P. Adventices du colza et orobanche rameuse. Journée internationale sur la lutte contre les mauvaises herbes. In Proceedings of the AFPP-Vingtième Conférence du COLUMA, Dijon, France, 11–12 December 2007; pp. 326–345. [Google Scholar]
- León-Rivera, I.; Mirón-López, G.; Estrada-Soto, S.; Aguirre-Crespo, F.; Gutiérrez, M.; Molina-Salinas, G.M.; Hurtado, G.; Navarrete-Vázquez, G.; Montiel, E. Glycolipid ester-type heterodimers from Ipomoea tyrianthina and their pharmacological activity. Bioorg. Med. Chem. Lett. 2009, 19, 4652–4656. [Google Scholar] [CrossRef]
- León-Rivera, I.; Villeda-Hernández, J.; Campos-Peña, V.; Aguirre-Moreno, A.; Estrada-Soto, S.; Navarrete-Vázquez, G.; Rios, M.Y.; Aguilar-Guadarrama, B.; Castillo-España, P.; Rivera-Leyva, J.C. Evaluation of the neuroprotective activity of stansin 6, a resin glycoside from Ipomoea stans. Bioorg. Med. Chem. Lett. 2014, 24, 3541–3545. [Google Scholar] [CrossRef]
- Li, J.H.; Pan, J.T.; Yin, Y.Q. Two novel resin glycosides isolated from Ipomoea cairica with α-glucosidase inhibitory activity. Chin. J. Nat. Med. 2016, 14, 0227–0231. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soriano, G.; Fernández-Aparicio, M.; Masi, M.; Vilariño-Rodríguez, S.; Cimmino, A. Complex Mixture of Arvensic Acids Isolated from Convolvulus arvensis Roots Identified as Inhibitors of Radicle Growth of Broomrape Weeds. Agriculture 2022, 12, 585. https://doi.org/10.3390/agriculture12050585
Soriano G, Fernández-Aparicio M, Masi M, Vilariño-Rodríguez S, Cimmino A. Complex Mixture of Arvensic Acids Isolated from Convolvulus arvensis Roots Identified as Inhibitors of Radicle Growth of Broomrape Weeds. Agriculture. 2022; 12(5):585. https://doi.org/10.3390/agriculture12050585
Chicago/Turabian StyleSoriano, Gabriele, Mónica Fernández-Aparicio, Marco Masi, Susana Vilariño-Rodríguez, and Alessio Cimmino. 2022. "Complex Mixture of Arvensic Acids Isolated from Convolvulus arvensis Roots Identified as Inhibitors of Radicle Growth of Broomrape Weeds" Agriculture 12, no. 5: 585. https://doi.org/10.3390/agriculture12050585
APA StyleSoriano, G., Fernández-Aparicio, M., Masi, M., Vilariño-Rodríguez, S., & Cimmino, A. (2022). Complex Mixture of Arvensic Acids Isolated from Convolvulus arvensis Roots Identified as Inhibitors of Radicle Growth of Broomrape Weeds. Agriculture, 12(5), 585. https://doi.org/10.3390/agriculture12050585








