Abstract
The present study determines the chemical composition of Schinus molle essential oil and its mortality and repellent effect on Bactericera cockerelli immature stage and Sitophilus zeamais adults. Twenty-four compounds were identified and the most abundant were o-Cymene (29.04), 1R-α-Pinene (15.52), camphene (14.00), and β-myrcene (11.54). On the fifth-instar psyllid nymph, the LC50 and LC90 at 48 h were 442.67 and 864.29 ppm, and for the fourth-instar were 273.41 and 534.67 ppm. The maize-weevil registered an LC50 and LC90 of 343.25 and 986.96 ppm for the fifteenth day. A selection index (Si) of 0.37 with 800 ppm was registered, showing the highest repellent activity, while with the lowest concentration (50 ppm), non-repellent activity was recorded. However, all concentrations above 100 ppm showed repellency against the maize weevil. The study reveals, for the first time, the essential oil’s insecticidal effects on the fourth and fifth nymphal stage of the potato/tomato psyllid B. cockerelli and the usefulness of the essential oil as a repellent against adult of S. zeamais. The Si effect on maize weevil was grouped into categories.
1. Introduction
Food production must rise in the next twenty years to satisfy demand from the world’s growing population. The FAO [1] estimated that agriculture would have to produce almost 50% more food, fodder, and biofuel than it produced 10 years ago. A large number of pests have disturbed agriculture crop yield [2]. Meanwhile, food security is at risk with broad economic, social, and environmental consequences [3]. Crop damage caused by agricultural pests is a worldwide problem.
The tomato/potato psyllid Bactericera cockerelli (Sulc) (Hemiptera: Triozidae) is an important pest in Solanaceae crops [4,5], its presence in extreme cases can cause total losses [6,7]; this insect is distributed in Central and North American [8,9,10,11]. In Mexico, it is reported in the tomatoes/potatoes production areas of Villa de Arista, San Luis Potosí; Yurécuaro, Michoacán; La Laguna Region in the states of Durango and Coahuila; San Quentin, Baja California; Morelos; Puebla; Guanajuato; Nayarit; Sinaloa, and the State of Mexico [12]. B. cockerelli causes damage by infecting plants during feeding-sucking activities on leaves and stems, resulting in chlorosis and transmitting phytoplasmas-bacterial plant pathogens that can cause devastating yield losses of up to 80% [13,14,15]. The psyllid B. cockerelli is a vector of Candidatus Liberibacter (psyllaurous) solanacearum [6,16] and is a causal agent of permanent tomato disease [14], Zebra chip [17], and potato purple-tip [18]. The maize weevil Sitophilus zeamais (Motschulsky) (Coleoptera: Dryophthoridae) is an economically important insect in agricultural and stored grains; it is widespread worldwide and causes large economic losses [19,20,21,22,23]. Weevil infestations can cause reduced weight and quality of grain [24,25,26]. Deterioration of maize affects the moisture content, leading to fungi colonization, generating total or partial darkening of the germ or the entire grain heating. These affections cause biochemical changes and mycotoxins production that affects public health when maize is employed as food for animals or humans [21,27,28].
In Mexico, farmers depend on synthetic chemical insecticides but derived of their improper use, they have a negative influence on the natural environment [29,30,31]. For example, in Coahuila and San Luis Potosi State, farmers make twelve applications of thiacloprid and imidacloprid during the growing season for tomato/potato psyllid management, which generate multiple mechanisms of resistance of the insect [12]. For maize weevils’ management, they used phosphine [32] and methyl bromide [33], commonly used regardless of their inherent risks [34]. To minimize pesticide use, non-synthetic, friendly chemical strategies have been sought, profitable, and with low or no residual effects [35,36,37,38].
Plant-derived natural products are an alternative to synthetic chemical insecticides [35,36]. Plants synthesize secondary metabolites that are decisive in the resistance and defense against insects, the damage caused by herbivores, and infections caused by microorganisms, among others [39,40]. The main secondary metabolites belong to three chemical groups: phenolic compounds (coumarins, flavonoids, and tannins), nitrogen-containing compounds (alkaloids, mainly), and carbon and hydrogen compounds (terpenoids) [41,42,43,44]. Secondary metabolites have a wide range of biological activities [42]. They act by disrupting the cellular and physiological processes responsible for homeostasis, which, as a result, has an insect-static effect or leads to death. Other main effects studied on secondary metabolites include inhibition of feeding, interruption of development, reduction of fertility, deformations or malformations in consecutive generations, interference with vital enzyme activity, effect on the nervous system, blockage of metabolic pathways, behavioral effects, and reductions in insects’ population [45,46,47,48,49,50,51,52,53].
Schinus molle (Sessé & Moc.) (Anacardiaceae) is an aromatic plant species used for pest control; it is native to the Andean region of South America, mainly Peru, although it extends from Ecuador and Central America to Southern California and western Texas in the United States; it is a cosmopolitan plant widely distributed in Mexico, commonly known as “pirul” [54,55]. It also is used to treat disease and enhance general health and wellbeing [56]. The insecticide effect of leaves and fruit essential oil as a fumigant and repellent has been shown on Triatoma infestans (Olmedo & Carcavallo) (Hemiptera: Reduviidae) [41]. Its insecticide and attractive activity in rice weevil Sitophilus oryzae (L.) (Coleoptera: Curculionidae) have also been tested [57] and against the eucalyptus defoliator Gonipterus plantensis (Gyllenhaal) (Coleoptera: Curculionidae) [58].
Using “pirul” essential oil against crop pests has been demonstrated, suggesting the hypothesis that this plant contains secondary metabolites that can be used for the control of the tomato/potato psyllid and the maize weevil to protect stored grains. Therefore, the present study determines the chemical composition of “pirul” essential oil and its mortality and repellent effect on B. cockerelli immature stages and S. zeamais adults.
2. Materials and Methods
The experiment took place at the Laboratory of Applied Entomology (LAE) of the Center for Sustainable Development Studies and Wildlife Use (CEDESU), Autonomous University of Campeche (UAC), in Campeche, Mexico (19°48′05.48″ N, 90°30′16.23″ W).
2.1. Plant Material Collection
Freshly green collected samples of S. molle leaves were obtained from the Research Center for Integral Regional Development, Oaxaca Unit (CIIDIR-OAX) of the National Polytechnic Institute, in Santa Cruz Xoxocotlán, Oaxaca, Mexico (17°1′39.92″ N, 96°43′13.24″ W). The plant material was selected for aromatic properties, availability, and frequency of use among the inhabitants [59,60]. A sample specimen was deposited in the CIIDIR-OAX herbarium for future reference. The plant was washed with chlorinated water, placed on newspaper for drying undercover, and pulverized using a mechanical mill (Fritsch®, Pulverisette 19, Idar-Oberstein, Germany) with a variable speed of 300–3000 rpm.
2.2. Insect Mass Rearing
About 3500 unsexed adults and nymphs of B. cockerelli were collected from organic tomato crops in the municipality of Zimatlan de Alvarez, Oaxaca, Mexico (16°52′23.85″ N 96°46′31.07″ W). Nymphs and adults were placed in plastic containers with 5000 mL capacity and tomato plant leaves with moist cotton to prevent dehydration during transport to the laboratory. Subsequently, the organisms were placed in 8-week-old tomato plants for the oviposition of the psyllids inside 0.5 × 0.5 × 0.5 m entomological cages with anti-aphid mesh covers. Infested tomato plants (after 9 d) were shaken from the cage and moved to another cage (1.5 × 1.5 × 1.5 m), where the insects completed their development. Healthy tomato plants were incorporated to achieve new generations during the development of the bioassays.
Unsexed adults of maize weevil were obtained from infested corn kernels from farmers in San José de las Huertas, Ejutla de Crespo, Oaxaca, Mexico (16°33′54.0″ N, 96°42′24.9″ W). One hundred pairs of adult weevils were placed in 20 L plastic jars containing 2500 g of seeds. Subsequently, the jars were covered with screw caps with holes (2 cm) and a 40 mesh anti aphids net placed in a ventilated place. All adults were removed through sieving after the oviposition period of 14 d and each jar was left undisturbed under laboratory conditions until the F1 progeny emerged, which was used in the experiments [61].
Both colonies were kept in the LAE-CEDESU-UAC at 25 ± 1.5 °C, under a photocycle of 12:12 (L:D) h and relative humidity of 70 ± 10%. The specimens were identified and confirmed based on the keys described by Munyaneza [62], Munyaneza and Henne [63], and Haines [64].
2.3. Extraction of Essential Oil
The essential oil extraction was carried out in an aromatic water recirculation system using a Clevenger-type device adapted with a conventional microwave (Samsung MW1235WB, 1.1 ft3, 2450 MHz, Compton, CA, USA), using a 1:10 (p/v) ratio of plant material in water. The essential oil was quantified every 10 min (radiation time) and allowed to settle for 5 min. The essential oil layer was separated from the aqueous phase with a separating funnel and the oil obtained was dehydrated with anhydrous sodium sulfate (Na2SO4) and stored in an amber bottle at 4 °C for bioassays.
2.4. Identification of Volatile Compounds
The headspace solid-phase microextraction (HS-SPME) was used to identify volatile organic compounds. Fibers coated with polydimethylsiloxane of 100 µm were used. The fibers were conditioned before use by heating them in the chromatographic injector port, performing a complete execution on the system. A sample of 8 µL of essential oil was deposited in a 5 mL headspace vial and was enriched for 4 min on an SPME fiber. The analysis was performed on an Agilent HP 6890 with a 5973 GCMS System (Wilmington, DE, USA). The compounds were separated into an Agilent J&W HP-5ms capillary column (Wilmington, DE, USA). The enriched fiber was placed in the GC injector. The oven temperature was set at 40 to 250 °C with increasing intervals of 10 °C/min. The execution was completed in 10 min. The relative percentages of essential oil compounds were obtained using helium as a carrier gas with a flow rate of 1 mL/min. The compounds were identified by the GC retention time and the mass spectrum library of the National Institute of Standards and Technology (NIST) 02; also, the spectra were compared with those stored in the NIST 14 library.
2.5. Tomato/Potato Psyllid Mortality
The bioassay was performed individually for fourth and fifth instar nymphs using the standard leaf dip technique [65]. The bioassays were carried out with three repetitions using six concentrations obtained by serial dilution method of essential oil in 70% ethanol with Tween-20 (0.01% of polysorbate) to prepare a serial dilution of test concentrations. Serial dilutions at concentrations of 50, 100, 200, 400, 600, and 800 ppm were performed, a negative control (100 μL of 70% ethanol upon the leaves) and a positive control using Imidacloprid 35% (CONFIDOR 350 SC®, Bayer México, Mexico City, Mexico) (5 μL/cm2) was established [27,66,67]. Control groups were used to ensure that the results were because of the independent variable.
The leaves were immersed for 10 s in the different treatments (20 leaves per treatment) and were allowed to dry individually inside Petri dishes with filter paper (Whatman #1) at room temperature on paper moistened with distilled water. In each petri dish, groups of twenty individuals of fourth and fifth instar nymphs were placed individually on the leaves for each treatment. The nymphs were placed with an entomological brush. Data mortality was recorded at 24 and 48 h.
2.6. Mortality of Maize Weevil
Ten (10) grams of maize grains were used, which were placed in 9 cm diameter Petri dishes. The essential oil was diluted with 5 mL of ethanol to achieve test solutions at 50, 100, 200, 400, 600, and 800 ppm. In each Petri dish, 0.5 mL of the test solutions were applied until all grains were covered. After 20 min of evaporating the ethanol, 20 adults of 10–12 d old unsexed were placed [68]. The weevil mortality was recorded at 1, 5, 10, and 15 d after application. A negative control without application of essential oil and with 100 µL acetone was established [27,66,67] and piperonyl butoxide with deltamethrin as pyrethroid insecticide at 10 µL/cm2 (K-Obiol 2.5, Bayer Mexico) was used as a positive control. The mortality was expressed as the percentage concerning the dead or living insects found in the control group, where dead insects were those that did not show movement. The bioassays were carried out with four repetitions.
2.7. Maize Weevil Selection Index
The experiment was conducted using an apparatus comprising five circular plastic containers, with a central flask connected to the other four containers by plastic cylinders (10-cm long, 1-cm in diameter) on a diagonal [61,69,70,71]. Containers 1 and 2 were filled with 50 g of maize grain treated with essential oil, and containers 3 and 4 with 50 g of maize grain without treatment application (ethanol control), in container number 5 in the center of the device., 20 adults fasting for 24 h without sexing were released. A commercial repellent, N, N-diethyl-3-methylbenzamide (DEET), was used as a positive control. The total number of insects per container after 24 h were recorded, and calculated the Selection Index (Si) as proposed by Mazzonetto [72] and Mazzonetto and Vendramin [69]; Si = (2 × G)/(G + C), where Si is the selection index, C is the number of insects on the untreated containers (%), and G is the number of insects on the treated container (%).
The repellent/attraction effect (selection index) of essential oil on maize weevil were grouped into categories proposed by Arivoli et al. [73] with some modifications:
o—Neutral activity (Si = 1)
−—No-repellent activity (Si > 1.00)
+—Low repellent activity (0.75 ≥ Si ≥ 0.99)
++—Middle repellent activity (0.50 ≥ Si ≥ 0.74)
+++—High repellent activity (0.25 ≥ Si ≥ 0.49)
++++—Very high repellent activity (0.00 ≥ Si ≥ 0.24)
2.8. Statistical Analyzes
The bioassays were carried out individually under a completely randomized experimental design for each insect pest and variable studied. Data were tested for normality of errors (Shapiro–Wilk test) and homogeneity of variances (Bartlett test). One-way analysis of variance (ANOVA) and comparison of means were performed using Tukey’s test (p < 0.05) as a post hoc test performed with the help of Minitab version 18.1. Mortality data from each concentration assay were subjected to Probit analysis and LC50 and LC90 values were estimated [41,57,58]. Student’s t-test was used to compare significant differences between the means of the number of adult weevils in the treated and untreated maize grain. The data are presented in tables with the values of the means and standard deviation for the mortality and selection index variables, while the lethal concentrations are expressed as means and standard error of the mean with the fiducial confidence intervals (95.0%) to determine a range of values that meet reliability standards.
3. Results
3.1. Compounds Identification
The volatile compounds obtained through microwave-assisted hydrodistillation of powdered dried leaves of S. molle are shown in Table 1. By comparing the mass spectra of each compound with the data stored in the NIST 14 library, 24 chemical compounds representing 99.74% of their composition were identified. The most abundant compounds were o-Cymene (29.04), 1R-α-Pinene (15.52), camphene (14.00), and β-myrcene (11.54). Among the least present compounds, we have β-Pinene (8.17), α-Phellandrene (7.26), β-Phellandrene (5.39), and Tricyclene (4.19); the remaining compounds represent 4.63% of the total and their concentration ranges between 1.01 and 0.04% (Figure 1).

Table 1.
Compounds obtained from Schinus molle leaves essential oil analyzed by GC–MS.
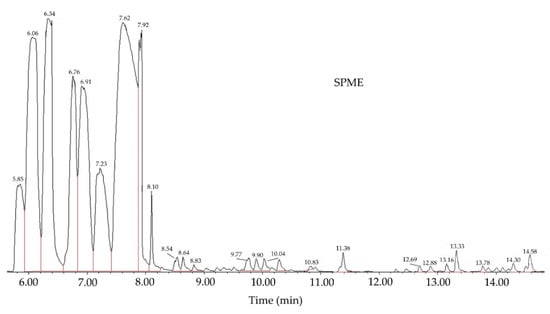
Figure 1.
Chromatogram of Schinus molle essential oil obtained by HS-SPME/GC-MS.
The yield of essential oil extracted from the leaves of S. molle by microwave-assisted microdistillation was 0.79% (1:10 w/v) with an isolation time of 30 min.
3.2. Tomato/Potato Psyllid Biological Response
Both instar-stages of tomato-potato psyllid were susceptible to the essential oil. There was a directly proportional relationship between treatment-concentrations and psyllid mortality. The highest concentration (800 ppm) on the fourth and fifth-instar nymph recorded mortality of 97.50 ± 2.89 (F = 393.87, df = 7, 24, p < 0.001, r2 = 0.9914) and 85.00 ± 4.08% (F = 384.13, df = 7, 24, p < 0.001, r2 = 0.9912) at 48 h; while with the lowest one (50 ppm) 7.50 ± 2.89 (F = 393.87, df = 7, 24, p < 0.001, r2 = 0.9914) and 5.00 ± 4.08% (F = 384.13, df = 7, 24, p < 0.001, r2 = 0.9912) mortality was registered (Table 2).

Table 2.
Mortality (%) of Bactericera cockerelli nymphal stages treated with Schinus molle essential oil.
On fifth-instar nymph, the LC50 and LC90 at 24 h were 523.81 ± 27.69 (473.31–583.95) and 1029.90 ± 63.01 ppm, and for 48 h were 442.67 ± 21.71 and 864.29 ± 45.09 ppm. The lethal concentrations LC50 and LC90 on fourth-instar nymph to 24 h were 329.43 ± 16.96 and 662.34 ± 31.08 ppm, while at 48 h were 273.41 ± 14.33 and 534.67 ± 25.63 ppm (Table 3).

Table 3.
Lethal concentrations and regression analysis of Bactericera cockerelli nymphal stages treated with Schinus molle essential oil.
3.3. Mortality of Maize Weevil
The highest concentration (800 ppm) for the tenth- and fifteenth-day registered mortality was 66.67 ± 5.77 (F = 64.90, df = 7, 16, p < 0.001, r2 = 0.966) and 80.00 ± 10.00% (F = 21.34, df = 7, 16, p < 0.001, r2 = 0.9033), while with the lowest one (50 ppm) on fifteenth day, 30.00 ± 10.00% mortality were achieved (Table 4).

Table 4.
Mortality (%) of the maize weevil Sitophilus zeamais treated with Schinus molle essential oil.
For the fifth day, low biological activity was recorded with an LC50 and LC90 of 781.49 ± 116.35 and 1641.29 ± 300.86 ppm, which increased gradually until the fifteenth day, in which was registered an LC50 and LC90 of 343.25 ± 46.46 and 986.96 ± 125.86. During this time frame, no egg-laying was observed (Table 5).

Table 5.
Lethal concentrations and regression analysis of Sitophilus zeamais adults treated with Schinus molle essential oil.
3.4. Maize Weevil Selection Index (Si)
The number of maize weevils on treated grains decreases while essential oil concentration increases. A Si of 0.37 ± 0.12 (F = 13.78, df = 7, 16, p < 0.001, r2 = 0.8577) with 800 ppm concentration were recorded denoting a high repellent activity (+++, 0.25 ≥ Si ≥ 0.49); while with the lowest one (50 ppm) a Si of 1.07 ± 0.15 (F = 18.77, df = 6, 14, p < 0.001, r2 = 0.8894) were registered, showing no-repellent activity, instead all essential oil concentrations above 100 ppm showed repellent-effect on the maize weevil (Table 6).

Table 6.
A selection index of Sitophilus zeamais on treated and untreated maize grain with the Schinus molle essential oil.
4. Discussion
Plants produce a vast array of secondary metabolites. These compounds have important ecological functions, protecting plants against insect attacks [74,75]. The exploration of bio-insecticide alternatives involves two aspects of the application: one is subsistence agriculture, which tries to seek independence of the farmer by giving pest-fighting alternatives through plants in the same environment; and the second is to seek new molecules with insecticidal properties that could be synthesized in laboratories [35,76]. For this reason, it is why the need arises to explore the chemical composition of plant essential oils employed by the population, as their appropriateness becomes safer and more reliable.
The essential oil chemical composition in our study differs in the number of founded compounds from other studies, such as those implemented by Doleski et al. [77] in Brazil with fresh leaves of “pirul”, in which they identified nineteen compounds represented by Bicyclogermacrene (20.50%), β-caryophyllene (19.70%), Spathulenol (19.20%), Globulol (9.50%), Germacrene-D (7.40%), Caryophyllene oxide (5.30%), and terpinen-4-ol (1,20%), while Machado et al. [78] and Benzi et al. [57] mentioned twenty-two compounds as Limonene (15.68%), α-Phellandrene (13.80%), Elemol (9.00%), β-Cubebene (7.30%), Camphene (5.31%), δ-Cadinene (5.26%), γ-Eudesmol (3.61%), α-Pinene (3.56%) y β-Eudesmol (2.80%). These differences may be because of several factors, such as the type of material used for extraction (fresh or dried leaves), phenological age of the plant, developmental and climatic conditions, and the cutting season of the leaves [79,80]. So, the differences between chemical compositions suggest different chemotypes, which become a direct factor in the efficiency of the essential oil in integrated pest management.
The efficacy of S. molle as a biopesticide has been shown in several studies, but in these, they used solvents of diverse polarities to obtain bioactive molecules. Huerta et al. [81] assessed the toxicity of ethanolic and aqueous extracts of fresh leaves from S. molle on adults of Xanthogaleruca luteola Müller (Coleoptera: Chrysomelidae), a pest of elms (Ulmus spp., Ulmaceae), with a ratio of 2.0 to 4.7% in weight/volume (w/v) with ethanol and 2.5 to 5.6% w/v with water, achieving of 73.6 to 100% and 15.3 to 27.8% mortality; similarly, ethanol and aqueous extracts from leaves of “pirul” have been evaluated on third-stage larvae of Gonipterus platensis (Marelli) (Coleoptera: Curculionidae) with 0.5 to 3.4% w/v, achieved mortality data between 47.10 to 100.00% [58]. The greatest insecticidal effect of S. molle essential oil has been reported on Haematobia irritans (Linneus) (Diptera: Muscidae), with 96% mortality [82].
Hussein et al. [83] demonstrated the effectiveness of the S. molle essential oil on Aphis nerii Boyer de Fonscolombe (Hemiptera: Aphididae) by calculating a lethal concentration of 1.0 mg/L and an inhibitory effect on the activity of acetylcholinesterase with 0.5 mg/L. Zahran et al. [84] report unfavorable results of the effectiveness of the essential oil in the larvae of Culex pipiens Linnaeus (Diptera: Culicidae), although, in adults, they report high toxicity with an LC50 of 2.45 mg/L compared to other essential oils within the same study.
De Batista et al. [85] demonstrated the efficacy of the S. molle, a non-polar extract and essential oil, on Ctenocephalides felis Bouché (Siphonaptera: Pulicidae), reaching up to 100% mortality at a concentration of 800 µg/cm2. The above-mentioned shows that the set of chemicals that make up this species, both in extract and essential oil, can have a synergistic effect since 100% effectiveness is achieved, compared to that found when individual metabolites are evaluated.
Some studies address the synergism of secondary plant compounds. Arceo-Medina et al. [86] evaluated the null acaricidal activity of the various compounds found in Petiveria alliacea Linnaeus (Caryophyllales: Petiveriaceae) against Rhipicephalus microplus Canestrini (Ixodida: Ixodidae), observing the null acaricidal activity of the compounds individually; while when combining these compounds exhibited a synergistic increase in activity, with a high mortality rate (≥92%) on larvae and adults. Likewise, it was found that when two compounds (Bornyl acetate and camphene) from Valeriana officinalis var. latifolia Linnaeus (Dipsacales: Caprifoliaceae) were mixed, a synergistic effect was presented, increasing the toxicity of stored grain beetles [87].
In this study, we found compounds showing their biological effectiveness against other organisms, even in low percentages. For example, Ávila et al. [88] found that δ-Cadinene has a positive effect against Spodoptera frugiperda Walker (Lepidoptera: Noctuidae), and it was more active (LC50 = 9.4 μL/L) when the essential oil got from Piper septuplinervium (Miq.) C. DC. (Piperaceae) leaves. Xiao et al. [89] showed similar results for Psoroptes cuniculi Delafond (Sarcoptiformes: Psoroptidae) management, showing 80% mortality after three hours of application. The above shows that it could involve this metabolite in the positive result shown in our study against B. cockerelli and S. zeamais.
Sesquiterpene potential has also been reported, such as the β-caryophyllene showing contact and fumigant activity against adults of Megoura japonica Matsumura (Hemiptera: Aphididae) recorded an LC50 of 0.072 µg/L and 8.82 mg/L [90]; likewise, De-Carvalho et al. [91] mentioned that β-caryophyllene and gurjinene founded in Cordia verbenacea DC. (Lamiales: Boraginaceae) have an acaricidal effect against R. microplus, another compound identified in our study. Therefore, it is fundamental to continue with individual compounds assessments to confirm isolated biological activity or synergism.
The repellent effect of S. molle was confirmed by Benzi et al. [57] were, who mentioned that ethanolic extract of leaves at 0.04 and 0.4% w/v had repellent effects (65.00 and 71.47%) on adults of S. oryzae Linnaeus (Coleoptera: Curculionidae), while the derivates of the fruit lacked repellent activity. In our study, we found promising results with a lower concentration of essential oil than those found by these authors, we can express the essential oil showed better activity than solvents extract because of the compound’s evaporation process during the solvent extraction in the rotary evaporator or natural conditions. The potentiality of the chemical constituents from “pirul” is clear, and for the moment, we prove the efficacy of this natural product on maize weevil adults and report for the first time the use of this essential oil for the management of the fourth and fifth nymphal stage of the potato/tomato psyllid. The fourth instar nymphs of the psyllid are more susceptible than the fifth instar nymphs. This may be because of the waxy secretions that envelop the most developed nymphs, protecting the insect from the penetration of the product [92].
Terpenoids mostly dominate essential oils, and their main mode of action against insects is neurotoxicity [93]. Rizvi et al. [94] mention that essential oil significantly inhibits acetylcholinesterase activity in insects; however, experiments on other pest organisms can be developed [95].
When terpenoids come into contact with insects, they slow down passaging food through the intestine, reducing digestibility by inhibiting the secretion of digestive enzymes (proteases) and forming conglomerates of terpenoids and proteins of low digestibility. Consequently, the insect presents physical weakening (starvation) and low growth and development rate. Coumarins are conjugated with the enzyme transaminase and cytochrome P450, thus inhibiting the insect detoxification system [96]. Likewise, monoterpenoids have hydrophilic-hydrophobic properties, penetrating insect cuticles and interfering with their physiological functions [97].
5. Conclusions
The study reveals for the first-time efficiency of Schinus molle essential oil insecticidal agents against two economic importance crops pests, on fourth and fifth instar nymphs of B. cockerelli and S. zeamais adults. Likewise, this plant-derived natural essential oil can be used as a repellent for the maize weevil; it is an efficient alternative for managing agricultural crop pests, being a feasible and ecological option for the environment.
For future perspectives, larger laboratory and field studies will be needed to investigate the “pirul” essential oil effects to cover the whole possible insects’ stages (egg, nymph/larva, and adult) and non-target organisms, likewise test for all possible insecticidal effect (toxicity, repellency or anti-feeding activity). The use of botanical insecticides will help the development of sustainable agriculture.
Author Contributions
Conceptualization, C.G.-E. and D.H.-G.; methodology, C.G.-E., N.A.-H., J.J.-P. and D.H.-G.; software, C.G.-E., N.A.-H. and J.J.-P.; validation, C.G.-E., N.A.-H., N.L.-V. and A.L.-C.; formal analysis, C.G.-E., N.A.-H., D.H.-G. and F.L.-V.; investigation, C.G.-E., N.A.-H. and J.J.-P.; resources, D.H.-G., J.A.-R., L.A.A.-P. and L.L.-R.; data curation, C.G.-E. and D.H.-G.; writing—original draft preparation, C.G.-E., D.H.-G., N.A.-H. and J.J.-P.; writing— review and editing, C.G.-E., D.H.-G., N.A.-H., N.L.-V., L.L.-R., F.L.-V., J.A.-R., A.L.-C., N.S.G.-D., J.A.-R. and L.A.A.-P.; supervision, C.G.-E. and D.H.-G.; project administration, C.G.-E.; funding acquisition, D.H.-G. and N.L.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the associated data are available in the manuscript.
Acknowledgments
We extend our gratitude to the National Polytechnic Institute (IPN) CIIDIR Oaxaca for their valuable support and the National Council of Science and Technology (CONACYT Mexico) for 270428 research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. The Future of Food and Agriculture: Trends and Challenges. Roma. Available online: https://www.fao.org/3/i6583e/i6583e.pdf (accessed on 13 January 2022).
- Manchikanti, P. Bioavailability and environmental safety of nanobiopesticides. In Nano-Biopesticides Today and Future Perspectives; Opender, K., Ed.; Academic Press: Jalandhar, India, 2019; pp. 207–222. [Google Scholar]
- Heeb, L.; Jenner, E.; Cock, M.J.W. Climate-smart pest management: Building resilience of farms and landscapes to changing pest threats. J. Pest Sci. 2019, 92, 951–969. [Google Scholar] [CrossRef]
- Greenway, G.A.; Rondon, S. Economic Impacts of Zebra Chip in Idaho, Oregon, and Washington. Am. J. Potato Res. 2018, 95, 362–367. [Google Scholar] [CrossRef]
- Haapalainen, M. Biology and epidemics of Candidatus Liberibacter species, psyllid transmitted plant-pathogenic bacteria. Ann. Appl. Biol. 2014, 165, 172–198. [Google Scholar] [CrossRef]
- Liefting, L.W.; Sutherlan, P.W.; Ward, L.I.; Paice, K.L.; Weir, B.S.; Clover, G.R.G. A new, ‘Candidatus Liberibacter’ species associated with diseases of solanaceous crops. Plant Dis. 2009, 93, 208–214. [Google Scholar] [CrossRef]
- Munyaneza, J.E. Zebra chip disease, Candidatus Liberibacter, and potato psyllid: A global threat to the potato industry. Am. J. Potato Res. 2015, 92, 230–235. [Google Scholar] [CrossRef]
- Abdullah, N.M.M. Life history of the potato psyllid Bactericera cockerelli (Homoptera: Psyllidae) in controlled environment agriculture in Arizona. Afr. J. Agric. Res. 2008, 3, 60–67. [Google Scholar]
- Davidson, M.M.; Teulon, D.A.J.; Scott, I.A.W.; Workman, P. A review of the potato psyllid (Bactericera cockerelli); a new pest of potato in New Zealand. Crop Food Res. 2022, 2008, 14. [Google Scholar]
- Swisher, K.; Sengoda, V.; Dixon, J.; Munyaneza, J.; Murphy, A.; Rondon, S.; Thompson, B.; Karasev, A.; Wenninger, E.; Olsen, N.; et al. Assessing potato psyllid haplotypes in potato crops in Pacific Northwestern United States. Am. J. Pot. Res. 2014, 91, 485–491. [Google Scholar] [CrossRef]
- Wenninger, E.J.; Carroll, A.; Dahan, J.; Karasev, A.V.; Thornton, M.; Miller, J.; Nolte, P.; Olsen, N.; Price, W. Phenology of the potato psyllid, Bactericera cockerelli (Hemiptera; Triozidae), and ‘Candidatus Liberibacter solanacearum’ in commercial potato fields in Idaho. Environ. Entomol. 2017, 46, 1179–1188. [Google Scholar] [CrossRef]
- Vega, G.M.T.; Rodríguez, M.J.C.; Díaz, G.R.O.; Bujanos, M.; Mota, S.D.; Martínez, C.J.L.; Lagunes, T.A.; Garzón, T.A. Susceptibilidad a insecticidas en dos poblaciones mexicanas del salerillo, Bactericera cockerelli (Sulc) (Hemiptera: Triozidae). Agrociencia 2008, 42, 463–471. (In Spanish) [Google Scholar]
- Almeyda, L.I.H.; Sánchez, J.A.; Garzón, T.J.A. Vectores causante de punta morada de la papa en Coahuila y Nuevo León, México. Agric. Tec. Mex. 2008, 34, 141–150. (In Spanish) [Google Scholar]
- Garzón-Tiznado, J.A.; Cárdenas-Valenzuela, O.G.; Bujanos-Muñiz, R.; Marín-Jarillo, A.; Becerra-Flora, A.; Velarde-Felix, S.; Reyes-Moreno, C.; González-Chavira, M.; Martínez-Carrillo, J.L. Asociación de Hemiptera: Triozidae con la enfermedad “Permanete del Tomate” en México. Agric. Tec. Mex. 2009, 35, 61–72. (In Spanish) [Google Scholar]
- Rivera-Martínez, R.; Acosta-Guadarrama, A.D.; Ramírez-Dávila, J.F.; Figueroa-Figueroa, D.K.; Maldonado-Zamora, F.I.; Lara-Díaz, A.V. Distribución espacial de las poblaciones de adultos de Bactericera cockerelli Sulc. en cultivo de tomate de cáscara (Physalis ixocarpa Brot.). South. Entomol. 2017, 42, 1057–1068. (In Spanish) [Google Scholar] [CrossRef]
- Villegas-Rodríguez, F.; Díaz-Gómez, O.; Casas-Flores, J.S.; Monreal-Vargas, C.T.; Tamayo-Mejía, F.; Aguilar-Medel, S. Actividad de dos hongos entomopatógenos, identificados molecularmente, sobre Bactericera cockerelli. Rev. Colomb. Entomol. 2017, 43, 27–33. (In Spanish) [Google Scholar] [CrossRef]
- Munyaneza, J.E.; Crosslin, J.M.; Upton, J.E. Association of Bactericera cockerelli (Homoptera: Psyllidae) with “Zebra Chip”, a new potato disease in southwestern United States and Mexico. J. Econ. Entomol. 2007, 100, 656–663. [Google Scholar] [CrossRef]
- Salas-Marina, M.A. Eficiencia de Insectos Vectores en la Transmisión de Fitoplasma de la Punta Morada de la Papa. Master’s Thesis, UAAAN, Coahuila, Mexico, 2006. (In Spanish). [Google Scholar]
- Silva, G.; Lagunes, A.; Rodríguez, J. Control de Sitophilus zeamais (Coleoptera: Curculionidae) con polvos vegetales solos y en mezcla con carbonato de calcio en maíz almacenado. Cienc. Investig. Agrar. 2003, 30, 153–160. (In Spanish) [Google Scholar] [CrossRef]
- Ortiz, U.A.; Silva, A.G.; Urbina, P.A.; Zapata, Z.M.N.; Rodríguez, M.C.; Lagunes, T.A. Bioactivity of tepa (Laureliopsis philippiana (Looser) Shodde) powder to Sitophilus zeamais Motschuslky control in laboratory. Chil. J. Agric. Res. 2012, 72, 68–73. [Google Scholar] [CrossRef]
- Suleiman, R.A.; Rosentrater, K.A.; Bern, C.J. Effects of Deterioration Parameters on Storage of Maize: An ASABE Meeting Presentation; Paper No. 131593351; Iowa State University: Kansas City, MO, USA, 2013. [Google Scholar]
- Mbata, G.N.; Ivey, C.; Shapiro-Ilan, D. The potential for using entomopathogenic nematodes and fungi in the management of the maize weevil, Sitophilus zeamais (Motschulsky) (Coleoptera: Curculionidae). Biol. Control. 2018, 125, 39–43. [Google Scholar] [CrossRef]
- Bohinc, T.; Horvat, A.; Andric, G.; Prazic Golic, M.; Kljajic, P.; Trdan, S. Comparison of three different wood ashes and diatomaceous earth in controlling the maize weevil under laboratory conditions. J. Stored Prod. Res. 2018, 79, 1–8. [Google Scholar] [CrossRef]
- García-Lara, S.; Espinosa, C.C.; Bergvinson, D. Manual de Plagas en Granos Almacenados y Tecnologías Alternas Para su Manejo y Control; CIMMYT: Mexico City, Mexico, 2007. (In Spanish) [Google Scholar]
- Adebayo, O.J.; Omoloye, A.O.A. Rearing the maize weevil, Sitophilus zeamais, on an artificial maize–cassava diet. J. Insect Sci. 2012, 12, 69. [Google Scholar]
- Abdullahi, N.; Umar, I.; Tukur, Z.; Babura, S.R. Comparative efficacy of the bark and root powders of Acacia nilotica against maize weevil Sitophilus zeamais (Motsculsky) (Coleoptera: Curculionidae) in Kano State of Nigeria. Afr. J. Agric. Res. 2014, 9, 588–592. [Google Scholar]
- González, H.B.; Armstrong, P.R.; Maghirang, R.G. Simultaneous monitoring of stored grain with relative humidity, temperature, and carbon dioxide sensors. Appl. Eng. Agric. 2009, 25, 595–604. [Google Scholar] [CrossRef]
- Lawrence, J.; Maier, D.E. Aeration strategy simulations for wheat storage in the subtropical region of north India. Trans. ASABE 2011, 54, 1395–1405. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, I.; Imadi, S.R.; Shazadi, K.; Gul, A.; Hakeem, K.R. Effects of Pesticides on Environment. In Plant, Soil and Microbes; Hakeem, K., Akhtar, M., Abdullah, S., Eds.; Springer International: Cham, Switzerland, 2016; pp. 253–269. [Google Scholar]
- Hassaan, M.A.; Nemr, A.E. Pesticides pollution: Classifications, human health impact, extraction and treatment techniques. Egypt. J. Aquat. Res. 2020, 46, 207–220. [Google Scholar] [CrossRef]
- Boyer, S.; Zhang, H.; Lemperiere, G. A review of control methods and resistance mechanisms in stored-product insects. Bull. Entomol. Res. 2012, 102, 213–229. [Google Scholar] [CrossRef]
- Bell, C. Fumigation in the 21st century. Crop Prot. 2000, 19, 563–569. [Google Scholar] [CrossRef]
- Negatu, B.; Kromhout, H.; Mekonnen, Y.; Vermeulen, R. Use of Chemical Pesticides in Ethiopia: A cross-sectional comparative study on Knowledge, Attitude and Practice of farmers and farm workers in three farming systems. Ann. Occup. Hyg. 2016, 60, 551–566. [Google Scholar] [CrossRef]
- Silva, G.; Lagunes, A.; Rodríguez, J.; Rodríguez, D. Insecticidas vegetales: Una vieja y nueva alternativa para el manejo de plagas. Manejo Integr. Plagas. Agroecol. 2002, 66, 4–12. (In Spanish) [Google Scholar]
- Tofel, K.H.; Kosma, P.; Stahler, M.; Adler, C.; Nukenine, E.N. Insecticidal products from Azadirachta indica and Plectranthus glandulosus growing in Cameroon for the protection of stored cowpea and maize against their major insect pests. Ind. Crop. Prod. 2017, 110, 58–64. [Google Scholar] [CrossRef]
- Arthur, F.H. Structural pest management for stored product insects. In Recent Advances in Stored Product Protection; Athanassiou, C.G., Arthur, F.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 65–81. [Google Scholar]
- Athanassiou, C.G.; Arthur, F.H. Bacterial insecticides and inert material. In Recent Advances in Stored Product Protection; Athanassiou, C.G., Arthur, F.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 83–98. [Google Scholar]
- Montesino, M. Insecticidas botánicos como alternativa para el manejo de plagas en sistemas agroforestales. Agric. Org. 2009, 1, 24–26. (In Spanish) [Google Scholar]
- Duplais, C.; Papon, N.; Courdavault, V. Tracking the Origin and Evolution of Plant Metabolites. Trends Plant Sci. 2020, 25, 1182–1184. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, A.A.; Werdin-González, J.O.; Sánchez-Chopa, C. Biological activity of Schinus molle on Triatoma infestans. Fitoterapia 2006, 77, 381–383. [Google Scholar] [CrossRef] [PubMed]
- Chowański, S.; Adamski, Z.; Marciniak, P.; Rosiński, G.; Büyükgüzel, E.; Büyükgüzel, K.; Falabella, P.; Scrano, L.; Ventrella, E.; Lelario, F.; et al. A Review of Bioinsecticidal Activity of Solanaceae Alkaloids. Toxins 2016, 8, 60. [Google Scholar] [CrossRef]
- Hanson, J.R. Natural Products: The Secondary Metabolites; The Royal Society of Chemistry: Cambridge, UK, 2003; pp. 1–148. [Google Scholar]
- Kuete, V. Bioactivity of Plant Constituents against Vancomycin-Resistant Enterococci. In Fighting Multidrug Resistance with Herbal Extracts; Rai, M.K., Kon, K.V., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 23–30. [Google Scholar]
- Wink, M. Interference of alkaloids with neuroreceptors and ion channels. Stud. Nat. Prod. Chem. 2000, 21, 3–122. [Google Scholar]
- Adeyemi, M.M.H. The potential of secondary metabolites in plant material as deterrents against insect pests: A review. Afr. J. Pure Appl. Chem. 2010, 4, 243–246. [Google Scholar]
- López, M.D.; Pascual-Villalobos, M.J. Mode of inhibition of acetylcholinesterase by monoterpenoids and implications for pest control. Ind. Crop. Prod. 2010, 31, 284–288. [Google Scholar] [CrossRef]
- Rattan, R.S. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot. 2010, 29, 913–920. [Google Scholar] [CrossRef]
- Wink, M. Evolution of secondary metabolites in legumes (Fabaceae). S. Afr. J. Bot. 2013, 89, 164–175. [Google Scholar] [CrossRef]
- Nenaah, G.E. Chemical composition, toxicity and growth inhibitory activities of essential oils of three Achillea species and their nano-emulsions against Tribolium castaneum (Herbst). Ind. Crop. Prod. 2014, 53, 252–260. [Google Scholar] [CrossRef]
- Teichert, I.; Nowrousian, M.; Pöggeler, S.; Kück, U. The filamentous fungus Sordaria macrospora as a genetic model to study fruiting body development. Adv. Genet. 2014, 87, 199–244. [Google Scholar] [PubMed]
- Maazoun, A.M.; Hlel, T.B.; Hamdi, S.H.; Belhadj, F.; Jemâa, J.M.B.; Marzouki, M.N. Screening for insecticidal potential and acetylcholinesterase activity inhibition of Urginea maritima bulbs extract for the control of Sitophilus oryzae (L.). J. Asia-Pac. Entomol. 2017, 20, 752–760. [Google Scholar] [CrossRef]
- Rosado-Aguilar, J.A.; Arjona-Cambranes, K.; Torres-Acosta, J.F.J.; Rodríguez-Vivas, R.I.; Bolio-González, M.E.; Ortega-Pacheco, A.; Alzina-López, A.; Gutiérrez-Ruiz, E.J.; Gutiérrez-Blanco, E.; Aguilar-Caballero, A.J. Plant products and secondary metabolites with acaricide activity against ticks. Vet. Parasitol. 2017, 238, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Zunino, M.P.; López, M.L.; Zygadlo, J.A. Medicinal plants of Argentina. Pharmacological properties and phytochemistry. In Advances in Phytochemisty; Imperato, F., Ed.; Research Singpost: Trivandrum, India, 2003; pp. 209–245. [Google Scholar]
- Viturro, C.; Bandoni, A.; Dellacassa, E.; Serafini, L.A.; Elder, H. Problemática Schinus en Latinoamérica. In Normalización de Productos Naturales Obtenidos de Especies de la Flora Aromática Latinoamericana: Proyecto CYTED IV.20E; Dellacassa, Ed.; EDIPUCRS: Porto Alegre, Brazil, 2010; pp. 205–280. [Google Scholar]
- Ruffa, M.J.; Ferraro, G.; Wagner, M.L.; Calcagno, M.L.; Campos, R.H.; Cavallaro, L. Cytotoxic effect of argentine medicinal plant extracts on human hepatocellular carcinoma cell line. J. Ethnopharmacol. 2002, 79, 335–339. [Google Scholar] [CrossRef]
- Benzi, V.; Stefanazzi, N.; Ferrero, A.A. Biological activity of essential oils from leaves and fruits of pepper tree (Schinus molle L.) to control rice weevil (Sitophilus oryzae L.). Chil. J. Agric. Res. 2009, 69, 154–159. [Google Scholar] [CrossRef]
- Chiffelle, I.; Huerta, A.; Sandoval, C.A.; Araya, J.E. Insecticide effect of leaf extracts from Schinus molle on larva of Gonipterus platensis. Rev. Fac. Nac. Agron. Medellín 2017, 70, 8263–8270. [Google Scholar]
- Bouchra, C.; Achouri, M.; Idrissi-Hassani, L.; Hmamouchi, M. Chemical composition and antifungal activity of essential oils of seven Moroccan Labiatae against Botrytis cinerea Pers. Fr. J. Ethnopharmacol. 2003, 89, 165–169. [Google Scholar] [CrossRef]
- Ansari, N.D.; Hasanzadeh, N.; Bagher, M.R.; Ghasemi, A. Antibacterial Activity and Chemical Compositions of Chamaemelum nobile Essential Oil/Extracts against Pseudomonas tolaasii, the Causative Agent of Mushroom Brown Blotch. Annu. Biol. Res. 2012, 3, 2602–2608. [Google Scholar]
- Hernández-Cruz, J.; Luna-Cruz, A.; Loera-Alvarado, E.; Villanueva-Sánchez, E.; Landero-Valenzuela, N.; Zárate-Nicolás, B.H.; Diego-Nava, F.; Granados-Echegoyen, C.A. Effiiency of the Essential Oil of Porophyllum linaria (Asteraceae) a Mexican Endemic Plant Against Sitophilus zeamais (Coleoptera: Curculionidae). J. Insect Sci. 2019, 6, iez079. [Google Scholar] [CrossRef]
- Munyaneza, J.E. Zebra chip disease of potato: Biology, epidemiology, and management. Am. J. Potato Res. 2012, 89, 329–350. [Google Scholar] [CrossRef]
- Munyaneza, J.E.; Henne, D.C. Leafhopper and psyllid pests of potato. In Insect Pests of Potato: Global Perspectives on Biology and Management; Giordanengo, P., Vincent, C., Alyokhin, A., Eds.; Academic Press: San Diego, CA, USA, 2012; pp. 65–102. [Google Scholar]
- Haines, C.P. Insects and arachnids of tropical stored products: Their biology and identification. In A Training Manual, 2nd ed.; Haines, C.P., Ed.; Natal University Press: Pietermaritzburg, South Africa; Natural Resources Institute: Kent, UK, 1991. [Google Scholar]
- Akhtara, Y.; Murray, B.I.; Chi-Hoon, L.; Aang-Guei, L.; Hoi-Seon, L. Toxicity of quinones against two-spotted spider mite and three species of aphids in laboratory and greenhouse conditions. Ind. Crop. Prod. 2012, 37, 536–541. [Google Scholar] [CrossRef]
- Lagunes, T.A.; Rodríguez, C. Búsqueda de Tecnología Apropiada Para el Combate de Plagas del Maíz Almacenado en Condiciones Rústicas; CONACYT/Colegio de Postgraduados: Texcoco, México, 1989; p. 150. (In Spanish) [Google Scholar]
- Oliveira, T.A.; Ronchi-Teles, B.; Fonseca, C.R.V.; Silva, S.L.R.; Santos, P.A.; Núñez, C.V. Insecticidal activity of Vitex cymosa (Lamiaceae) and Eschweilera pedicellata (Lecythidaceae) extracts against Sitophilus zeamais adults (Curculionidae). Emir. J. Food Agric. 2012, 24, 49–56. [Google Scholar] [CrossRef]
- Fazolin, M.; Estrela, J.L.V.; Catani, V.; Alécio, M.; De Lima, M.S. Atividade inseticida do óleo essencial de Tanaecium nocturnum (Barb. Rodr.) Bur. and K. Shum (Bignoneaceae) sobre Sitophilus zeamais Motsch. (Coleoptera: Curculionidae). Acta Amazon. 2007, 37, 599–604. [Google Scholar] [CrossRef]
- Mazzonetto, F.; Vendramin, J.D. Efeito de pos de origem vegetal sobre Acanthoscelides obtectus (Say) (Coleoptera: Bruchidae) em feijao armazenado. Neotrop. Entomol. 2003, 31, 145–149. [Google Scholar] [CrossRef]
- Fouad, H.A.; Faroni, L.R.D.; Ribeiro, R.C.; Tavares, W.D.S.; Petacci, F. Extraction and repellent activity of Lepidoploa aurea and Memora nodosa against stored grain and by product pests. Vie. Milieu. Life. Environ. 2012, 62, 11–15. [Google Scholar]
- Viteri-Jumbo, L.O.; Faroni, L.R.A.; Oliveira, E.E.; Pimentel, M.A.; Silva, G.N. Potential use of clove and cinnamon essential oils to control the bean weevil, Acanthoscelides obtectus Say, in small storage units. Ind. Crop. Prod. 2014, 56, 27–34. [Google Scholar] [CrossRef]
- Mazzonetto, F. Efecto de Genótipos de Feijoeiro e de pós de Origen Vegetal Sobre Zabrotes subfasciatus (Boh.) e Acanthoscelides obtectus (Say) (Col.: Bruchidae). Piracicaba, 134. Ph.D. Thesis, Universidad de Sao Paulo, São Paulo, Brazil, 2002. [Google Scholar]
- Arivoli, S.; Tennyson, S. Ovicidal activity of plant extract against Spodoptera litura (Fab.) (Lepidoptera: Noctuidae). Bull. Environ. Pharmacol. Life Sci. 2013, 2, 140–145. [Google Scholar]
- Elumalai, K.; Mahboob, S.; Al-Ghanim, K.A.; Al-Misned, F.J.; Pandiyan, P.M.; Baabu, K.; Krishnappa, K.; Govindarajan, M. Entomofaunal survey and larvicidal activity of greener silver nanoparticles: A perspective for novel eco-friendly mosquito control. Saudi J. Biol. Sci. 2020, 27, 2917–2928. [Google Scholar] [CrossRef]
- Azeem, M.; Zaman, T.; Abasi, M.A.; Abid, M.; Mozuratis, R.; Alwahibi, S.M.; Elshikh, S.M. Pesticidal potential of some wild plant essential oils against grain pests Tribolium castaneum (Herbst, 1797) and Aspergillus flavus (Link, 1809). Arab. J. Chem. 2022, 15, 103482. [Google Scholar] [CrossRef]
- Diemer, N.; Staudacher, P.; Atuhaire, A.; Fuhrimann, S.; Inauen, J. Smallholder farmers’ information behavior differs for organic versus conventional pest management strategies: A qualitative study in Uganda. J. Clean. Prod. 2020, 257, 120. [Google Scholar] [CrossRef]
- Doleski, M.P.S.; Ferreira, C.C.H.; Calil, B.J.; Palermo, M.M. Chemical composition of the Schinus molle L. essential oil and their biological activities. Rev. Cuba. Farm. 2015, 49, 132–143. [Google Scholar]
- Machado, D.C.; Rama, V.; Rehman, U.J.; Maia, S.N.L.H.B.; Meneghetti, K.E.; Almeida, P.V.; Silva, Z.R.; Farago, V.P.; Khan, A.I. Schinus molle: Anatomy of leaves and stems, chemical composition and insecticidal activities of volatile oil against bed bug (Cimex lectularius). Braz. J. Pharmacogn. 2019, 20, 1–10. [Google Scholar] [CrossRef]
- Yang, L.; Kui-Shan, W.; Xiao, R.; Ying-Xian, Z.; Feng, W.; Qiang, W. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Hayasundara, N.D.B.; Arampath, P. Effect of variety, location & maturity stage at harvesting, on essential oil chemical composition, and weight yield of Zingiber officinale roscoe grown in Sri Lanka. Heliyon 2021, 7, 06560. [Google Scholar]
- Huerta, A.; Chiffelle, I.; Puga, K.; Azúa, F.; Araya, J. Toxicity and repellence of aqueous and ethanolic extracts from Schinus molle on elm leaf beetle Xanthogaleruca luteola. Crop Prot. 2010, 29, 1118–1123. [Google Scholar] [CrossRef]
- López, A.; Castro, S.; Andina, J.M.; Ures, X.; Munguía, B.; Llabot, M.J.; Elder, H.; Dellacassa, E.; Palma, S.; Domínguez, L. Insecticidal activity of microencapsulated Schinus molle essential oil. Ind. Crop. Prod. 2014, 53, 209–216. [Google Scholar] [CrossRef]
- Hussein, H.S.; Tawfeek, M.E.; Abdelgaleil, S.A.M. Chemical composition, aphicidal and antiacetilcholinesterase activities of essential oils against Aphis nerii Boyer de Fonscolombe. J. Asia-Pac. Entomol. 2021, 24, 259–265. [Google Scholar] [CrossRef]
- Zahran, H.E.D.; Abou-Taleb, H.K.; Abdegaleil, S.A.M. Adulticidal, larvicidal and biochemical properties of essential oils against Culex pipiens L. J. Asia-Pac. Entomol. 2017, 20, 133–139. [Google Scholar] [CrossRef]
- De Batista, C.L.; Cid, P.Y.; De Almeida, P.A.; Prudencio, E.R.; Riger, J.C.; De Souza, A.A.M.; Coumendouros, K.; Chaves, A.S.D. In vitro efficacy of essential oils and extracts of Schinus molle L. against Ctenocephalides felis felis. Parasitology 2016, 143, 627–638. [Google Scholar] [CrossRef]
- Arceo-Medina, G.N.; Rosado-Aguilar, J.A.; Rodríguez-Vivas, R.I.; Borgez-Argaez, R. Sinergystic and antagonistic action of fatty acids: Sulphides and stilbenes against acaricide-resistant Ripicephalus microplus ticks. Vet. Parasitol. 2016, 228, 121–125. [Google Scholar] [CrossRef]
- Feng, Y.X.; Wang, Y.; Geng, Z.F.; Zhang, D.; Almaz, B.; Du, S.S. Contact toxicity and repellent efficacy of valerianaceae spp. to three stored-product insects and synergistic interactions between two major compounds camphene and bornyl acetato. Ecotoxicol. Environ. Saf. 2020, 190, 110106. [Google Scholar] [CrossRef]
- Ávila, M.M.C.; Cuca, S.L.E.; Cerón, S.J.A. Chemical composition and insecticidal properties of essential oils of Piper septuplinervium and P. subtomentosum (Piperaceae). Nat. Prod. Commun. 2014, 9, 1527–1530. [Google Scholar]
- Xiao, G.; Xiaofei, S.; Bing, L.; Xu, Z.Z.; Hao, W.; Jiyu, Z. Acaricidal activities of the essential oil from Rhododendron nivale Hook. f. and its main compound, cadinene against Psoroptes cuniculi. Vet. Parasitol. 2017, 236, 51–54. [Google Scholar]
- Shujie, M.; Ran, J.; Menglei, G.; Kaitao, Q.; Lihui, Z. Insecticidal activity of essential oil from Cephalotaxus sinensis and its main components against various agricultural pests. Ind. Crop. Prod. 2020, 150, 112403. [Google Scholar]
- De-Carvalho, C.K.N.; Costa, J.L.M.; Fernandes, L.D.; Marques, C.K.; Souza, D.E.; Moreira, D.I.; Sérgio, T.M.; Oiram, F.F.; Cardoso, D.R.; Joseph, M.S. Acaricidal activity of cashew nut shell liquid associated with essential oils from Cordia verbenacea and Psidium guajava on Rhipicephalus microplus. J. Essent. Oil Res. 2019, 31, 297–304. [Google Scholar] [CrossRef]
- Garrido, A.; Castañer, M.; Del Busto, T.; Malagon, J. Toxicidad de diversos plaguicidas sobre los estados inmaduros de Aleurothrixus fioccosus (Mask.) e incidencia sobre el insencto útil Cales noacki How. Bol. San Veg. Plagas 1990, 16, 173–181. (In Spanish) [Google Scholar]
- Yeom, H.-J.; Kang, J.S.; Kim, G.-H.; Park, I.-K. Insecticidal and acetylcholine esterase inhibition activity of Apiaceae plant essential oils and their constituents against adults of German cockroach (Blattella germanica). J. Agric. Food Chem. 2012, 60, 7194–7203. [Google Scholar] [CrossRef]
- Rizvi, S.A.H.; Ling, S.; Tian, F.; Xie, F.; Zeng, X. Toxicity and enzyme inhibition activities of the essential oil and dominant constituents derived from Artemisia absinthium L. against adult Asian citrus psyllid Diaphorina citri Kuwayama (Hemiptera: Psyllidae). Ind. Crop. Prod. 2018, 121, 468–475. [Google Scholar] [CrossRef]
- Pavela, R.; Maggi, F.; Giordani, C.; Cappellacci, L.; Petrelli, R.; Canale, A. Insecticidal activity of two essential oils used in perfumery (ylang ylang and frankincense). Nat. Prod. Res. 2020, 35, 4746–4752. [Google Scholar] [CrossRef]
- Wen, Z.; Zeng, R.S.; Niu, G.; Berenbaum, M.R.; Schuler, M.A. Ecological significance of induction of broad-substrate cytochrome P450s by natural and synthetic inducers in Helicoverpa zea. J. Chem. Ecol. 2009, 35, 183–189. [Google Scholar] [CrossRef]
- Tak, J.H.; Jovel, E.; Isman, M.B. Comparative and synergistic activity of Rosmarinus officinalis L. essential oil constituents against the larvae and an ovarian cell line of the cabbage looper, Trichoplusia ni (Lepidoptera: Noctuidae). Pest. Manag. Sci. 2016, 72, 474–480. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).