Deep Soil Water Content and Forage Production in a Tropical Agroforestry System
Abstract
:1. Introduction
2. Materials and Methods
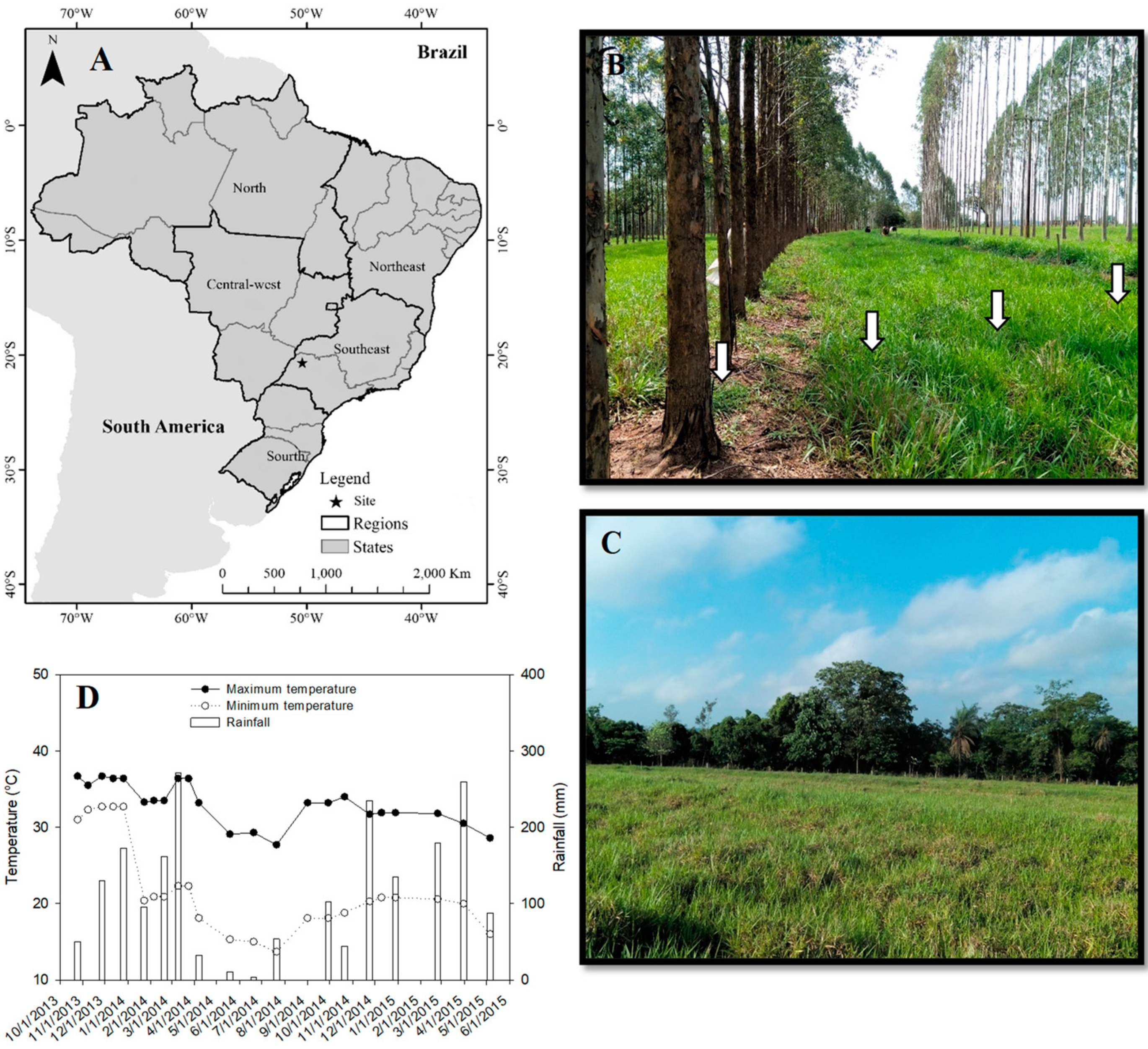
2.1. Characterization of the Experimental Area
2.2. Experimental Design and Treatments
2.3. Site History
2.4. Soil Water Content
2.5. Forage Production
2.6. Statistical Analysis
3. Results
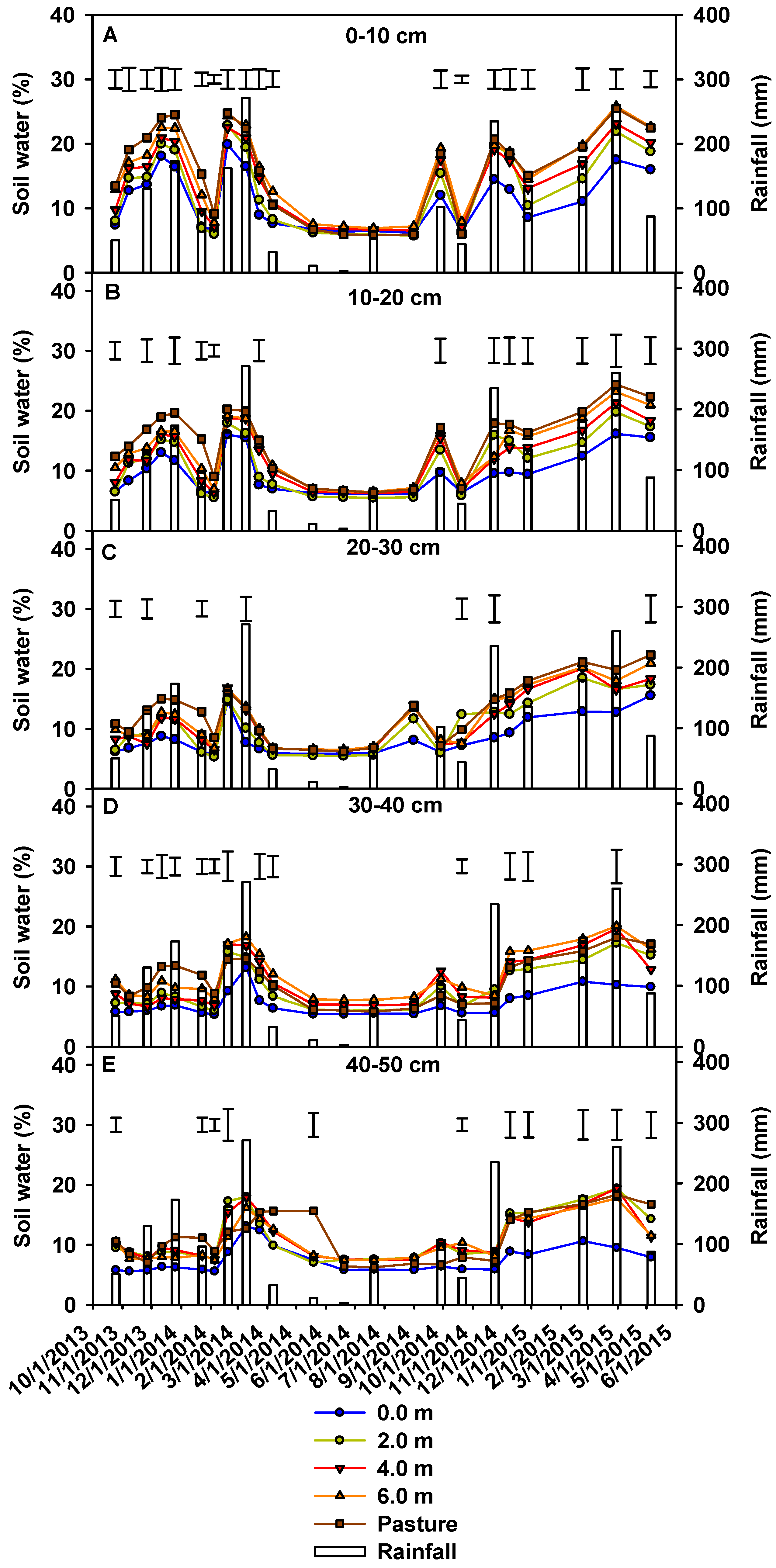
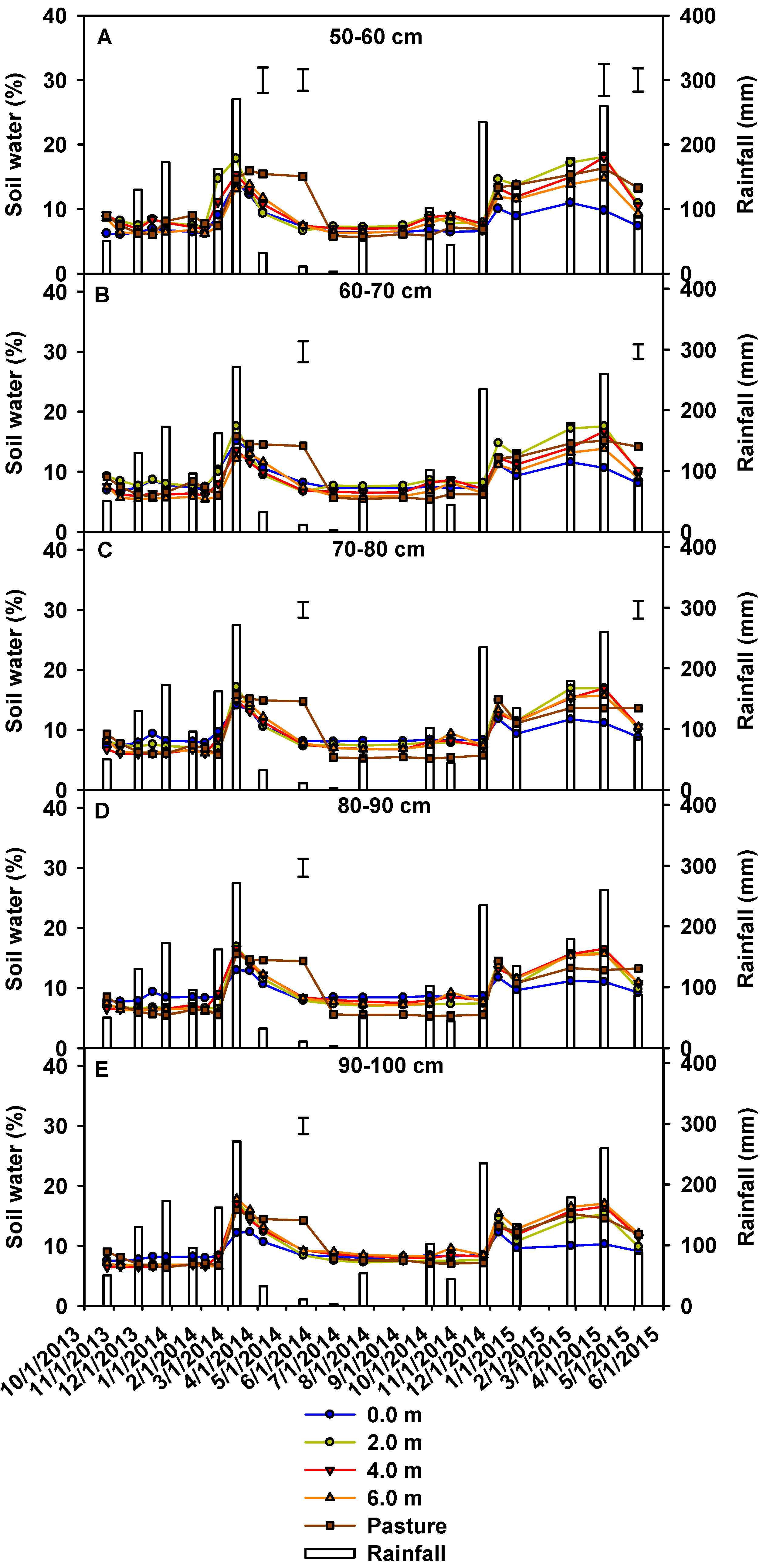
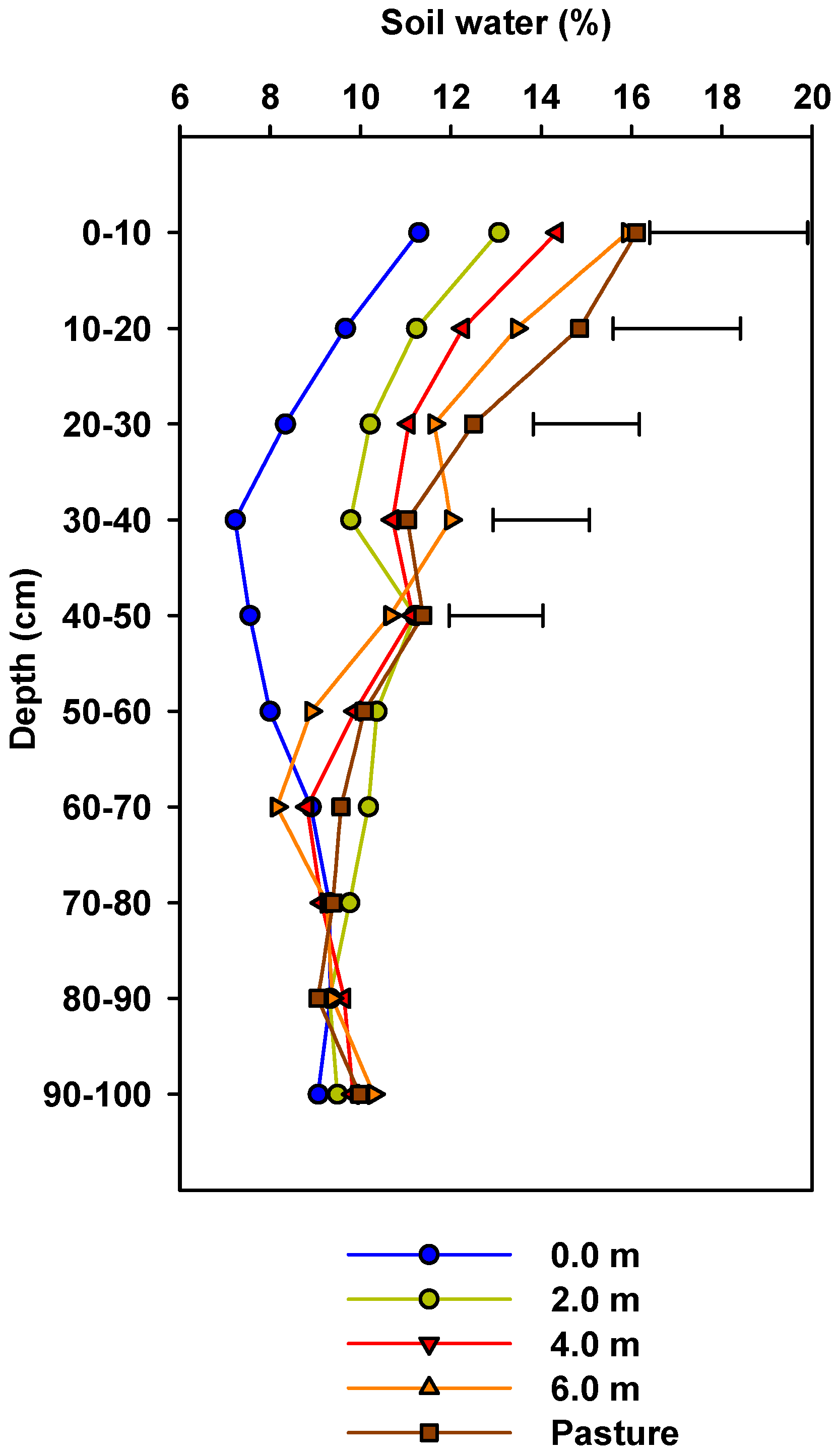
3.1. Soil Water Content
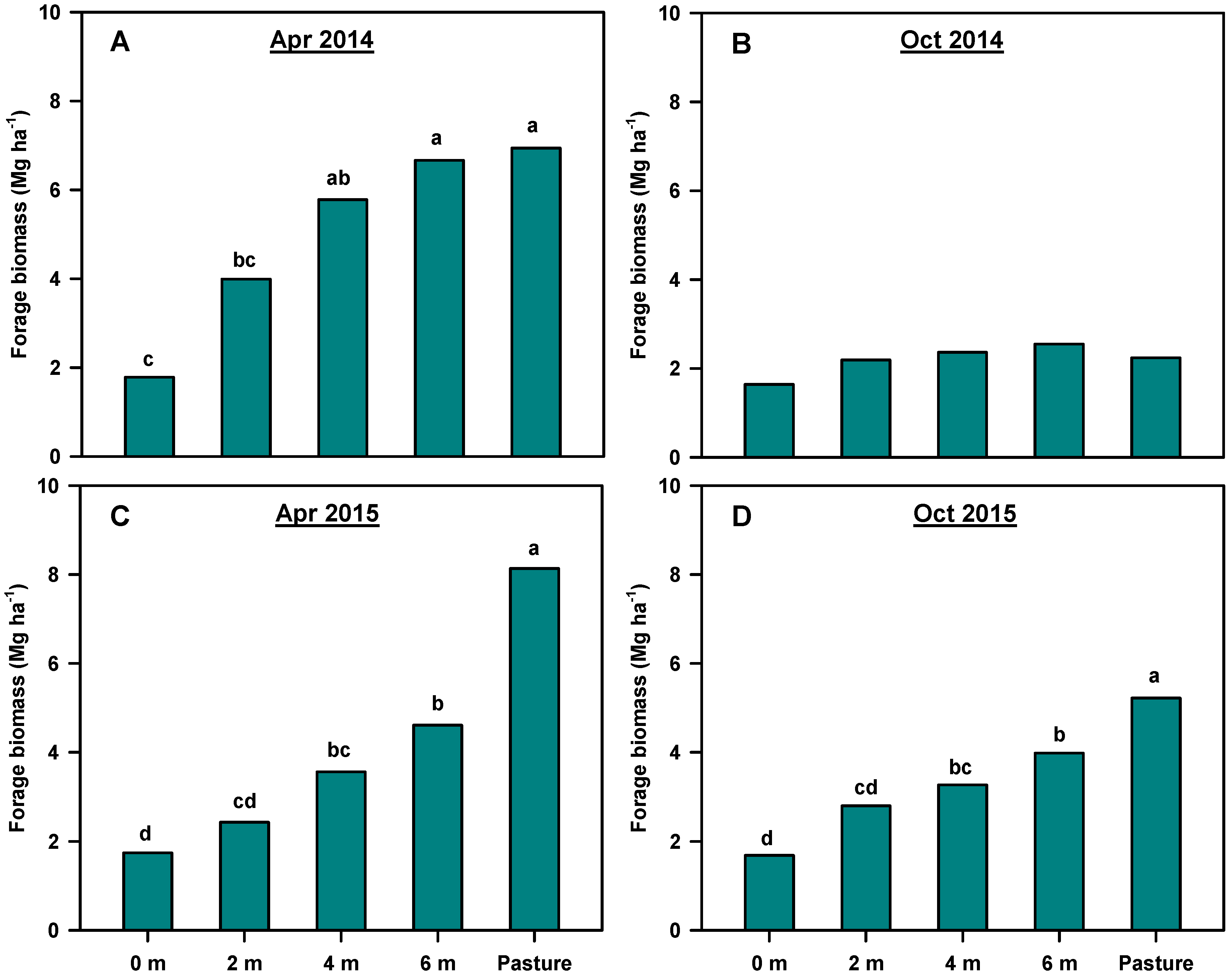
3.2. Forage Biomass
4. Discussion
Forage Biomass
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nii-Annang, S.; Grünewald, H.; Freese, D.; Hüttl, R.F.; Dilly, O. Microbial activity, organic C accumulation and 13 C abundance in soils under alley cropping systems after 9 years of recultivation of quaternary deposits. Biol. Fertil. Soils 2009, 45, 531–538. [Google Scholar] [CrossRef]
- Sarto, M.V.; Borges, W.L.; Sarto, J.R.; Pires, C.A.; Rice, C.W.; Rosolem, C.A. Soil microbial community and activity in a tropical integrated crop-livestock system. Appl. Soil Ecol. 2020, 145, 103350. [Google Scholar] [CrossRef]
- Udawatta, R.P.; Garrett, H.E.; Kallenbach, R. Agroforestry buffers for nonpoint source pollution reductions from agricultural watersheds. J. Environ. Qual. 2011, 40, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.L.; Lima, H.V.; Campanha, M.M.; Gilkes, R.J.; Oliveira, T.S. Soil physical quality of Luvisols under agroforestry, natural vegetation and conventional crop management systems in the Brazilian semi-arid region. Geoderma 2011, 167, 61–70. [Google Scholar] [CrossRef]
- Lemaire, G.; Franzluebbers, A.; de Faccio Carvalho, P.C.; Dedieu, B. Integrated crop–livestock systems: Strategies to achieve synergy between agricultural production and environmental quality. Agric. Ecosyst. Environ. 2014, 190, 4–8. [Google Scholar] [CrossRef]
- Maia, S.M.F.; Xavier, F.A.S.; Oliveira, T.S.; Mendonça, E.S.; Araújo Filho, J.A. Organic carbon pools in a Luvisol under agroforestry and conventional farming systems in the semi-arid region of Ceará, Brazil. Agrofor. Syst. 2007, 71, 127–138. [Google Scholar] [CrossRef]
- Udawatta, R.P.; Anderson, S.H. CT-measured pore characteristics of surface and subsurface soils influenced by agroforestry and grass buffers. Geoderma 2008, 145, 381–389. [Google Scholar] [CrossRef]
- Seobi, T.; Anderson, S.H.; Udawatta, R.P.; Gantzer, C.J. Influence of grass and agroforestry buffer strips on soil hydraulic properties for an Albaqualf. Soil Sci. Soc. Am. J. 2005, 69, 893–901. [Google Scholar] [CrossRef] [Green Version]
- Vieira Junior, N.A.; Evers, J.; Vianna, M.S.; Pedreira, B.C.; Pezzopane, J.R.M.; Marina, F.R. Understanding the arrangement of Eucalyptus-Marandu palisade grass silvopastoral systems in Brazil. Agric. Syst. 2022, 196, 103316. [Google Scholar] [CrossRef]
- Santos, D.C.; Júnior, R.G.; Vilela, L.; Maciel, G.A.; Souza Franca, A.F. Implementation of silvopastoral systems in Brazil with Eucalyptus urograndis and Brachiaria brizantha: Productivity of forage and an exploratory test of the animal response. Agric. Ecosyst. Environ. 2018, 266, 174–180. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Y.; Cao, Q.; Shen, Y.; Zhang, B. Modeling the coupling processes of evapotranspiration and soil water balance in agroforestry systems. Agric. Water Manag. 2021, 250, 106839. [Google Scholar] [CrossRef]
- Bosi, C.; Pezzopane, J.R.M.; Sentelhas, P.C. Soil water availability in a full sun pasture and in a silvopastoral system with eucalyptus. Agrofor. Syst. 2019, 94, 429–440. [Google Scholar] [CrossRef]
- Pezzopane, J.R.M.; Bosi, C.; Nicodemo, M.L.F.; Santos, P.M.; Cruz, P.G.D.; Parmejiani, R.S. Microclimate and soil moisture in a silvopastoral system in southeastern Brazil. Bragantia 2015, 74, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Pezzopane, J.R.M.; Santos, P.M.; Cruz, P.G.; Bosi, C.; Sentelhas, P.C. An integrated agrometeorological model to simulate Marandu productivity. Field Crop. Res. 2018, 224, 13–21. [Google Scholar] [CrossRef]
- Lin, B.B. The role of agroforestry in reducing water loss through soil evaporation and crop transpiration in coffee agroecosystems. Agric. Meteorol. 2010, 150, 510–518. [Google Scholar] [CrossRef]
- Bosi, C.; Pezzopane, J.R.M.; Sentelhas, P.C.; Santos, P.M.; Nicodemo, M.L.F. Produtividade e características biométricas do capim-braquiária em sistema silvipastoril. Pesqui. Agropecu. Bras. 2014, 49, 449–456. [Google Scholar] [CrossRef]
- Borges, W.L.; Calonego, J.C.; Rosolem, C.A. Impact of crop-livestock-forest integration on soil quality. Agrofor. Syst. 2019, 93, 2111–2119. [Google Scholar] [CrossRef]
- Quinkenstein, A.; Woellecke, J.; Böhm, C.; Grünewald, H.; Freese, D.; Schneider, B.U.; Hüttl, R.F. Ecological benefits of the alley cropping agroforestry system in sensitive regions of Europe. Environ. Sci. Technol. 2009, 12, 1112–1121. [Google Scholar] [CrossRef]
- Livesley, S.J.; Gregory, P.J.; Buresh, R.J. Competition in tree row agroforestry systems. 1. Distribution and dynamics of fine root length and biomass. Plant Soil 2000, 227, 149–161. [Google Scholar] [CrossRef]
- Geremia, E.V.; Crestani, S.; Mascheroni, J.D.C.; Carnevalli, R.A.; Mourão, G.B.; Silva, S.C. Sward structure and herbage intake of Brachiaria brizantha cv. Piatã in a crop-livestock-forestry integration area. Livest. Sci. 2018, 212, 83–92. [Google Scholar] [CrossRef]
- Gomes, F.J.; Pedreira, C.G.; Bosi, C.; Cavalli, J.; Holschuch, S.G.; Mourão, G.B.; Pedreira, B.C. Shading effects on Marandu palisadegrass in a silvopastoral system: Plant morphological and physiological responses. Agron. J. 2019, 111, 2332–2340. [Google Scholar] [CrossRef]
- Siriri, D.; Ong, C.K.; Wilson, J.; Boffa, J.M.; Black, C.R. Tree species and pruning regime affect crop yield on bench terraces in SW Uganda. Agrofor. Syst. 2010, 78, 65–77. [Google Scholar] [CrossRef] [Green Version]
- Bosi, C.; Pezzopane, J.R.M.; Sentelhas, P.C. Silvopastoral system with Eucalyptus as a strategy for mitigating the effects of climate change on Brazilian pasturelands. An. Acad. Bras. Ciênc. 2020, 92, e20180425. [Google Scholar] [CrossRef] [Green Version]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; de Moraes Gonçalves, J.L. Modeling monthly mean air temperature for Brazil. Theor. Appl. Climatol. 2013, 113, 407–427. [Google Scholar] [CrossRef]
- Staff, S.S. Keys to Soil Taxonomy, 12th ed; Natural Resources Conservation Service, United States Department of Agriculture: Washington, DC, USA, 2014. [Google Scholar]
- Van Raij, B.V.; Quaggio, J.A. Métodos de Análise de Solo Para Fins de Fertilidade; Copersucar Ltda: Sao Paulo, Brazil, 1983; p. 81. [Google Scholar]
- Grossman, R.B.; Reinsch, T.G. Bulk Density Linear Extensibility. In Methods of Soil Analysis; Dane, J.H., Topp, C.G., Eds.; Soil Science Society of America: Madison, WI, USA, 2002; pp. 207–210. [Google Scholar]
- Gee, G.W.; Or, D. Particle-size analysis. In Methods of Soil Analysis Madison; Dane, J.H., Topp, G.C., Eds.; Soil Science Society of America: Madison, WI, USA, 2002; pp. 255–293. [Google Scholar]
- Germon, A.; Guerrini, I.A.; Bordron, B.; Bouillet, J.P.; Nouvellon, Y.; de Moraes Gonçalves, J.L.; Laclau, J.P. Consequences of mixing Acacia mangium and Eucalyptus grandis trees on soil exploration by fine-roots down to a depth of 17 m. Plant Soil 2018, 424, 203–220. [Google Scholar] [CrossRef] [Green Version]
- Jose, S.; Gillespie, A.R.; Seifert, J.R.; Biehle, D.J. Defining competition vectors in a temperate alley cropping system in the midwestern USA: 2. Competition for water. Agrofor. Syst. 2000, 48, 41–59. [Google Scholar] [CrossRef]
- Reynolds, P.E.; Simpson, J.A.; Thevathasan, N.V.; Gordon, A.M. Effects of tree competition on corn and soybean photosynthesis, growth, and yield in a temperate tree-based agroforestry intercropping system in southern Ontario, Canada. Ecol. Eng. 2007, 29, 362–371. [Google Scholar] [CrossRef]
- Laclau, J.P.; Arnaud, M.; Bouillet, J.P.; Ranger, J. Spatial distribution of Eucalyptus roots in a deep sandy soil in the Congo: Relationships with the ability of the stand to take up water and nutrients. Tree Physiol. 2001, 21, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Radersma, S.; Ong, C.K. Spatial distribution of root length density and soil water of linear agroforestry systems in sub-humid Kenya: Implications for agroforestry models. For. Ecol. Manag. 2004, 188, 77–89. [Google Scholar] [CrossRef]
- Prasad, J.V.N.S.; Korwar, G.R.; Rao, K.V.; Mandal, U.K.; Rao, C.A.R.; Rao, G.R.; Rao, M.R. Tree row spacing affected agronomic and economic performance of Eucalyptus-based agroforestry in Andhra Pradesh, Southern India. Agrofor. Syst. 2010, 78, 253–267. [Google Scholar] [CrossRef]
- Odhiambo, H.O.; Ong, C.K.; Deans, J.D.; Wilson, J.; Khan, A.A.H.; Sprent, J.I. Roots, soil water and crop yield: Tree crop interactions in a semi-arid agroforestry system in Kenya. Plant Soil 2001, 235, 221–233. [Google Scholar] [CrossRef] [Green Version]
- Pezzopane, J.R.M.; Bonani, W.L.; Bosi, C.; da Rocha, E.L.F.; de Campos Bernardi, A.C.; Oliveira, P.P.A.; de Faria Pedroso, A. Reducing competition in a crop–livestock–forest integrated system by thinning eucalyptus trees. Exp. Agric. 2020, 56, 574–586. [Google Scholar] [CrossRef]
- Benavides, R.; Douglas, G.B.; Osoro, K. Silvopastoralism in New Zealand: Review of effects of evergreen and deciduous trees on pasture dynamics. Agrofor. Syst. 2009, 76, 327–350. [Google Scholar] [CrossRef]
- Albrecht, A.; Kandji, S.T. Carbon sequestration in tropical agroforestry systems. Agric. Ecosyst. Environ. 2003, 99, 15–27. [Google Scholar] [CrossRef]
- Wang, T.; Wedin, D.A.; Franz, T.E.; Hiller, J. Effect of vegetation on the temporal stability of soil moisture in grass-stabilized semi-arid sand dunes. J. Hydrol. 2015, 521, 447–459. [Google Scholar] [CrossRef]
- Shem, K.; Catherine, M.; Ong, C. Gas exchange responses of Eucalyptus, C. africana and G. robusta to varying soil moisture content in semi-arid (Thika) Kenya. Agrofor. Syst. 2009, 75, 239–249. [Google Scholar] [CrossRef]
- Gonçalves, J.L.M.; Mendes, K.C.F.S.; Sasaki, C.M. Mineralização de nitrogênio em ecossistemas florestais naturais e implantados do Estado de São Paulo. Rev. Bras. Cienc. Solo 2001, 25, 601–616. [Google Scholar] [CrossRef]
- Bouillet, J.P.; Laclau, J.P.; Arnaud, M.; M’Bou, A.T.; Saint-André, L.; Jourdan, C. Changes with age in the spatial distribution of roots of Eucalyptus clone in Congo: Impact on water and nutrient uptake. For. Ecol. Manag. 2002, 171, 43–57. [Google Scholar] [CrossRef]
- Guenni, O.S.S.F.R.; Seiter, S.; Figueroa, R. Growth responses of three Brachiaria species to light intensity and nitrogen supply. TG Trop. Grassl. 2008, 42, 75. [Google Scholar]
- Pollock, K.M.; Mead, D.J.; McKenzie, B.A. Soil moisture and water use by pastures and silvopastures in a sub-humid temperate climate in New Zealand. Agrofor. Syst. 2009, 75, 223–238. [Google Scholar] [CrossRef]
- Paciullo, D.S.C.; de Castro, C.R.T.; de Miranda Gomide, C.A.; Maurício, R.M.; Pires, M.D.F.Á.; Müller, M.D.; Xavier, D.F. Performance of dairy heifers in a silvopastoral system. Livest. Sci. 2011, 141, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Sarto, M.V.M.; Borges, W.L.B.; Bassegio, D.; Rice, C.; Rosolem, C.A. Maize and sorghum root growth and yield when intercropped with forage grasses. Agron. J. 2021, 113, 1–16. [Google Scholar] [CrossRef]
- Neely, C.B.; Rouquette, F.M.; Morgan, C.L., Jr.; Smith, G.R.; Hons, F.M.; Rooney, W.L. Integrating legumes as cover crops and intercrops into grain sorghum production systems. Agron. J. 2018, 110, 1363–1378. [Google Scholar] [CrossRef]
- Silva, P.C.G.; Tiritan, C.S.; Echer, F.R.; Santos Cordeiro, C.F.; Rebonatti, M.D.; Santos, C.H. No-tillage and crop rotation increase crop yields and nitrogen stocks in sandy soils under agroclimatic risk. Field Crops Res. 2020, 258, 107947. [Google Scholar] [CrossRef]
- Oonyu, J. Upland rice growing: A potential solution to declining crop yields and the degradation of the Doho wetlands, Butaleja district, Uganda. Afr. J. Agric. Res. 2011, 6, 2774–2783. [Google Scholar]
- Sarto, M.V.; Borges, W.L.; Sarto, J.R.; Rice, C.W.; Rosolem, C.A. Deep soil carbon stock, origin, and root interaction in a tropical integrated crop–livestock system. Agrofor. Syst. 2020, 94, 1865–1877. [Google Scholar] [CrossRef]
- Sarto, M.V.; Borges, W.L.; Sarto, J.R.; Rice, C.W.; Rosolem, C.A. Root and shoot interactions in a tropical integrated crop–livestock–forest system. Agrofor. Syst. 2020, 181, 102796. [Google Scholar] [CrossRef]
- Pezzopane, J.R.M.; Bosi, C.; de Campos Bernardi, A.C.; Muller, M.D.; de Oliveira, P.P. A Managing eucalyptus trees in agroforestry systems: Productivity parameters and PAR transmittance. Agric. Ecosyst. Environ. 2021, 312, 107350. [Google Scholar] [CrossRef]
- Mead, R.; Willey, R. The concept of a ‘land equivalent ratio’and advantages in yields from intercropping. Exp. Agric. 1980, 16, 217–228. [Google Scholar] [CrossRef] [Green Version]
- Hübner, R.; Kühnel, A.; Lu, J.; Dettmann, H.; Wang, W.; Wiesmeier, M. Soil carbon sequestration by agroforestry systems in China: A meta-analysis. Agric. Ecosyst. Environ. 2021, 315, 107437. [Google Scholar] [CrossRef]
- Souza, W.D.; Barbosa, O.R.; Marques, J.D.A.; Costa, M.A.T.; Gasparino, E.; Limberger, E. Microclima em sistemas silvipastoris com eucalipto em renques com diferentes alturas. Rev. Bras. Zootec. 2010, 39, 685–694. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, E.B.; Jayasundara, S.; de Oliveira Bordonal, R.; Berchielli, T.T.; Reis, R.A.; Wagner-Riddle, C.; La Scala, N., Jr. Greenhouse gas balance and carbon footprint of beef cattle in three contrasting pasture-management systems in Brazil. J. Clean. Prod. 2017, 142, 420–431. [Google Scholar] [CrossRef] [Green Version]
| Soil Depth | P (Resin) | SOM a | pHCaCl2 | K+ | Ca2+ | Mg2+ | H + Al | Al3+ | BS b |
|---|---|---|---|---|---|---|---|---|---|
| cm | mg dm−3 | g kg−1 | ———— mmolc dm−3———— | % | |||||
| 0–5 | 2.89 | 12.2 | 4.9 | 1.57 | 19.4 | 13.7 | 24 | 0.36 | 55 |
| 5–10 | 0.78 | 11.7 | 4.7 | 1.32 | 7.0 | 4.1 | 31 | 1.12 | 29 |
| 10–20 | 0.33 | 11.5 | 4.9 | 1.04 | 6.2 | 3.1 | 32 | 1.39 | 25 |
| 20–40 | 0.35 | 10.4 | 4.7 | 0.71 | 7.5 | 3.3 | 31 | 1.28 | 27 |
| 40–60 | 0.30 | 9.8 | 5.1 | 0.88 | 8.0 | 3.3 | 22 | 2.47 | 35 |
| 60–80 | 0.44 | 9.8 | 5.2 | 0.80 | 8.2 | 3.5 | 21 | 1.69 | 26 |
| 80–100 | 0.44 | 7.2 | 5.3 | 0.57 | 9.1 | 3.8 | 19 | 1.40 | 41 |
| Soil depth | Sandy | Silt | Clay | Ds c | Texture | ||||
| cm | ———————g kg−1——————— | Mg m−3 | |||||||
| 0–5 | 816 | 102 | 82 | 1.58 | Loamy sand | ||||
| 5–10 | 820 | 73 | 107 | 1.60 | Loamy sand | ||||
| 10–20 | 809 | 68 | 123 | 1.60 | Loamy sand | ||||
| 20–40 | 783 | 75 | 142 | 1.60 | Sandy loam | ||||
| 40–60 | 776 | 69 | 155 | 1.55 | Sandy loam | ||||
| 60–80 | 746 | 62 | 192 | 1.53 | Sandy loam | ||||
| 80–100 | 728 | 64 | 208 | 1.47 | Sandy loam | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarto, M.V.M.; Borges, W.L.B.; Bassegio, D.; Nunes, M.R.; Rice, C.W.; Rosolem, C.A. Deep Soil Water Content and Forage Production in a Tropical Agroforestry System. Agriculture 2022, 12, 359. https://doi.org/10.3390/agriculture12030359
Sarto MVM, Borges WLB, Bassegio D, Nunes MR, Rice CW, Rosolem CA. Deep Soil Water Content and Forage Production in a Tropical Agroforestry System. Agriculture. 2022; 12(3):359. https://doi.org/10.3390/agriculture12030359
Chicago/Turabian StyleSarto, Marcos Vinicius Mansano, Wander Luis Barbosa Borges, Doglas Bassegio, Márcio Renato Nunes, Charles W. Rice, and Ciro Antonio Rosolem. 2022. "Deep Soil Water Content and Forage Production in a Tropical Agroforestry System" Agriculture 12, no. 3: 359. https://doi.org/10.3390/agriculture12030359
APA StyleSarto, M. V. M., Borges, W. L. B., Bassegio, D., Nunes, M. R., Rice, C. W., & Rosolem, C. A. (2022). Deep Soil Water Content and Forage Production in a Tropical Agroforestry System. Agriculture, 12(3), 359. https://doi.org/10.3390/agriculture12030359







