Bta-miR-125a Regulates Milk-Fat Synthesis by Targeting SAA1 mRNA in Bovine Mammary Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Plasmid Construction and Site-Directed Mutagenesis
2.3. Luciferase Reporter Assays
2.4. Transient Transfection in MAC-T Cells
2.5. RNA Isolation and Quantitative RT-PCR (qRT-PCR)
2.6. Western Blot Analysis
2.7. Flow Cytometry
2.8. Oil Red O Staining
2.9. Triglyceride Assay
2.10. Data Analysis
3. Results
3.1. Bta-miR-125a Mimic Suppressed the Gene Expression of SAA1 mRNA by Targeting a Specific Sequence in Its 3′-UTR
3.2. Bta-miR-125a Inhibits SAA1 Expression in MAC-T Cells
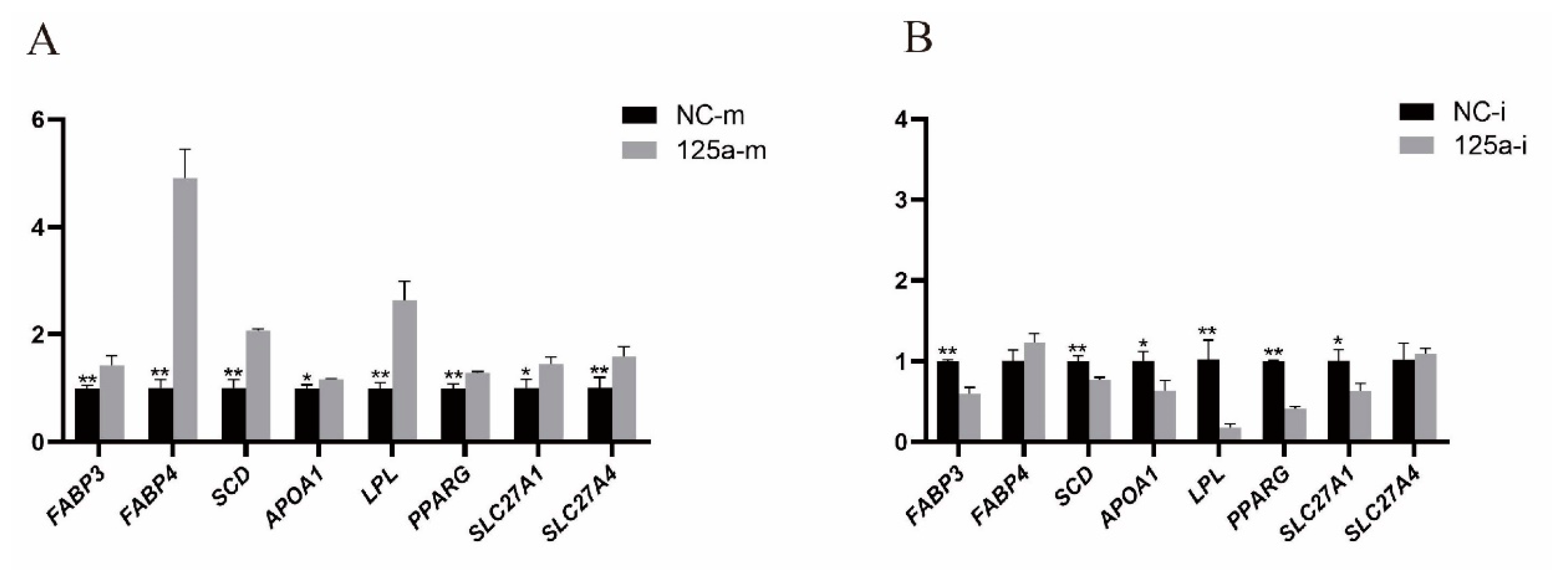
3.3. Bta-miR-125a Regulates Expression of Lipid-Related Genes in MAC-T
3.4. Bta-miR-125a Enhances Lipogenesis in Bovine Mammary Epithelial Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melfsen, A.; Holstermann, M.; Haeussermann, A.; Molkentin, J.; Susenbeth, A.; Hartung, E. Accuracy and application of milk fatty acid estimation with diffuse reflectance near-infrared spectroscopy. J. Dairy Res. 2018, 85, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chu, S.; Wang, X.; Fan, Y.; Zhan, T.; Arbab, A.A.I.; Li, M.; Zhang, H.; Mao, Y.; Loor, J.J.; et al. MicroRNA-106b Regulates Milk Fat Metabolism via ATP Binding Cassette Subfamily A Member 1 (ABCA1) in Bovine Mammary Epithelial Cells. J. Agric. Food Chem. 2019, 67, 3981–3990. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Saghatelian, A.; Chong, L.W.; Zhang, C.L.; Cravatt, B.F.; Evans, R.M. Maternal PPAR gamma protects nursing neonates by suppressing the production of inflammatory milk. Genes Dev. 2007, 21, 1895–1908. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Q.; Zhou, C.H.; Cong, S.; Han, D.X.; Wang, C.J.; Tian, Y.; Zhang, J.B.; Jiang, H.; Yuan, B. Lipopolysaccharide inhibits triglyceride synthesis in dairy cow mammary epithelial cells by upregulating miR-27a-3p, which targets the PPARG gene. J. Dairy Sci. 2021, 104, 989–1001. [Google Scholar] [CrossRef]
- Yu, L.; Wu, W.K.; Li, Z.J.; Liu, Q.C.; Li, H.T.; Wu, Y.C.; Cho, C.H. Enhancement of doxorubicin cytotoxicity on human esophageal squamous cell carcinoma cells by indomethacin and 4-[5-(4-chlorophenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide (SC236) via inhibiting P-glycoprotein activity. Mol. Pharmacol. 2009, 75, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Digre, A.; Nan, J.; Frank, M.; Li, J.P. Heparin interactions with apoA1 and SAA in inflammation-associated HDL. Biochem. Biophys. Res. Commun. 2016, 474, 309–314. [Google Scholar] [CrossRef]
- Yamazaki, K.; Kuromitsu, J.; Tanaka, I. Microarray analysis of gene expression changes in mouse liver induced by peroxisome proliferator- activated receptor alpha agonists. Biochem. Biophys. Res. Commun. 2002, 290, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Ray, B.K.; Ray, A. Rabbit serum amyloid a gene: Cloning, characterization and sequence analysis. Biochem. Biophys. Res. Commun. 1991, 180, 1258–1264. [Google Scholar] [CrossRef]
- Li, D.; Xie, P.; Zhao, S.; Zhao, J.; Yao, Y.; Zhao, Y.; Ren, G.; Liu, X. Hepatocytes derived increased SAA1 promotes intrahepatic platelet aggregation and aggravates liver inflammation in NAFLD. Biochem. Biophys. Res. Commun. 2021, 555, 54–60. [Google Scholar] [CrossRef]
- Gan, X.W.; Wang, W.S.; Lu, J.W.; Ling, L.J.; Zhou, Q.; Zhang, H.J.; Ying, H.; Sun, K. De novo Synthesis of SAA1 in the Placenta Participates in Parturition. Front. Immunol. 2020, 11, 1038. [Google Scholar] [CrossRef]
- Siegmund, S.V.; Schlosser, M.; Schildberg, F.A.; Seki, E.; De Minicis, S.; Uchinami, H.; Kuntzen, C.; Knolle, P.A.; Strassburg, C.P.; Schwabe, R.F. Serum Amyloid a Induces Inflammation, Proliferation and Cell Death in Activated Hepatic Stellate Cells. PLoS ONE 2016, 11, e0150893. [Google Scholar] [CrossRef]
- Yang, S.; Gao, Y.; Zhang, S.; Zhang, Q.; Sun, D. Identification of Genetic Associations and Functional Polymorphisms of SAA1 Gene Affecting Milk Production Traits in Dairy Cattle. PLoS ONE 2016, 11, e0162195. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, A.S.; de Beer, M.C.; Steel, D.M.; Rits, M.; Lelias, J.M.; Lane, W.S.; de Beer, F.C. Identification of novel members of the serum amyloid A protein superfamily as constitutive apolipoproteins of high density lipoprotein. J. Biol. Chem. 1992, 267, 3862–3867. [Google Scholar] [CrossRef]
- De Buck, M.; Gouwy, M.; Wang, J.M.; Van Snick, J.; Opdenakker, G.; Struyf, S.; Van Damme, J. Structure and Expression of Different Serum Amyloid A (SAA) Variants and their Concentration-Dependent Functions During Host Insults. Curr. Med. Chem. 2016, 23, 1725–1755. [Google Scholar] [CrossRef]
- Del Pozo-Acebo, L.; Hazas, M.; Tomé-Carneiro, J.; Gil-Cabrerizo, P.; San-Cristobal, R.; Busto, R.; García-Ruiz, A.; Dávalos, A. Bovine Milk-Derived Exosomes as a Drug Delivery Vehicle for miRNA-Based Therapy. Int. J. Mol. Sci. 2021, 22, 1105. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.P.; Shih, I.H.; Jones-Rhoades, M.W.; Bartel, D.P.; Burge, C.B. Prediction of mammalian microRNA targets. Cell 2003, 115, 787–798. [Google Scholar] [CrossRef]
- Lim, L.P.; Glasner, M.E.; Yekta, S.; Burge, C.B.; Bartel, D.P. Vertebrate microRNA genes. Science 2003, 299, 1540. [Google Scholar] [CrossRef]
- Silveri, L.; Tilly, G.; Vilotte, J.L.; Le Provost, F. MicroRNA involvement in mammary gland development and breast cancer. Reprod. Nutr. Dev. 2006, 46, 549–556. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, X.; Zhang, L.; Jin, J.; Xia, A.; Wang, C.; Cui, Y.; Qu, B.; Li, Q.; Sheng, C. Comparative transcriptome analysis to investigate the potential role of miRNAs in milk protein/fat quality. Sci. Rep. 2018, 8, 6250. [Google Scholar] [CrossRef]
- Ibarra, I.; Erlich, Y.; Muthuswamy, S.K.; Sachidanandam, R.; Hannon, G.J. A role for microRNAs in maintenance of mouse mammary epithelial progenitor cells. Genes Dev. 2007, 21, 3238–3243. [Google Scholar] [CrossRef]
- Li, M.; Li, Q.; Gao, X. Expression and function of leptin and its receptor in dairy goat mammary gland. J. Dairy Res. 2010, 77, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Patel, S.H.; Ginestier, C.; Ibarra, I.; Martin-Trevino, R.; Bai, S.; McDermott, S.P.; Shang, L.; Ke, J.; Ou, S.J.; et al. MicroRNA93 regulates proliferation and differentiation of normal and malignant breast stem cells. PLoS Genet. 2012, 8, e1002751. [Google Scholar] [CrossRef]
- Ucar, A.; Vafaizadeh, V.; Jarry, H.; Fiedler, J.; Klemmt, P.A.; Thum, T.; Groner, B.; Chowdhury, K. miR-212 and miR-132 are required for epithelial stromal interactions necessary for mouse mammary gland development. Nat. Genet. 2010, 42, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Pan, Q.; Yang, Y.; Gao, Y.; Liu, X.; Li, W.; Han, Y.; Yuan, X.; Qu, Y.; Zhao, Z. miR-224 Affects Mammary Epithelial Cell Apoptosis and Triglyceride Production by Downregulating ACADM and ALDH2 Genes. DNA Cell Biol. 2017, 36, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, P.; Yu, H.; Yang, Y.; Xia, L.; Yang, R.; Fang, X.; Zhao, Z. miR-21-3p Targets Elovl5 and Regulates Triglyceride Production in Mammary Epithelial Cells of Cow. DNA Cell Biol. 2019, 38, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Li, H.M.; Wang, C.M.; Li, Q.Z.; Gao, X.J. MiR-15a decreases bovine mammary epithelial cell viability and lactation and regulates growth hormone receptor expression. Molecules 2012, 17, 12037–12048. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Hou, Y.; Yang, S.; Xie, Y.; Zhang, S.; Zhang, Y.; Zhang, Q.; Lu, X.; Liu, G.E.; Sun, D. Transcriptional profiling of mammary gland in Holstein cows with extremely different milk protein and fat percentage using RNA sequencing. BMC Genom. 2014, 15, 226. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, S.; Zhang, Q.; Guo, X.; Wu, C.; Yao, M.; Sun, D. Comprehensive MicroRNA Expression Profile of the Mammary Gland in Lactating Dairy Cows with Extremely Different Milk Protein and Fat Percentages. Front. Genet. 2020, 11, 548268. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Liu, Q.; Zhang, X.; Ren, Y.; Lei, X.; Li, S.; Chen, Q.; Deng, K.; Wang, P.; Zhang, H.; et al. Identification and analysis of the expression of microRNA from lactating and nonlactating mammary glands of the Chinese swamp buffalo. J. Dairy Sci. 2017, 100, 1971–1986. [Google Scholar] [CrossRef]
- Perruchot, M.H.; Arévalo-Turrubiarte, M.; Dufreneix, F.; Finot, L.; Lollivier, V.; Chanat, E.; Mayeur, F.; Dessauge, F. Mammary Epithelial Cell Hierarchy in the Dairy Cow Throughout Lactation. Stem Cells Dev. 2016, 25, 1407–1418. [Google Scholar] [CrossRef]
- Xia, L.; Zhao, Z.; Yu, X.; Lu, C.; Jiang, P.; Yu, H.; Li, X.; Yu, X.; Liu, J.; Fang, X.; et al. Integrative analysis of miRNAs and mRNAs revealed regulation of lipid metabolism in dairy cattle. Funct. Integr. Genom. 2021, 21, 393–404. [Google Scholar] [CrossRef]
- Rejman, J.J.; Oliver, S.P.; Muenchen, R.A.; Turner, J.D. Proliferation of the MAC-T bovine mammary epithelial cell line in the presence of mammary secretion whey proteins. Cell Biol. Int. Rep. 1992, 16, 993–1001. [Google Scholar] [CrossRef]
- Johnson, T.L.; Fujimoto, B.A.; Jiménez-Flores, R.; Peterson, D.G. Growth hormone alters lipid composition and increases the abundance of casein and lactalbumin mRNA in the MAC-T cell line. J. Dairy Res. 2010, 77, 199–204. [Google Scholar] [CrossRef]
- Wang, T.; Lee, H.; Zhen, Y. Responses of MAC-T Cells to Inhibited Stearoyl-CoA Desaturase 1 during cis-9, trans-11 Conjugated Linoleic Acid Synthesis. Lipids 2018, 53, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Jéru, I.; Hayrapetyan, H.; Duquesnoy, P.; Cochet, E.; Serre, J.L.; Feingold, J.; Grateau, G.; Sarkisian, T.; Jeanpierre, M.; Amselem, S. Involvement of the modifier gene of a human Mendelian disorder in a negative selection process. PLoS ONE 2009, 4, e7676. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.Z.; Lee, M.J.; Hu, H.; Pollin, T.I.; Ryan, A.S.; Nicklas, B.J.; Snitker, S.; Horenstein, R.B.; Hull, K.; Goldberg, N.H.; et al. Acute-phase serum amyloid A: An inflammatory adipokine and potential link between obesity and its metabolic complications. PLoS Med. 2006, 3, e287. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Kuo, W.H.; Chen, C.Y.; Lin, H.Y.; Wu, H.T.; Liu, B.H.; Chen, C.H.; Mersmann, H.J.; Chang, K.J.; Ding, S.T. Docosahexaenoic acid regulates serum amyloid A protein to promote lipolysis through down regulation of perilipin. J. Nutr. Biochem. 2010, 21, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Benditt, E.P.; Eriksen, N. Amyloid protein SAA is associated with high density lipoprotein from human serum. Proc. Natl. Acad. Sci. USA 1977, 74, 4025–4028. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Ye, R.D. Serum amyloid A1: Structure, function and gene polymorphism. Gene 2016, 583, 48–57. [Google Scholar] [CrossRef]
- Du, J.; Xu, Y.; Zhang, P.; Zhao, X.; Gan, M.; Li, Q.; Ma, J.; Tang, G.; Jiang, Y.; Wang, J.; et al. MicroRNA-125a-5p Affects Adipocytes Proliferation, Differentiation and Fatty Acid Composition of Porcine Intramuscular Fat. Int. J. Mol. Sci. 2018, 19, 501. [Google Scholar] [CrossRef] [PubMed]
- Perretta, L.; Ouldibbat, L.; Hagadorn, J.I.; Brumberg, H.L. High versus low medium chain triglyceride content of formula for promoting short-term growth of preterm infants. Cochrane Database Syst. Rev. 2021, 2, Cd002777. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, M.C.; Healy, A.M.; Harte, D.; Walshe, K.G.; Torgerson, P.R.; Doherty, M.L. Milk amyloid A: Correlation with cellular indices of mammary inflammation in cows with normal and raised serum amyloid A. Res. Vet. Sci. 2006, 80, 155–161. [Google Scholar] [CrossRef]
- Kho, Y.; Kim, S.; Yoon, B.S.; Moon, J.H.; Kim, B.; Kwak, S.; Woo, J.; Oh, S.; Hong, K.; Kim, S.; et al. Induction of serum amyloid A genes is associated with growth and apoptosis of HC11 mammary epithelial cells. Biosci. Biotechnol. Biochem. 2008, 72, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Khatib, H. Comparison of transcriptomic landscapes of bovine embryos using RNA-Seq. BMC Genom. 2010, 11, 711. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, F.; Wang, Y.; Yu, G.; Jia, B.L. Silencing of SAA1 inhibits palmitate- or high-fat diet induced insulin resistance through suppression of the NF-κB pathway. Mol. Med. 2019, 25, 17. [Google Scholar] [CrossRef]
- HafezQorani, S.; Lafzi, A.; de Bruin, R.G.; van Zonneveld, A.J.; van der Veer, E.P.; Son, Y.A.; Kazan, H. Modeling the combined effect of RNA-binding proteins and microRNAs in post-transcriptional regulation. Nucleic Acids Res. 2016, 44, e83. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.Y.; Hou, X.M.; Qu, B.; Zhang, N.; Li, N.; Cui, Y.J.; Li, Q.Z.; Gao, X.J. Functional analysis of FABP3 in the milk fat synthesis signaling pathway of dairy cow mammary epithelial cells. Vitr. Cell. Dev. Biol. Anim. 2014, 50, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, W.; Tang, K.; Wang, Y.; Zan, L.; Yang, W. Bta-miR-34b regulates milk fat biosynthesis by targeting mRNA decapping enzyme 1A (DCP1A) in cultured bovine mammary epithelial cells1. J. Anim. Sci. 2019, 97, 3823–3831. [Google Scholar] [CrossRef]
- Jiao, B.L.; Zhang, X.L.; Wang, S.H.; Wang, L.X.; Luo, Z.X.; Zhao, H.B.; Khatib, H.; Wang, X. MicroRNA-221 regulates proliferation of bovine mammary gland epithelial cells by targeting the STAT5a and IRS1 genes. J. Dairy Sci. 2019, 102, 426–435. [Google Scholar] [CrossRef]
- Wang, M.; Li, L.; Liu, R.; Song, Y.; Zhang, X.; Niu, W.; Kumar, A.K.; Guo, Z.; Hu, Z. Obesity-induced overexpression of miRNA-24 regulates cholesterol uptake and lipid metabolism by targeting SR-B1. Gene 2018, 668, 196–203. [Google Scholar] [CrossRef]
- Xu, Y.; Du, J.; Zhang, P.; Zhao, X.; Li, Q.; Jiang, A.; Jiang, D.; Tang, G.; Jiang, Y.; Wang, J.; et al. MicroRNA-125a-5p Mediates 3T3-L1 Preadipocyte Proliferation and Differentiation. Molecules 2018, 23, 317. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Hengbo, S.; Jun, L.; Jun, L.; Wangsheng, Z.; Huibin, T.; Huaiping, S. PPARG modulated lipid accumulation in dairy GMEC via regulation of ADRP gene. J. Cell. Biochem. 2015, 116, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Ren, C.; Fan, Y.; Liu, Z.; Zhang, G.; Zhang, Y.; You, P.; Wang, F. miR-27a is an important adipogenesis regulator associated with differential lipid accumulation between intramuscular and subcutaneous adipose tissues of sheep. Domest. Anim. Endocrinol. 2020, 71, 106393. [Google Scholar] [CrossRef] [PubMed]
- Bionaz, M.; Loor, J.J. Gene networks driving bovine milk fat synthesis during the lactation cycle. BMC Genom. 2008, 9, 366. [Google Scholar] [CrossRef]
- Kadegowda, A.K.; Bionaz, M.; Piperova, L.S.; Erdman, R.A.; Loor, J.J. Peroxisome proliferator-activated receptor-gamma activation and long-chain fatty acids alter lipogenic gene networks in bovine mammary epithelial cells to various extents. J. Dairy Sci. 2009, 92, 4276–4289. [Google Scholar] [CrossRef] [PubMed]
- Minuti, A.; Bionaz, M.; Lopreiato, V.; Janovick, N.A.; Rodriguez-Zas, S.L.; Drackley, J.K.; Loor, J.J. Prepartum dietary energy intake alters adipose tissue transcriptome profiles during the periparturient period in Holstein dairy cows. J. Anim. Sci. Biotechnol. 2020, 11, 1–14. [Google Scholar] [CrossRef]





| Name | RT Primer (5′ to 3′) | Forward Primer (5′ to 3′) | Reversed Primer (5′ to 3′) | Tm (°C) |
|---|---|---|---|---|
| bta-miR-125a | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAG CACAGGTT | GGGCTTCCCTGAGACCCTTT | CTCAACTGGTGTCGTGGAGTC | 60 |
| U6 | TIANGEN: CD201–0145 | 60 | ||
| Gene Name | Forward Primer Sequence | Reverse Primer Sequence | Amplicon (bp) | Tm (°C) |
|---|---|---|---|---|
| SAA1 | AGTCCACAGCCAGTGGATGT | ATCTCTGAATATTTTCTCTGGCATC | 2433 | 60 |
| GAPDH | AGATGGTGAAGGTCGGAGTG | CGTTCTCTGCCTTGACTGTG | 189 | 60 |
| MARVELD1 | GGCCAGCTGTAAGATCATCACA | TCTGATCACAGACAGAGCACCAT | 100 | 60 |
| FABP3 | GAACTCGACTCCCAGCTTGAA | AAGCCTACCACAATCATCGAAG | 103 | 59 |
| FABP4 | TGGATAGTGCAGCCAGTGTGA | TCCAGTGTGATGCGGTGTGTA | 109 | 60 |
| SCD | TCCTGTTGTTGTGCTTCATCC | GGCATAACGGAATAAGGTGGC | 101 | 60 |
| APOA1 | CGGCGGCTTCTCTTGTATAGC | TTCAAGCGTGAGCTGAAACG | 83 | 60 |
| LPL | ACACAGCTGAGGACACTTGCC | GCCATGGATCACCACAAAGG | 101 | 58 |
| PPARG | CCAAATATCGGTGGGAGTCG | ACAGCGAAGGGCTCACTCTC | 101 | 58 |
| SLC27A1 | GTACCAGCACGAAAGGCTCAA | ATCACACGGCGCTCTTCAA | 120 | 58 |
| SLC27A4 | CACGGAGGAACTTCAGATGTGA | GGCCCCGCTATACTGACTATGA | 127 | 59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, X.; Yuan, T.; Fang, Z.; Feng, J.; Wu, C. Bta-miR-125a Regulates Milk-Fat Synthesis by Targeting SAA1 mRNA in Bovine Mammary Epithelial Cells. Agriculture 2022, 12, 344. https://doi.org/10.3390/agriculture12030344
Cui X, Yuan T, Fang Z, Feng J, Wu C. Bta-miR-125a Regulates Milk-Fat Synthesis by Targeting SAA1 mRNA in Bovine Mammary Epithelial Cells. Agriculture. 2022; 12(3):344. https://doi.org/10.3390/agriculture12030344
Chicago/Turabian StyleCui, Xiaogang, Tianqi Yuan, Zhengyu Fang, Jiao Feng, and Changxin Wu. 2022. "Bta-miR-125a Regulates Milk-Fat Synthesis by Targeting SAA1 mRNA in Bovine Mammary Epithelial Cells" Agriculture 12, no. 3: 344. https://doi.org/10.3390/agriculture12030344
APA StyleCui, X., Yuan, T., Fang, Z., Feng, J., & Wu, C. (2022). Bta-miR-125a Regulates Milk-Fat Synthesis by Targeting SAA1 mRNA in Bovine Mammary Epithelial Cells. Agriculture, 12(3), 344. https://doi.org/10.3390/agriculture12030344






