The Changes in Soil Microorganisms and Soil Chemical Properties Affect the Heterogeneity and Stability of Soil Aggregates before and after Grassland Conversion
Abstract
:1. Introduction
2. Materials and Methods
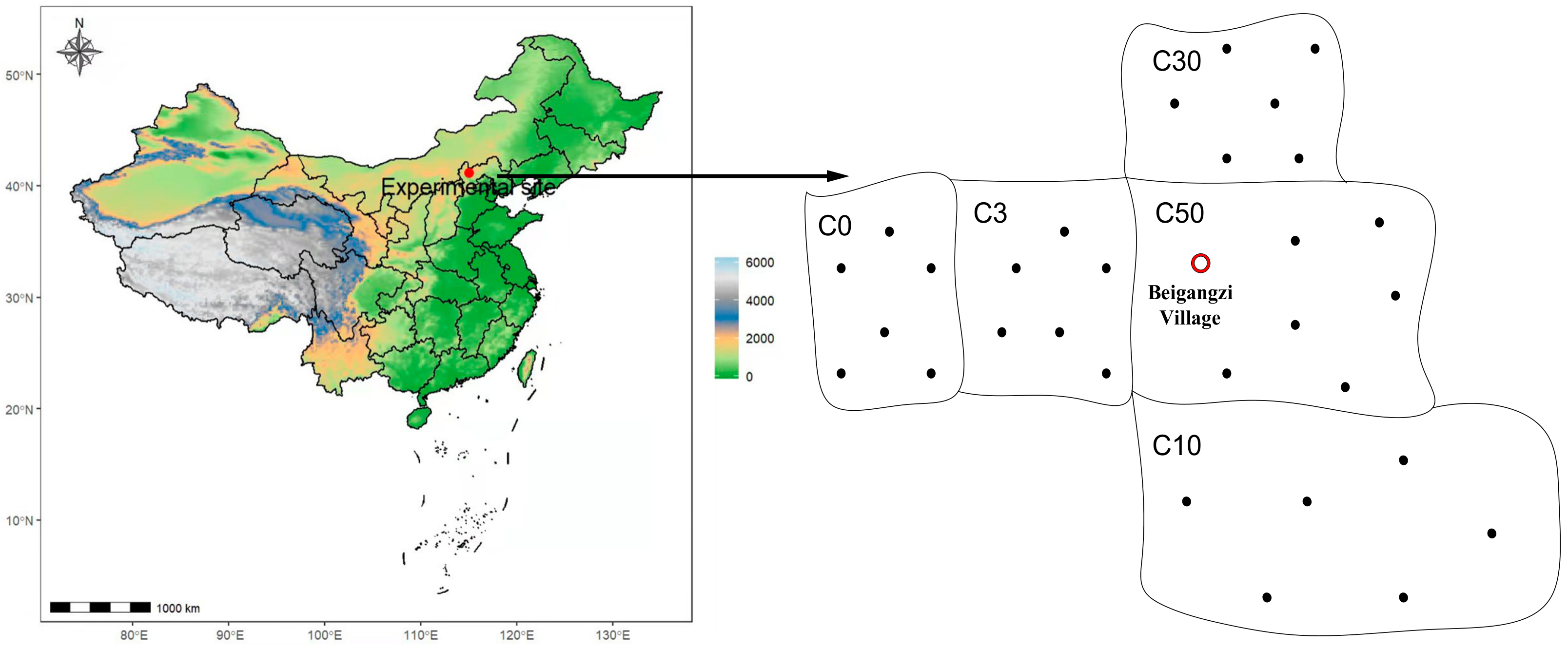
2.1. Study Site and Experimental Design
2.2. Distribution and Stability of Aggregates
2.3. Physical and Chemical Properties of Soil
2.4. The Main Biological Factors and Enzymes
2.5. Statistical Analysis
3. Results
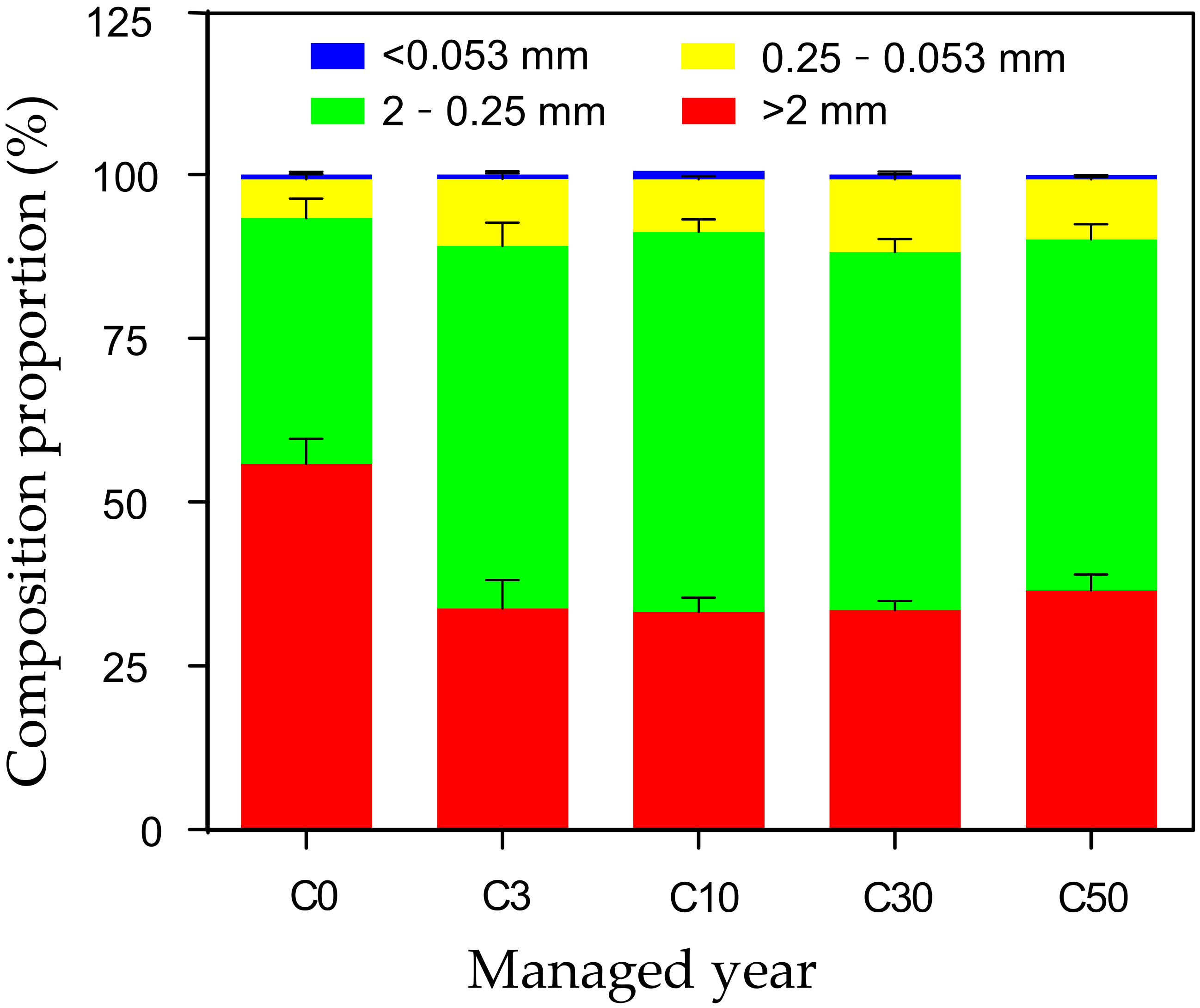
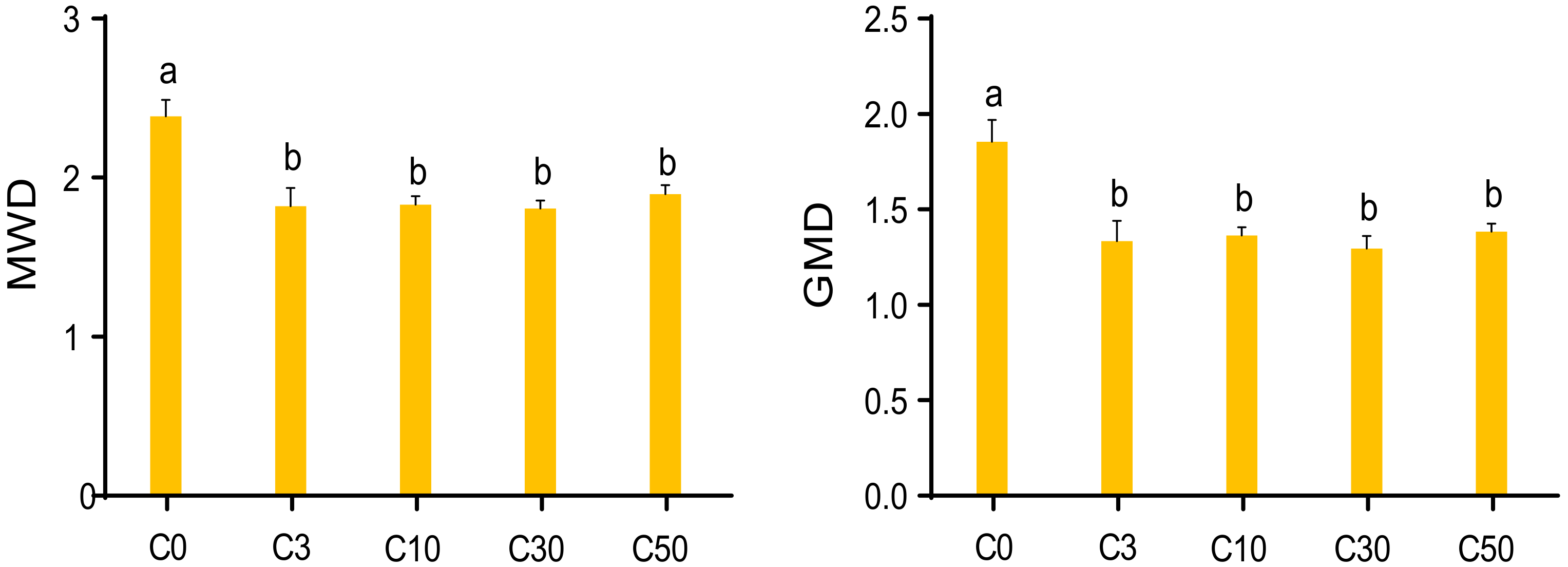
3.1. Distribution and Stability of Soil Aggregates in Conversion Grasslands with Different Managed Years
3.2. Changes in Soil Physicochemical Properties in Conversion Grasslands with Different Managed Years
3.3. Changes in Main Biological Factors and Enzymes in Conversion Grasslands with Different Managed Years
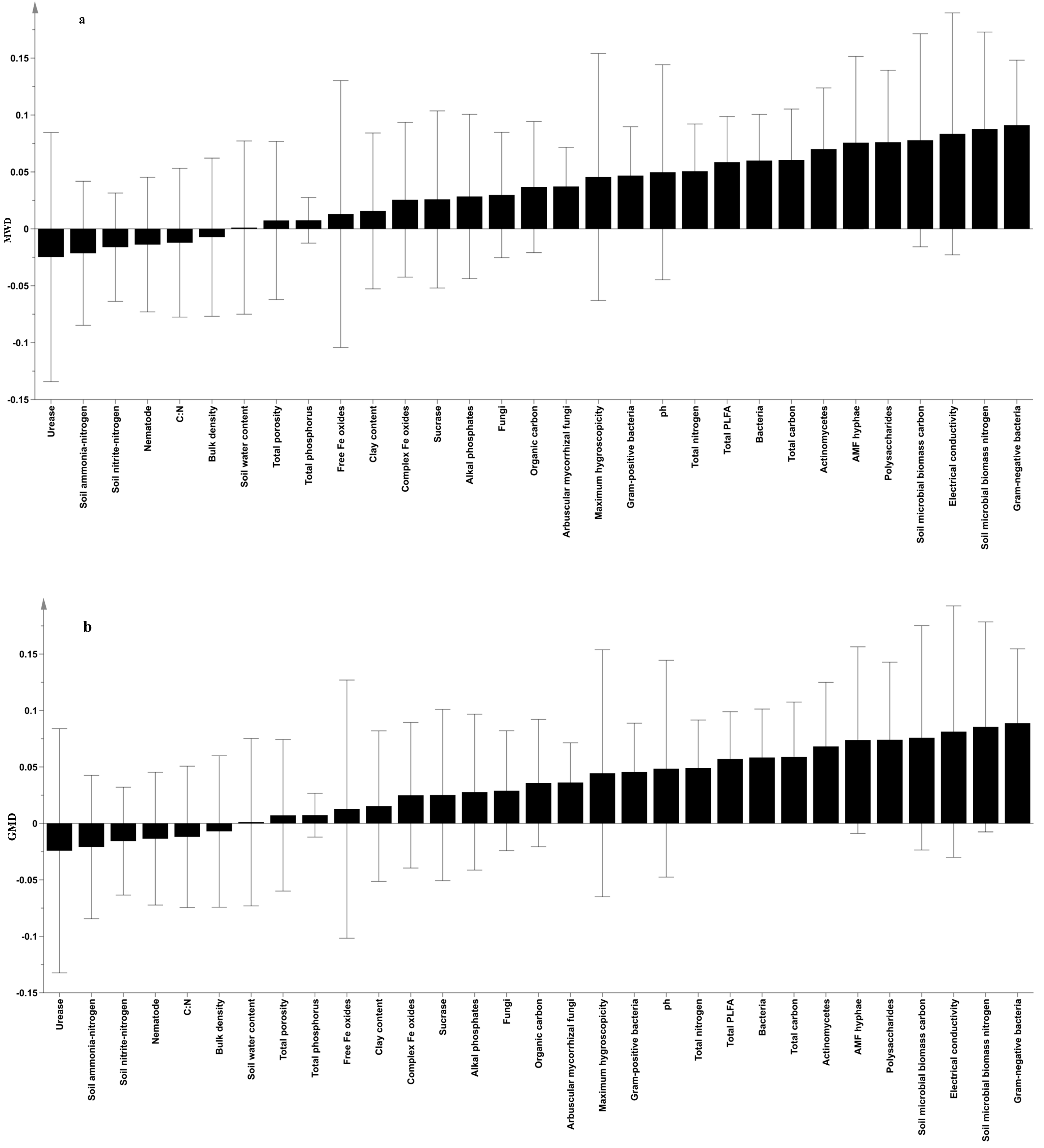
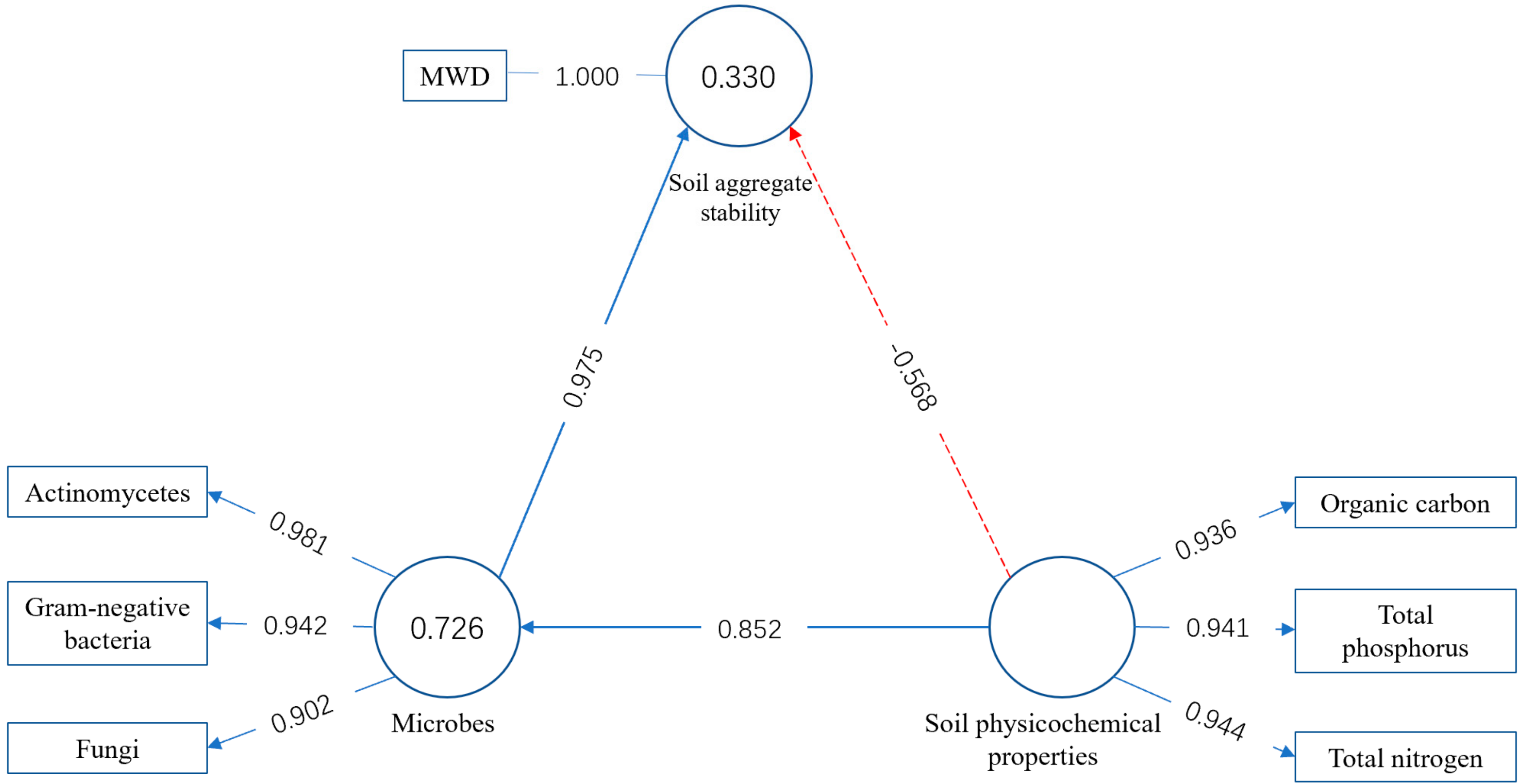
3.4. Relationship between Soil Aggregate Stability and Soil Physicochemical Properties, Main Biological Factors and Enzymes
4. Discussion
4.1. Distribution and Stability of Soil Aggregates under Different Conversion Years
4.2. Effects of Soil Physical and Chemical Properties on Soil Aggregate Stability
4.3. Effects of the Main Biological Factors on Soil Aggregate Stability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, H.L.; Wang, Y.M.; Dou, X.; Xu, M.Y.; Wang, K. Progress and perspective of agro-pasturage ecotone. Acta Ecologica Sinica 2009, 29, 4420–4425. (In Chinese) [Google Scholar]
- Xu, K.X.; Su, Y.J.; Liu, J.; Hu, T.Y.; Jin, S.C.; Ma, Q.; Zhai, Q.P.; Wang, R.; Zhang, J.; Li, Y.M. Estimation of degraded grassland aboveground biomass using machine learning methods from terrestrial laser scanning data. Ecol. Indic. 2020, 108, 105747. [Google Scholar] [CrossRef]
- Zhao, H.L.; Zhao, X.Y.; Zhang, T.H.; Zhou, R.L. Boundary line on Agro-pasture zigzag zone in north China and its problems on eco-environment. Adv. Earth Sci. 2002, 17, 739–747. (In Chinese) [Google Scholar]
- Ni, S.X. Progress in the research on land evaluation in China during the latest ten years. J. Nat. Res. 2003, 18, 672–683. (In Chinese) [Google Scholar]
- Qin, H.L.; Gao, W.S.; He, W.Q. Emergy analysis of the farming system in the semiarid area where farming and animal husbandry crisscross—With Wuchuan county as an example. Agric. Res. Arid. Areas 2005, 23, 157–161. (In Chinese) [Google Scholar]
- Hosseini, F.; Mosaddeghi, M.R.; Hajabbasi, M.A.; Sabzalian, M.R. Influence of tall fescue endophyte infection on structural stability as quantified by high energy moisture characteristic in a range of soils. Geoderma 2015, 249, 87–99. [Google Scholar] [CrossRef]
- Wang, Y.D.; Hu, N.; Ge, T.D.; Kuzyakov, Y.; Wang, Z.L.; Li, Z.F.; Tang, Z.; Chen, Y.; Wu, C.Y.; Lou, Y.L. Soil aggregation regulates distributions of carbon, microbial community and enzyme activities after 23-year manure amendment. Appl. Soil Ecol. 2017, 111, 65–72. [Google Scholar] [CrossRef]
- Zhu, G.Y.; Deng, L.; Shangguan, Z.P. Effects of soil aggregate stability on soil N following land use changes under erodible environment. Agric. Ecosyst. Environ. 2018, 262, 18–28. [Google Scholar] [CrossRef]
- Fallahzade, J.; Karimi, A.; Naderi, M.; Shirani, H. Soil mechanical properties and wind erosion following conversion of desert to irrigated croplands in central Iran. Soil Tillage Res. 2020, 204, 104665. [Google Scholar] [CrossRef]
- Li, J.; Yuan, X.L.; Ge, L.; Li, Q.; Li, Z.G.; Wang, L.; Liu, Y. Rhizosphere effects promote soil aggregate stability and associated organic carbon sequestration in rocky areas of desertification. Agric. Ecosyst. Environ. 2020, 304, 107126. [Google Scholar] [CrossRef]
- Liu, R.; Zhou, X.H.; Wang, J.W.; Shao, J.J.; Fu, Y.L.; Liang, C.; Yan, E.R.; Chen, X.Y.; Wang, X.H.; Bai, S.H. Differential magnitude of rhizosphere effects on soil aggregation at three stages of subtropical secondary forest successions. Plant Soil 2019, 436, 365–380. [Google Scholar] [CrossRef]
- Zhu, G.Y.; Shangguan, Z.P.; Deng, L. Soil aggregate stability and aggregate-associated carbon and nitrogen in natural restoration grassland and Chinese red pine plantation on the Loess Plateau. Catena 2017, 149, 253–260. [Google Scholar] [CrossRef]
- Kaiser, M.; Kleber, M.; Berhe, A.A. How air-drying and rewetting modify soil organic matter characteristics: An assessment to improve data interpretation and inference. Soil Biol. Biochem. 2015, 80, 324–340. [Google Scholar] [CrossRef]
- Dagesse, D. Effect of freeze-drying on soil aggregate stability. Soil Sci. Soc. Am. J. 2011, 75, 2111–2121. [Google Scholar] [CrossRef]
- Chenu, C.; Le Bissonnais, Y.; Arrouays, D. Organic matter influence on clay wettability and soil aggregate stability. Soil Sci. Soc. Am. J. 2000, 64, 1479–1486. [Google Scholar] [CrossRef]
- Bai, T.S.; Wang, P.; Ye, C.L.; Hu, S.J. Form of nitrogen input dominates N effects on root growth and soil aggregation: A meta-analysis. Soil Biol. Biochem. 2021, 157, 108251. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Pérès, G.; Cluzeau, D.; Menasseri, S.; Soussana, J.; Bessler, H.; Engels, C.; Habekost, M.; Gleixner, G.; Weigelt, A.; Weisser, W.; et al. Mechanisms linking plant community properties to soil aggregate stability in an experimental grassland plant diversity gradient. Plant Soil 2013, 373, 285–299. [Google Scholar] [CrossRef]
- Kooch, Y.; Rostayee, F.; Hosseini, S.M. Effects of tree species on topsoil properties and nitrogen cycling in natural forest and tree plantations of northern Iran. Catena 2016, 144, 65–73. [Google Scholar] [CrossRef]
- Bonfante-Fasolo, P.; Gianinazzi-Pearson, V. Ultrastructural aspects of endomycorrhiza in the ericaceae. III. Morphology of the dissociated symbionts and modifications occurring during their reassociation in Axenic culture. New Phytol. 1982, 91, 691–704. [Google Scholar] [CrossRef]
- Caesar-TonThat, T.C.; Cochran, V.L. Soil aggregate stabilization by a saprophytic lignin-decomposing basidiomycete fungus I. Microbiological aspects. Biol. Fertil. Soils 2000, 32, 374–380. [Google Scholar] [CrossRef]
- Cheng, L.J.; Lai, H.X.; Li, S.J.; Zhou, M.K. Effect of microorganism upon the formation of soil aggregates. Acta Agric. Boreali-Occident. Sin. 1994, 22, 93–97. (In Chinese) [Google Scholar]
- Yao, Y.F.; Ge, N.N.; Yu, S.; Wei, X.R.; Wang, X.; Jin, J.W.; Liu, X.T.; Shao, M.G.; Wei, Y.C.; Kang, L. Response of aggregate associated organic carbon, nitrogen and phosphorous to re-vegetation in agro-pastoral ecotone of northern China. Geoderma 2019, 341, 172–180. [Google Scholar] [CrossRef]
- Chivenge, P.; Vanlauwe, B.; Gentile, R.; Six, J. Organic resource quality influences short-term aggregate dynamics and soil organic carbon and nitrogen accumulation. Soil Biol. Biochem. 2011, 43, 657–666. [Google Scholar] [CrossRef]
- Duan, X.W.; Xie, Y.; Feng, Y.J.; Gao, X.F. A study of permanent wilting point in the northeast black soil regions. J. Soil Water Conserv. 2008, 22, 212–216. [Google Scholar] [CrossRef]
- Saha, D.; Kukal, S.S. Soil structural stability and water retention characteristics under different land uses of degraded lower himalayas of north-west India. Land Degrad. Dev. 2015, 26, 263–271. [Google Scholar] [CrossRef]
- Song, H.; Li, Z.; Du, B.; Wang, G.; Ding, Y. Bacterial communities in sediments of the shallow Lake Dongping in China. J. Appl. Microbiol. 2012, 112, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2013. (In Chinese) [Google Scholar]
- Ge, Z.B.; Yin, T.T.; Zhou, Q.Q.; Zhang, J.; Sheng, X.F.; He, L.Y. Reduced cadmium and lead uptake by leafy vegetables and soil remediation in the presence of the biofilm-producing Bacillus strains. J. Nanjing Agric. Univ. 2020, 43, 80–88. [Google Scholar] [CrossRef]
- Wei, Y.J.; Wu, X.L.; Xia, J.W.; Shen, X.; Cai, C.F. Variation of soil aggregation along the weathering gradient: Comparison of grain size distribution under different disruptive forces. PLoS ONE 2016, 11, e160960. [Google Scholar] [CrossRef]
- Liu, W.Z. Plant Pathogenic Nematology; China Agriculture Press: Beijing, China, 2003. (In Chinese) [Google Scholar]
- Bao, T.L.; Gao, L.Q.; Wang, S.S.; Yang, X.Q.; Ren, W.; Zhao, Y.G. Moderate disturbance increases the PLFA diversity and biomass of the microbial community in biocrusts in the Loess Plateau region of China. Plant Soil 2020, 451, 499–513. [Google Scholar] [CrossRef]
- Hill, G.T.; Mitkowski, N.A.; Aldrich-Wolfe, L.; Emele, L.R.; Jurkonie, D.D.; Fricke, A.; Maldonado-Ramirez, S.; Lynch, S.T.; Nelson, E.B. Methods for assessing the composition and diversity of soil microbial communities. Appl. Soil Ecol. 2000, 15, 25–36. [Google Scholar] [CrossRef]
- Djukic, I.; Zehetner, F.; Mentler, A.; Gerzabek, M.H. Microbial community composition and activity in different Alpine vegetation zones. Soil Biol. Biochem. 2010, 42, 155–161. [Google Scholar] [CrossRef]
- Guo, J.X.; Li, J.S.; Liu, K.S.; Tang, S.M.; Zhai, X.J.; Wang, K. Analysis of soil microbial dynamics at a cropland-grassland interface in an agro-pastoral zone in a temperate steppe in northern China. Catena 2018, 170, 257–265. [Google Scholar] [CrossRef]
- Picariello, E.; Baldantoni, D.; De Nicola, F. Acute effects of PAH contamination on microbial community of different forest soils. Environ. Pollut. 2020, 262, 114378. [Google Scholar] [CrossRef]
- Yan, Y.J.; Dai, Q.H.; Hu, G.; Jiao, Q.; Mei, L.; Fu, W.B. Effects of vegetation type on the microbial characteristics of the fissure soil-plant systems in karst rocky desertification regions of SW China. Sci. Total Environ. 2020, 712, 136543. [Google Scholar] [CrossRef]
- Mills, A.A.; Price, G.W.; Fillmore, S.A.E. Responses of nematode, bacterial, and fungal populations to high frequency applications and increasing rates of biosolids in an agricultural soil. Appl. Soil Ecol. 2020, 148, 103481. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, J.J.; Meng, Y.L.; Li, J.B.; Gaston, L.A.; Fultz, L.M.; Delaune, R.D. Potential use of biochar and rhamnolipid biosurfactant for remediation of crude oil-contaminated coastal wetland soil: Ecotoxicity assessment. Chemosphere 2020, 253, 126617. [Google Scholar] [CrossRef]
- Lopes, L.D.; Fernandes, M.F. Changes in microbial community structure and physiological profile in a kaolinitic tropical soil under different conservation agricultural practices. Appl. Soil Ecol. 2020, 152, 103545. [Google Scholar] [CrossRef]
- Jastrow, J.D.; Miller, R.M.; Lussenhop, J. Contributions of interacting biological mechanisms to soil aggregate stabilization in restored prairie. Soil Biol. Biochem. 1998, 30, 905–916. [Google Scholar] [CrossRef]
- Jakobsen, I.; Abbott, L.K.; Robson, A.D. External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum L. 1. Spread of hyphae and phosphorus inflow into roots. New Phytol. 1992, 120, 371–380. [Google Scholar] [CrossRef]
- An, S.; Zheng, F.; Zhang, F.; Van Pelt, S.; Hamer, U.; Makeschin, F. Soil quality degradation processes along a deforestation chronosequence in the Ziwuling area, China. Catena 2008, 75, 248–256. [Google Scholar] [CrossRef]
- Gianfreda, L.; Sannino, F.; Ortega, N.; Nannipieri, P. Activity of free and immobilized urease in soil: Effects of pesticides. Soil Biol. Biochem. 1994, 26, 777–784. [Google Scholar] [CrossRef]
- Waruru, B.K.; Shepherd, K.D.; Ndegwa, G.M.; Sila, A.M. Estimation of wet aggregation indices using soil properties and diffuse reflectance near infrared spectroscopy: An application of classification and regression tree analysis. Biosyst. Eng. 2016, 152, 148–164. [Google Scholar] [CrossRef]
- Schaller, F.W.; Stockinger, K.R. A comparison of 5 methods for expressing aggregation data. Soil Sci. Soc. Am. Proc. 1953, 17, 310–345. [Google Scholar] [CrossRef]
- Kemper, W.D.; Rosenau, R.C. Aggregate Stability and Size Distribution. In Methods of Soil Analysis: Part I, Physical and Mineralogical Methods; Agronomy Monograph No 9; American Society of Agronomy: Madison, WI, USA, 1986; pp. 425–442. [Google Scholar]
- Cebrián-Piqueras, M.A.; Trinogga, J.; Trenkamp, A.; Minden, V.; Maier, M.; Mantilla-Contreras, J. Digging into the roots: Understanding direct and indirect drivers of ecosystem service trade-offs in coastal grasslands via plant functional traits. Environ. Monit. Assess. 2021, 193, 271. [Google Scholar] [CrossRef]
- Motiei, A.; Ogonowski, M.; Reichelt, S.; Gorokhova, E. Ecotoxicological assessment of suspended solids: The importance of biofilm and particle aggregation. Environ. Pollut. 2021, 280, 116888. [Google Scholar] [CrossRef]
- Celik, I. Land-use effects on organic matter and physical properties of soil in a southern Mediterranean highland of Turkey. Soil Tillage Res. 2005, 83, 270–277. [Google Scholar] [CrossRef]
- Hurisso, T.T.; Davis, J.G.; Brummer, J.E.; Stromberger, M.E.; Mikha, M.M.; Haddi, M.L.; Booher, M.R.; Paul, E.A. Rapid changes in microbial biomass and aggregate size distribution in response to changes in organic matter management in grass pasture. Geoderma 2013, 193–194, 68–75. [Google Scholar] [CrossRef]
- Blair, N.; Faulkner, R.D.; Till, A.R.; Korschens, M.; Schulz, E. Long-term management impacts on soil C, N and physical fertility Part II: Bad Lauchstadt static and extreme FYM experiments. Soil Tillage Res. 2006, 91, 39–47. [Google Scholar] [CrossRef]
- Blair, N.; Faulkner, R.D.; Till, A.R.; Poulton, P.R. Long-term management impacts on soil C, N and physical fertility Part I: Broadbalk experiment. Soil Tillage Res. 2006, 91, 30–38. [Google Scholar] [CrossRef]
- Souza, E.D.; Costa, S.E.G.V.A.; Anghinoni, I.; Carneiro, M.A.C.; Martins, A.P.; Bayer, C. Soil quality indicators in a Rhodic Paleudult under long term tillage systems. Soil Tillage Res. 2014, 139, 28–36. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Elliott, E.T. Carbon and nitrogen distribution in aggregates from cultivated and native grassland soils. Soil Sci. Soc. Am. J. 1993, 57, 1071–1076. [Google Scholar] [CrossRef] [Green Version]
- Veum, K.S.; Goyne, K.W.; Kremer, R.; Motavalli, P.P. Relationships among water stable aggregates and organic matter fractions under conservation management. Soil Sci. Soc. Am. J. 2012, 76, 2143. [Google Scholar] [CrossRef]
- Carter, M.R.; Stewart, B.A. Structure and Organic Matter Storage in Agricultural Soils; CRC Press Inc.: Boca Raton, FL, USA, 1996. [Google Scholar]
- Barral, M.T.; Arias, M.; Guerif, J. Effect of iron and organic matter on the porosity and structural stability of soil aggregates. Soil Tillage Res. 1998, 46, 261–272. [Google Scholar] [CrossRef]
- De Gryze, S.; Six, J.; Brits, C.; Merckx, R. A quantification of short-term macroaggregate dynamics: Influences of wheat residue input and texture. Soil Biol. Biochem. 2004, 37, 55–66. [Google Scholar] [CrossRef]
- Ran, Y.G.; Ma, M.H.; Liu, Y.; Zhu, K.; Yi, X.M.; Wang, X.X.; Wu, S.J.; Huang, P. Physicochemical determinants in stabilizing soil aggregates along a hydrological stress gradient on reservoir riparian habitats: Implications to soil restoration. Ecol. Eng. 2020, 143, 105664. [Google Scholar] [CrossRef]
- Albalasmeh, A.A.; Ghezzehei, T.A. Interplay between soil drying and root exudation in rhizosheath development. Plant Soil 2014, 374, 739–751. [Google Scholar] [CrossRef]
- Denef, K.; Six, J.; Bossuyt, H.; Frey, S.D.; Elliott, E.T.; Merckx, R.; Paustian, K. Influence of dry-wet cycles on the interrelationship between aggregate, particulate organic matter, and microbial community dynamics. Soil Biol. Biochem. 2001, 33, 1599–1611. [Google Scholar] [CrossRef]
- Egan, G.; Crawley, M.J.; Fornara, D.A. Effects of long-term grassland management on the carbon and nitrogen pools of different soil aggregate fractions. Sci. Total Environ. 2018, 613, 810–819. [Google Scholar] [CrossRef]
- Igwe, C.A.; Zarei, M.; Stahr, K. Colloidal stability in some tropical soils of southeastern Nigeria as affected by iron and aluminium oxides. Catena 2009, 77, 232–237. [Google Scholar] [CrossRef]
- Zhao, J.S.; Chen, S.; Hu, R.G.; Li, Y.Y. Aggregate stability and size distribution of red soils under different land uses integrally regulated by soil organic matter, and iron and aluminum oxides. Soil Tillage Res. 2017, 167, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Yan, X.; Zhou, H.; Zhang, Y.Z.; Sun, H. Assessing the contributions of sesquioxides and soil organic matter to aggregation in an Ultisol under long-term fertilization. Soil Tillage Res. 2015, 146, 89–98. [Google Scholar] [CrossRef]
- Duiker, S.W.; Rhoton, F.E.; Torrent, J.; Smeck, N.E.; Lal, R. Iron (hydr)oxide crystallinity effects on soil aggregation. Soil Sci. Soc. Am. J. 2003, 67, 606–611. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, L.; Liang, C.H.; Xi, F.M.; Pei, Z.J.; Du, L.Y. Soil aggregate stability and Iron and aluminium oxide contents under different fertiliser treatments in a long-term solar greenhouse experiment. Pedosphere 2016, 26, 760–767. [Google Scholar] [CrossRef]
- Spohn, M.; Giani, L. Water-stable aggregates, glomalin-related soil protein, and carbohydrates in a chronosequence of sandy hydromorphic soils. Soil Biol. Biochem. 2010, 42, 1505–1511. [Google Scholar] [CrossRef]
- Galloway, A.F.; Pedersen, M.J.; Merry, B.; Marcus, S.E.; Blacker, J.; Benning, L.G.; Field, K.J.; Knox, J.P. Xyloglucan is released by plants and promotes soil particle aggregation. New Phytol. 2018, 217, 1128–1136. [Google Scholar] [CrossRef] [Green Version]
- Wick, A.F.; Daniels, W.L.; Nash, W.L.; Burger, J.A. Aggregate recovery in reclaimed coal mine soils of SW virginia. Land Degrad. Dev. 2016, 27, 965–972. [Google Scholar] [CrossRef]
- Kavdır, Y.; Ekinci, H.; Yuksel, O.; Mermut, A.R. Soil aggregate stability and 13C CP/MAS-NMR assessment of organic matter in soils influenced by forest wildfires in Çanakkale, Turkey. Geoderma 2005, 129, 219–229. [Google Scholar] [CrossRef]
- Singh, S.; Singh, J.S. Water-stable aggregates and associated organic matter in forest, savanna, and cropland soils of a seasonally dry tropical region, India. Biol. Fertil. Soils 1996, 22, 76–82. [Google Scholar] [CrossRef]
- Fonte, S.J.; Yeboah, E.; Ofori, P.; Quansah, G.W.; Vanlauwe, B.; Six, J. Fertilizer and residue quality effects on organic matter stabilization in soil aggregates. Soil Sci. Soc. Am. J. 2009, 73, 961–966. [Google Scholar] [CrossRef] [Green Version]
- Bossuyt, H.; Denef, K.; Six, J.; Frey, S.D.; Merckx, R.; Paustian, K. Influence of microbial populations and residue quality on aggregate stability. Appl. Soil Ecol. 2001, 16, 195–208. [Google Scholar] [CrossRef]
- Zhong, Z.K.; Han, X.H.; Xu, Y.D.; Zhang, W.; Fu, S.Y.; Liu, W.C.; Ren, C.J.; Yang, G.H.; Ren, G.X. Effects of land use change on organic carbon dynamics associated with soil aggregate fractions on the Loess Plateau, China. Land Degrad. Dev. 2019, 30, 1070–1082. [Google Scholar] [CrossRef]
- Xu, H.D.; Yuan, H.J.; Yu, M.K.; Cheng, X.R. Large macroaggregate properties are sensitive to the conversion of pure plantation to uneven-aged mixed plantations. Catena 2020, 194, 104724. [Google Scholar] [CrossRef]
- Piazza, G.; Pellegrino, E.; Moscatelli, M.C.; Ercoli, L. Long-term conservation tillage and nitrogen fertilization effects on soil aggregate distribution, nutrient stocks and enzymatic activities in bulk soil and occluded microaggregates. Soil Tillage Res. 2020, 196, 104482. [Google Scholar] [CrossRef]
- Sall, S.N.; Masse, D.; Diallo, N.H.; Sow, T.M.B.; Hien, E.; Guisse, A. Effects of residue quality and soil mineral N on microbial activities and soil aggregation in a tropical sandy soil in Senegal. Eur. J. Soil Biol. 2016, 75, 62–69. [Google Scholar] [CrossRef]
- Lehmann, A.; Zheng, W.S.; Ryo, M.; Soutschek, K.; Roy, J.; Rongstock, R.; Maass, S.; Rillig, M.C. Fungal traits important for soil aggregation. Front. Microbiol. 2020, 10, 2904. [Google Scholar] [CrossRef]
- Zelles, L. Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: A review. Biol. Fertil. Soils 1999, 29, 111–129. [Google Scholar] [CrossRef]
- Blaud, A.; Lerch, T.Z.; Chevallier, T.; Nunan, N.; Chenu, C.; Brauman, A. Dynamics of bacterial communities in relation to soil aggregate formation during the decomposition of 13C-labelled rice straw. Appl. Soil Ecol. 2012, 53, 1–9. [Google Scholar] [CrossRef]
- Cui, H.; Ou, Y.; Lv, D.; Wang, L.X.; Liang, A.Z.; Yan, B.X.; Li, Y.X. Aggregate-related microbial communities and nutrient stoichiometry under different croplands. Ecol. Process. 2020, 9, 33. [Google Scholar] [CrossRef]
- Lehmann, A.; Zheng, W.S.; Rillig, M.C. Soil biota contributions to soil aggregation. Nat. Ecol. Evol. 2017, 1, 1828–1835. [Google Scholar] [CrossRef]
- McCalla, T.M. Influence of some microbial groups on stablizing soil structure against falling water drops. Soil Sci. Soc. Am. J. 1947, 11, 260–263. [Google Scholar] [CrossRef] [Green Version]
- Kohler, J.; Roldan, A.; Campoy, M.; Caravaca, F. Unraveling the role of hyphal networks from arbuscular mycorrhizal fungi in aggregate stabilization of semiarid soils with different textures and carbonate contents. Plant Soil 2017, 410, 273–281. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Mallik, A.; Zhang, J.C.; Huang, Y.Q.; Zhou, L.W. Effects of arbuscular mycorrhizal fungi on inoculated seedling growth and rhizosphere soil aggregates. Soil Tillage Res. 2019, 194, 104340. [Google Scholar] [CrossRef]
- Kanazawa, S.; Filip, Z. Distribution of microorganisms, total biomass, and enzyme activities in different particles of brown soil. Microb. Ecol. 1986, 12, 205–215. [Google Scholar] [CrossRef]
- Allison, S.D.; Jastrow, J.D. Activities of extracellular enzymes in physically isolated fractions of restored grassland soils. Soil Biol. Biochem. 2006, 38, 3245–3256. [Google Scholar] [CrossRef]
- Shi, B.K.; Zhang, J.M.; Wang, C.L.; Ma, J.Y.; Sun, W. Responses of hydrolytic enzyme activities in saline-alkaline soil to mixed inorganic and organic nitrogen addition. Sci. Rep. 2018, 8, 4543. [Google Scholar] [CrossRef] [Green Version]
| Soil Physicochemical Parameter | Managed Year | ||||
|---|---|---|---|---|---|
| C0 | C3 | C10 | C30 | C50 | |
| Bulk density (g cm−3) | 1.25 ± 0.05 a | 1.28 ± 0.03 a | 1.28 ± 0.07 a | 1.23 ± 0.04 a | 1.26 ± 0.03 a |
| Total soil porosity (%) | 52.72 ± 1.74 a | 51.74 ± 1.22 a | 51.63 ± 2.74 a | 53.70 ± 1.39 a | 52.30 ± 1.03 a |
| Clay content (%) | 25.92 ± 1.99 a,b | 29.07 ± 1.34 a | 27.60 ± 1.76 a | 22.95 ± 0.92 b | 18.63 ± 2.77 b |
| Soil water content (%) | 13.60 ± 1.42 a,b | 13.17 ± 1.17 a,b | 12.69 ± 0.50 a,b | 16.52 ± 1.38 a | 10.57 ± 0.58 b |
| Maximum hygroscopicity (%) | 7.91 ± 0.68 a | 8.92 ± 0.38 a | 5.67 ± 0.24 b | 5.81 ± 0.33 b | 5.07 ± 0.17 b |
| pH | 9.55 ± 0.19 a | 9.21 ± 0.11 a | 8.70 ± 0.02 a,b | 8.62 ± 0.03 b | 8.06 ± 0.10 c |
| Electrical conductivity (μs cm−1) | 388.92 ± 77.20 a,b | 143.54 ± 22.23 a,b | 103.19 ± 4.23 b | 123.67 ± 6.51 b | 253.28 ± 25.44 a |
| Soil organic carbon (g kg−1) | 24.56 ± 2.45 a,b,c | 15.44 ± 1.33 b,c | 16.74 ± 0.60 c | 19.56 ± 0.46 b | 34.37 ± 1.71 a |
| Total carbon (g kg−1) | 39.62 ± 3.23 a | 28.06 ± 1.87 b | 20.63 ± 1.38 b | 26.60 ± 1.31 b | 43.15 ± 0.96 a |
| Total nitrogen (g kg−1) | 2.65 ± 0.28 a,b | 1.41 ± 0.08 b | 1.61 ± 0.06 b | 1.79 ± 0.10 b | 3.34 ± 0.08 a |
| C:N | 15.20 ± 0.61 a,b,c | 20.06 ± 1.36 a | 12.82 ± 0.64 b,c | 14.92 ± 0.37 a,b | 12.91 ± 0.12 c |
| Soil ammonia-nitrogen (mg kg−1) | 13.48 ± 0.96 b | 10.74 ± 0.93 b | 12.44 ± 0.93 b | 23.51 ± 0.54 a | 24.49 ± 0.46 a |
| Soil nitrite-nitrogen (mg kg−1) | 9.98 ± 1.56 b | 10.18 ± 0.98 b | 9.22 ± 0.43 b | 14.42 ± 1.35 b | 54.55 ± 2.53 a |
| Total phosphorus (g kg−1) | 0.64 ± 0.02 b | 0.49 ± 0.04 c | 0.47 ± 0.02 c | 0.63 ± 0.02 b | 1.13 ± 0.04 a |
| Polysaccharides (mg g−1) | 1.82 ± 0.21 a | 1.18 ± 0.08 b | 1.00 ± 0.12 b | 1.09 ± 0.11 b | 1.47 ± 0.07 a,b |
| Complex Fe oxides (g kg−1) | 0.18 ± 0.02 a | 0.14 ± 0.01 a | 0.18 ± 0.02 a | 0.17 ± 0.02 a | 0.19 ± 0.02 a |
| Free Fe oxides (g kg−1) | 6.87 ± 0.50 a,b | 6.11 ± 0.34 a,b | 7.45 ± 0.18 a | 7.54 ± 0.13 a | 6.26 ± 0.12 b |
| Soil Biological Parameter | Managed Year | ||||
|---|---|---|---|---|---|
| C0 | C3 | C10 | C30 | C50 | |
| Nematode (individuals/100 g dry soil) | 420.33 ± 26.80 a | 448.67 ± 7.90 a | 506.83 ± 17.78 a | 479.00 ± 23.82 a | 555.67 ± 39.89 a |
| Soil microbial biomass carbon (mg kg−1) | 1870.83 ± 177.26 a | 1639.64 ± 165.25 a,b | 966.88 ± 60.84 b | 983.34 ± 70.83 b | 858.12 ± 20.04 b |
| Soil microbial biomass nitrogen (mg kg−1) | 177.53 ± 22.30 a | 131.44 ± 25.91 a,b | 81.39 ± 6.56 a,b | 70.14 ± 6.89 b | 62.92 ± 4.78 b |
| Total PLFA (nmol g−1) | 43.15 ± 5.40 a | 23.11 ± 2.94 b | 23.55 ± 1.49 b | 25.02 ± 1.80 b | 53.21 ± 1.84 a |
| Bacteria (nmol g−1) | 32.39 ± 3.98 a | 17.43 ± 2.36 b | 17.53 ± 1.12 b | 18.05 ± 1.27 b | 39.30 ± 1.10 a |
| Fungi (nmol g−1) | 3.55 ± 0.62 b | 2.69 ± 0.13 b | 2.34 ± 0.19 b | 2.80 ± 0.22 b | 5.12 ± 0.38 a |
| Actinomycetes (nmol g−1) | 5.70 ± 0.69 a | 2.74 ± 0.27 b | 2.88 ± 0.26 b | 3.44 ± 0.29 b | 6.33 ± 0.16 a |
| Gram-negative bacteria(G−) (nmol g−1) | 14.24 ± 1.78 a | 6.26 ± 1.23 b | 4.84 ± 0.47 b | 4.98 ± 0.57 b | 12.93 ± 0.88 a |
| Gram-positive bacteria (G+) (nmol g−1) | 9.23 ± 1.11 b | 4.51 ± 0.64 c | 4.92 ± 0.32 c | 6.25 ± 0.45 c | 12.70 ± 0.41 a |
| Arbuscular mycorrhizal fungi (nmol g−1) | 1.50 ± 0.26 a,b | 0.69 ± 0.09 b | 0.80 ± 0.09 b | 0.73 ± 0.09 b | 2.46 ± 0.28 a |
| AMF hyphae (m g−1) | 1.33 ± 0.03 a | 0.99 ± 0.02 b | 0.99 ± 0.02 b | 0.90 ± 0.03 b | 0.63 ± 0.06 c |
| Urease (NH4+-N mg/ (100 g·24 h)) | 21.56 ± 2.03 a,b | 22.85 ± 0.64 b | 21.70 ± 0.46 b | 23.23 ± 0.51 b | 26.10 ± 0.19 a |
| Alkal phosphates (phenol μg/(g·24 h)) | 2283.83 ± 232.35 a,b | 2129.67 ± 44.94 b | 2082.17 ± 36.77 b | 2139.67 ± 61.30 a,b | 2390.50 ± 29.63 a |
| Sucrase (glucose mg/g·24 h) | 5.22 ± 0.39 b | 3.10 ± 0.43 c | 4.98 ± 0.27 b | 5.18 ± 0.37 b | 7.24 ± 0.24 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, C.; Liu, K.; Dou, P.; Li, J.; Wang, K. The Changes in Soil Microorganisms and Soil Chemical Properties Affect the Heterogeneity and Stability of Soil Aggregates before and after Grassland Conversion. Agriculture 2022, 12, 307. https://doi.org/10.3390/agriculture12020307
Ren C, Liu K, Dou P, Li J, Wang K. The Changes in Soil Microorganisms and Soil Chemical Properties Affect the Heterogeneity and Stability of Soil Aggregates before and after Grassland Conversion. Agriculture. 2022; 12(2):307. https://doi.org/10.3390/agriculture12020307
Chicago/Turabian StyleRen, Cheng, Kesi Liu, Pengpeng Dou, Jiahuan Li, and Kun Wang. 2022. "The Changes in Soil Microorganisms and Soil Chemical Properties Affect the Heterogeneity and Stability of Soil Aggregates before and after Grassland Conversion" Agriculture 12, no. 2: 307. https://doi.org/10.3390/agriculture12020307
APA StyleRen, C., Liu, K., Dou, P., Li, J., & Wang, K. (2022). The Changes in Soil Microorganisms and Soil Chemical Properties Affect the Heterogeneity and Stability of Soil Aggregates before and after Grassland Conversion. Agriculture, 12(2), 307. https://doi.org/10.3390/agriculture12020307







