The Synergetic Effect of Soil Amendments on Reducing Bioavailable Heavy Metals and Greenhouse Gas Emissions from Upland Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Amendments
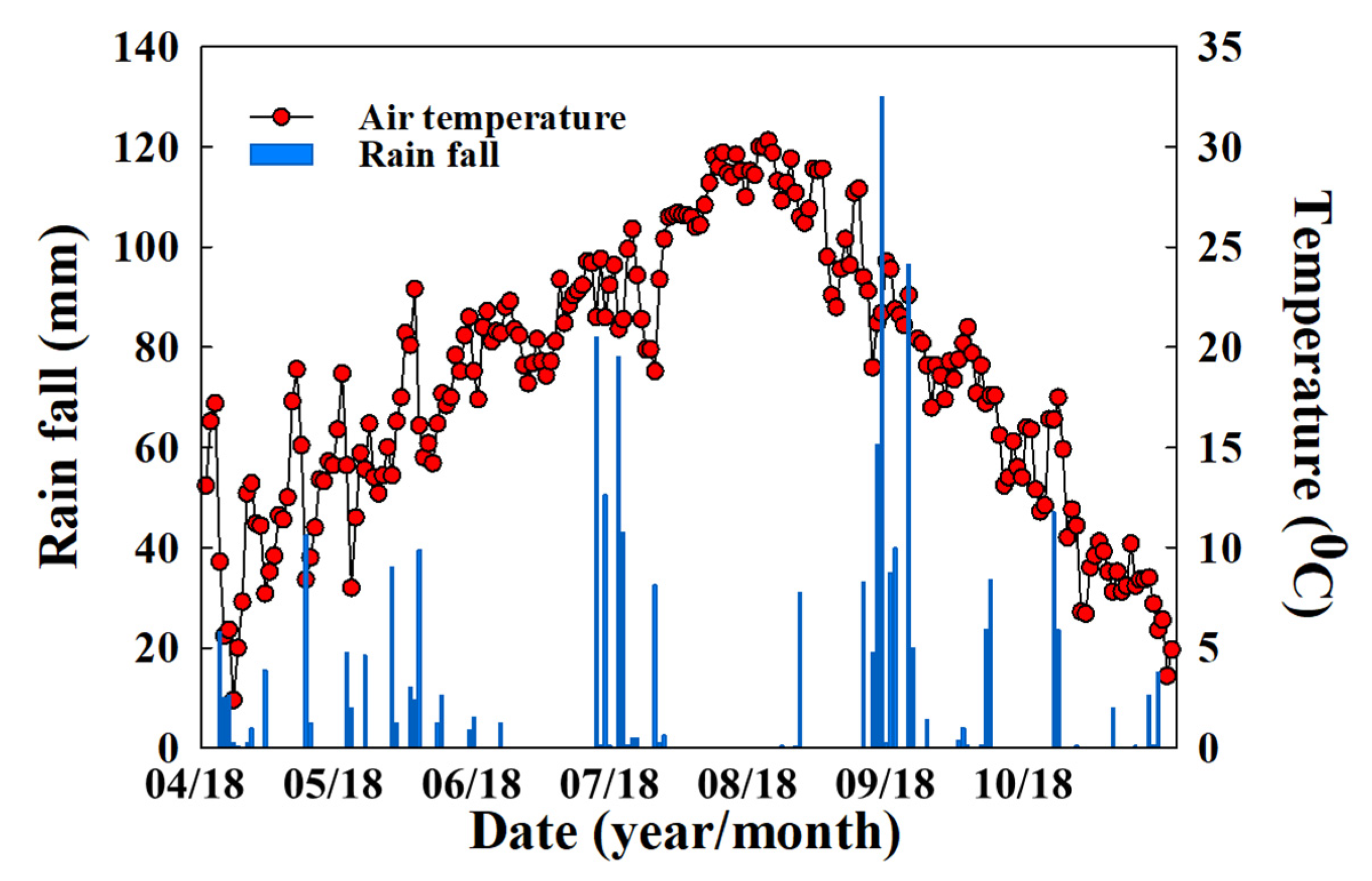
2.2. Site Description and Experimental Field Setup
2.3. Soil and Plant Sampling
2.4. Chemical and Heavy Metal Analyses of Soil and Plants
2.5. Monitoring Greenhouse Gas Emissions
2.6. Statistical Analysis
3. Results and Discussion
3.1. Properties of Amendments and Upland Soil
3.2. Effect of Amendments on Soil Chemical Properties
3.3. The Effect of Amendments on Reducing the Bioavailable Heavy Metals in Soil
3.4. The Effect of Amendments on Heavy Metal Bioaccumulation
3.5. The Effect of Amendments on Greenhouse Gas Emission Reduction and Crop Yield
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal(loid)s contaminated soils--to mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.K.; Kim, J.W.; Kim, H.S.; Lee, S.P.; Yang, J.E.; Kim, S.C. Bottom Ash Modification via Sintering Process for Its Use as a Potential Heavy Metal Adsorbent: Sorption Kinetics and Mechanism. Materials 2021, 14, 3060. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Lim, S.S.; Park, H.J.; Yang, H.I.; Park, S.I.; Kwak, J.H.; Choi, W.J. Fly ash and zeolite decrease metal uptake but do not improve rice growth in paddy soils contaminated with Cu and Zn. Environ. Int. 2019, 129, 551–564. [Google Scholar] [CrossRef]
- Chapuis-Lardy, L.; Wrage, N.; Metay, A.; Chotte, J.-L.; Bernoux, M. Soils, a sink for N2O? A review. Glob. Chang. Biol. 2007, 13, 1–17. [Google Scholar] [CrossRef]
- Feng, Z.; Zhu, L. Impact of biochar on soil N2O emissions under different biochar-carbon/fertilizer-nitrogen ratios at a constant moisture condition on a silt loam soil. Sci. Total Environ. 2017, 584–585, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Farrell, M.; Perkins, W.T.; Hobbs, P.J.; Griffith, G.W.; Jones, D.L. Migration of heavy metals in soil as influenced by compost amendments. Environ. Pollut. 2010, 158, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Rosen, V.; Chen, Y. Effects of compost application on soil vulnerability to heavy metal pollution. Environ. Sci. Pollut. Res. Int. 2018, 25, 35221–35231. [Google Scholar] [CrossRef]
- Kumarathilaka, P.; Seneweera, S.; Meharg, A.; Bundschuh, J. Arsenic speciation dynamics in paddy rice soil-water environment: Sources, physico-chemical, and biological factors—A review. Water Res 2018, 140, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Oertel, C.; Matschullat, J.; Zurba, K.; Zimmermann, F.; Erasmi, S. Greenhouse gas emissions from soils—A review. Geochemistry 2016, 76, 327–352. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.H.; Kim, S.U.; Han, H.R.; Hur, D.Y.; Owens, V.N.; Kumar, S.; Hong, C.O. Mitigation of global warming potential and greenhouse gas intensity in arable soil with green manure as source of nitrogen. Environ. Pollut. 2021, 288, 117724. [Google Scholar] [CrossRef]
- Ruangcharus, C.; Kim, S.U.; Yoo, G.Y.; Choi, E.J.; Kumar, S.; Kang, N.; Hong, C.O. Nitrous oxide emission and sweet potato yield in upland soil: Effects of different type and application rate of composted animal manures. Environ. Pollut. 2021, 279, 116892. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, X.-y.; Lei, M.; Yang, J.; Ma, J.; Qiao, P.-w.; Chen, T.-b. Migration and transformation of arsenic: Contamination control and remediation in realgar mining areas. Appl. Geochem. 2017, 77, 44–51. [Google Scholar] [CrossRef]
- Yao, A.; Ju, L.; Ling, X.; Liu, C.; Wei, X.; Qiu, H.; Tang, Y.; Morel, J.L.; Qiu, R.; Li, C.; et al. Simultaneous attenuation of phytoaccumulation of Cd and As in soil treated with inorganic and organic amendments. Environ. Pollut. 2019, 250, 464–474. [Google Scholar] [CrossRef]
- Derakhshan Nejad, Z.; Jung, M.C.; Kim, K.H. Remediation of soils contaminated with heavy metals with an emphasis on immobilization technology. Environ. Geochem. Health 2018, 40, 927–953. [Google Scholar] [CrossRef]
- Huang, J.H.; Hsu, S.H.; Wang, S.L. Effects of rice straw ash amendment on Cu solubility and distribution in flooded rice paddy soils. J. Hazard. Mater. 2011, 186, 1801–1807. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ogata, F.; Nakamura, T.; Kawasaki, N. Synthesis of novel zeolites produced from fly ash by hydrothermal treatment in alkaline solution and its evaluation as an adsorbent for heavy metal removal. J. Environ. Chem. Eng. 2020, 8, 103687. [Google Scholar] [CrossRef]
- Xu, D.; Ji, P.; Wang, L.; Zhao, X.; Hu, X.; Huang, X.; Zhao, H.; Liu, F. Effect of modified fly ash on environmental safety of two soils contaminated with cadmium and lead. Ecotoxicol. Environ. Saf. 2021, 215, 112175. [Google Scholar] [CrossRef]
- Alam, Q.; Dezaire, T.; Gauvin, F.; Delsing, A.C.A.; Brouwers, H.J.H. Valorization of bottom ash fines by surface functionalization to reduce leaching of harmful contaminants. J. Environ. Manag. 2020, 271, 110884. [Google Scholar] [CrossRef] [PubMed]
- Chindaprasirt, P.; Jaturapitakkul, C.; Chalee, W.; Rattanasak, U. Comparative study on the characteristics of fly ash and bottom ash geopolymers. Waste Manag. 2009, 29, 539–543. [Google Scholar] [CrossRef]
- Levandowski, J.; Kalkreuth, W. Chemical and petrographical characterization of feed coal, fly ash and bottom ash from the Figueira Power Plant, Paraná, Brazil. Int. J. Coal Geol. 2009, 77, 269–281. [Google Scholar] [CrossRef]
- Bethanis, S.; Cheeseman, C.R.; Sollars, C.J. Properties and microstructure of sintered incinerator bottom ash. Ceram. Int. 2002, 28, 881–886. [Google Scholar] [CrossRef]
- Geetha, S.; Ramamurthy, K. Properties of sintered low calcium bottom ash aggregate with clay binders. Constr. Build. Mater. 2011, 25, 2002–2013. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Mañosa, J.; Maldonado-Alameda, A.; Quina, M.J.; Chimenos, J.M. Rapid sintering of weathered municipal solid waste incinerator bottom ash and rice husk for lightweight aggregate manufacturing and product properties. J. Clean. Prod. 2019, 232, 713–721. [Google Scholar] [CrossRef]
- Luo, H.; Wu, Y.; Zhao, A.; Kumar, A.; Liu, Y.; Cao, B.; Yang, E.-H. Hydrothermally synthesized porous materials from municipal solid waste incineration bottom ash and their interfacial interactions with chloroaromatic compounds. J. Clean. Prod. 2017, 162, 411–419. [Google Scholar] [CrossRef]
- Pena, R.; Guerrero, A.; Goni, S. Hydrothermal treatment of bottom ash from the incineration of municipal solid waste: Retention of Cs(I), Cd(II), Pb(II) and Cr(III). J. Hazard. Mater. 2006, 129, 151–157. [Google Scholar] [CrossRef]
- Chiang, Y.W.; Ghyselbrecht, K.; Santos, R.M.; Meesschaert, B.; Martens, J.A. Synthesis of zeolitic-type adsorbent material from municipal solid waste incinerator bottom ash and its application in heavy metal adsorption. Catal. Today 2012, 190, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Ipsilantis, I.; Coyne, M.S. Soil microbial community response to hexavalent chromium in planted and unplanted soil. J. Environ. Qual. 2007, 36, 638–645. [Google Scholar] [CrossRef]
- Linn, D.M.; Doran, J.W. Effect of water-filled pore space on carbon dioxide and nitrous oxide production in tilled and nontilled soils. Soil Sci. Soc. Am. J. 1984, 48, 1267–1272. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.U.; Lee, H.H.; Moon, S.M.; Han, H.R.; Hong, C.O. Nitrous oxide emissions and maize yield as influenced by nitrogen fertilization and tillage operations in upland soil. Appl. Biol. Chem. 2021, 64, 18. [Google Scholar] [CrossRef]
- Luo, H.; He, D.; Zhu, W.; Wu, Y.; Chen, Z.; Yang, E.H. Humic acid-induced formation of tobermorite upon hydrothermal treatment with municipal solid waste incineration bottom ash and its application for efficient removal of Cu(II) ions. Waste Manag. 2019, 84, 83–90. [Google Scholar] [CrossRef]
- Reddy, K.R.; Vardanyan, L.; Hu, J.; Villapando, O.; Bhomia, R.K.; Smith, T.; Harris, W.G.; Newman, S. Soil phosphorus forms and storage in stormwater treatment areas of the Everglades: Influence of vegetation and nutrient loading. Sci. Total Environ. 2020, 725, 138442. [Google Scholar] [CrossRef] [PubMed]
- Aftabtalab, A.; Rinklebe, J.; Shaheen, S.M.; Niazi, N.K.; Moreno-Jimenez, E.; Schaller, J.; Knorr, K.H. Review on the interactions of arsenic, iron (oxy)(hydr)oxides, and dissolved organic matter in soils, sediments, and groundwater in a ternary system. Chemosphere 2022, 286, 131790. [Google Scholar] [CrossRef]
- Stopelli, E.; Duyen, V.T.; Mai, T.T.; Trang, P.T.K.; Viet, P.H.; Lightfoot, A.; Kipfer, R.; Schneider, M.; Eiche, E.; Kontny, A.; et al. Spatial and temporal evolution of groundwater arsenic contamination in the Red River delta, Vietnam: Interplay of mobilisation and retardation processes. Sci. Total Environ. 2020, 717, 137143. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ma, L.; Liang, Y.; Gao, B.; Harris, W. Simultaneous immobilization of lead and atrazine in contaminated soils using dairy-manure biochar. Environ. Sci. Technol. 2011, 45, 4884–4889. [Google Scholar] [CrossRef]
- Li, H.; Xu, H.; Zhou, S.; Yu, Y.; Li, H.; Zhou, C.; Chen, Y.; Li, Y.; Wang, M.; Wang, G. Distribution and transformation of lead in rice plants grown in contaminated soil amended with biochar and lime. Ecotoxicol. Environ. Saf. 2018, 165, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, G.; Zheng, H.; Li, F.; Ngo, H.H.; Guo, W.; Liu, C.; Chen, L.; Xing, B. Investigating the mechanisms of biochar’s removal of lead from solution. Bioresour. Technol. 2015, 177, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Yuan, S.; Hong, M.; Zhang, L.; Huang, Q. Mechanism of negative surface charge formation on biochar and its effect on the fixation of soil Cd. J. Hazard. Mater. 2020, 384, 121370. [Google Scholar] [CrossRef] [PubMed]
- He, L.L.; Huang, D.Y.; Zhang, Q.; Zhu, H.H.; Xu, C.; Li, B.; Zhu, Q.H. Meta-analysis of the effects of liming on soil pH and cadmium accumulation in crops. Ecotoxicol. Environ. Saf. 2021, 223, 112621. [Google Scholar] [CrossRef]
- Cao, X.; Gao, X.; Zeng, X.; Ma, Y.; Gao, Y.; Baeyens, W.; Jia, Y.; Liu, J.; Wu, C.; Su, S. Seeking for an optimal strategy to avoid arsenic and cadmium over-accumulation in crops: Soil management vs cultivar selection in a case study with maize. Chemosphere 2021, 272, 129891. [Google Scholar] [CrossRef]
- Cao, X.; Bai, L.; Zeng, X.; Zhang, J.; Wang, Y.; Wu, C.; Su, S. Is maize suitable for substitution planting in arsenic-contaminated farmlands? Plant Soil Environ. 2019, 65, 425–434. [Google Scholar] [CrossRef]
- Davidson, E.A.; LKeller, M.; Erickson, H.E.; Verchot, L.V.; Veldkamp, E. Testing a conceptual model of soil emissions of nitrous and nitric oxides. Bioscience 2000, 50, 667–680. [Google Scholar] [CrossRef]
- Kim, D.G.; Vargas, R.; Bond-Lamberty, B.; Turetsky, M.R. Effects of soil rewetting and thawing on soil gas fluxes: A review of current literature and suggestions for future research. Biogeosciences 2012, 9, 2459–2483. [Google Scholar] [CrossRef] [Green Version]
- da Silva Cardoso, A.; Junqueira, J.B.; Reis, R.A.; Ruggieri, A.C. How do greenhouse gas emissions vary with biofertilizer type and soil temperature and moisture in a tropical grassland? Pedosphere 2020, 30, 607–617. [Google Scholar] [CrossRef]
- Hu, J.; Inglett, K.S.; Wright, A.L.; Reddy, K.R. Nitrous Oxide Production and Reduction in Seasonally-Flooded Cultivated Peatland Soils. Soil Sci. Soc. Am. J. 2016, 80, 783–793. [Google Scholar] [CrossRef]
- Tarin, M.W.K.; Khaliq, M.A.; Fan, L.; Xie, D.; Tayyab, M.; Chen, L.; He, T.; Rong, J.; Zheng, Y. Divergent consequences of different biochar amendments on carbon dioxide (CO2) and nitrous oxide (N2O) emissions from the red soil. Sci. Total Environ. 2021, 754, 141935. [Google Scholar] [CrossRef]
- Abalos, D.; Liang, Z.; Dörsch, P.; Elsgaard, L. Trade-offs in greenhouse gas emissions across a liming-induced gradient of soil pH: Role of microbial structure and functioning. Soil Biol. Biochem. 2020, 150, 108006. [Google Scholar] [CrossRef]
- Nobile, C.; Denier, J.; Houben, D. Linking biochar properties to biomass of basil, lettuce and pansy cultivated in growing media. Sci. Hortic. 2020, 261, 109001. [Google Scholar] [CrossRef] [Green Version]

| pH | EC | OM | Av. P2O5 | Total Heavy Metal Concentration | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| As | Cd | Pb | Cu | Zn | Ni | |||||
| (1:5) | dS/m | % | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | |
| BA | 8.02 ± 0.61 b | 0.19 ± 0.05 c | 0.05 ± 0.01 c | 30.04 ± 3.2 b | 1.26 ± 0.61 | 0.22 ± 0.02 | 7.41 ± 3.37 | 5.13 ± 0.05 | 14.52 ± 4.49 | 15.22 ± 0.27 |
| SM10 | 8.68 ± 0.11 a | 1.29 ± 0.03 b | 0.25 ± 0.10 b | 117.9 ± 11.2 a | 10.4 ± 0.96 | 0.66 ± 0.14 | 4.23 ± 0.22 | 17.23 ± 0.72 | 31.45 ± 1.41 | 13.43 ± 1.03 |
| SM10 + L | 9.00 ± 0.04 a | 1.25 ± 0.07 b | 0.35 ± 0.01 a | 102.2 ± 1.1 a | ||||||
| SM10 + FeO | 8.15 ± 0.05 b | 1.62 ± 0.05 a | 0.21 ± 0.10 b | 36.90 ± 11.9 b | ||||||
| Sand | Silt | Clay | Texture | pH | EC | OM | Av. P2O5 | Total Heavy Metal Concentration | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| As | Cd | Pb | |||||||||
| % | % | % | dS/m | % | mg/kg | mg/kg | mg/kg | mg/kg | |||
| Soil | 36.2 | 50.4 | 13.4 | Silt loam | 7.23 ± 0.31 | 1.46 ± 0.12 | 3.66 ± 0.87 | 1131.8 ± 26.31 | 94.9 ± 4.28 | 4.2 ± 1.01 | 338.5 ± 22.5 |
| Optimum range/threshold value | 6.0–6.5 | <2.0 | 2.0–3.0 | 300–500 | 25 | 4 | 200 | ||||
| Treatment | pH | EC | OM | Av. P2O5 |
|---|---|---|---|---|
| (1:5) | dS/m | % | mg/kg | |
| Control | 7.30 ± 0.01 c | 0.51 ± 0.01 b | 3.49 ± 0.22 a | 1246.8 ± 46.1 a |
| BA | 7.60 ± 0.06 bc | 0.40 ± 0.01 c | 2.86 ± 0.44 bc | 844.1 ± 42.2 b |
| SM | 7.37 ± 0.08 c | 0.61 ± 0.20 a | 3.03 ± 0.25 b | 1110.2 ± 31.8 a |
| SM + L | 7.96 ± 0.19 ab | 0.66 ± 0.04 a | 1.94 ± 0.06 d | 857.4 ± 46.2 b |
| SM + FeO | 8.19 ± 0.36 a | 0.64 ± 0.01 a | 2.44 ± 0.06 c | 1170.6 ± 64.7 a |
| Heavy Metals | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| As | Cd | Pb | |||||||
| mg/kg | mg/kg | mg/kg | |||||||
| Before | After | Reduction Efficiency (%) | Before | After | Reduction Efficiency (%) | Before | After | Reduction Efficiency (%) | |
| Control | 2.12 ± 0.11 a | 3.45 ± 0.06 a | 0.35 ± 0.03 a | 0.41 ± 0.01 a | 55.53 ± 3.09 b | 60.04 ± 0.89 a | |||
| BA | 2.66 ± 0.06 a | 1.39 ± 0.17 c | 47.7 | 0.36 ± 0.01 a | 0.44 ± 0.01 a | −22.2 | 70.40 ± 0.55 a | 63.76 ± 0.92a | 9.4 |
| SM | 2.29 ± 0.06 a | 1.93 ± 0.05 bc | 15.7 | 0.31 ± 0.01 a | 0.41 ± 0.01 a | −32.3 | 52.36 ± 1.32 b | 46.00 ± 0.84 b | 12.1 |
| SM + L | 2.61 ± 0.07 a | 2.15 ± 0.08 b | 17.6 | 0.37 ± 0.03 a | 0.08 ± 0.01 b | 64.6 | 64.63 ± 4.98 a | 37.88 ± 0.31 b | 41.4 |
| SM + FeO | 2.20 ± 0.04 a | 0.46 ± 0.03 d | 79.1 | 0.31 ± 0.01 a | 0.36 ± 0.01 a | 55.4 | 55.49 ± 1.53 b | 35.77 ± 2.85 b | 35.5 |
| Heavy Metals | ||||||
|---|---|---|---|---|---|---|
| As | Cd | Pb | ||||
| mg/kg | mg/kg | mg/kg | ||||
| Corn | Root | Corn | Root | Corn | Root | |
| Control | 0.32 ± 0.04 ab | 9.18 ± 1.46 b | 0.01 ± 0.01 a | 0.64 ± 0.10 b | 3.02 ± 0.06 a | 19.68 ± 1.51 b |
| BA | 0.40 ± 0.20 a | 18.01±1.72 a | 0.02 ± 0.01 a | 0.94 ± 0.05 a | 1.63 ± 0.24 b | 34.38 ± 3.29 a |
| SM | 0.46 ± 0.16 a | 10.59 ± 0.60 b | 0.02 ± 0.01 a | 0.64 ± 0.04 b | 1.89 ± 0.16 b | 19.89 ± 1.31 b |
| SM + L | 0.31 ± 0.10 ab | 17.88 ± 3.38 a | 0.01 ± 0.01 a | 0.78 ± 0.10 ab | 1.15 ± 0.06 c | 34.89 ± 5.69 a |
| SM + FeO | 0.13 ± 0.07 b | 22.55 ± 2.17 a | 0.02 ± 0.01 a | 1.23 ± 0.04 a | 1.02 ± 0.06 c | 38.98 ± 3.52 a |
| N2O-N | CO2-C | |
|---|---|---|
| kg/ha/yr | ton/ha/yr | |
| Control | 46.97 ± 9.33 a | 35.19 ± 3.49 a |
| BA | 20.34 ± 3.25 b | 29.82 ± 0.84 a |
| SM | 13.80 ± 3.81 b | 33.33 ± 1.76 a |
| SM + L | 16.64 ± 8.91 b | 33.02 ± 1.74 a |
| SM + FeO | 13.77 ± 7.91 b | 29.20 ± 2.43 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Y.-K.; Kim, J.-W.; Kim, H.-S.; Yang, J.-E.; Kim, S.-C. The Synergetic Effect of Soil Amendments on Reducing Bioavailable Heavy Metals and Greenhouse Gas Emissions from Upland Soil. Agriculture 2022, 12, 246. https://doi.org/10.3390/agriculture12020246
Hong Y-K, Kim J-W, Kim H-S, Yang J-E, Kim S-C. The Synergetic Effect of Soil Amendments on Reducing Bioavailable Heavy Metals and Greenhouse Gas Emissions from Upland Soil. Agriculture. 2022; 12(2):246. https://doi.org/10.3390/agriculture12020246
Chicago/Turabian StyleHong, Young-Kyu, Jin-Wook Kim, Hyuck-Soo Kim, Jae-E. Yang, and Sung-Chul Kim. 2022. "The Synergetic Effect of Soil Amendments on Reducing Bioavailable Heavy Metals and Greenhouse Gas Emissions from Upland Soil" Agriculture 12, no. 2: 246. https://doi.org/10.3390/agriculture12020246
APA StyleHong, Y.-K., Kim, J.-W., Kim, H.-S., Yang, J.-E., & Kim, S.-C. (2022). The Synergetic Effect of Soil Amendments on Reducing Bioavailable Heavy Metals and Greenhouse Gas Emissions from Upland Soil. Agriculture, 12(2), 246. https://doi.org/10.3390/agriculture12020246






