Abstract
In the Czech Republic, demethylation inhibitors (DMIs) are used both as fungicides in controlling phoma stem canker and as growth regulators. This heavy use can result in the development of resistant isolates. A total of 45 and 286 Leptosphaeria maculans isolates were tested in vitro, using the mycelial growth and microtiter plate assays, respectively. The objective was to determine the sensitivity of L. maculans isolates collected in the Czech Republic to the fungicides tetraconazole, metconazole, and prochloraz. The mean EC50 values with the mycelial growth plate method were 1.33, 0.78, and 0.40 µg mL−1 for tetraconazole, metconazole, and prochloraz, respectively. The mean EC50 values for the microtiter plate assay were 3.01, 0.44, and 0.19 µg mL−1 for tetraconazole, metconazole, and prochloraz, respectively. All three fungicides also had high variation factors that may be due to inserts in the ERG11 promoter region. In addition, cross sensitivity among the three fungicides was observed. Overall, the high variation factors and the PCR (polymerase chain reaction) results observed in this study could signify the presence of resistant isolates in L. maculans Czech populations, especially in isolates tested for sensitivity to tetraconazole.
1. Introduction
Since their introduction into the agricultural fungicide market in the 1970s, demethylation inhibitor (DMI) fungicides (Fungicide Resistance Action Committee (FRAC) group 3) have been a highly effective and inexpensive way of controlling a broad range of fungi [1]. They target the cytochrome P450 enzyme 14-α-demethylase, which is essential for converting lanosterol to ergosterol encoded by the ERG11 (also known as CYP51) gene. By inhibiting the sterol C14-demethylation step during sterol formation in higher fungi, they deplete the amount of ergosterol in the cell and increase the accumulation of 14α-demethylated sterols. Thus, the fungal membrane structure is disrupted, preventing active membrane transport, resulting in fungistasis [2,3].
In the Czech Republic, oilseed rape cultivation accounts for about 15% of the total arable area, making it the second-most frequently grown crop [4]. However, phoma stem canker (also termed blackleg disease) has become a major limiting factor in oilseed rape production [5]. Phoma stem canker is caused by the dothideomycete fungi Leptosphaeria maculans (Desm.) Ces. & De Not. (anamorph Phoma lingam (Tode) Desm.) [6] and Leptosphaeria biglobosa Shoemaker & H. Brun [7]. Yield losses are usually about 10%, but in some seasons can be between 30 and 50% [6]. Control is mainly based on the use of cultural management strategies, resistant cultivars, and fungicides [8]. In the Czech Republic, fungicides registered for control of oilseed rape diseases mainly belong to the DMI fungicide group [9]. Fungicides are generally applied in autumn, but sometimes also in spring if oilseed rape is planted intensively. Many triazoles appear in distinct isomeric forms, which means they can also act as growth regulators [10,11]. In Europe, DMIs, including metconazole and tebuconazole, are currently used in oilseed rape cultivation as both fungicides and plant growth regulators [11,12,13,14,15]. Because of the site-specific mechanism of action of DMI fungicides, there is the risk that intensive use and overexposure will lead to resistance problems, making it hard to control pathogen populations [16,17].
In fungicide resistance management, it is important to continuously monitor fungicide efficacy, changes in pathogen sensitivity, and effectiveness of fungicide regimes to determine the development of fungicide resistance over time [18]. These would allow for the recommendation and implementation of antiresistance strategies, thus delaying the selection pressure of a fungicide class [18]. There have only been a few studies on the sensitivity of L. maculans to fungicides, despite their vast use. Previous sensitivity studies in the UK have reported that although L. maculans and L. biglobosa differ in their sensitivity to DMI fungicides, they are still effective in controlling phoma stem canker [19,20]. In Australia, however, Van de Wouw et al. [21] and Yang et al. [22] found resistant isolates in L. maculans populations. There have been no studies on the sensitivity of L. maculans isolates to DMI fungicides in the Czech Republic.
There are three main mechanisms of resistance to DMI fungicides: point mutations within the ERG11 gene, overexpression of the ERG11 gene, and overexpression of genes encoding efflux pumps [23]. Huang et al. [24] and Van de Wouw et al. [21] also investigated whether differences in sensitivity among L. maculans isolates are a result of changes within the ERG11, but no mutations were detected. A recent study of six resistant Australian L. maculans isolates, however, showed a 275 bp insertion in two isolates and three long terminal repeat retrotransposons (5263 bp, 5267 bp, and 5248 bp) inserted in the promoter region of three isolates [22]. The objective of this study, therefore, was to determine the sensitivity of L. maculans isolates collected in the Czech Republic to three DMI fungicides, tetraconazole, metconazole, and prochloraz, using the mycelium growth plate and microtiter plate assays.
2. Materials and Methods
2.1. Sample Collection and Pathogen Isolation
A total of 310 L. maculans isolates were isolated from symptomatic oilseed rape leaves collected from fields of farmers and research stations in 9 regions and 43 localities in the Czech Republic during five growing seasons, 2014–2017, and 2019 (Figure 1). Thirty-one of these isolates were collected by the Agricultural Research Institute Kroměříž between 2014 and 2017 from 4 regions and 25 localities. In 2017, Krukanice and Lužany localities were sprayed with Caramba 0.5 L ha−1 (metconazole 60 g L−1) and Staré Smrkovice locality with Horizon 2 × 0.5 L ha−1 (tebuconazole 250 g L−1). The spray history of the other localities is unknown. Necrotic leaf discs excised from oilseed rape leaves showing phoma leaf symptoms were immersed in 20% bleach solution (1% NaOCl) for 3 min, rinsed in sterile double-distilled water three times, and placed in a humid chamber. Following isolation, single pycnidium isolates were collected under a stereomicroscope with an inoculation needle and placed onto a Petri dish with PDA (potato dextrose agar) amended with chloramphenicol (100 µg mL−1). After incubation in darkness at 20 °C for growth, the isolates were subcultured onto a new growth medium (PDA or V8 vegetable juice agar).
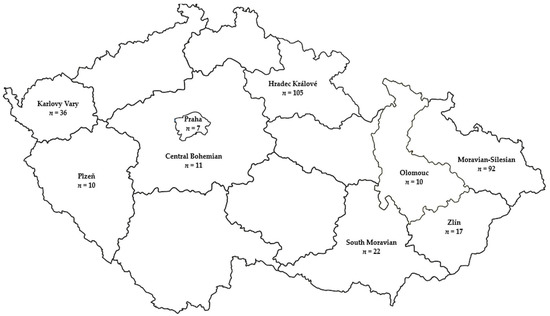
Figure 1.
Map of the Czech Republic showing 310 Leptosphaeria maculans isolates collected from nine regions between 2014–2017 and 2019. The n represents number of isolates collected per region.
2.2. Confirmation of L. maculans Isolates
All isolates were confirmed as L. maculans using both morphological and genetic characteristics [25]. Morphological characterization of L. maculans was based on the presence or absence of yellow pigment on the PDA plates. Isolates with an absence of yellow pigment were categorized as L. maculans [25]. Genetic characterization was carried out using species-specific primers by Liu et al. [26] and Mahuku et al. [27]. Fresh mycelia were first ground in liquid nitrogen using a mortar and pestle. Genomic DNA was then extracted using the GenElute Plant Genomic DNA Miniprep Kit (Sigma-Aldrich®, St. Louis MO, USA), according to the manufacturers’ instructions. PCR solutions (25 µL) were made up of sterile double-distilled water, DNA template (1 µL, ~100 ng), 1 × buffer for the Taq polymerase (Thermo Fisher Scientific, Waltham, MA, USA), 2.5 mM MgCl2, 0.25 µm each of dNTP (Thermo Fisher Scientific, Waltham, MA, USA), 0.4 μM each of the relevant primers (Sigma-Aldrich®, St. Louis, MO, USA), and 1 U Taq polymerase (Thermo Fisher Scientific, Waltham, MA, USA). Amplification conditions, according to Liu et al. [26], were 95 °C for 2 min, 30 cycles at 95 °C for 15 s, 70 °C for 30 s, 72 °C for 1 min, and a final extension at 72 °C for 10 min (DNA Engine Thermal Cycler Bio-Rad Laboratories, Hertfordshire, UK), while amplification conditions with the pair of primers of Mahuku et al. [27] were 95 °C for 2 min, 35 cycles at 95 °C for 60 s, 55 °C for 30 s, 72 °C for 60 s, and a final extension at 72 °C for 4 min (DNA Engine Thermal Cycler Bio-Rad Laboratories, Hertfordshire, UK). The PCR products were separated on 1% agarose gels containing ethidium bromide at 0.5 μg mL−1, 1 × TBE buffer at 5 V cm−1 for 60 min. The gels were visually analyzed under UV light (InGenius LHR and GeneSnap software, Syngene Synoptics Ltd., Cambridge, UK).
2.3. Fungicides
Technical-grade tetraconazole, metconazole, and prochloraz (Pestanal®, Sigma-Aldrich, St. Louis, MO, USA) were dissolved in dimethylsulfoxide (DMSO) to produce a stock solution (10 mg mL−1).
2.4. In Vitro Sensitivity of L. maculans isolates to Tetraconazole, Metconazole, and Prochloraz Using the Mycelium Growth Plate Method
In vitro assessment of inhibition of mycelial growth in the first collected 45 L. maculans isolates was determined using the mycelial growth plate method (Table 1). For each isolate, a mycelial plug (5 mm in diameter) was removed from the margin of a two-week-old colony and placed upside down on cooling fungicide-amended V8 juice agar after autoclaving at six concentrations (0, 0.001, 0.01, 0.1, 1.0, and 10 µg mL−1). Isolates were incubated in the darkness (20 °C) for 14 days. The experiment was conducted with three replicates for each isolate. The average fungal growth rate was then measured at two perpendicular directions and expressed as a percentage of growth inhibition. The minimum inhibitory concentration of each fungicide was determined for each isolate as the lowest concentration that inhibited 100% of the growth of a pathogen isolate.

Table 1.
EC50 range for 45 Leptosphaeria maculans isolates from seven regions in the Czech Republic from 2014 to 2017 used in the mycelial growth inhibition assay against DMI fungicides (tetraconazole, metconazole, and prochloraz). N represents the number of isolates collected per region.
2.5. In Vitro Sensitivity of Conidiospores of L. maculans to Tetraconazole, Metconazole, and Prochloraz Using the Microtiter Plate Method
In vitro assessment of inhibition of conidiospores in 286 L. maculans isolates (including 21 isolates from the mycelium growth plate assay method) was carried out using methods modified from Pijls et al. [28] and Sewell et al. [20] (Table 2). First, fresh fungal mycelia of each isolate grown in a 9 cm Petri dish were crushed together with agar using a sterile L-shaped spreader and placed into 20% V8 juice agar. After 10 days under 16 h fluorescent light (Lumilux cool white, Osram GmbH, Munich, Germany) at 20 °C and 90% relative humidity, conidiospores were harvested by flooding the plates with 10–15 mL of sterile double-distilled water and gently scratching the surface of the Petri dish with a sterile glass rod. The suspension was filtered using three layers of autoclaved filter cloth (mesh size of 0.7 mm) to remove pycnidia and mycelia debris. The resulting conidiospore suspension was adjusted to a stock concentration of 1 × 107 spores mL−1 using a light microscope and a Bürker hemocytometer.

Table 2.
EC50 range for 286 Leptosphaeria maculans isolates from nine regions in the Czech Republic in 2016, 2017, and 2019 used in the microtiter plate assay against DMI fungicides (tetraconazole, metconazole, and prochloraz). N represents the number of isolates collected per region.
For the fungicide sensitivity test, 2 × potato dextrose broth (PDB) was amended with technical grade tetraconazole, metconazole, and prochloraz at twelve increasing concentrations (0, 0.098, 0.195, 0.39, 0.781, 1.562, 3.125, 6.25, 12.5, 25, 50, and 100 μg mL−1). Here, fungicide amended media (100 μL) was added to wells of a flat-bottom 96-well microtiter plate (GAMEDIUM spol. s.r.o., Jesenice u Prahy, Czech Republic). In addition, conidiospore suspensions (100 μL) containing about 1 × 106 spores were added to each well of a single row. There were four replicates for each isolate. The plates were then incubated at 20 °C for 4 days in the darkness. Subsequent growth and conidiospore germination, as indicated by absorbance, were measured with the TECAN sunrise plate reader (Tecan Austria GmbH, Grödig, AustriaCity, Country), software Magellan V7.2 at a wavelength of 630 nm in endpoint mode.
2.6. PCR Amplification of ERG11 Regulatory Region for Molecular Detection of Insertions Conferring Resistance to DMI
A subset of 55 isolates with varying sensitivities to the fungicides based on EC50 were selected to investigate, by PCR assay, the absence (sensitive isolates) or presence (resistant isolates) of inserts in the ERG11 promoter region conferring ERG11 overexpression. To amplify ERG11 promoter region from total DNA, PCR reactions were set up in a 25 μL volume as above and carried out using the primer pair EPS1-F/EPS6-R and amplification temperatures by Yang et al. [22].
2.7. Data Analysis
In the fungicide sensitivity experiments, the effective concentration at which 50% fungal growth was inhibited (EC50) for each isolate was calculated [29,30]. The EC50 of each fungicide was determined by nonlinear regression (curve-fit) using GraphPad Prism version 8.0 (GraphPad Software, La Jolla, CA, USA). The D’Agostino–Pearson method was used to test the normality of frequency distributions (GraphPad Prism version 8.0). Because the data violated the assumption of normality, a nonparametric form of correlation, Spearman’s rank correlation test, was performed on logEC50 values to test sensitivity associations between tetraconazole and metconazole, prochloraz and metconazole, and prochloraz and tetraconazole.
3. Results
3.1. Mycelium Growth Plate Assay
The mean, minimum, and maximum EC50 values of all isolates tested for sensitivity to tetraconazole were 1.33, 0.24, and 4.81 µg mL−1, respectively (variation factor: 20.04). At the highest concentration of 10 µg mL−1, only 11% of the isolates were completely inhibited by tetraconazole, thus making it difficult to determine the minimum inhibitory concentration for the other 89%. Seven percent of the isolates had EC50 values below 1 µg mL−1.
L. maculans isolates tested for sensitivity to metconazole had EC50 values ranging from 0.06 to 1.73 µg mL−1, representing a 28.83-fold variation factor (EC50 mean = 0.78 µg mL−1). Seventy-one percent of the isolates had a minimum inhibitory concentration between 1 and 10 µg mL−1, while the other 29% had minimum inhibitory concentrations greater than 10 µg mL−1. Forty-nine percent of the isolates had EC50 values below 1 µg mL−1.
The mean, minimum, and maximum EC50 values of all isolates tested for sensitivity to prochloraz were 0.40, 0.02, and 1.46 µg mL−1, respectively (variation factor: 73). Seven percent of the isolates had a minimum inhibitory concentration between 0.1 and 1 µg mL−1; 82% of the isolates had a minimum inhibitory concentration between 1 and 10 µg mL−1; while the remaining 11% had a minimum inhibitory concentration above 10 µg mL−1. Seventy-three percent of the isolates had EC50 values below 1 µg mL−1. Data are summarized in Table 1 and Figure 2.
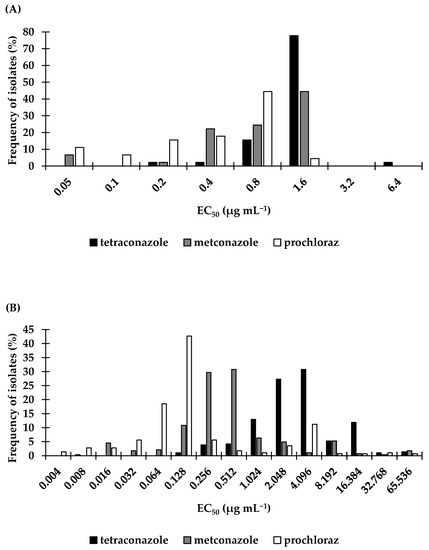
Figure 2.
Frequency distribution of sensitivity of Leptosphaeria maculans isolates collected between 2014 and 2017 from the Czech Republic to the DMI fungicides; tetraconazole, metconazole, and prochloraz using in vitro methods to determine the effective concentration which inhibits (A) mycelium (B) conidiospores by 50% compared to the nonamended control (EC50 µg mL−1). Individual isolates are grouped in class intervals when following interval is twofold the previous interval. Values on the x-axis indicate the midpoint of the interval.
For all 45 L. maculans isolates, the frequency distribution curves for the three fungicides were lognormally distributed when tested using the D’Agostino–Pearson normality test.
3.2. Microtiter Plate Assay
For the 286 L. maculans isolates carried out with the microtiter plate assay, tetraconazole had EC50 values ranging from 0.00659 to 59.51 µg mL−1 with a variation factor of 9022.02-fold; metconazole had EC50 values ranging from 0.0136 to 70.69 µg mL−1 with a variation factor of 5197.80-fold, and prochloraz had EC50 values ranging from 0.00298 to 56.04 µg mL−1 with a variation factor of 18824.32-fold. The mean EC50 values for tetraconazole, metconazole, and prochloraz were 3.01, 0.44, and 0.19 µg mL−1, respectively (Table 2, Figure 2). Frequency distributions of the mean EC50 values were lognormally distributed for all three fungicides when tested using the D’Agostino–Pearson normality test.
3.3. Cross Sensitivity
Spearman correlation analysis for mycelium growth plate assay indicated there was no correlation between tetraconazole and metconazole (r = 0.1809, p = 0.2343) or prochloraz and tetraconazole (r = 0.1385, p = 0.3643). However, there was significant correlation between prochloraz and metconazole (r = 0.6673, p < 0.0001) (Figure 3).
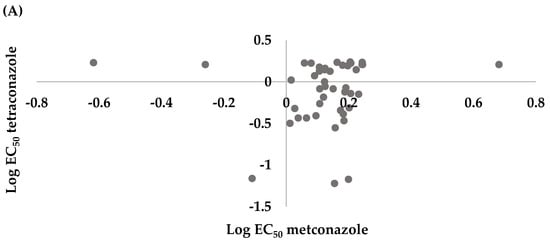
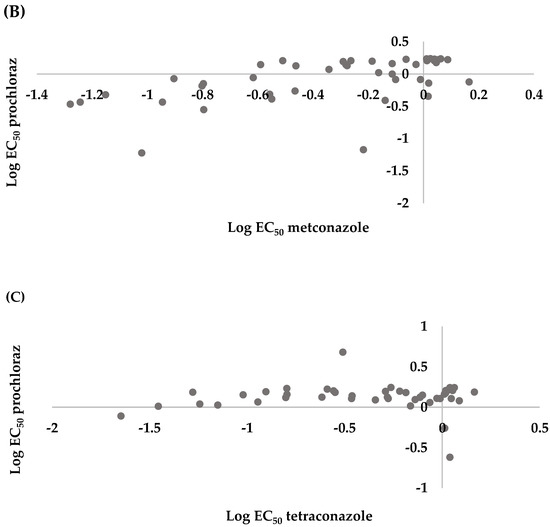
Figure 3.
Cross resistance between (A) tetraconazole and metconazole (r = 0.1809, p = 0.2343), (B) prochloraz and metconazole (r = 0.6673, p < 0.0001), and (C) prochloraz and tetraconazole (r = 0.1385, p = 0.3643) of 45 mycelial Leptosphaeria maculans isolates collected from oilseed rape plants obtained in the Czech Republic.
Spearman correlation analysis for the microtiter plate assay indicated there was a positive correlation between tetraconazole and metconazole (r = 0.3625, p < 0.0001), prochloraz and metconazole (r = 0.4623, p < 0.0001), and prochloraz and tetraconazole (r = 0.3516, p < 0.0001) (Figure 4).
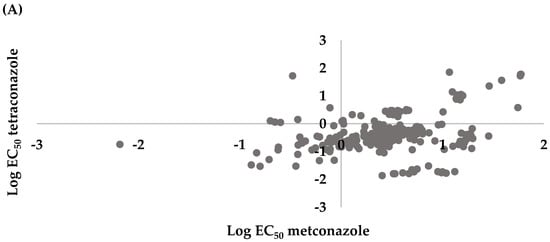
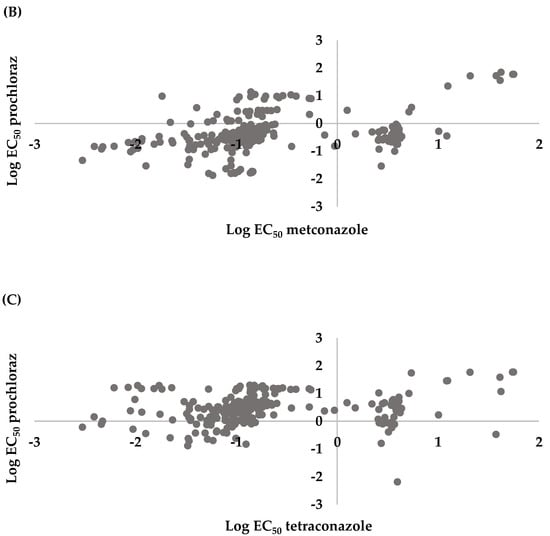
Figure 4.
Cross resistance between (A) tetraconazole and metconazole (r = 0.3625, p < 0.0001), (B) prochloraz and metconazole (r = 0.4623, p < 0.0001), and (C) prochloraz and tetraconazole (r = 0.3516, p < 0.0001) of 286 Leptosphaeria maculans conidial isolates collected from oilseed rape plants obtained in the Czech Republic.
3.4. Differences between Fungicide Sensitivity Monitoring Methods
The variation factors for all three fungicides in the microtiter plate assay were significantly higher than the variation factors for all three fungicides in the mycelium growth assay.
3.5. Promoter Insertions
Amplification of the ERG11 promoter region of the 55 isolates with variable sensitivity to all three DMI fungicides showed that 42 isolates (76%) yielded the PCR product size of 1099 bp, representing sensitive isolates, and the other 13 isolates had a PCR product size between 1200 and 1500 bp, representing resistant isolates, indicating an insertion in ERG11 promoter region [22] (Figure 5). Preliminary sequencing of six sensitive and four resistant isolates revealed that sequences with an insert occur within resistant isolates (data not shown). For tetraconazole, the mean EC50 for the sensitive L. maculans isolates was 2.4025 μg mL−1, compared with 7.8981 μg mL−1 for the resistant isolates. The mean EC50 value of the metconazole-sensitive isolates was 0.8464 μg mL−1 and of metconazole-resistant isolates was 3.7139 μg mL−1. For prochloraz, the mean EC50 values were 0.5637 and 1.6884 μg mL−1 for sensitive and resistant isolates, respectively.
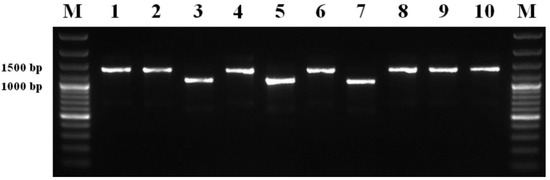
Figure 5.
Amplification of the ERG11 promoter region in 10 selected Leptosphaeria maculans isolates using the primers EPS1-F and EPS6-R. Sensitive isolates of L. maculans are shown in lanes 3, 5, 7; resistant isolates with an insertion in ERG11 promoter region are shown in lanes 1–2, 4, 6, 8–10, lane M: GeneRuler 100 bp Plus DNA Ladder (Thermo Fisher Scientific, Waltham, MA, USA).
4. Discussion
DMI fungicides are widely used in agriculture to control plant diseases. They have both preventive and curative properties [31]. These broad-spectrum fungicides have been used successfully for many years in controlling plant pathogens [31], including phoma stem canker [9]. Nevertheless, DMIs are categorized as medium-risk fungicides by FRAC. There have only been a few studies on resistance to fungicides, which have been mostly focused on L. maculans sensitivity to strobilurins (quinone outside inhibitor; QoI) [32,33] and other DMI fungicides [20,21,22,24,34]. However, similar to other phytopathogenic fungi [35,36,37,38,39,40,41], resistance to DMI fungicides in L. maculans has also been documented [21,22]. This is the first study on the sensitivity of L. maculans isolates to DMI fungicides in the Czech Republic. QoI and SDHI fungicides are also used by Czech farmers, but so far only QoI resistance has been reported among L. maculans isolates (data not shown).
In vitro fungicide sensitivity studies are used to determine changes in the sensitivity of fungal isolates to a particular active ingredient over time. Testing is typically carried out using mycelial growth assays where the growth of isolates on fungicide amended medium is compared to the growth on nonamended medium [42,43,44]. This method, though, is usually cumbersome and time-consuming [45]. On the other hand, the microtiter plate assay method allows many isolates to be quickly and simultaneously tested over several fungicide concentrations [28]. In this study, both the mycelium growth assay and the microtiter plate methods were used to test for the sensitivity of L. maculans isolates to three DMI fungicides (tetraconazole, metconazole, and prochloraz) registered for use in the Czech Republic for oilseed rape production. In contrast to the mycelium growth plate method, the microtiter plate method was able to test a large sample size (286 isolates).
Both the mycelium growth plate method and the microtiter plate assay showed that, of the three fungicides, tetraconazole had the highest mean EC50. Here, the mean EC50 for L. maculans isolates to the three fungicides were similar to the mean EC50 of resistant isolates in some studies, and in other studies similar to the mean EC50 of sensitive isolates. This made it difficult to determine the sensitivity of the isolates to these fungicides based on mean EC50 alone. However, this is not surprising because none of the other studies were based on the sensitivity of L. maculans isolates to tetraconazole, metconazole, and prochloraz, but rather to flusilazole, fluquinconazole, prothioconazole, and tebuconazole [20,21,22,24,34]. Resistance to DMI fungicides is quantitative, meaning it develops in a stepwise gradual progression [18]. It results from the modification of many interacting genes [18]. Therefore, depending on the number of gene changes, isolates exhibit a range of sensitivity to the fungicide [18]. Our current study showed that when tested for mycelial growth inhibition, L. maculans isolates had variation factors of 20.04, 28.83, and 73 for tetraconazole, metconazole, and prochloraz isolates, respectively. With the microtiter plate method and a larger number of isolates, this variation factor was much higher, at 9022.02 (tetraconazole), 5197.80 (metconazole), and 18,824.32 (prochloraz). Because metconazole and tetraconazole are triazole fungicides commonly used in the Czech Republic as both fungicides and growth regulators, the high mean EC50 values and variation factors suggest high-intensity use of these fungicides may have led to the selection of resistance in some isolates.
Prochloraz is an imidazole fungicide registered in the Czech Republic against both oilseed rape and cereal diseases [9]. Nevertheless, in oilseed rape plants, this fungicide is more commonly used in managing sclerotinia stem rot [4]. Even though Sclerotinia sclerotiorum is the main target organism for fungicide application, both pathogens usually occur on the same oilseed rape plant and field and are affected by a fungicide containing prochloraz at the same time [9]. L. maculans tested for sensitivity to prochloraz in the current study had a high mean EC50 and variation factor, which also suggests a selection pressure with prochloraz. One reason for this could be through the inadvertent exposure of L. maculans populations to prochloraz residues used in controlling cereal diseases, as oilseed rape plants are usually grown in rotation with cereals [9]. Another reason for the high mean EC50 values and variation factor could be the presence of cross resistance between prochloraz, metconazole, and tetraconazole shown in this study. Cross resistance usually occurs between fungicides with similar modes of action [46]. Thus, fungal isolates which are resistant to one fungicide will often be resistant to another closely related fungicide, even if they have not been exposed to the other closely related fungicide [46], as has been seen between DMI fungicides in Cercospora beticola isolates [47], and between tebuconazole and difenoconazole in Didymella bryoniae [48]. In addition, Ishii et al. [49] demonstrated cross resistance between mefentrifluconazole and other DMI fungicides: propiconazole, difenoconazole, and tebuconazole. However, cross resistance was only observed with all three fungicides in the isolates tested with the microtiter plate method and between metconazole and prochloraz in the isolates tested with the mycelium growth plate method. The lack of cross resistance between tetraconazole and metconazole and between prochloraz and tetraconazole could be because of the small sample size (45 isolates) tested with this method. Czech farmers have reported a decrease in the efficacy of triazole fungicides (personal communication). However, it is unknown whether this is as a result of the emergence of L. maculans resistance to this fungicide group or because of improper fungicide use. Results based on EC50 values show that reduced sensitivity of L. maculans to triazole fungicides used to control of phoma stem canker could be related to the decreased efficacy observed under field conditions.
Molecular mechanisms of DMI resistance have been studied where overexpression of the ERG11 gene is sometimes associated with inserts upstream of the ERG11 gene [50]. The use of molecular markers for rapid PCR-based detection have been recently developed [50], where similar PCR tests have been used to find inserts in isolates of Venturia inaequalis and Blumeriella jaapii resistant to myclobutanil [51,52]. Yang et al. [22] designed primers to test for insertions in the coding and promoter regions of ERG11. Using these primers, 76% of the isolates tested were shown to have PCR products between 1200 and 1500 bp, suggesting resistance in these isolates. However, these results are not conclusive, as further testing on other isolates and more sequencing data would be needed to confirm the size of the insert.
5. Conclusions
This study has shown that tetraconazole, metconazole, and prochloraz have not effectively inhibited in vitro L. maculans populations in the Czech Republic. This insensitivity could be a result of an insertion in the promoter region of the ERG11 gene. In addition, cross resistance occurring between DMI fungicides was observed in this study. Therefore, it is recommended that DMI fungicides be used either in rotation with other fungicide classes or as tank mixes to reduce the chances of fungicide resistance developing, as sole reliance on one fungicide class is also not advisable in modern agriculture. However, because in vitro assays only provide insight into possible resistant development, they are not entirely accurate predictors of field efficacy, and future studies would be needed to confirm the field performance of commercially formulated products.
Author Contributions
Conceptualization, O.F. and J.M.; methodology, O.F. and J.M.; validation, J.M. and P.R.; formal analysis, O.F.; investigation, O.F. and J.M.; resources, J.M. and P.R.; data curation, O.F.; writing—original draft preparation, O.F. and J.M.; writing—review and editing, O.F., J.M. and P.R.; visualization, O.F.; supervision, J.M. and P.R.; project administration, J.M.; funding acquisition, J.M. and P.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Ministry of Agriculture within the framework of the National Agency for Agricultural Research, Project No QK1710397.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Eva Plachká and Pavel Matušinský for collecting and providing samples used in this study, Barbora Slavětinská for her laboratory assistance, and Tomáš Hlavsa for statistical analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Russell, P.E. Fungicide Resistance: Occurrence and Management. J. Agric. Sci. 1995, 124, 317–323. [Google Scholar] [CrossRef]
- Parker, J.E.; Warrilow, A.G.; Price, C.L.; Mullins, J.G.; Kelly, D.E.; Kelly, S.L. Resistance to Antifungals that Target CYP51. J. Chem. Biol. 2014, 7, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Marichal, P.; Gorrens, J.; Laurijssens, L.; Vermuyten, K.; Van Hove, C.; Le Jeune, L.; Verhasselt, P.; Sanglard, D.; Borgers, M.; Ramaekers, F.C.; et al. Accumulation of 3-Ketosteroids Induced by Itraconazole in Azole-Resistant Clinical Candida albicans Isolates. Antimicrob. Agents Chemother. 1999, 43, 2663–2670. [Google Scholar] [CrossRef]
- Czech Statistical Office. Areas under Crops. Available online: https://vdb.czso.cz/vdbvo2/faces/en/index.jsf?page=vystup-objektparametry&katalog=30840&z=T&sp=A&skupId=346&pvo=ZEM02A (accessed on 22 October 2021).
- Mazáková, J.; Urban, J.; Zouhar, M.; Ryšánek, P. Analysis of Leptosphaeria Species Complex Causing Phoma Leaf Spot and Stem Canker of Winter Oilseed Rape (Brassica napus) in the Czech Republic. Crop Pasture Sci. 2017, 68, 254–264. [Google Scholar] [CrossRef]
- Fitt, B.D.L.; Brun, H.; Barbetti, M.J.; Rimmer, S.R. World-Wide Importance of Phoma Stem Canker (Leptosphaeria maculans and L. biglobosa) on Oilseed Rape (Brassica napus). Eur. J. Plant Path. 2006, 114, 3–15. [Google Scholar] [CrossRef]
- Shoemaker, R.A.; Brun, H. The Teleomorph of the Weakly Aggressive Segregate of Leptosphaeria maculans. Can. J. Bot. 2001, 79, 412–419. [Google Scholar] [CrossRef]
- West, J.S.; Kharbanda, P.D.; Barbetti, M.J.; Fitt, B.D.L. Epidemiology and Management of Leptosphaeria maculans (Phoma Stem Canker) on Oilseed Rape in Australia, Canada and Europe. Plant Path. 2001, 50, 10–27. [Google Scholar] [CrossRef]
- Central Institute for Supervising and Testing in Agriculture. Available online: http://eagri.cz/public/app/eagriapp/POR/Vyhledavani.aspx?type=0&vyhledat=A&stamp=1566295194615 (accessed on 23 July 2021).
- Berry, P.M.; Spink, J.H. Understanding the Effect of a Triazole with Anti-Gibberellin Activity on the Growth and Yield of Oilseed Rape (Brassica napus). J. Agric. Sci. 2009, 147, 273–285. [Google Scholar] [CrossRef]
- Davis, T.D.; Steffens, G.L.; Sankhla, N. Triazole Plant Growth Regulators. Hortic. Rev. 1988, 10, 63–105. [Google Scholar]
- Fletcher, R.A.; Hofstra, G.; Gao, J. Comparative Fungitoxic and Plant Growth Regulating Properties of Triazole Derivatives. Plant Cell Physiol. 1986, 27, 367–371. [Google Scholar]
- Luster, D.G.; Miller, P.A. Triazole Plant Growth Regulator Binding to Native and Detergent-Solubilized Plant Microsomal Cytochrome P450. Pestic. Biochem. Physiol. 1993, 46, 27–39. [Google Scholar] [CrossRef]
- Coules, A.E.; Lunn, G.D.; Rossal, S. Disease and Canopy Control in Oilseed Rape Using Triazole Fungicides. In Proceedings of the BCPC Conference: Pests and Diseases, Brighton, UK, 18–21 November 2002. [Google Scholar]
- Zamani-Noor, N.; Knüfer, J. Effects of Host Plant Resistance and Fungicide Application on Phoma Stem Canker, Growth Parameters and Yield of Winter Oilseed Rape. Crop Prot. 2018, 112, 313–321. [Google Scholar] [CrossRef]
- Hollomon, D.W. Fungicide Resistance: Facing the Challenge. Plant Prot. Sci. 2015, 51, 170–176. [Google Scholar] [CrossRef]
- Ma, Z.; Michailides, T.J. Advances in Understanding Molecular Mechanisms of Fungicide Resistance and Molecular Detection of Resistant Genotypes in Phytopathogenic Fungi. Crop Prot. 2005, 24, 853–863. [Google Scholar] [CrossRef]
- Brent, K.J.; Hollomon, D.W. Fungicide Resistance in Crop Pathogens: How Can it Be Managed, 2nd ed.; Fungicide Resistance Action Committee; Crop Life International: Brussels, Belgium, 2007; p. 60. [Google Scholar]
- Eckert, M.R.; Rossall, S.; Selley, A.; Fitt, B.D.L. Effects of Fungicides on in Vitro Spore Germination and Mycelial Growth of the Phytopathogens Leptosphaeria maculans and L. biglobosa (Phoma Stem Canker of Oilseed Rape). Pest Manag. Sci. 2010, 66, 396–405. [Google Scholar] [CrossRef]
- Sewell, T.R.; Hawkins, N.J.; Stotz, H.U.; Huang, Y.J.; Kelly, S.L.; Kelly, D.E.; Fraaije, B.; Fitt, B.D.L. Azole Sensitivity in Leptosphaeria Pathogens of Oilseed Rape: The Role of Lanosterol 14α-Demethylase. Sci. Rep. 2017, 7, 15849. [Google Scholar] [CrossRef] [PubMed]
- Van de Wouw, A.P.; Elliott, V.L.; Chang, S.; López-Ruiz, F.J.; Marcroft, S.J.; Idnurm, A. Identification of Isolates of the Plant Pathogen Leptosphaeria maculans with Resistance to the Triazole Fungicide Fluquinconazole Using a Novel in Planta Assay. PLoS ONE 2017, 12, e0188106. [Google Scholar]
- Yang, Y.; Marcroft, S.J.; Forsyth, L.M.; Zhao, J.; Ziqin, L.; Van de Wouw, A.P.; Idnurm, A. Sterol Demethylation Inhibitor Fungicide Resistance in Leptosphaeria maculans is Caused by Modifications in the Regulatory Region of ERG11. Plant Dis. 2020, 104, 1280–1290. [Google Scholar] [CrossRef]
- Price, C.L.; Parker, J.E.; Warrilow, A.G.S.; Kelly, D.E.; Kelly, S.L. Azole Fungicides Understanding Resistance Mechanisms in Agricultural Fungal Pathogens. Pest Manag. Sci. 2015, 8, 1054–1058. [Google Scholar] [CrossRef]
- Huang, Y.J.; Hood, J.; Eckert, M.R.; Stonard, J.F.; Cools, H.J.; King, G.J.; Rosall, S.; Ashworth, F.; Fitt, B.D.L. Effects of Fungicide on Growth of Leptosphaeria maculans and L. biglobosa in Relation to Development of Phoma Stem Canker on Oilseed Rape (Brassica napus). Plant Path. 2011, 60, 607–620. [Google Scholar] [CrossRef]
- Williams, R.H.; Fitt, B.D.L. Differentiating A and B Groups of Leptosphaeria maculans, Causal Agent of Stem Canker (Blackleg) of Oilseed Rape. Plant Pathol. 1999, 48, 161–175. [Google Scholar] [CrossRef]
- Liu, S.Y.; Liu, Z.; Fitt, B.D.L.; Evans, N.; Foster, S.J.; Huang, Y.-J.; Latunde-Dada, A.O.; Lucas, J.A. Resistance to Leptosphaeria maculans (Phoma Stem Canker) in Brassica napus (Oilseed Rape) Induced by L. biglobosa and Chemical Defence Activators in Field and Controlled Environments. Plant Pathol. 2006, 55, 401–412. [Google Scholar] [CrossRef]
- Mahuku, G.S.; Hall, R.; Goodwin, P.H. Co-Infection and Induction of Systemic Acquired Resistance by Weakly and Highly Virulent Isolates of Leptosphaeria maculans in Oilseed Rape. Physiol. Mol. Plant Pathol. 1996, 49, 61–72. [Google Scholar] [CrossRef]
- Pijls, C.F.N.; Shaw, M.W.; Parker, A.A. Rapid Test to Evaluate in Vitro Sensitivity of Septoria tritici to Flutriafol, Using a Microtitre Plate Reader. Plant Pathol. 1994, 43, 726–732. [Google Scholar] [CrossRef]
- Wong, F.P.; Midland, S.L. Sensitivity Distributions of California Populations of Colletotrichum cereale to the DMI Fungicides Propiconazole, Myclobutanil, Tebuconazole, and Triadimefon. Plant Dis. 2007, 91, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.P.; Wilcox, W.F. Sensitivity to Azoxystrobin among Isolates of Uncinula necator: Baseline Distribution and Relationship to Myclobutanil Sensitivity. Plant Dis. 2002, 86, 394–404. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ishii, H.; Hollomon, D.W. Fungicide Resistance in Plant Pathogens: Principles and a Guide to Practical Management, 1st ed.; Springer: Berlin/Heilderberg, Germany, 2015; p. 490. [Google Scholar]
- Fraser, M.; Hwang, S.-F.; Ahmed, H.U.; Akhavan, A.; Stammler, G.; Barton, W.; Strelkov, S.E. Sensitivity of Leptosphaeria maculans to Pyraclostrobin in Alberta, Canada. Can. J. Plant Sci. 2016, 97, 83–91. [Google Scholar] [CrossRef]
- Wang, Y.; Akhavan, A.; Hwang, S.-F.; Strelkov, S.E. Decreased Sensitivity of Leptosphaeria maculans to Pyraclostrobin in Alberta, Canada. Plant Dis. 2020, 104, 2462–2468. [Google Scholar] [CrossRef]
- Van de Wouw, A.P.; Scanlan, J.L.; Marcroft, S.J.; Smith, A.J.; Sheedy, E.M.; Perndt, N.W.; Harrison, C.E.; Forsyth, L.M.; Idnurm, A. Fungicide Sensitivity and Resistance in the Blackleg Fungus, Leptosphaeria maculans, across Canola Growing Regions in Australia. Crop Pasture Sci. 2021, 72, 994–1007. [Google Scholar] [CrossRef]
- Délye, C.; Laigret, F.; Corio-Costet, M.F. A Mutation in the 14 Alpha-Demethylase Gene of Uncinula necator that Correlates with Resistance to a Sterol Biosynthesis Inhibitor. Appl. Environ. Microbiol. 1997, 63, 2966–2970. [Google Scholar] [CrossRef] [PubMed]
- Erickson, E.O.; Wilcox, W.F. Distributions of Sensitivities to Three Sterol Demethylation Inhibitor Fungicides among Populations of Uncinula necator Sensitive and Resistant to Triadimefon. Phytopathology 1997, 87, 784–791. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fraaije, B.A.; Cools, H.J.; Kim, S.H.; Motteram, J.; Clark, W.S.; Lucas, J.A. A Novel Substitution I381V in the Sterol 14α-Demethylase (CYP51) of Mycosphaerella graminicola Is Differentially Selected by Azole Fungicides. Mol. Plant Pathol. 2007, 8, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Ghosoph, J.M.; Schmidt, L.S.; Margosan, D.A.; Smilanick, J.L. Imazalil Resistance Linked to a Unique Insertion Sequence in the PdCYP51 Promoter Region of Penicillium digitatum. Postharvest Biol. Technol. 2007, 44, 9–18. [Google Scholar] [CrossRef]
- Omrane, S.; Sghyer, H.; Audéon, C.; Lanen, C.; Duplaix, C.; Walker, A.S.; Fillinger, S. Fungicide Efflux and the MgMFS1 Transporter Contribute to the Multidrug Resistance Phenotype in Zymoseptoria tritici Field Isolates. Environ. Microbiol. 2015, 17, 2805–2823. [Google Scholar] [CrossRef] [PubMed]
- Stergiopoulos, I.; Van Nistelrooy, J.G.; Kema, G.H.; De Ward, M.A. Multiple Mechanisms Account for Variation in Base-Line Sensitivity to Azole Fungicides in Field Isolates of Mycosphaerella graminicola. Pest Manag. Sci. 2003, 59, 1333–1343. [Google Scholar] [CrossRef]
- Dyer, P.S.; Hansen, J.; Delaney, A.; Lucas, J.A. Genetic Control of Resistance to the Sterol 14alpha-Demethylase Inhibitor Fungicide Prochloraz in the Cereal Eyespot Pathogen Tapesia yallundae. Appl. Environ. Microbial. 2000, 66, 4599–4604. [Google Scholar] [CrossRef]
- Smith, F.D.; Parker, D.M.; Köller, W. Sensitivity Distribution of Venturia ynaequalis to Sterol Demethylation Inhibitor Flusilazole: Baseline Sensitivity and Implications for Resistance Monitoring. Phytopathology 1991, 81, 392–396. [Google Scholar] [CrossRef]
- Wang, W.; Fang, Y.; Imran, M.; Hu, Z.; Zhang, S.; Huang, Z.; Liu, X. Characterization of the Field Fludioxonil Resistance and Its Molecular Basis in Botrytis cinerea from Shanghai Province in China. Microorganisms 2021, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Cools, H.J.; Nishimura, K.; Borghi, L.; Kikuhara, K.; Yamaoka, Y. DMI-Fungicide Resistance in Venturia nashicola, the Causal Agent of Asian Pear Scab—How Reliable Are Mycelial Growth Tests in Culture? Microorganisms 2021, 9, 1377. [Google Scholar] [CrossRef] [PubMed]
- Wedge, D.E.; Curry, K.J.; Kreiser, B.; Curry, A.; Abril, M.; Smith, B.J. Fungicide Resistance Profiles for 13 Botrytis cinerea Isolates from Strawberry in Southeastern Louisiana. Int. J. Fruit Sci. 2013, 13, 413–429. [Google Scholar] [CrossRef]
- Brent, K.J.; Hollomon, D.W. Fungicide Resistance: The Assessment of Risk Monograph, 2nd ed.; Global Crop Protection Federation: Brussels, Belgium, 1998; p. 53. [Google Scholar]
- Karaoglanidis, G.S.; Thanassoulopoulos, C.C. Cross-Resistance Patterns among Sterol Biosynthesis Inhibiting Fungicides (SBIs) in Cercospora beticola. Eur. J. Plant Pathol. 2003, 109, 929–934. [Google Scholar] [CrossRef]
- Thomas, A.; Langston, D.B., Jr.; Stevenson, K.L. Baseline Sensitivity and Cross-Resistance to Succinate-Dehydrogenase-Inhibiting and Demethylation Inhibiting Fungicides in Didymella bryoniae. Plant Dis. 2012, 96, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Bryson, P.K.; Kayamori, M.; Miyamoto, T.; Yamaoka, Y.; Schnabel, G. Cross-Resistance to the New Fungicide Mefentrifluconazole in DMI-Resistant Fungal Pathogens. Pestic. Biochem. Physiol. 2021, 171, 104737. [Google Scholar]
- Patel, R.; Mendrick, C.; Knapp, C.; Grist, R.; McNicholas, P. Clinical Evaluation of the Sensititre Yeast One Plate for Testing Susceptibility of Filamentous Fungi to Posaconazole. J. Clin. Microbiol. 2007, 45, 2000–2001. [Google Scholar] [CrossRef]
- Schnabel, G.; Jones, A.L. The 14α−Demethylase (CYP51A1) Gene Is Overexpressed in Venturia inaequalis Strains Resistant to Myclobutanil. Phytopathology 2001, 91, 102–110. [Google Scholar] [CrossRef]
- Ma, Z.; Proffer, T.J.; Jacobs, J.L.; Sundin, G.W. Overexpression of the 14alpha-Demethylase Target Gene (CYP51) Mediates Fungicide Resistance in Blumeriella jaapii. Appl. Environ. Microbiol. 2006, 72, 2581–2585. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).