Influence of the Rootstock and the Ploidy Level of the Scion and the Rootstock on Sweet Orange (Citrus sinensis) Peel Essential Oil Yield, Composition and Aromatic Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Essential Oil Analysis
2.2.1. Raw Material
2.2.2. Hydrodistillation
2.2.3. Gas Chromatography (GC) Analysis
2.2.4. Gas Chromatography–Mass Spectrometry Analysis (GC–MS)
2.2.5. Identification of Components
2.2.6. Sensorial Analysis
2.3. Statistical Analysis
3. Results
3.1. Influence of the Rootstock on Essential Oil Yield, Composition and Aromatic Profile
3.2. Influence of Ploidy Level on Essential Oil Yield, Composition and Aromatic Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mudge, K.; Janick, J.; Scofield, S.; Goldschmidt, E.E. A History of Grafting. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 437–493. ISBN 978-0-470-59377-6. [Google Scholar]
- Castle, W.S. A Career Perspective on Citrus Rootstocks, Their Development, and Commercialization. HortScience 2010, 45, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Moreno, P.; Ambrós, S.; Albiach-Martí, M.R.; Guerri, J.; Peña, L. Citrus Tristeza Virus: A Pathogen That Changed the Course of the Citrus Industry. Mol. Plant Pathol. 2008, 9, 251–268. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Muñoz, N.; Velázquez, K.; Vives, M.C.; Ruiz-Ruiz, S.; Pina, J.A.; Flores, R.; Moreno, P.; Guerri, J. The Resistance of Sour Orange to Citrus Tristeza Virus Is Mediated by Both the Salicylic Acid and RNA Silencing Defence Pathways: Study of the Resistance of Sour Orange to CTV. Mol. Plant Pathol. 2017, 18, 1253–1266. [Google Scholar] [CrossRef] [PubMed]
- Bownman, K.D.; Joubert, J. Citrus rootstocks. In The Genus Citrus; Talon, M., Caruso, M., Gmitter, J.F.G., Eds.; Elsevier WP: Duxford, UK, 2020; pp. 105–127. ISBN 978-0-12-812217-4. [Google Scholar]
- Hussain, S.; Curk, F.; Anjum, M.A.; Pailly, O.; Tison, G. Performance Evaluation of Common Clementine on Various Citrus Rootstocks. Sci. Hortic. 2013, 150, 278–282. [Google Scholar] [CrossRef]
- Caruso, M.; Continella, A.; Modica, G.; Pannitteri, C.; Russo, R.; Salonia, F.; Arlotta, C.; Gentile, A.; Russo, G. Rootstocks Influence Yield Precocity, Productivity, and Pre-Harvest Fruit Drop of Mandared Pigmented Mandarin. Agronomy 2020, 10, 1305. [Google Scholar] [CrossRef]
- Bitters, W.P.; Scora, R.W. The Influence of Citrus Rootstocks upon the Volatile Rind Oil Content of Valencia Orange (Citrus Sinensis Osbeck). Bot. Gaz. 1970, 131, 105–109. [Google Scholar] [CrossRef]
- Verzera, A.; Trozzi, A.; Gazea, F.; Cicciarello, G.; Cotroneo, A. Effects of Rootstock on the composition of Bergamot (Citrus Bergamia Risso et Poiteau) Essential oil. J. Agric. Food Chem. 2003, 51, 206–210. [Google Scholar] [CrossRef]
- Pedruzzi, L. Influence of rootstock on essential oil composition of mandarins. Acta Farm. Bonaer. 2004, 23, 4. [Google Scholar]
- Darjazi, B.B. The effect of sour orange, Swingle citrumelo and Troyer citrange rootstocks on the peel components of kumquat (Fortunella Margarita). J. Med. Plants By-Prod. 2017, 1, 111–116. [Google Scholar]
- Zouaghi, G.; Najar, A.; Aydi, A.; Claumann, C.A.; Zibetti, A.W.; Ben Mahmoud, K.; Jemmali, A.; Bleton, J.; Moussa, F.; Abderrabba, M.; et al. Essential oil components of citrus cultivar ‘MALTAISE DEMI SANGUINE’ (Citrus sinensis) as affected by the effects of rootstocks and viroid infection. Int. J. Food Prop. 2019, 22, 438–448. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Hernández, M.G.; Sánchez-Bravo, P.; Hernández, F.; Carbonell-Barrachina, Á.A.; Pastor-Pérez, J.J.; Legua, P. Determination of the volatile profile of lemon peel oils as affected by rootstock. Foods 2020, 9, 241. [Google Scholar] [CrossRef] [Green Version]
- Darjazi, B.B. The effects of rootstock on the volatile flavor Components of Page mandarin [(Citrus reticulata Var Dancy × Citrus paradisi Var Dancan) × Citrus clementina] Flower and Leaf. Afr. J. Agric. Res. 2011, 6, 1884–1896. [Google Scholar] [CrossRef]
- Benjamin, G.; Tietel, Z.; Porat, R. Effects of Rootstock/Scion Combinations on the Flavor of Citrus Fruit. J. Agric. Food Chem. 2013, 61, 11286–11294. [Google Scholar] [CrossRef] [PubMed]
- Allario, T.; Brumos, J.; Colmenero-Flores, J.M.; Iglesias, D.J.; Pina, J.A.; Navarro, L.; Talon, M.; Ollitrault, P.; Morillon, R. Tetraploid Rangpur Lime Rootstock Increases Drought Tolerance via Enhanced Constitutive Root Abscisic Acid Production: Tetraploid Rootstock Increases Drought Tolerance. Plant Cell Environ. 2013, 36, 856–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvez, L.; Dereeper, A.; Mournet, P.; Froelicher, Y.; Bruyère, S.; Morillon, R.; Ollitrault, P. Intermediate Inheritance with Disomic Tendency in Tetraploid Intergeneric Citrus × Poncirus Hybrids Enhances the Efficiency of Citrus Rootstock Breeding. Agronomy 2020, 10, 1961. [Google Scholar] [CrossRef]
- Dambier, D.; Benyahia, H.; Pensabene-Bellavia, G.; Aka Kaçar, Y.; Froelicher, Y.; Belfalah, Z.; Lhou, B.; Handaji, N.; Printz, B.; Morillon, R.; et al. Somatic hybridization for citrus rootstock breeding: An effective tool to solve some important issues of the mediterranean citrus industry. Plant Cell Rep. 2011, 30, 883–900. [Google Scholar] [CrossRef]
- Grosser, J.W. New somatic hybrid rootstock candidates for tree-size control and high juice quality. Proc. Fla. State Hort. Soc. 2011, 124, 131–135. [Google Scholar]
- Grosser, J.W.; Barthe, G.A.; Castle, B.; Gmitter, F.G.J.; Lee, O. The development of improved tetraploid citrus rootstocks to facilitate advanced production systems and sustainable citriculture in Florida. Acta Hortic. 2015, 1065, 319–327. [Google Scholar] [CrossRef]
- Ruiz, M.; Pensabene-Bellavia, G.; Quiñones, A.; García-Lor, A.; Morillon, R.; Ollitrault, P.; Primo-Millo, E.; Navarro, L.; Aleza, P. Molecular characterization and stress tolerance evaluation of new allotetraploid somatic hybrids between Carrizo citrange and Citrus macrophylla W. Rootstocks. Front. Plant Sci. 2018, 9, 901. [Google Scholar] [CrossRef]
- Saleh, B.; Allario, T.; Dambier, D.; Ollitrault, P.; Morillon, R. Tetraploid citrus rootstocks are more tolerant to salt stress than diploid. Comptes Rendus Biol. 2008, 331, 703–710. [Google Scholar] [CrossRef]
- Aleza, P.; Juárez, J.; Ollitrault, P.; Navarro, L. Production of tetraploid plants of non-apomictic citrus genotypes. Plant Cell Rep. 2009, 28, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Barrett, H.C.; Hutchison, D.J. Spontaneous tetraploidy in apomictic seedlings of citrus. Econ. Bot. 1978, 32, 27–45. [Google Scholar] [CrossRef]
- Grosser, J.; Gmitter, F.; Bowman, K. New Rootstocks in the Citrus Breeding Pipeline. Citrus Ind. July 2020, 2020, 8–11. [Google Scholar]
- Aleza, P.; Froelicher, Y.; Schwarz, S.; Agustí, M.; Hernández, M.; Juárez, J.; Luro, F.; Morillon, R.; Navarro, L.; Ollitrault, P. Tetraploidization events by chromosome doubling of nucellar cells are frequent in apomictic citrus and are dependent on genotype and environment. Ann. Bot. 2011, 108, 37–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cameron, J.W.; Scora, R.W. A comparison of rind oil components of diploid and tetraploid citrus by gas-liquid chromatography. Taxon 1968, 17, 128–135. [Google Scholar] [CrossRef]
- Hussain, S.; Curk, F.; Dhuique-Mayer, C.; Urban, L.; Ollitrault, P.; Luro, F.; Morillon, R. Autotetraploid trifoliate orange (Poncirus trifoliata) rootstocks do not impact clementine quality but reduce fruit yields and highly modify rootstock/scion physiology. Sci. Hortic. 2012, 134, 100–107. [Google Scholar] [CrossRef]
- Hussain, S.; Curk, F.; Ollitrault, P.; Morillon, R.; Luro, F. Facultative apomixis and chromosome doubling are sources of heterogeneity in citrus rootstock trials: Impact on clementine production and breeding selection. Sci. Hortic. 2011, 130, 815–819. [Google Scholar] [CrossRef]
- Luro, F.; Bloquel, E.; Tomu, B.; Costantino, G.; Tur, I.; Riolacci, S.; Varamo, F.; Ollitrault, P.; Froelicher, Y.; Curk, F.; et al. The INRACIRAD Citrus Germplasm Collection of San Giuliano, Corsica; Publications du centre Jean Bérard: Naples, Italy, 2017; pp. 243–261. [Google Scholar]
- Bicchi, C.; Liberto, E.; Matteodo, M.; Sgorbini, B.; Mondello, L.; Zellner, B.; Zellner d’Acampora, B.; Costa, R.; Rubiolo, P. Quantitative analysis of essential oils: A complex task. Flavour Fragr. J. 2008, 23, 382–391. [Google Scholar] [CrossRef]
- Luro, F.; Viglietti, G.; Marchi, E.; Costantino, G.; Scarpa, G.M.; Tomi, F.; Paoli, M.; Curk, F.; Ollitrault, P. Genetic, Morphological and chemical investigations reveal the genetic origin of Pompia (C. Medica Tuberosa Risso & Poiteau)—An old endemic Sardinian citrus fruit. Phytochemistry 2019, 168, 112083. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 21 October 2021).
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1984; pp. 3–700. ISBN 978-0-471-87092-0. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7.2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 21 October 2021).
- Christensen, R.H.B.; Brockhoff, P.B.; Kuznetsova, A.; Birot, S.; Stachlewska, K.A. Thurstonian Models for Sensory Discrimination. R Package Version 1.5-2. Available online: https://github.com/perbrock/sensR (accessed on 21 October 2021).
- González-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Blázquez, M.A.; Granell, A. Volatile Compounds in citrus essential oils: A comprehensive review. Front. Plant Sci. 2019, 10, 12. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Bohlmann, J.; Meyer-Gauen, G.; Croteau, R. Plant terpenoid synthases: Molecular biology and phylogenetic analysis. Proc. Natl. Acad. Sci. USA 1998, 95, 4126–4133. [Google Scholar] [CrossRef] [Green Version]
- Castle, W.S. Rootstock as a fruit quality factor in citrus and deciduous tree crops. N. Z. J. Crop Hortic. Sci. 1995, 23, 383–394. [Google Scholar] [CrossRef]
- Scoost, R.K.; Cameron, J.W. Tree and fruit characters of citrus triploids from tetraploid by diploid crosses. Hilgardia 1969, 39, 569–579. [Google Scholar] [CrossRef] [Green Version]
- Weiss-Schneeweiss, H.; Emadzade, K.; Jang, T.-S.; Schneeweiss, G.M. Evolutionary consequences, constraints and potential of polyploidy in plants. Cytogenet. Genome Res. 2013, 140, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Haberer, G.; Matthes, M.; Rattei, T.; Mayer, K.F.X.; Gierl, A.; Torres-Ruiz, R.A. Impact of natural genetic variation on the transcriptome of autotetraploid Arabidopsis thaliana. PNAS 2010, 107, 17809–17814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allario, T.; Brumos, J.; Colmenero-Flores, J.M.; Tadeo, F.; Froelicher, Y.; Talon, M.; Navarro, L.; Ollitrault, P.; Morillon, R. Large changes in anatomy and physiology between diploid Rangpur lime (Citrus limonia) and Its autotetraploid are not associated with large changes in leaf gene expression. J. Exp. Bot. 2011, 62, 2507–2519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elston, A.; Lin, J.; Rouseff, R. Determination of the Role of Valencene in Orange Oil as a Direct Contributor to Aroma Quality. Flavour Fragr. J. 2005, 20, 381–386. [Google Scholar] [CrossRef]
- Dugo, G.; Verzera, A.; d’Alcontres, I.S.; Cotroneo, A.; Trozzi, A.; Mondello, L. On the Genuineness of Citrus Essential Oils. Part XLIII. The Composition of the Volatile Fraction of Italian Sweet Orange Oils (Citrus sinensis (L.) Osbeck). J. Essent. Oil Res. 1994, 6, 101–137. [Google Scholar] [CrossRef]
- Dugo, P.; Mondello, L.; Cogliandro, E.; Verzera, A.; Dugo, G. On the genuineness of citrus essential oils. 51. Oxygen heterocyclic compounds of bitter orange oil (Citrus aurantium L.). J. Agric. Food Chem. 1996, 44, 544–549. [Google Scholar] [CrossRef]
- Rodríguez, A.; Peris, J.E.; Redondo, A.; Shimada, T.; Costell, E.; Carbonell, I.; Rojas, C.; Peña, L. Impact of D-limonene synthase up- or down-regulation on sweet orange fruit and juice odor perception. Food Chem. 2017, 217, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Rouseff, R.L.; Ruiz Perez-Cacho, P.; Jabalpurwala, F. Historical review of citrus flavor research during the past 100 years. J. Agric. Food Chem. 2009, 57, 8115–8124. [Google Scholar] [CrossRef] [PubMed]

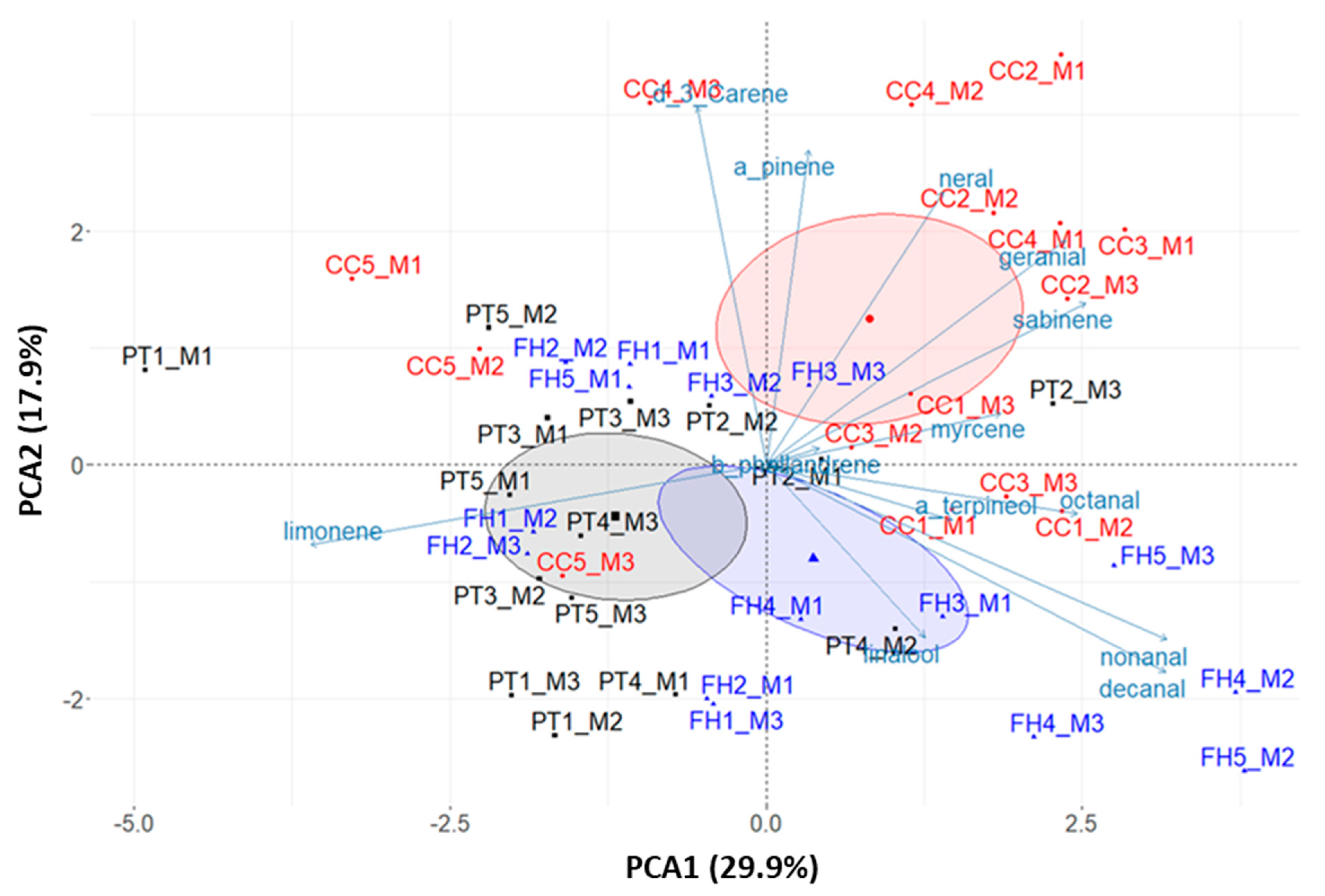
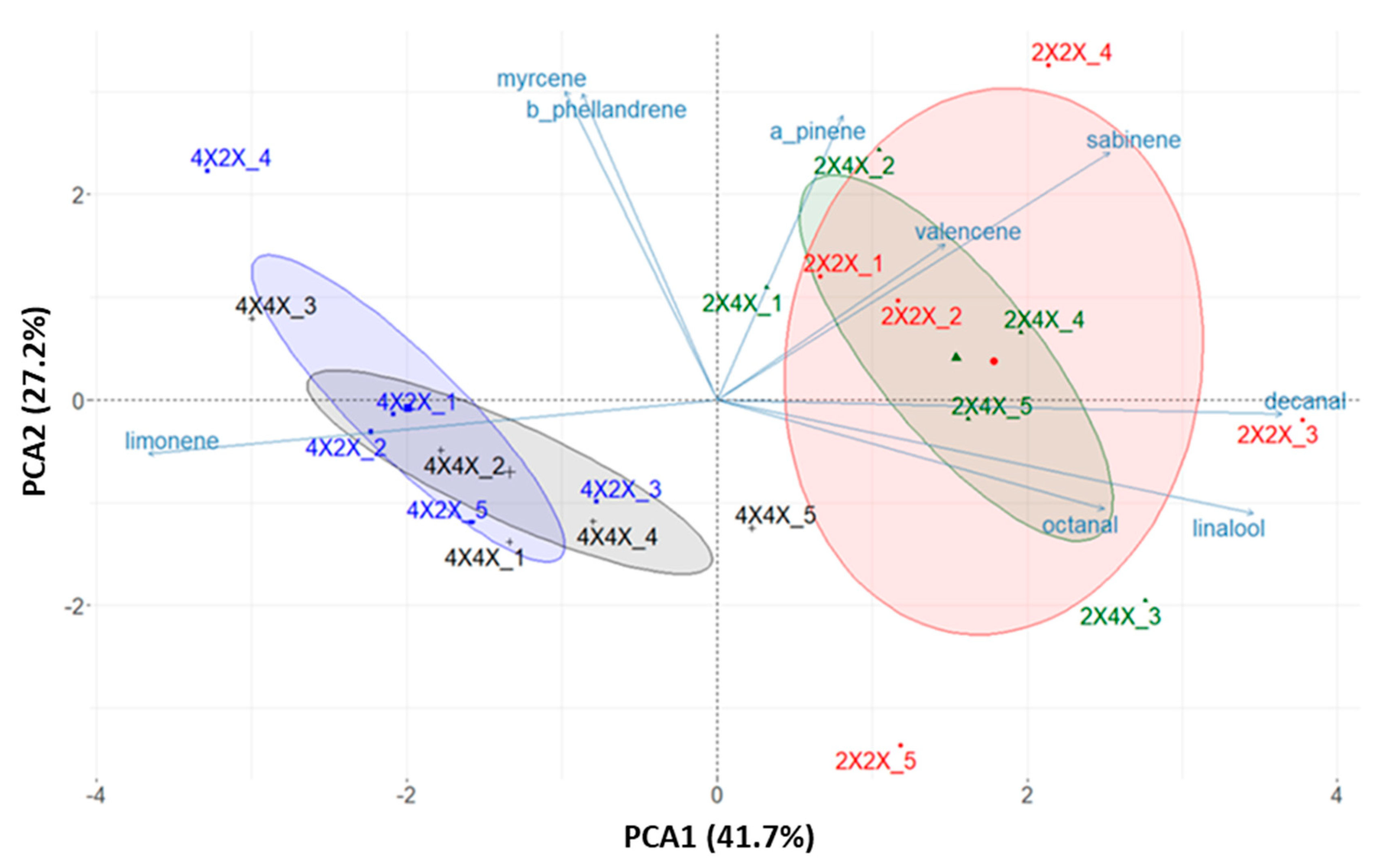
| ROOTSTOCK | ||||||
|---|---|---|---|---|---|---|
| Carrizo Citrange | FLHORAG1 | P. trifoliata | ||||
| Compound | Ria 1 | Rip 2 | Mean ± sd 3 | Mean ± sd | Mean ± sd | Method 4 |
| 2-hexenal | 825 | 1225 | 0.02 ± 0.02 ab | 0.02 ± 0.02 a | 0.00 ± 0.01 b | RI, MS |
| α-thujene | 922 | 1017 | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.05 ± 0.03 a | RI, MS |
| α-pinene | 930 | 1017 | 0.48 ± 0.01 a | 0.47 ± 0.01 ab | 0.47 ± 0.02 b | RI, MS |
| sabinene | 965 | 1125 | 0.41 ± 0.10 a | 0.29 ± 0.07 b | 0.17 ± 0.07 c | RI, MS |
| β-pinene | 970 | 1114 | 0.01 ± 0.02 a | 0.00 ± 0.01 a | 0.01 ± 0.02 a | RI, MS |
| myrcene * | 980 | 1163 | 2.13 ± 0.06 ab | 2.14 ± 0.04 a | 2.09 ± 0.06 b | RI, MS |
| octanal * | 980 | 1294 | 0.64 ± 0.15 a | 0.59 ± 0.10 a | 0.62 ± 0.14 a | RI, MS |
| δ-3-carene | 1005 | 1150 | 0.29 ± 0.09 a | 0.25 ± 0.09 a | 0.23 ± 0.04 a | RI, MS |
| p-cymene | 1011 | 1275 | 0.04 ± 0.03 a | 0.00 ± 0.00 b | 0.05 ± 0.05 a | RI, MS |
| limonene * | 1023 | 1208 | 94.02 ± 0.41 b | 94.29 ± 0.37 ab | 94.37 ± 0.39 a | RI, MS |
| β-phellandrene * | 1023 | 1215 | 0.21 ± 0.01 b | 0.23 ± 0.01 a | 0.19 ± 0.02 c | RI, MS |
| (E)-β-ocimene | 1036 | 1253 | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | RI, MS |
| γ-terpinene | 1048 | 1248 | 0.00 ± 0.01 a | 0.00 ± 0.01 a | 0.02 ± 0.03 a | RI, MS |
| trans sabinene hydrate | 1055 | 1467 | 0.01 ± 0.02 a | 0.01 ± 0.01 ab | 0.00 ± 0.00 b | RI, MS |
| terpinolene | 1078 | 1286 | 0.01 ± 0.02 a | 0.01 ± 0.01 a | 0.00 ± 0.01 a | RI, MS |
| nonanal | 1082 | 1397 | 0.09 ± 0.01 a | 0.09 ± 0.01 a | 0.08 ± 0.02 a | RI, MS |
| linalool | 1083 | 1549 | 0.49 ± 0.06 b | 0.60 ± 0.07 a | 0.53 ± 0.07 b | RI, MS |
| cis-limonene-1,2-epoxide | 1117 | 1450 | 0.03 ± 0.04 b | 0.00 ± 0.00 b | 0.07 ± 0.07 a | RI, MS |
| trans-limonene-1,2-epoxide | 1121 | 1462 | 0.13 ± 0.05 b | 0.00 ± 0.01 c | 0.24 ± 0.13 a | RI, MS |
| citronellal | 1131 | 1492 | 0.00 ± 0.02 a | 0.00 ± 0.01 a | 0.00 ± 0.00 a | RI, MS |
| nonan-1-ol | 1155 | 1301 | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.01 ± 0.02 a | RI |
| terpinen-4-ol | 1162 | 1604 | 0.02 ± 0.03 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | RI, MS |
| α-terpineol | 1173 | 1698 | 0.11 ± 0.02 a | 0.10 ± 0.04 a | 0.10 ± 0.02 a | RI, MS |
| decanal | 1184 | 1501 | 0.58 ± 0.09 b | 0.68 ± 0.12 a | 0.53 ± 0.08 b | RI, MS |
| neral | 1216 | 1685 | 0.12 ± 0.05 a | 0.04 ± 0.04 b | 0.08 ± 0.06 a | RI, MS |
| geranial | 1243 | 1735 | 0.13 ± 0.06 a | 0.10 ± 0.04 a | 0.10 ± 0.03 a | RI, MS |
| β-elemene | 1386 | 1590 | 0.02 ± 0.03 ab | 0.04 ± 0.04 a | 0.01 ± 0.02 b | RI, MS |
| geranyl α-terpinene | 1941 | 2219 | 0.00 ± 0.00 b | 0.03 ± 0.03 a | 0.00 ± 0.00 b | RI |
| Total | 100.00 | 100.00 | 100.00 | |||
| Olefins | 97.62 a | 97.77 a | 97.65 a | |||
| Oxygenated | 2.38 a | 2.23 a | 2.35 a | |||
| Aliphatic aldehydes | 1.33 a | 1.38 a | 1.23 a | |||
| PLOIDY OF SCION/ROOTSTOCK | |||||||
|---|---|---|---|---|---|---|---|
| 2X/2X | 2X/4X | 4X/2X | 4X/4X | ||||
| Compound | Ria 1 | Rip 2 | Mean ± sd 3 | Mean ± sd | Mean ± sd | Mean ± sd | Method 4 |
| hexanal | 776 | 1087 | 0.00 ± 0.00 a | 0.01 ± 0.02 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | RI, MS |
| 2-hexenal | 828 | 1225 | 0.01 ± 0.01 a | 0.03 ± 0.03 a | 0.03 ± 0.03 a | 0.01 ± 0.01 a | RI, MS |
| α-thujene | 922 | 1017 | 0.01 ± 0.02 a | 0.02 ± 0.04 a | 0.00 ± 0.00 a | 0.02 ± 0.04 a | RI, MS |
| α-pinene | 930 | 1017 | 0.51 ± 0.07 a | 0.53 ± 0.01 a | 0.51 ± 0.02 a | 0.51 ± 0.01 a | RI, MS |
| sabinene | 965 | 1125 | 0.73 ± 0.19 a | 0.65 ± 0.16 ab | 0.46 ± 0.04 bc | 0.40 ± 0.13 c | RI, MS |
| β-pinene | 970 | 1114 | 0.03 ± 0.03 a | 0.02 ± 0.02 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | RI, MS |
| myrcene * | 980 | 1163 | 1.94 ± 0.07 a | 1.96 ± 0.07 a | 1.96 ± 0.05 a | 1.94 ± 0.06 a | RI, MS |
| octanal * | 980 | 1294 | 0.15 ± 0.03 a | 0.17 ± 0.05 a | 0.10 ± 0.05 a | 0.13 ± 0.06 a | RI, MS |
| δ-3-carene | 1005 | 1150 | 0.09 ± 0.07 a | 0.09 ± 0.03 a | 0.04 ± 0.05 a | 0.02 ± 0.03 a | RI, MS |
| p-cymene | 1011 | 1275 | 0.10 ± 0.10 a | 0.07 ± 0.12 a | 0.02 ± 0.04 a | 0.03 ± 0.05 a | RI, MS |
| limonene * | 1023 | 1208 | 94.49 ± 0.48 a | 94.6 ± 0.29 a | 95.77 ± 0.10 b | 95.63 ± 0.29 b | RI, MS |
| β-phellandrene * | 1023 | 1215 | 0.21 ± 0.02 a | 0.22 ± 0.02 a | 0.22 ± 0.01 a | 0.21 ± 0.02 a | RI, MS |
| γ-terpinene | 1048 | 1248 | 0.05 ± 0.06 a | 0.03 ± 0.05 a | 0.02 ± 0.04 a | 0.05 ± 0.07 a | RI, MS |
| nonanal | 1082 | 1397 | 0.01 ± 0.02 a | 0.01 ± 0.01 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | RI, MS |
| linalool | 1083 | 1549 | 0.87 ± 0.13 a | 0.89 ± 0.10 a | 0.63 ± 0.13 b | 0.70 ± 0.09 ab | RI, MS |
| cis-limonene-1,2-epoxide | 1117 | 1450 | 0.07 ± 0.07 a | 0.07 ± 0.11 a | 0.00 ± 0.00 a | 0.03 ± 0.07 a | RI, MS |
| trans-limonene-1,2-epoxide | 1121 | 1462 | 0.01 ± 0.03 a | 0.02 ± 0.05 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | RI, MS |
| citronellal | 1131 | 1492 | 0.01 ± 0.02 a | 0.01 ± 0.02 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | RI, MS |
| terpinen-4-ol | 1162 | 1604 | 0.06 ± 0.06 a | 0.04 ± 0.04 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | RI, MS |
| α-terpineol | 1173 | 1698 | 0.07 ± 0.04 a | 0.05 ± 0.04 ab | 0.00 ± 0.01 b | 0.01 ± 0.02 b | RI, MS |
| decanal | 1184 | 1501 | 0.27 ± 0.03 a | 0.26 ± 0.04 a | 0.17 ± 0.03 b | 0.19 ± 0.02 b | RI, MS |
| trans-carveol | 1198 | 1836 | 0.00 ± 0.00 a | 0.00 ± 0.01 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | RI, MS |
| neral | 1216 | 1685 | 0.07 ± 0.06 a | 0.05 ± 0.07 a | 0.01 ± 0.01 a | 0.02 ± 0.04 a | RI, MS |
| geranial | 1243 | 1735 | 0.09 ± 0.02 a | 0.09 ± 0.01 a | 0.00 ± 0.01 b | 0.02 ± 0.03 b | RI, MS |
| valencene | 1486 | 1716 | 0.15 ± 0.04 a | 0.11 ± 0.04 a | 0.08 ± 0.05 a | 0.07 ± 0.04 a | RI, MS |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | |||
| Olefins | 98.31 a | 98.30 a | 99.06 b | 98.89 b | |||
| Oxygenated | 1.69 a | 1.70 a | 0.94 b | 1.11 b | |||
| Aliphatic aldehydes | 0.44 a | 0.47 a | 0.30 c | 0.33 bc | |||
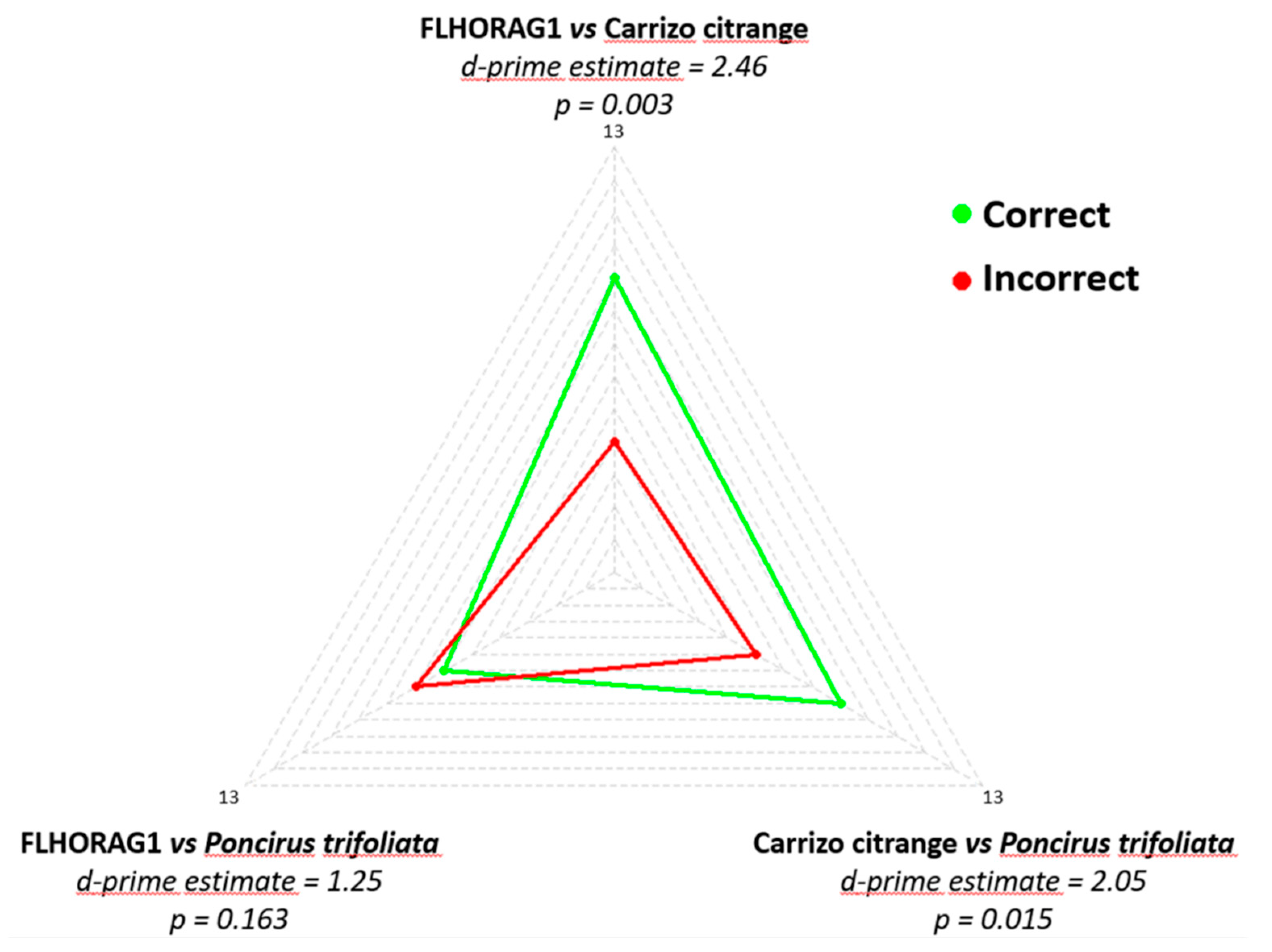
| Reference | Test | Ploidy of Scion/Rootstock | |||
|---|---|---|---|---|---|
| 2X/2X | 2X/4X | 4X/2X | 4X/4X | ||
| 2X/2X | Identical | 8 | 1 | 5 | 0 |
| Different | 2 | 9 | 5 | 10 | |
| p-value | 0.003 | 0.175 | 0 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrer, V.; Paymal, N.; Quinton, C.; Costantino, G.; Paoli, M.; Froelicher, Y.; Ollitrault, P.; Tomi, F.; Luro, F. Influence of the Rootstock and the Ploidy Level of the Scion and the Rootstock on Sweet Orange (Citrus sinensis) Peel Essential Oil Yield, Composition and Aromatic Properties. Agriculture 2022, 12, 214. https://doi.org/10.3390/agriculture12020214
Ferrer V, Paymal N, Quinton C, Costantino G, Paoli M, Froelicher Y, Ollitrault P, Tomi F, Luro F. Influence of the Rootstock and the Ploidy Level of the Scion and the Rootstock on Sweet Orange (Citrus sinensis) Peel Essential Oil Yield, Composition and Aromatic Properties. Agriculture. 2022; 12(2):214. https://doi.org/10.3390/agriculture12020214
Chicago/Turabian StyleFerrer, Vincent, Noémie Paymal, Carole Quinton, Gilles Costantino, Mathieu Paoli, Yann Froelicher, Patrick Ollitrault, Félix Tomi, and François Luro. 2022. "Influence of the Rootstock and the Ploidy Level of the Scion and the Rootstock on Sweet Orange (Citrus sinensis) Peel Essential Oil Yield, Composition and Aromatic Properties" Agriculture 12, no. 2: 214. https://doi.org/10.3390/agriculture12020214
APA StyleFerrer, V., Paymal, N., Quinton, C., Costantino, G., Paoli, M., Froelicher, Y., Ollitrault, P., Tomi, F., & Luro, F. (2022). Influence of the Rootstock and the Ploidy Level of the Scion and the Rootstock on Sweet Orange (Citrus sinensis) Peel Essential Oil Yield, Composition and Aromatic Properties. Agriculture, 12(2), 214. https://doi.org/10.3390/agriculture12020214







