Low Doses of Anatase and Rutile Nanoparticles Differently Modulate Photosynthesis and Regulatory Genes: A Contribution to the Nanoagroindustry
Abstract
:1. Introduction
2. Materials and Methods
2.1. NPs Supply, Characterization, and Solution Preparation
2.2. Plant Material and Type of Substrate for Seed Exposure
2.3. Plant Growth and Elemental Contents
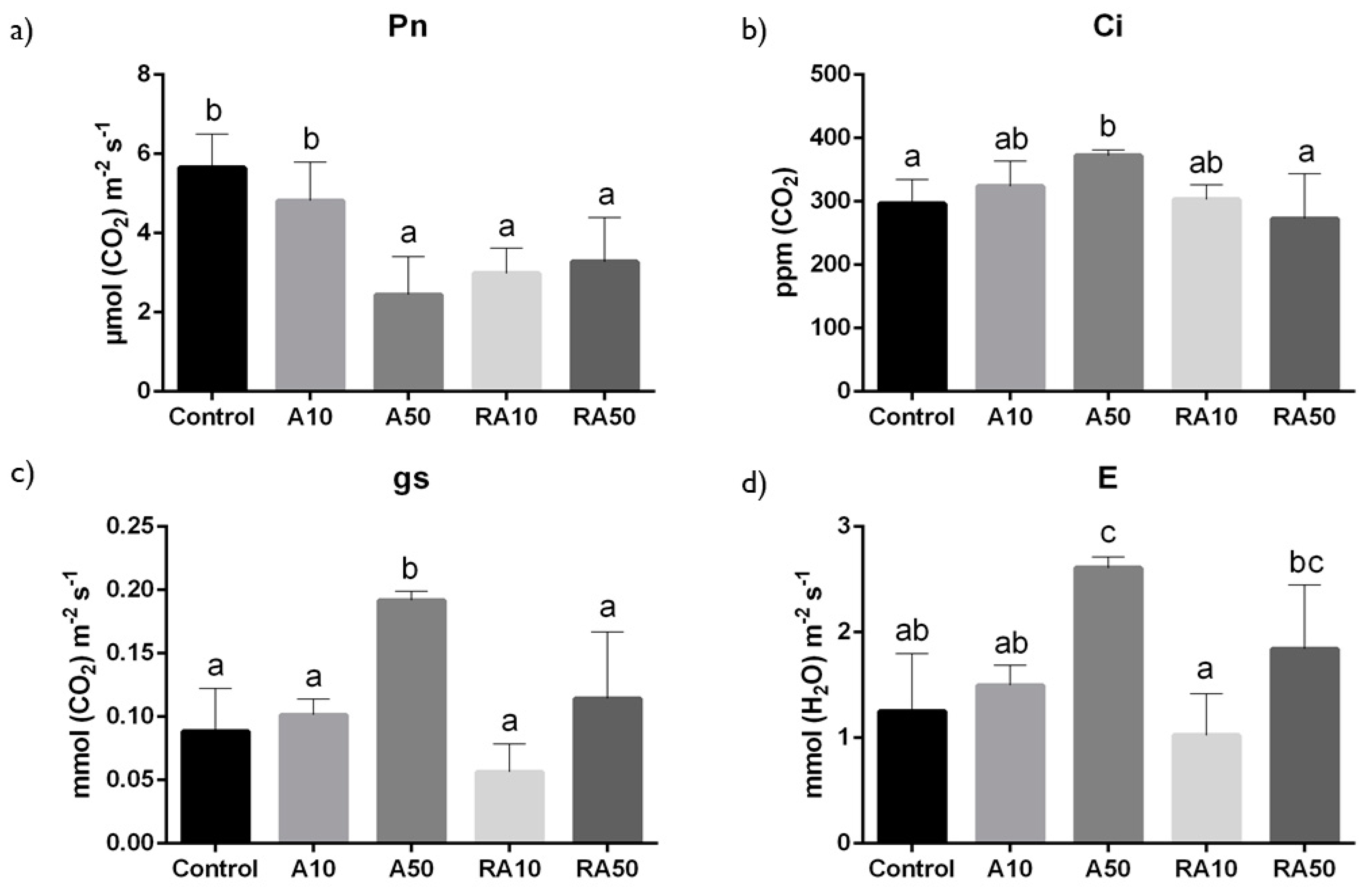
2.4. Gas Exchange and PSII Efficiency
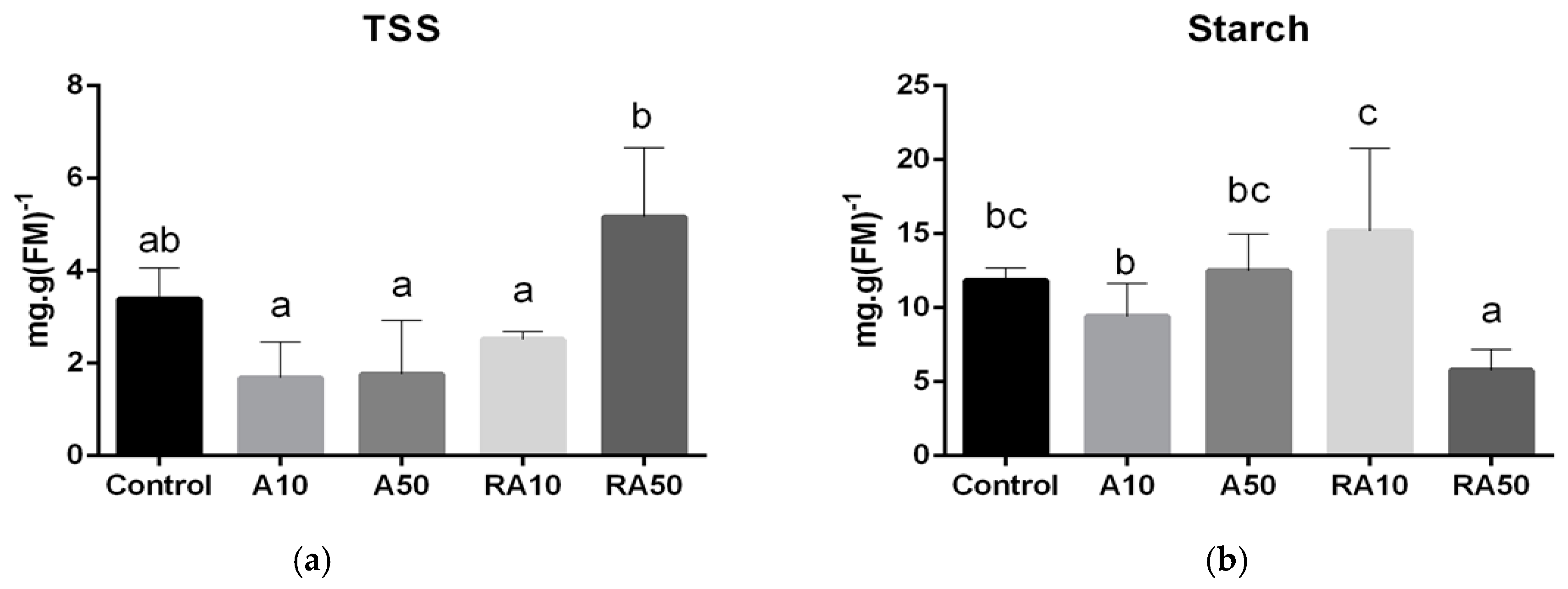
2.5. Carbohydrate Content
2.6. Gene Expression
2.7. Statistical Analysis
3. Results
3.1. Germination, Growth, and Nutrition
3.2. Photosynthetic Performance
3.3. Gene Expression
3.4. PCA Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asadishad, B.; Chahal, S.; Akbari, A.; Cianciarelli, V.; Azodi, M.; Ghoshal, S.; Tufenkji, N. Amendment of Agricultural Soil with Metal Nanoparticles: Effects on Soil Enzyme Activity and Microbial Community Composition. Environ. Sci. Technol. 2018, 52, 1908–1918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kah, M.; Hofmann, T. Nanopesticide research: Current trends and future priorities. Environ. Int. 2014, 63, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Mailapalli, D.R. Nanopesticides for Pest Control. Sustain. Agric. Rev. 2020, 8, 43–74. [Google Scholar] [CrossRef]
- Prasad, R.; Bhattacharyya, A.; Nguyen, Q. Nanotechnology in Sustainable Agriculture: Recent Developments, Challenges, and Perspectives. Front. Microbiol. 2017, 8, 165–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariz-Ponte, N.; Sario, S.; Mendes, R.J.; Correia, C.V.; Moutinho-Pereira, J.; Correia, C.M.; Santos, C. TiSiO4 nanoparticles can stimulate plant growth and the photosynthetic pigments on lettuce crop. Agriculture/Pol’nohospodárstvo 2020, 66, 148–160. [Google Scholar] [CrossRef]
- Giraldo, J.P.; Landry, M.P.; Faltermeier, S.M.; McNicholas, T.P.; Iverson, N.M.; Boghossian, A.A.; Reuel, N.F.; Hilmer, A.J.; Sen, F.; Brew, J.A.; et al. Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat. Mater. 2014, 13, 400–408. [Google Scholar] [CrossRef] [Green Version]
- Lyu, S.; Wei, X.; Chen, J.; Wang, C.; Wang, X.; Pan, D. Titanium as a Beneficial Element for Crop Production. Front. Plant Sci. 2017, 8, 597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastogi, A.; Zivcak, M.; Sytar, O.; Kalaji, H.M.; He, X.; Mbarki, S.; Brestic, M. Impact of Metal and Metal Oxide Nanoparticles on Plant: A Critical Review. Front. Chem. 2017, 5, 78. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Chen, J.; Dou, R.; Gao, X.; Mao, C.; Wang, L. Assessment of the Phytotoxicity of Metal Oxide Nanoparticles on Two Crop Plants, Maize (Zea mays L.) and Rice (Oryza sativa L.). Int. J. Environ. Res. 2015, 12, 15100–15109. [Google Scholar] [CrossRef] [Green Version]
- Mariz-Ponte, N.; Dias, C.M.; Silva, A.M.; Santos, C.; Silva, S. Low levels of TiO2-nanoparticles interact antagonistically with Al and Pb alleviating their toxicity. Plant Physiol. Biochem. 2021, 167, 1–10. [Google Scholar] [CrossRef]
- Cox, A.; Venkatachalam, P.; Sahi, S.; Sharma, N. Silver and titanium dioxide nanoparticle toxicity in plants: A review of current research. Plant Physiol. Biochem. 2016, 107, 147–163. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oi, L.E.; Choo, M.Y.; Lee, H.V.; Ong, H.C.; Abd Hamid, S.B.; Juan, J.C. Recent advances of titanium dioxide (TiO2) for green organic synthesis. Rsc Adv. 2016, 6, 108741–108754. [Google Scholar] [CrossRef]
- Silva, S.; Oliveira, H.; Craveiro, S.C.; Calado, A.J.; Santos, C. Pure anatase and rutile + anatase nanoparticles differently affect wheat seedlings. Chemosphere 2016, 151, 68–75. [Google Scholar] [CrossRef]
- Silva, S.; Pinto, G.; Santos, C. Low doses of Pb affected Lactuca sativa photosynthetic performance. Photosynthetica 2017, 55, 50–57. [Google Scholar] [CrossRef]
- Larue, C.; Khodja, H.; Herlin-Boime, N.; Brisset, F.; Flank, A.M.; Fayard, B.; Chaillou, S.; Carrière, M. Investigation of titanium dioxide nanoparticles toxicity and uptake by plants. J. Phys. Conf. Ser. 2011, 304, 012057. [Google Scholar] [CrossRef]
- Arshad, M.; Nisar, S.; Gul, I.; Nawaz, U.; Irum, S.; Ahmad, S.; Sadat, H.; Mian, I.A.; Ali, S.; Rizwan, M.; et al. Multi-element uptake and growth responses of Rice (Oryza sativa L.) to TiO2 nanoparticles applied in different textured soils. Ecotox. Environ. Safe. 2021, 215, 112149. [Google Scholar] [CrossRef] [PubMed]
- Jacob, D.L.; Borchardt, J.D.; Navaratnam, L.; Otte, M.L.; Bezbaruah, A.N. Uptake and translocation of Ti from nanoparticles in crops and wetland plants. Int. J Phytoremediat. 2013, 15, 142–153. [Google Scholar] [CrossRef]
- Deng, Y.; Petersen, E.J.; Challis, K.E.; Rabb, S.A.; Holbrook, R.D.; Ranville, J.F.; Nelson, B.C.; Xing, B. Multiple method analysis of TiO2 nanoparticle uptake in rice (Oryza sativa L.) plants. Environ. Sci. Technol. 2017, 51, 10615–10623. [Google Scholar] [CrossRef] [Green Version]
- Amini, S.; Maali-Amiri, R.; Mohammadi, R.; Kazemi-Shahandashti, S.S. cDNA-AFLP analysis of transcripts induced in chickpea plants by TiO2 nanoparticles during cold stress. Plant Physiol. Biochem. 2017, 111, 39–49. [Google Scholar] [CrossRef]
- Landa, P.; Vankova, R.; Andrlova, J.; Hodek, J.; Marsik, P.; Storchova, H.; White, J.C.; Vanek, T. Nanoparticle-specific changes in Arabidopsis thaliana gene expression after exposure to ZnO, TiO2, and fullerene soot. J. Hazard Mater. 2012, 241, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, N.G.; Bejai, S.; Meijer, J.; Seisenbaeva, G.A.; Kessler, V.G. Nano titania aided clustering and adhesion of beneficial bacteria to plant roots to enhance crop growth and stress management. Sci. Rep. 2015, 5, 10146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Missaoui, T.; Smiri, M.; Chmingui, H.; Hafiane, A. Effects of nanosized titanium dioxide on the photosynthetic metabolism of fenugreek (Trigonella foenum-graecum L.). Comptes Rendus Biol. 2017, 340, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Maroufpoor, N.; Mousavi, M.; Hatami, M.; Rasoulnia, A.; Lajayer, B.A. Mechanisms involved in stimulatory and toxicity effects of nanomaterials on seed germination and early seedling growth. In Advances in Phytonanotechnology; From Synthesis to Application; Academic Press: Cambridge, MS, USA, 2019; pp. 153–181. [Google Scholar]
- Abbasi Khalaki, M.; Moameri, M.; Asgari Lajayer, B.; Astatkie, T. Influence of nano-priming on seed germination and plant growth of forage and medicinal plants. Plant Growth Regul. 2021, 93, 13–28. [Google Scholar] [CrossRef]
- Lei, Z.; Mingyu, S.; Xiao, W.; Chao, L.; Chunxiang, Q.; Liang, C.; Hao, H.; Xiaoqing, L.; Fashui, H. Antioxidant stress is promoted by nano-anatase in spinach chloroplasts under UV-B radiation. Biol. Trace Elem. Res. 2008, 121, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Naeem, M.S.; Wang, X.; Liu, L.; Chen, C.; Ma, N.; Zhang, C. Nano-TiO2 is not phytotoxic as revealed by the oilseed rape growth and photosynthetic apparatus ultra-structural response. PLoS ONE 2015, 10, e0143885. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Liu, C.; Qu, C.; Zheng, L.; Yang, F.; Su, M.; Hong, F. Was improvement of spinach growth by nano-TiO2 treatment related to the changes of RuBisCO activase? Biometals 2008, 21, 211–217. [Google Scholar] [CrossRef]
- Tan, W.; Du, W.; Darrouzet-Nardi, A.J.; Hernandez-Viezcas, J.A.; Ye, Y.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Effects of the exposure of TiO2 nanoparticles on basil (Ocimum basilicum) for two generations. Sci. Total Environ. 2018, 26, 240–248. [Google Scholar] [CrossRef]
- Wu, B.; Zhu, L.; Le, X.C. Metabolomics analysis of TiO2 nanoparticles induced toxicological effects on rice (Oryza sativa L.). Environ. Pollut. 2017, 230, 302–310. [Google Scholar] [CrossRef]
- Rastogi, A. Industrial Nanoparticles and Their Influence on Gene Expression in Plants. In Nanomaterials in Plants, Algae and Microorganisms; Academic Press: London, UK, 2019; pp. 89–101. [Google Scholar]
- Kataria, S.; Jain, M.; Rastogi, A.; Živčák, M.; Brestic, M.; Liu, S.; Tripathi, D.K. Role of nanoparticles on photosynthesis: Avenues and applications. In Nanomaterials in Plants, Algae and Microorganisms; Academic Press: London, UK, 2019; pp. 103–127. [Google Scholar]
- Sharma, S.; Sahu, B.; Srinivasan, S.; Singh, M.; Govindasamy, J.; Shanmugam, V. Effect of galvanotaxic graphene oxide on chloroplast activity: Interaction quantified with Biolayer-Interferometry coupled confocal microscopy. Carbon 2020, 162, 147–156. [Google Scholar] [CrossRef]
- Hu, J.; Wu, X.; Wu, F.; Chen, W.; Zhang, X.; White, J.C.; Li, J.; Wan, Y.; Liu, J.; Wang, X. TiO2 nanoparticle exposure on lettuce (Lactuca sativa L.): Dose-dependent deterioration of nutritional quality. Environ. Sci. Nano 2020, 7, 501–513. [Google Scholar] [CrossRef]
- Giorgetti, L. Effects of nanoparticles in plants: Phytotoxicity and genotoxicity assessment. In Nanomaterials in Plants, Algae and Microorganisms; Academic Press: London, UK, 2019; pp. 65–87. [Google Scholar]
- Boxall, A.B.; Tiede, K.; Chaudhry, Q. Engineered nanomaterials in soils and water: How do they behave and could they pose a risk to human health? Nanomedicine 2007, 2, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Santos, C.; Pinto, G.; Silva, A.M.; Silva, S. Titanium dioxide nanoparticles impaired both photochemical and non-photochemical phases of photosynthesis in wheat. Protoplasma 2019, 256, 69–78. [Google Scholar] [CrossRef]
- Klughammer, C.; Schreiber, U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl. Notes 2008, 1, 201–247. [Google Scholar]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Irigoyen, J.J.; Einerich, D.W.; Sánchez-Díaz, M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativd) plants. Physiol. Plant. 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Osaki, M.; Shinano, T.; Tadano, T. Redistribution of carbon and nitrogen compounds from the shoot to the harvesting organs during maturation in field crops. Soil Sci. Plant Nutr. 1991, 37, 117–128. [Google Scholar] [CrossRef]
- Borowski, J.M.; Galli, V.; da Silva Messias, R.; Perin, E.C.; Buss, J.H.; e Silva, S.D.D.A.; Rombaldi, C.V. Selection of candidate reference genes for real-time PCR studies in lettuce under abiotic stresses. Planta 2014, 239, 1187–1200. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wang, X.P.; Yang, X.Y.; Chen, S.Y.; Li, Q.Q.; Wang, W.; Hou, C.J.; Gao, X.; Wang, L.; Wang, S.C. Zinc Oxide Nanoparticles Affect Biomass Accumulation and Photosynthesis in Arabidopsis. Front. Plant Sci. 2016, 6, 1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Servin, A.D.; Morales, M.I.; Castillo-Michel, H.; Hernandez-Viezcas, J.A.; Munoz, B.; Zhao, L.; Nunez, J.E.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Synchrotron verification of TiO2 accumulation in cucumber fruit: A possible pathway of TiO2 nanoparticle transfer from soil into the food chain. Environ. Sci. Technol. 2013, 47, 11592–11598. [Google Scholar] [CrossRef] [PubMed]
- Song, U.; Jun, H.; Waldman, B.; Roh, J.; Kim, Y.; Yi, J.; Lee, E.J. Functional analyses of nanoparticle toxicity: A comparative study of the effects of TiO2 and Ag on tomatoes (Lycopersicon esculentum). Ecotoxicol. Environ. Saf. 2013, 93, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Song, U.; Shin, M.; Lee, G.; Roh, J.; Kim, Y.; Lee, E. Functional Analysis of TiO2 Nanoparticle Toxicity in Three Plant Species. Biol. Trace Elem. Res. 2013, 155, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Raliya, R.; Nair, R.; Chavalmane, S.; Wang, W.N.; Biswas, P. Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics 2015, 7, 1584–1594. [Google Scholar] [CrossRef] [PubMed]
- Zhori, A.; Meco, M.; Brandl, H.; Bachofen, R. In situ chlorophyll fluorescence kinetics as a tool to quantify effects on photosynthesis in Euphorbia cyparissias by a parasitic infection of the rust fungus Uromyces pisi. BMC Res. Notes 2015, 8, 698. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Hong, F.; Liu, C.; Zheng, L.; Su, M.; Wu, X.; Yang, F.; Wu, C.; Yang, P. Mechanism of nano-anatase TiO2 on promoting photosynthetic carbon reaction of spinach. Biol. Trace Elem. Res. 2006, 111, 239–253. [Google Scholar] [CrossRef]
- Schreiber, U.; Bilger, W.; Neubauer, C. Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. In Ecophysiology of Photosynthesis; Springer: Berlin/Heidelberg, Germany, 1995; pp. 40–70. [Google Scholar] [CrossRef]
- Killi, D.; Haworth, M. Diffusive and metabolic constraints to photosynthesis in quinoa during drought and salt stress. Plants 2017, 6, 49. [Google Scholar] [CrossRef] [Green Version]
- Bradfield, S.J. Influence of TiO2 Engineered Nanoparticles on Photosynthetic Efficiency and Contaminant Uptake. Master’s Thesis, Southern Illinois University at Carbondale, Carbondale, IL, USA, 2015. [Google Scholar]
- Conway, J.R.; Beaulieu, A.L.; Beaulieu, N.L.; Mazer, S.J.; Keller, A.A. Environmental stresses increase photosynthetic disruption by metal oxide nanomaterials in a soil-grown plant. ACS Nano 2015, 9, 11737–11749. [Google Scholar] [CrossRef] [Green Version]
- Linglan, M.; Chao, L.; Chunxiang, Q.; Sitao, Y.; Jie, L.; Fengqing, G.; Fashui, H. RuBisCO activase mRNA expression in spinach: Modulation by nanoanatase treatment. Biol. Trace Elem. Res. 2008, 122, 168–178. [Google Scholar] [CrossRef]
- Couée, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Asli, S.; Neumann, P.M. Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport. Plant Cell Environ. 2009, 32, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Tumburu, L.; Andersen, C.; Rygiewicz, P.; Reichman, J. Phenotypic and genomic responses to titanium dioxide and cerium oxide nanoparticles in Arabidopsis germinants. Environ. Toxicol. Chem. 2015, 34, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.P.; McIntyre, C.L.; Glassop, D.; Shorter, R. Use of expression analysis to dissect alterations in carbohydrate metabolism in wheat leaves during drought stress. Plant Mol. Biol. 2008, 67, 197–214. [Google Scholar] [CrossRef] [PubMed]


| Gene Designation | Description | GenBank ID | Primers (5′–3′) |
|---|---|---|---|
| β tub * | β-tubulin, btub1 | AB232704.1 | F: AAATGTGGGACGCAAAGAAC R: TCATCCACCTCTTTCGTGCT |
| apt1 * | adenine phosphoribosyl transferase 1-like | XM_023900216.1 | F: CGCCATTTACAAGCTTCATATTC R: ATCCCTGGCTTCGGAAAG |
| petB | cytochrome b6 | NC_007578.1:74837–76254 | F: ACAGGTGTGGTTCTGGGTGT R: GTGGATTGTCCCACACTAGCA |
| petA | cytochrome f | NC_007578.1:62045–63007 | F: GATACGAAATAACCATAGCGGATG R: ATCCCTGGCTTCGGAAAG |
| psbA | photosystem II protein D1 | NC_007578.1:c1540-479 | F: GTGTAGCTTGTTACATGGGTCGT R: TCCTAGAGGCATACCATCAGAAAAG |
| psaA | photosystem I P700 chlorophyll a apoprotein A1 | NC_007578.1:c41453-39201 | F: ATGGCTAAGCGATCCGACT R: TCCAGATGCTCGCCAAAT |
| psaC | photosystem I subunit VII; | NC_007578.1:c116733-116488 | F: TGTATCGGGTGTACGCAATG R: CAGGCGGATTCACATCTCTT |
| atpA | ATP synthase CF1 alpha subunit | NC_007578.1:28282–29808 | F: TGTAGCTATTGGTCAAAAAGCATCT R: GCCAGAGCTGCTCCTGTATAA |
| atpB | ATP synthase CF1 beta subunit | NC_007578.1:c54296-52800 | F: AACGAGAGGGATGGACGTAAT R: GTATAAAGGCAGGCGCAGAT |
| ndhA | NADH dehydrogenase subunit 1 | NC_007578.1:c121081-118937 | F: GCGCAGTCAAAATATGGTTTTT R: CGGTTTGATAACCTGCTACTAATT |
| ndhD | NADH dehydrogenase subunit 4 | NC_007578.1:c116370-114868 | F: ACGTCTTGTTTATCTCGACCAAA R: TGAGTGGTTTTGTTGCAGAAGT |
| rbcL | RuBisCO large subunit | AY874437.1 | F: ATTTTGGCAGCATTTCGAGT R: CATCGGTCCACACAGTTGTC |
| Control | A10 | A50 | RA10 | RA50 | ||
|---|---|---|---|---|---|---|
| Length (cm) | Aerial part | 6.85 ± 0.521 b | 5.83 ± 0.650 ab | 5.35 ± 1.150 a | 6.42 ± 1.020 ab | 6.27 ± 0.535 ab |
| Root part | 3.88 ± 1.036 ab | 3.52 ± 0.776 a | 3.82 ± 2.062 ab | 2.33 ± 0.816 a | 5.68 ± 0.788 b | |
| Fresh matter (mg) | Aerial part | 195.5 ± 64.61 ab | 259.6 ± 91.45 b | 151.0 ± 74.41 ab | 261.2 ± 49.61 b | 97.4 ± 17.17 a |
| Root part | 28.3 ± 1.71 ab | 35.8 ± 12.38 ab | 21.6 ± 10.11 a | 45.0 ± 9.77 b | 20.0 ± 4.69 a | |
| Dry matter (mg) | Aerial part | 4.5 ± 1.10 a | 7.2 ± 3.27 a | 5.0 ± 2.24 a | 7.8 ± 2.39 a | 3.8 ± 1.30 a |
| Root part | 1.0 ± 0.00 a | 1.2 ± 0.45 a | 1.0 ± 0.00 a | 1.2 ± 0.45 a | 1.25 ± 0.25 a |
| mg·gDM−1 | Control | A10 | A50 | RA10 | RA50 |
|---|---|---|---|---|---|
| Ca | 2.45 ± 0.291 ab | 3.78 ± 0.334 c | 3.62 ± 0.076 c | 2.70 ± 0.882 b | 1.84 ± 0.399 a |
| K | 47.49 ± 9.588 a | 42.52 ± 2.348 a | 43.74 ± 3.123 a | 42.73 ± 7.857 a | 37.01 ± 6.476 a |
| Fe | 0.10 ± 0.029 a | 0.17 ± 0.022 b | 0.16 ± 0.037 b | 0.14 ± 0.031 ab | 0.14 ± 0.025 ab |
| Mg | 7.68 ± 0.517 a | 8.05 ± 1.588 a | 8.90 ± 0.879 a | 8.00 ± 1.268 a | 7.33 ± 0.635 a |
| P | 16.74 ± 0.273 b | 14.40 ± 0.622 ab | 13.44 ± 1.209 ab | 14.76 ± 3.902 ab | 12.38 ± 2.162 a |
| Mn | 0.68 ± 0.052 a | 0.84 ± 0.139 b | 0.91 ± 0.055 b | 0.85 ± 0.090 b | 0.90 ± 0.064 b |
| Ti | Nd. | Nd. | 2.5 × 10−6 | Nd. | 6.7 × 10−6 |
| µmol gFM−1 | Control | A10 | A50 | RA10 | RA50 |
|---|---|---|---|---|---|
| Chla | 0.124 ± 0.013 a | 0.122 ± 0.015 a | 0.126 ± 0.008 a | 0.162 ± 0.016 b | 0.126 ± 0.016 a |
| Chlb | 0.053 ± 0.005 a | 0.052 ± 0.005 a | 0.055 ± 0.005 a | 0.070 ± 0.007 b | 0.054 ± 0.008 a |
| Chla/b ratio | 2.324 ± 0.010 a | 2.317 ± 0.037 a | 2.321 ± 0.064 a | 2.320 ± 0.015 a | 2.333 ± 0.061 a |
| Anthocyanins | 0.008 ± 0.002 a | 0.007 ± 0.002 a | 0.009 ± 0.002 a | 0.008 ± 0.001 a | 0.009 ± 0.004 a |
| Carotenoids | 0.051 ± 0.005 a | 0.052 ± 0.007 a | 0.055 ± 0.004 a | 0.068 ± 0.008 a | 0.054 ± 0.008 a |
| Parameter | Control | A10 | A50 | RA10 | RA50 |
|---|---|---|---|---|---|
| F0 | 70.3 ± 10.31 a | 62.6 ± 6.72 a | 68.7 ± 15.37 a | 75.2 ± 18.24 a | 69.8 ± 8.12 a |
| Fm | 433.8 ± 84.34 a | 362.0 ± 44.93 a | 420.5 ± 116.63 a | 466.2 ± 145.08 a | 437.0 ± 74.75 a |
| Fv | 363.4 ± 75.08 a | 299.38 ± 39.58 a | 351.8 ± 101.33 a | 391.0 ± 127.38 a | 367.3 ± 66.82 a |
| Fv/Fm | 0.84 ± 0.014 a | 0.83 ± 0.011 a | 0.83 ± 0.011 a | 0.83 ± 0.024 a | 0.84 ± 0.012 a |
| F0′ | 27.0 ± 8.77 b | 9.4 ± 5.19 a | 26.0 ± 9.86 b | 17.8 ± 7.52 ab | 26.0 ± 13.60 b |
| Fm’ | 151.9 ± 35.43 b | 70.1 ± 36.38 a | 133.2 ± 46.89 ab | 123.7 ± 34.59 ab | 148.4 ± 59.51 b |
| Fv’ | 124.8 ± 28.27 b | 60.7 ± 32.37 a | 107.2 ± 37.98 ab | 105.8 ± 32.46 ab | 122.4 ± 46.66 b |
| Fv’/Fm’ | 0.82 ± 0.032 a | 0.86 ± 0.039 a | 0.80 ± 0.028 a | 0.85 ± 0.061 a | 0.83 ± 0.033 a |
| ΦPSII | 0.44 ± 0.085 a | 0.54 ± 0.067 a | 0.48 ± 0.133 a | 0.46 ± 0.070 a | 0.49 ± 0.074 a |
| qP | 0.54 ± 0.113 a | 0.63 ± 0.058 a | 0.59 ± 0.144 a | 0.54 ± 0.081 a | 0.59 ± 0.078 a |
| NPQ | 1.91 ± 0.417 a | 6.02 ± 4.201 b | 2.28 ± 0.617 a | 2.83 ± 1.039 ab | 2.19 ± 0.782 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariz-Ponte, N.; Sario, S.; Mendes, R.J.; Couto, M.; Gimranov, E.; Santos, M.; Correia, C.V.; Gomes, A.; Oliveira-Pinto, P.R.; Amorim, I.; et al. Low Doses of Anatase and Rutile Nanoparticles Differently Modulate Photosynthesis and Regulatory Genes: A Contribution to the Nanoagroindustry. Agriculture 2022, 12, 190. https://doi.org/10.3390/agriculture12020190
Mariz-Ponte N, Sario S, Mendes RJ, Couto M, Gimranov E, Santos M, Correia CV, Gomes A, Oliveira-Pinto PR, Amorim I, et al. Low Doses of Anatase and Rutile Nanoparticles Differently Modulate Photosynthesis and Regulatory Genes: A Contribution to the Nanoagroindustry. Agriculture. 2022; 12(2):190. https://doi.org/10.3390/agriculture12020190
Chicago/Turabian StyleMariz-Ponte, Nuno, Sara Sario, Rafael J. Mendes, Márcio Couto, Emil Gimranov, Marino Santos, Cristiana V. Correia, Anicia Gomes, Paulo R. Oliveira-Pinto, Isabel Amorim, and et al. 2022. "Low Doses of Anatase and Rutile Nanoparticles Differently Modulate Photosynthesis and Regulatory Genes: A Contribution to the Nanoagroindustry" Agriculture 12, no. 2: 190. https://doi.org/10.3390/agriculture12020190
APA StyleMariz-Ponte, N., Sario, S., Mendes, R. J., Couto, M., Gimranov, E., Santos, M., Correia, C. V., Gomes, A., Oliveira-Pinto, P. R., Amorim, I., Dias, M. C., Ferreira de Oliveira, J. M. P., & Santos, C. (2022). Low Doses of Anatase and Rutile Nanoparticles Differently Modulate Photosynthesis and Regulatory Genes: A Contribution to the Nanoagroindustry. Agriculture, 12(2), 190. https://doi.org/10.3390/agriculture12020190










