Abstract
Corn stover is one of the most agricultural residue abundances over the world; however, it is extremely prevented from microbial and enzymatic degradation into monomers because of the complex chemical and physical structure. In order to degrade corn stover, a large variety of enzymes with different specificities are required. However, each enzyme has its own reaction condition such as optimal pH and temperature to express its maximum activity. We hypothesize that the best sequence of an enzyme reaction could influence the degradation of corn stover. Therefore, the objective of this study was to investigate the effect of enzyme sequence action on the degradation of corn stover. A complete randomized design was used for this study. Four enzymes were used, cellulase (Cel) (pH 4.8 at 50 °C), hemicellulase (Hem) (pH 5 at 50 °C), pectinase (Pec) (pH 4 at 50 °C) and laccase (Lac) (pH 3 at 30 °C). This was subsequently submitted to enzyme sequence digestion following four steps (6 h incubation for each step) during which a single enzyme in each step was evaluated. The substrate (raw corn stover) was placed in sodium acetate buffer with an enzyme. The supernatant was then collected in each step for further chemical analysis. The results showed that there was a significant difference at p < 0.05 between treatments, suggesting that sequential action of fiber-degrading enzymes affected the chemical composition of corn stover. The best enzyme sequence (in terms of the total reducing sugar in different steps) was Hem-Cel-Pec-Lac (2.2 mg/mL) at p < 0.05; however, the worst enzyme sequence was Lac-Pec-Hem-Cel (0.8 mg/mL) at p < 0.05. Almost all the first steps in the process showed an increasing level of reducing sugar except the step which started with Lac where a lower reducing sugar level was observed. Similarly, xylose showed a higher level in all the processes in the first steps regardless of the enzyme type. It was observed that glucose production was totally dependent on the position of Cel in the enzyme sequence. Therefore, enzyme sequence action may be a useful method for corn stover to improve its degradation as feed stock.
1. Introduction
Corn stover is one of the most important lignocellulose biomasses all over the world. It is composed mainly of lignin (7.5%), hemicellulose (>26%) and cellulose (>34%). Additionally, it is used as a source of feedstock for the production of biofuel or as forage for ruminants [1,2,3,4]. Besides this, corn stover has a characteristic high carbohydrate content, ease of carbohydrate absorption as well as low lignification degree [5,6,7]. A large amount of corn stover is produced annually all the over the world [2,5,8]. However, almost 90% of this corn stover is burnt which causes severe environmental problems [2,9]. Many countries are producing a huge amount of corn stover. For instance, the U.S. and China produce about 245 and 22 million tons of dry corn stover annually, respectively [2,10,11], and that almost half of them are harvested and used as roughage or potential feedstock [2].
Despite the multi-purpose use of corn stover, it is extremely prevented from microbial and enzymatic degradation because of its complex chemical and physical structure. Therefore, it requires some initial treatments in order to enhance its degradability for more efficient utilization either as a source of biofuel production or feed for the livestock industry. Recent studies have indicated the importance of fiber-degrading enzymes on the degradation of corn stover [12,13]. Moreover, the end products of enzymatic degradation for lignocellulosic biomass primarily reducing sugars (mainly glucose, xylose) have been used for biofuel production and other industrial purposes [2,14,15,16]. A large variety of enzymes with different specificities are required to degrade corn stover such as cellulase, hemicellulase, pectinase and lactase [17]. However, different enzymes have their own reaction condition such as optimal pH and temperature to express its maximum activity. The reaction mixture of different enzymes together potentially could not achieve the maximum enzyme activity of all enzymes, and leads to low efficiency of fiber degradation and sugar production. There is no evidence about how enzyme sequence action affects the degradation of lignocellulosic biomass such as corn stover. Therefore, a new method is needed in order to better use these different varieties of enzymes. We hypothesize that the best enzyme sequence can maximize fiber degradation. Therefore, the objective of this study was to investigate the effect of enzyme sequence action on the degradation of corn stover.
2. Materials and Methods
2.1. Materials and Sample Preparation
The raw corn stover was collected after two months of harvesting time from a cornfield near Beijing, China. The raw corn stover was chopped and dried in air at room temperature for five days. This was further ground in a mill, sieved to obtain 2 mm sized pieces, and finally stored in sealed plastic bags at room temperature before use.
2.2. Experimental Design
A complete randomized design was used for this study. Number of treatments was 24 and each treatment had 3 replications. Four enzymes were used, including cellulase (Cel), hemicellulase (Hem), pectinase (Pec) and laccase (Lac). The four enzymes were placed and listed in sequential way by using permutations and combination theory (Table 1).

Table 1.
The order of fiber-degrading enzymes for four steps degradation of corn stover the enzymes used were cellulase (Cel), hemicellulase (Hem), pectinase (Pec) and laccase (Lac).
2.3. Enzymes and Enzyme Solution Preparation
The enzymes were obtained from Shanghai Yuanye Biotechnology, Co. Ltd. (Shanghai, China): Cellulase (S10041), Hemicellulase (S10045), Pectinase (S10007) and Laccase (S10189). All activities were measured at the optimal pH and temperature as suggested by the enzymes’ manufacturer. The optimal temperature for Cel, Hem and Pec were 50 °C and 30 °C for Lac. However, the optimal pH was different for each of the enzymes; 4.8 for Cel, 5 for Hem, 4 for Pec, and 3 for Lac. Hem and Pec activities were determined as described by Reynolds et al., 2018 [18]. Cel activity was determined as described by Adney and Baker, 1996 [19]. Lac activity was determined following the procedure as described by Muñiz-Mouro et al., 2018 [20].
Enzymes solutions were prepared by dissolving each enzyme (2 mg) separately in distilled water (50 mL), followed by an addition of a 2 mL enzyme solution to 10 mL buffer solution (0.5 M sodium acetate buffer) to reach a total volume to 12 mL. The optimal concentration of the four enzymes was determined in a pre-experiment (optimization experiments), where 0.2 mg/mL enzyme solution gave the best results in corn stover degradation. The activities of cellulase, hemicellulase, pectinase and laccase solutions are 0.34, 0.15, 3.37 and 0.001 U/mL, respectively.
2.4. Enzymatic Hydrolysis
Enzyme digestion was carried out in four steps by using a single enzyme in each step (Figure 1). Corn stover samples, (0.2 g) were placed in 50 mL centrifuge tubes and added to 12 mL solution (10 mL 0.5 M sodium acetate buffer plus 2 mL enzyme solution). The mixture was incubated in a water incubation shaking bath (Huamei, Beijing, China) (100× g) at different temperatures (each enzyme having a specific temperature) for 6 h. After the reaction, samples were placed in ice water/ice cubes to stop enzyme activity, centrifuged (Eppendorf, Hamburg, Germany) at 13,000× g and the supernatant subsequently separated from the solid phase. Afterwards, the second buffered enzyme solution was added to the solid phase for the second digestion step and so on till four digestions were completed. After a four-step digestion (6 h for each step by using four different fiber-degrading enzymes), total reducing sugar of the supernatant from each step was determined using the DNS method with a spectrophotometer (Jinghua, Shanghai, China) [21].
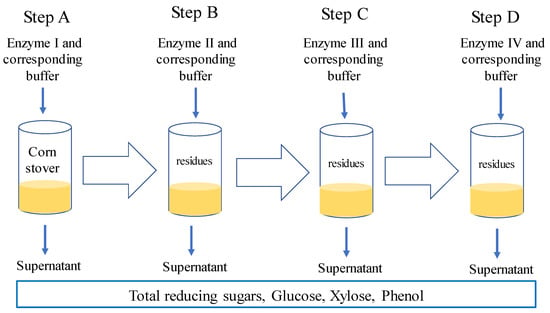
Figure 1.
Example of one enzyme sequence.
Xylose, glucose and total phenols concentrations in the supernatant from each step were determined using the Xylose Assay Kit and the Glucose Assay Kit and Total Phenol Assay Kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), respectively. Additionally, The D-Glucuronic/D-Galacturonic concentration in the supernatant from each step was measured using D-Glucuronic/D-Galacturonic Acid Assay Kit (Megazyme, Bray Business Park, Bray, Co. Wicklow, Ireland). All colorimetric analysis using these kits were carried out with a spectrophotometer (Jinghua, Shanghai, China).
2.5. Statistical Analysis
The ANOVA statistical analyses were done using the PROC GLM of SAS 9.4. The treatment was considered as fixed effect. The treatment means were compared using Tukey’s multiple comparisons procedure. Statistical significance was declared at p < 0.05. Additionally, the software Design-Expert 8.0.6 (Stat-Ease, Minneapolis, MN, USA) was used to determine the optimal parameters of steam explosion.
3. Results
3.1. The Effect of Enzyme Sequence Action on the Degradation of Corn Stover
Results of the data gathered showed that the best two enzyme sequence actions were Hem-Pec-Cel-Lac and Hem-Cel-Pec-Lac (Figure 2). There was a significant difference (p < 0.05) between the treatments. The total production levels for the best enzyme sequences were 2.2 mg/mL for the reducing sugar, 0.9 mg/mL for total glucose and 1.2 mg/mL for the total xylose, which were different to other sequences (p < 0.05). However, the worst enzyme sequence was Lac-Pec-Hem-Cel with production levels 0.8 mg/mL of the total reducing sugar, 0.7 mg/mL for the total glucose and 0.8 mg/mL of total xylose, which were different to other sequences (p < 0.05).
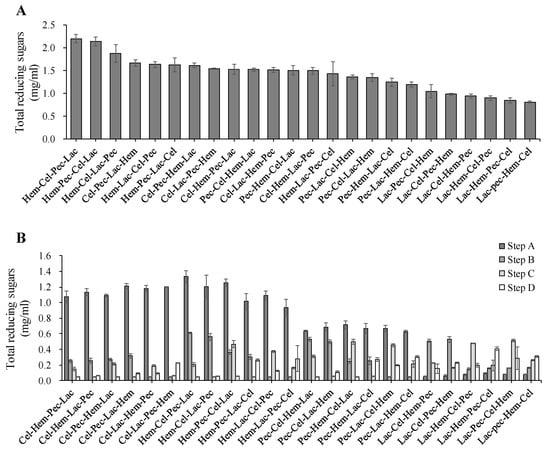
Figure 2.
The effect of enzyme sequence action on the release of reducing sugar. (A) Total reducing sugar production during four steps enzyme digestion for the corn stover (from the highest to the lowest). (B) Reducing sugar production levels during four steps enzyme digestion. The error bars represent standard error of the mean (n = 3).
3.2. The Production of Reducing Sugar
As mentioned above, the highest reducing sugar production was achieved by Hem-Pec-Cel-Lac and Hem-Cel-Pec-Lac (2.2 mg/mL) at p < 0.05 enzyme sequence (Figure 2), respectively, suggesting that the importance begging enzymes digestion was by Hem following by Pec or Cel (Pec and Cel can substitute their position) and then ending the enzyme sequence by Lac. However, the lowest level of the total reducing sugar was achieved by Lac-Pec-Hem-Cel (0.8 mg/mL) at p < 0.05 enzyme sequence which confirms our previous suggestion about the enzyme ordering of the sequence series. Most of the reducing sugars were produced in the first enzymes digestion except Lac which has the lowest production level, and this affected the other enzyme sequence hence the total production level of the reducing sugar (Figure 3). Both Cel and Hem produced half or more of the total reducing sugars when they were allocated in the first digestion step. However, Pec produced less compared to Cel and Hem. Moreover, glucose and xylose accounted for more than 90% of the total reducing sugar. Xylose showed higher than glucose for all enzyme sequences for the degradation of corn stover.
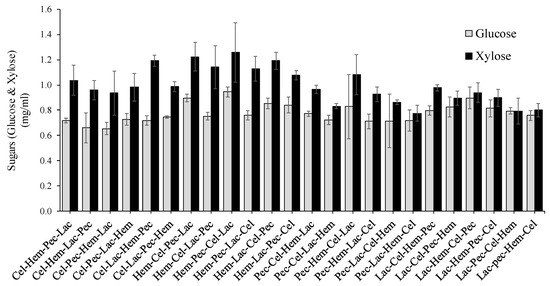
Figure 3.
Comparison between total glucose and xylose produced during four steps enzyme digestion for the corn stover. The error bars represent standard error of the mean (n = 3).
3.3. Glucose Production
The results showed that enzyme sequence action affects glucose production and there were significant differences at p < 0.05 between the treatments (Figure 3). The best enzyme sequence for total glucose production was Hem-Pec-Cel-Lac (0.94 mg/mL) at p < 0.05 while the lowest glucose production was observed by Cel-Pec-Hem-Lac (0.65 mg/mL) at p < 0.05. Additionally, the data revealed that glucose is produced in all digestion steps. Nonetheless, a high level of glucose production was associated with the location in the enzyme sequence (Figure 4). Moreover, a high glucose production level was achieved in the first step of digestion for all enzyme sequences except for the enzyme sequence that starts with Lac.
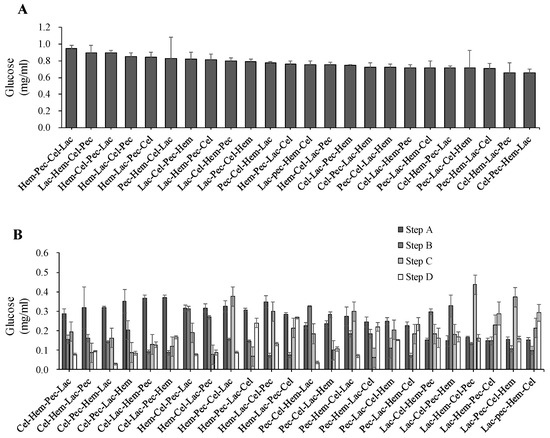
Figure 4.
The effect of enzyme sequence action on the release of glucose. (A) Total glucose production during four steps enzyme digestion for the corn stover (from the highest to the lowest). (B) Glucose production levels during four steps enzyme digestion. The error bars represent standard error of the mean (n = 3).
3.4. Xylose Production
The data revealed that enzyme sequence action affects xylose production and there were significant differences at p < 0.05 between the treatments (Figure 4). The best enzyme sequence for the total xylose production was Hem-Pec-Cel-Lac (1.2 mg/mL) at p < 0.05. The lowest xylose production was recorded by Pec-Lac-Hem-Cel (0.8 mg/mL) at p < 0.05. Most of the xylose (more than 50%) were produced in the first digestion step followed by the second digestion step regardless of the enzymes order in the sequence (Figure 5).
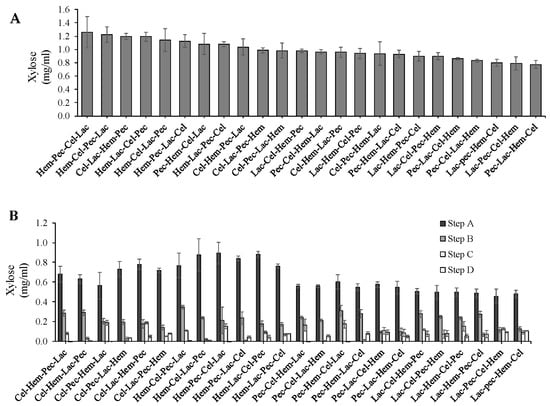
Figure 5.
The effect of enzyme sequence action on the release of xylose. (A) Total xylose production during four steps enzyme digestion for the corn stover (from the highest to the lowest). (B) Xylose production levels during four steps enzyme digestion. The error bars represent standard error of the mean (n = 3).
3.5. The Release of Phenolic Component
The enzyme sequence action affected the release of phenolics and there was a significant difference at p < 0.05 between the treatments (Figure 6). The highest phenol level was induced by Hem-Pec-Lac-Cel (11 mg/mL) at p < 0.05, whereas the lowest level was achieved by Hem-Cel-Lac-Pec (7 mg/mL) at p < 0.05. Additionally, more of the phenol was released in the first digestion step, followed by the second and so on, regardless of the enzymes order in the sequence (Figure 6).
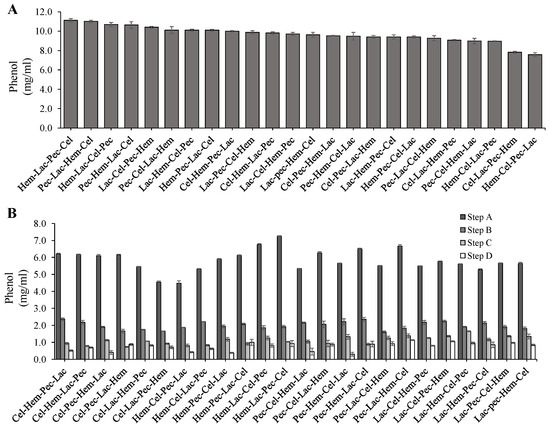
Figure 6.
The effect of enzyme sequence action on the release of Phenol. (A) Total phenol production during four steps enzyme digestion for the corn stover (from the highest to the lowest). (B) Phenol production levels during four steps enzyme digestion. The error bars represent standard error of the mean (n = 3).
4. Discussion
Enzymatic degradation of lignocellulosic biomass is one of the prominent technologies for the degradation of biomass into monomer sugars. Carbohydrates degradation within the biomass occurs by using different enzymes in a specific amount to convert the carbohydrates to their monomer sugars [17]. The enzymes worked in synergy to hydrolyze the substrate, thus indicating that enzymes mix activity is higher than the activities of single enzymes. However, each enzyme required a specific reaction condition (optimum pH and temperature) in order to express its maximum activity to convert the biomass into their monomer sugars. Therefore, the enzyme sequential action was proposed as a model for the optimization of enzymatic hydrolysis for biomass. The primary focus has been on the degradation of cellulose into glucose, but the researchers now are focusing on the production of both pentoses and hexoses as it increases the yield and improves the cost of the process [17,22], as well as the production for other chemicals such as phenols for industrial purposes. This has had an impact on the purpose of the process such as pretreatment type and that which is required for the biomass degradation. Different methods have been used for bioconversion of lignocellulosic materials, such as separate hydrolysis and fermentation, simultaneous saccharification and fermentation [23,24] and consolidated bioprocessing [25,26,27,28] All these processes may have an effect on biomass degradation. For instance, separate hydrolysis and fermentation required higher temperatures (50 °C). However, simultaneous saccharification requires lower temperatures (30–32 °C) [29].
We found that sequential action of fiber-degrading enzymes affected the releasing amount of reducing sugars from corn stover. The best enzyme sequence was Hem-Cel/Pec-Lac; however, the worst sequence was Lac-Pec-Hem-Cel. Almost all the first steps in the process showed an increasing level of reducing sugars. The fiber of corn stover is consisted with lignin, pectin, cellulose and hemicellulose [17]. The cellulose is in the core, and other constituent especially hemicellulose surrounding it. Different enzymes with different specificities are required for the degradation of lignocellulose biomass (corn stover) such as cellulase, hemicellulase, pectinase and laccase. The best enzyme sequence indicated that hemicellulase was the first important enzyme to explore the lignin, pectin and cellulose. It was interesting that laccase was in the last step because normally lactin surrounding the cellulose and thought to be the first step. Actually, laccase in the last step can mitigate the inhibition effect on cellulase activity caused by phenol acids induced by laccase [29]. This finding is essential to design the enzyme sequences, and to avoid the mixture of enzymes at one time due to phenol acids release induced by laccase in the industrial applications.
Cellulase enzyme is a combination of three different enzymes, namely exo-1,4-β-glucanases, endo-1,4-β-glucanases and β-glucosidases, which work simultaneously to degrade cellulose into glucose monomers substrate [17]. Endo-glucanases attacks cellulose chains in the middle and decreases the degree of polymerization. However, exo-glucanases cleave the ends of cellulose chains and eventually allows β-glucosidase to cleave cellobiose into glucose [17]. Several studies have been reported a synergistic effect between different cellulase components such as synergy between cellobiohydrolases, endo-glucanases and β-glucosidases and synergy between endo- and exo-glucanases [30,31,32,33]. High crystalline cellulose has the highest synergy degree, while high amorphous cellulose has lower degrees of synergy though this was opposite in some other studies [17]. The impact of pretreatments on the synergistic interactions between cellulases has been reported, though, the relationship between increased hydrolysis and increased synergy is not clear.
Hemicellulose is more complex in structure compared to cellulose and therefore needs a large variety of enzymes to degrade it effectively. Nevertheless, hemicellulase consists of depolymerizing enzymes that hydrolyzes the backbone and enzymes which have steric obstacles to the depolymerizing enzymes [17]. Moreover, hemicellulase consists of different enzymes such as end-oxylanases which degrade xylan backbone into smaller oligosaccharides and β-xylosidase, required to degrade xylo-oligosaccharides into xylose [34]. Therefore, synergy degree seems to be dependent on different factors such as the nature of cellulose substrate, enzymes and the conditions of reaction.
With regard to pectinase content, there are several enzymes such as polygalacturonase and pectate lyase which are involved in pectin degradation into its monomers [17]. Moreover, the complex structure of lignocellulose makes it hard to be degraded by enzymes. Cellulose is hampered by lignin and hemicelluloses whereas lignin has the greatest obstacle to cellulose hydrolysis compared to hemicellulose which has a lesser impact [17].
Lignin removal (either through pretreatment or lignin-degrading enzymes such as laccase) is a benefit for the exposure of cellulose and its degradation. Meanwhile, the cellulase and hemicellulose inhibitors, e.g., phenol acids, were released during lignin degradation by laccase. It is important to balance these two effects. But as seen in this study, laccase arranged to the last step is the best choice to get high cellulose degradation and reducing sugar production. These results revealed that the mixture of different enzymes together was not an efficient way to treat lignocellulose in the bioenergy industry again.
Some of the fiber-degrading enzymes are multifunctional enzymes (cross-specificity), which means they can have activity on more than one substrate. It has been reported that cellulase has activity on both cellulose and xylan [17]. There are some advantages associated with multifunctional enzymes. For instance, they are able to lower the number of enzymes required for the overall degradation. Therefore, using multifunctional enzymes in a sequential manner in the bioenergy or biomass industry will help to avoid overlapping between enzymes, which is beneficial for reducing the cost of enzymes, increasing hydrolysis efficiency and promoting the environment.
5. Conclusions
The reaction conditions of enzymatic degradation of corn stover were adjusted and enzyme sequence action for fiber-degrading enzymes was effective in improving degradation of corn stover. Enzyme sequence action altered the chemical composition of corn stover and affected its degradation. The enzyme sequence action conditions in the present study may be used to develop large-scale processes to improve the degradation of corn stover as feedstock for biofuel production or as forage for livestock.
Author Contributions
Conceptualization, S.Z. and J.W.; formal analysis, S.Z. and M.D.; data curation, N.Z.; writing—original draft preparation, S.Z.; writing—review and editing, M.D. and J.W.; visualization, S.Z.; funding acquisition, J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by The Scientific Research Project for Major Achievements of The Agricultural Science and Technology Innovation Program (ASTIP) (CAAS-ZDXT2019004), Modern Agro-Industry Technology Research System of the PR China, and the Agricultural Science and Technology Innovation Program (ASTIP-IAS12).
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
There no conflict of interest in this research.
References
- Li, J.; Zhang, R.; Siddhu, M.A.H.; He, Y.; Wang, W.; Li, Y.; Chen, C.; Liu, G. Enhancing methane production of corn stover through a novel way: Sequent pretreatment of potassium hydroxide and steam explosion. Bioresour. Technol. 2015, 181, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, G.; Zheng, N.; Wang, J.; Yu, Z. Steam explosion enhances digestibility and fermentation of corn stover by facilitating ruminal microbial colonization. Bioresour. Technol. 2018, 253, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Xu, X.; Gao, Y.; Yang, F.; Wang, G. Anaerobic fermentation of biogas liquid pretreated maize straw by rumen microorganisms in vitro. Bioresour. Technol. 2014, 153, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Li, W.-W.; Yu, H.-Q. Application of rumen microorganisms for anaerobic bioconversion of lignocellulosic biomass. Bioresour. Technol. 2013, 128, 738–744. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, Y.; Zou, X.; Yang, M.; Guo, L. Synergistic action between extracellular products from white-rot fungus and cellulase significantly improves enzymatic hydrolysis. Bioengineered 2018, 9, 178–185. [Google Scholar] [CrossRef]
- Chen, X.; Ning, X.Q.; Zhang, B.B.; Wang, Y.L.; Zeng, G.M.; Hu, C.B. Effect of Steam Explosion and Ionic Liquid Pretreatment Technology on the Enzymatic Hydrolysis of Corn Stalk. Asian J. Chem. 2012, 24, 1015–1018. [Google Scholar]
- Liu, L.; Sun, J.; Li, M.; Wang, S.; Pei, H.; Zhang, J. Enhanced enzymatic hydrolysis and structural features of corn stover by FeCl3 pretreatment. Bioresour. Technol. 2009, 100, 5853–5858. [Google Scholar] [CrossRef]
- Kumar, S.; Paritosh, K.; Pareek, N.; Chawade, A.; Vivekanand, V. De-construction of major Indian cereal crop residues through chemical pretreatment for improved biogas production: An overview. Renew. Sustain. Energy Rev. 2018, 90, 160–170. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Bi, Y.-Y.; Gao, C.-Y. The assessment and utilization of straw resources in China. Agric. Sci. China 2010, 9, 1807–1815. [Google Scholar] [CrossRef]
- Klopfenstein, T. Increasing the nutritive value of crop residues by chemical treatment. In Upgrading Residues and By-Products for Animals; CRC: Boca Raton, FL, USA, 2018; pp. 39–60. [Google Scholar]
- Perlack, R.D.; Eaton, L.M.; Turhollow, A.F., Jr.; Langholtz, M.H.; Brandt, C.C.; Downing, M.E.; Wright, L.; Kavkewitz, J.; Shamey, A.; Nelson, R.; et al. Agricultural Biomass and Wate Resources. In US Billion-Ton Update: Biomass Supply for a Bioenergy and Bioproducts Industry; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2011; pp. 52–84. [Google Scholar]
- Siddhu, M.A.H.; Li, J.; Zhang, J.; Huang, Y.; Wang, W.; Chen, C.; Liu, G. Improve the anaerobic biodegradability by copretreatment of thermal alkali and steam explosion of lignocellulosic waste. BioMed Res. Int. 2016, 2016, 2786598. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Wang, F.; Yin, S.; Ren, T.; Song, A. The Preparation and Ethanol Fermentation of High-Concentration Sugars from Steam-Explosion Corn Stover. Appl. Biochem. Biotechnol. 2015, 176, 613–624. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Pandit, R.; Paralikar, P.; Biswas, J.K.; da Silva, S.S. Emerging role of nanobiocatalysts in hydrolysis of lignocellulosic biomass leading to sustainable bioethanol production. Catal. Rev. Taylor Fr. 2019, 61, 1–26. [Google Scholar] [CrossRef]
- Detroy, R.W. Bioconversion of agricultural biomass to organic chemicals. In Organic Chemicals from Biomass; CRC: Boca Raton, FL, USA, 2018; pp. 19–43. [Google Scholar]
- Hassan, S.; Williams, G.A.; Jaiswal, A.K. Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresour. Technol. 2018, 262, 310–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Dyk, J.S.; Pletschke, B.I. A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymesfactors affecting enzymes, conversion and synergy. Biotechnol. Adv. 2012, 30, 1458–1480. [Google Scholar] [CrossRef]
- Reynolds, A.G.; Knox, A.; Di Profio, F. Evaluation of Macerating Pectinase Enzyme Activity under Various Temperature, pH and Ethanol Regimes. Beverages 2018, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Adney, B.; Baker, J. Measurement of Cellulase Activities. In Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 1996; pp. 1–11. [Google Scholar]
- Muñiz-Mouro, A.; Gullón, B.; Lú-Chau, T.; Moreira, M.; Lema, J.; Eibes, G. Laccase Activity as an Essential Factor in the Oligomerization of Rutin. Catalysts 2018, 8, 321. [Google Scholar] [CrossRef] [Green Version]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Merino, S.T.; Cherry, J. Progress and challenges in enzyme development for biomass utilization. Biofuels 2007, 108, 95–120. [Google Scholar]
- Maitan-Alfenas, G.P.; Visser, E.M.; Guimarães, V.M. Enzymatic hydrolysis of lignocellulosic biomass: Converting food waste in valuable products. Curr. Opin. Food Sci. 2015, 1, 44–49. [Google Scholar] [CrossRef]
- Öhgren, K.; Bura, R.; Lesnicki, G.; Saddler, J.; Zacchi, G. A comparison between simultaneous saccharification and fermentation and separate hydrolysis and fermentation using steam-pretreated corn stover. Process Biochem. 2007, 42, 834–839. [Google Scholar] [CrossRef]
- Elkins, J.G.; Raman, B.; Keller, M. Engineered microbial systems for enhanced conversion of lignocellulosic biomass. Curr. Opin. Biotechnol. 2010, 21, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Singh, A.; Himmel, M.E. Perspectives and new directions for the production of bioethanol using consolidated bioprocessing of lignocellulose. Curr. Opin. Biotechnol. 2009, 20, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Lynd, L.R.; Van Zyl, W.H.; McBride, J.E.; Laser, M. Consolidated bioprocessing of cellulosic biomass: An update. Curr. Opin. Biotechnol. 2005, 16, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Lynd, L.R.; Weimer, P.J.; Van Zyl, W.H.; Pretorius, I.S. Microbial cellulose utilization: Fundamentals and biotechnology. Microbiol. Mol. Biol. Rev. Am. Soc. Microbiol. 2002, 66, 506–577. [Google Scholar] [CrossRef] [Green Version]
- Andrić, P.; Meyer, A.S.; Jensen, P.A.; Dam-Johansen, K. Reactor design for minimizing product inhibition during enzymatic lignocellulose hydrolysis: I. Significance and mechanism of cellobiose and glucose inhibition on cellulolytic enzymes. Biotechnol Adv. 2010, 28, 308–324. [Google Scholar] [CrossRef]
- Agrawal, R.; Semwal, S.; Kumar, R.; Mathur, A.; Gupta, R.P.; Tuli, D.K.; Satlewal, A. Synergistic Enzyme Cocktail to Enhance Hydrolysis of Steam Exploded Wheat Straw at Pilot Scale. Front. Energy Res. 2018, 6, 122. [Google Scholar] [CrossRef]
- Pellegrini, V.O.A.; Bernardes, A.; Rezende, C.A.; Polikarpov, I. Cellulose fiber size defines efficiency of enzymatic hydrolysis and impacts degree of synergy between endo- and exoglucanases. Cellulose 2018, 25, 1865–1881. [Google Scholar] [CrossRef]
- Wang, S.; Li, F.; Wu, D.; Zhang, P.; Wang, H.; Tao, X.; Ye, J.; Nabi, M. Enzyme pretreatment enhancing biogas yield from corn stover: Feasibility, optimization, and mechanism analysis. J. Agric. Food Chem. 2018, 66, 10026–10032. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Huang, G.; Yang, Z.; Han, L. Understanding the synergistic effect and the main factors influencing the enzymatic hydrolyzability of corn stover at low enzyme loading by hydrothermal and/or ultrafine grinding pretreatment. Bioresour. Technol. 2018, 264, 327–334. [Google Scholar] [CrossRef]
- Michelin, M.; Ruiz, H.A.; de Lourdes, T.M.; Teixeira, J.A. Multi-step approach to add value to corncob: Production of biomass-degrading enzymes, lignin and fermentable sugars. Bioresour Technol. 2018, 247, 582–590. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).