RNA-Interference-Mediated Aphid Control in Crop Plants: A Review
Abstract
1. Introduction
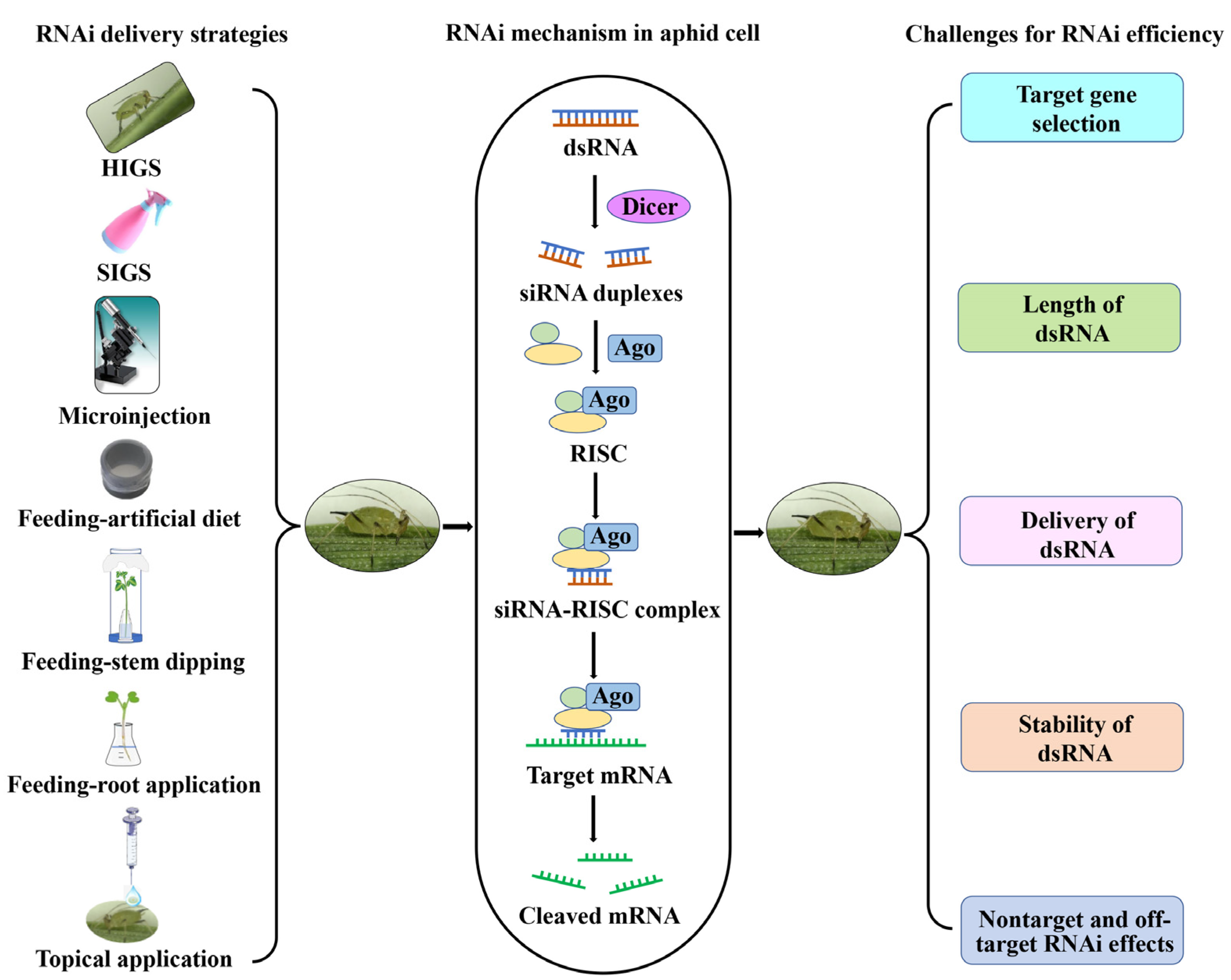
2. RNA-Interference-Based Aphid Control in Crop Plants
2.1. Host-Induced Gene Silencing
2.2. Host-Induced Gene Silencing Based Protection of Crop Plants from Aphids
2.3. Spray-Induced Gene Silencing
2.4. Spray-Induced Gene Silencing Based Aphid Control
2.5. Other Delivery-Method-Mediated Gene Silencing for Aphid Control
3. Challenges for Enhancing RNA Interference Efficiency
3.1. Target Gene Selection
3.2. Length of dsRNA
3.3. Delivery of dsRNA
3.4. The Stability of dsRNA
3.5. Nontarget and Off-Target RNAi Effects
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Budzinski, H.; Couderchet, M. Environmental and human health issues related to pesticides: From usage and environmental fate to impact. Environ. Sci. Pollut. Res. Int. 2018, 25, 14277–14279. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Zhao, H.; Wang, X.; Kang, Z. Prevalent pest management strategies for grain aphids: Opportunities and challenges. Front. Plant Sci. 2021, 12, 790919. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Yang, Q.; Xue, Z.; Liu, Y. RNA interference in fungi: Pathways, functions, and applications. Eukaryot. Cell 2011, 10, 1148–1155. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J.; Han, Y.; Fan, X.; Ding, S.-W. RNA interference functions as an antiviral immunity mechanism in mammals. Science 2013, 342, 231–234. [Google Scholar] [CrossRef]
- Nicolás, F.E.; Ruiz-Vázquez, R.M. Functional diversity of RNAi-associated sRNAs in fungi. Int. J. Mol. Sci. 2013, 14, 15348–15360. [Google Scholar] [CrossRef]
- Bologna, N.G.; Voinnet, O. The diversity, biogenesis, and activities of endogenous silencing small RNAs in Arabidopsis. Annu. Rev. Plant Biol. 2014, 65, 473–503. [Google Scholar] [CrossRef]
- Guo, Z.; Li, Y.; Ding, S.-W. Small RNA-based antimicrobial immunity. Nat. Rev. Immunol. 2019, 19, 31–44. [Google Scholar] [CrossRef]
- Song, L.; Fang, Y.; Chen, L.; Wang, J.; Chen, X. Role of non-coding RNAs in plant immunity. Plant Commun. 2021, 2, 100180. [Google Scholar] [CrossRef]
- Jain, R.G.; Robinson, K.E.; Asgari, S.; Mitter, N. Current scenario of RNAi-based hemipteran control. Pest Manag. Sci. 2021, 77, 2188–2196. [Google Scholar] [CrossRef]
- Napoli, C.; Lemieux, C.; Jorgensen, R. Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in trans. Plant Cell 1990, 2, 279–289. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Dalakouras, A.; Wassenegger, M.; McMillan, J.N.; Cardoza, V.; Maegele, I.; Dadami, E.; Runne, M.; Krczal, G.; Wassenegger, M. Induction of silencing in plants by high-pressure spraying of in vitro-synthesized small RNAs. Front. Plant Sci. 2016, 7, 1327. [Google Scholar] [CrossRef] [PubMed]
- San Miguel, K.; Scott, J.G. The next generation of insecticides: dsRNA is stable as a foliar-applied insecticide. Pest Manag. Sci. 2016, 72, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Numata, K.; Ohtani, M.; Yoshizumi, T.; Demura, T.; Kodama, Y. Local gene silencing in plants via synthetic ds RNA and carrier peptide. Plant Biotechnol. J. 2014, 12, 1027–1034. [Google Scholar] [CrossRef]
- Ghosh, S.K.B.; Hunter, W.B.; Park, A.L.; Gundersen-Rindal, D.E. Double strand RNA delivery system for plant-sap-feeding insects. PLoS ONE 2017, 12, e0171861. [Google Scholar] [CrossRef]
- Dalakouras, A.; Jarausch, W.; Buchholz, G.; Bassler, A.; Braun, M.; Manthey, T.; Krczal, G.; Wassenegger, M. Delivery of hairpin RNAs and small RNAs into woody and herbaceous plants by trunk injection and petiole absorption. Front. Plant Sci. 2018, 9, 1253. [Google Scholar] [CrossRef]
- Pitino, M.; Coleman, A.D.; Maffei, M.E.; Ridout, C.J.; Hogenhout, S.A. Silencing of aphid genes by dsRNA feeding from plants. PLoS ONE 2011, 6, e25709. [Google Scholar] [CrossRef]
- Xu, L.; Duan, X.; Lv, Y.; Zhang, X.; Nie, Z.; Xie, C.; Ni, Z.; Liang, R. Silencing of an aphid carboxylesterase gene by use of plant-mediated RNAi impairs Sitobion avenae tolerance of Phoxim insecticides. Transgenic Res. 2014, 23, 389–396. [Google Scholar] [CrossRef]
- Chung, S.H.; Jing, X.; Luo, Y.; Douglas, A.E. Targeting symbiosis-related insect genes by RNAi in the pea aphid-Buchnera symbiosis. Insect Biochem. Mol. Biol. 2018, 95, 55–63. [Google Scholar] [CrossRef]
- Koch, A.; Kogel, K.H. New wind in the sails: Improving the agronomic value of crop plants through RNAi-mediated gene silencing. Plant Biotechnol. J. 2014, 12, 821–831. [Google Scholar] [CrossRef]
- Christiaens, O.; Niu, J.; Taning, C.N.T. RNAi in insects: A revolution in fundamental research and pest control applications. Insects 2020, 11, 415. [Google Scholar] [CrossRef]
- Santala, J.; Valkonen, J.P. Sensitivity of small RNA-based detection of plant viruses. Front. Microbiol. 2018, 9, 939. [Google Scholar] [CrossRef]
- Sang, H.; Kim, J.-I. Advanced strategies to control plant pathogenic fungi by host-induced gene silencing (HIGS) and spray-induced gene silencing (SIGS). Plant Biotechnol. Rep. 2020, 14, 1–8. [Google Scholar] [CrossRef]
- Zand Karimi, H.; Innes, R.W. Molecular mechanisms underlying host-induced gene silencing. Plant Cell 2022, 34, 3183–3199. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Allen, R.; Davis, E.L.; Baum, T.J.; Hussey, R.S. Engineering broad root-knot resistance in transgenic plants by RNAi silencing of a conserved and essential root-knot nematode parasitism gene. Proc. Natl. Acad. Sci. USA 2006, 103, 14302–14306. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.A.; Bogaert, T.; Clinton, W.; Heck, G.R.; Feldmann, P.; Ilagan, O.; Johnson, S.; Plaetinck, G.; Munyikwa, T.; Pleau, M. Control of coleopteran insect pests through RNA interference. Nat. Biotechnol. 2007, 25, 1322–1326. [Google Scholar] [CrossRef]
- Nowara, D.; Gay, A.; Lacomme, C.; Shaw, J.; Ridout, C.; Douchkov, D.; Hensel, G.; Kumlehn, J.; Schweizer, P. HIGS: Host-induced gene silencing in the obligate biotrophic fungal pathogen Blumeria graminis. Plant Cell 2010, 22, 3130–3141. [Google Scholar] [CrossRef]
- Koch, A.; Kumar, N.; Weber, L.; Keller, H.; Imani, J.; Kogel, K.-H. Host-induced gene silencing of cytochrome P450 lanosterol C14α-demethylase–encoding genes confers strong resistance to Fusarium species. Proc. Natl. Acad. Sci. USA 2013, 110, 19324–19329. [Google Scholar] [CrossRef]
- Govindarajulu, M.; Epstein, L.; Wroblewski, T.; Michelmore, R.W. Host-induced gene silencing inhibits the biotrophic pathogen causing downy mildew of lettuce. Plant Biotechnol. J. 2015, 13, 875–883. [Google Scholar] [CrossRef]
- Jahan, S.N.; Åsman, A.K.; Corcoran, P.; Fogelqvist, J.; Vetukuri, R.R.; Dixelius, C. Plant-mediated gene silencing restricts growth of the potato late blight pathogen Phytophthora infestans. J. Exp. Bot. 2015, 66, 2785–2794. [Google Scholar] [CrossRef]
- Abdellatef, E.; Will, T.; Koch, A.; Imani, J.; Vilcinskas, A.; Kogel, K.H. Silencing the expression of the salivary sheath protein causes transgenerational feeding suppression in the aphid Sitobion avenae. Plant Biotechnol. J. 2015, 13, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Khan, S.A.; Hasse, C.; Ruf, S.; Heckel, D.G.; Bock, R. Full crop protection from an insect pest by expression of long double-stranded RNAs in plastids. Science 2015, 347, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Pitino, M.; Hogenhout, S.A. Aphid protein effectors promote aphid colonization in a plant species-specific manner. Mol. Plant Microbe Interact. 2013, 26, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Elzinga, D.A.; De Vos, M.; Jander, G. Suppression of plant defenses by a Myzus persicae (green peach aphid) salivary effector protein. Mol. Plant Microbe Interact. 2014, 27, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Coleman, A.D.; Wouters, R.H.; Mugford, S.T.; Hogenhout, S.A. Persistence and transgenerational effect of plant-mediated RNAi in aphids. J. Exp. Bot. 2015, 66, 541–548. [Google Scholar] [CrossRef]
- Faisal, M.; Abdel-Salam, E.M.; Alatar, A.A.; Saquib, Q.; Alwathnani, H.A.; Canto, T. Genetic transformation and siRNA-mediated gene silencing for aphid resistance in tomato. Agronomy 2019, 9, 893. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Y.; Tong, J.; Ge, P.; Wang, Q.; Zhao, Z.; Zhu-Salzman, K.; Hogenhout, S.A.; Ge, F.; Sun, Y. An aphid-secreted salivary protease activates plant defense in phloem. Curr. Biol. 2020, 30, 4826–4836. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, M.; Zhang, Q.; Fu, J.; Loiacono, F.V.; Yang, Y.; Wang, Z.; Li, S.; Chang, L.; Bock, R.; et al. Control of a sap-sucking insect pest by plastid-mediated RNA interference. Mol. Plant 2022, 15, 1176–1191. [Google Scholar] [CrossRef]
- Xu, L.; Hou, Q.; Zhao, Y.; Lu, L.; Li, B.; Ni, Z.; Liang, R. Silencing of a lipase maturation factor 2-like gene by wheat-mediated RNAi reduces the survivability and reproductive capacity of the grain aphid, Sitobion avenae. Arch. Insect Biochem. Physiol. 2017, 95, e21392. [Google Scholar] [CrossRef]
- Zhao, Y.; Sui, X.; Xu, L.; Liu, G.; Lu, L.; You, M.; Xie, C.; Li, B.; Ni, Z.; Liang, R. Plant-mediated RNAi of grain aphid CHS1 gene confers common wheat resistance against aphids. Pest Manag. Sci. 2018, 74, 2754–2760. [Google Scholar] [CrossRef]
- Hou, Q.; Xu, L.; Liu, G.; Pang, X.; Wang, X.; Zhang, Y.; You, M.; Ni, Z.; Zhao, Z.; Liang, R. Plant-mediated gene silencing of an essential olfactory-related Gqalpha gene enhances resistance to grain aphid in common wheat in greenhouse and field. Pest Manag. Sci. 2019, 75, 1718–1725. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sparks, C.; Jones, H.; Riley, M.; Francis, F.; Du, W.; Xia, L. Silencing an essential gene involved in infestation and digestion in grain aphid through plant-mediated RNA interference generates aphid-resistant wheat plants. Plant Biotechnol. J. 2019, 17, 852–854. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, V.; Bhattacharya, R. Host-mediated RNA interference targeting a cuticular protein gene impaired fecundity in the green peach aphid Myzus persicae. Pest Manag. Sci. 2018, 74, 2059–2068. [Google Scholar] [CrossRef] [PubMed]
- Thairu, M.W.; Skidmore, I.H.; Bansal, R.; Novakova, E.; Hansen, T.E.; Li-Byarlay, H.; Wickline, S.A.; Hansen, A.K. Efficacy of RNA interference knockdown using aerosolized short interfering RNAs bound to nanoparticles in three diverse aphid species. Insect Mol. Biol. 2017, 26, 356–368. [Google Scholar] [CrossRef]
- Yan, S.; Qian, J.; Cai, C.; Ma, Z.; Li, J.; Yin, M.; Ren, B.; Shen, J. Spray method application of transdermal dsRNA delivery system for efficient gene silencing and pest control on soybean aphid Aphis glycines. J. Pest Sci. 2020, 93, 449–459. [Google Scholar] [CrossRef]
- Biedenkopf, D.; Will, T.; Knauer, T.; Jelonek, L.; Furch, A.C.U.; Busche, T.; Koch, A. Systemic spreading of exogenous applied RNA biopesticides in the crop plant Hordeum vulgare. ExRNA 2020, 2, 12. [Google Scholar] [CrossRef]
- Liu, S.; Ladera-Carmona, M.J.; Poranen, M.M.; van Bel, A.J.E.; Kogel, K.-H.; Imani, J. Evaluation of dsRNA delivery methods for targeting macrophage migration inhibitory factor MIF in RNAi-based aphid control. J. Plant Dis. Prot. 2021, 128, 1201–1212. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Francis, F.; Xie, H.; Fan, J.; Wang, Q.; Liu, H.; Sun, Y.; Chen, J. The salivary effector protein Sg2204 in the greenbug Schizaphis graminum suppresses wheat defence and is essential for enabling aphid feeding on host plants. Plant Biotechnol. J. 2022, 20, 2187–2201. [Google Scholar] [CrossRef]
- Ding, B.Y.; Shang, F.; Zhang, Q.; Xiong, Y.; Yang, Q.; Niu, J.Z.; Smagghe, G.; Wang, J.J. Silencing of two insulin receptor genes disrupts nymph-adult transition of alate brown citrus aphid. Int. J. Mol. Sci. 2017, 18, 357. [Google Scholar] [CrossRef]
- Mou, X.; Yuan, G.R.; Jiang, H.B.; Liu, Z.; Wang, J.J. Functional characterization of two acetylcholinesterase genes in the brown citrus aphid, Aphis (Toxoptera) citricidus (Kirkaldy), using heterologous expression and RNA interference. Pestic. Biochem. Physiol. 2017, 138, 76–83. [Google Scholar] [CrossRef]
- Shang, F.; Niu, J.Z.; Ding, B.Y.; Zhang, Q.; Ye, C.; Zhang, W.; Smagghe, G.; Wang, J.J. Vitellogenin and its receptor play essential roles in the development and reproduction of the brown citrus aphid, Aphis (Toxoptera) citricidus. Insect Mol. Biol. 2018, 27, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Shang, F.; Ding, B.Y.; Ye, C.; Yang, L.; Chang, T.Y.; Xie, J.; Tang, L.D.; Niu, J.; Wang, J.J. Evaluation of a cuticle protein gene as a potential RNAi target in aphids. Pest Manag. Sci. 2020, 76, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Wang, Z.-W.; Sheng, Y.-L.; Wang, Z.-G.; Smagghe, G.; Christiaens, O.; Niu, J.; Wang, J. GNBP1 as a potential RNAi target to enhance the virulence of Beauveria bassiana for aphid control. J. Pest Sci. 2021, 95, 87–100. [Google Scholar] [CrossRef]
- Zheng, Y.; Hu, Y.; Yan, S.; Zhou, H.; Song, D.; Yin, M.; Shen, J. A polymer/detergent formulation improves dsRNA penetration through the body wall and RNAi-induced mortality in the soybean aphid Aphis glycines. Pest Manag. Sci. 2019, 75, 1993–1999. [Google Scholar] [CrossRef]
- Gong, Y.H.; Yu, X.R.; Shang, Q.L.; Shi, X.Y.; Gao, X.W. Oral delivery mediated RNA interference of a carboxylesterase gene results in reduced resistance to organophosphorus insecticides in the cotton aphid, Aphis gossypii Glover. PLoS ONE 2014, 9, e102823. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Pan, Y.; Yang, C.; Gao, X.; Xi, J.; Wu, Y.; Huang, X.; Zhu, E.; Xin, X.; Zhan, C.; et al. Over-expression of CYP6A2 is associated with spirotetramat resistance and cross-resistance in the resistant strain of Aphis gossypii Glover. Pestic. Biochem. Physiol. 2016, 126, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Rebijith, K.B.; Asokan, R.; Hande, H.R.; Kumar, N.K.; Krishna, V.; Vinutha, J.; Bakthavatsalam, N. RNA interference of odorant-binding protein 2 (OBP2) of the cotton aphid, Aphis gossypii (Glover), resulted in altered electrophysiological responses. Appl. Biochem. Biotechnol. 2016, 178, 251–266. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, H.; Pan, Y.; Gao, X.; Xi, J.; Zhang, J.; Shang, Q. Expression profile changes of cytochrome P450 genes between thiamethoxam susceptible and resistant strains of Aphis gossypii Glover. Pestic. Biochem. Physiol. 2018, 149, 1–7. [Google Scholar] [CrossRef]
- Pan, Y.; Chai, P.; Zheng, C.; Xu, H.; Wu, Y.; Gao, X.; Xi, J.; Shang, Q. Contribution of cytochrome P450 monooxygenase CYP380C6 to spirotetramat resistance in Aphis gossypii Glover. Pestic. Biochem. Physiol. 2018, 148, 182–189. [Google Scholar] [CrossRef]
- Ullah, F.; Gul, H.; Tariq, K.; Desneux, N.; Gao, X.; Song, D. Functional analysis of cytochrome P450 genes linked with acetamiprid resistance in melon aphid, Aphis gossypii. Pestic. Biochem. Physiol. 2020, 170, 104687. [Google Scholar] [CrossRef]
- Ullah, F.; Gul, H.; Tariq, K.; Hafeez, M.; Desneux, N.; Gao, X.; Song, D. RNA interference-mediated silencing of ecdysone receptor (EcR) gene causes lethal and sublethal effects on melon aphid, Aphis gossypii. Entomol. Gen. 2022, 42, 791–797. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, T.Y.; Ding, B.Y.; Niu, J.; Jiang, H.B.; Liu, T.X.; Wang, J.J. Crustacean cardioactive peptide and its receptor modulate the ecdysis behavior in the pea aphid, Acyrthosiphon pisum. J. Insect Physiol. 2022, 137, 104364. [Google Scholar] [CrossRef] [PubMed]
- Mutti, N.S.; Park, Y.; Reese, J.C.; Reeck, G.R. RNAi knockdown of a salivary transcript leading to lethality in the pea aphid, Acyrthosiphon pisum. J. Insect Sci. 2006, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Jaubert-Possamai, S.; Le Trionnaire, G.; Bonhomme, J.; Christophides, G.K.; Rispe, C.; Tagu, D. Gene knockdown by RNAi in the pea aphid Acyrthosiphon pisum. BMC Biotechnol. 2007, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Shakesby, A.J.; Wallace, I.S.; Isaacs, H.V.; Pritchard, J.; Roberts, D.M.; Douglas, A.E. A water-specific aquaporin involved in aphid osmoregulation. Insect Biochem. Mol. Biol. 2009, 39, 1–10. [Google Scholar] [CrossRef]
- Whyard, S.; Singh, A.D.; Wong, S. Ingested double-stranded RNAs can act as species-specific insecticides. Insect Biochem. Mol. Biol. 2009, 39, 824–832. [Google Scholar] [CrossRef]
- Mao, J.; Zeng, F. Feeding-based RNA interference of a gap gene is lethal to the pea aphid, Acyrthosiphon pisum. PLoS ONE 2012, 7, e48718. [Google Scholar] [CrossRef]
- Sapountzis, P.; Duport, G.; Balmand, S.; Gaget, K.; Jaubert-Possamai, S.; Febvay, G.; Charles, H.; Rahbe, Y.; Colella, S.; Calevro, F. New insight into the RNA interference response against cathepsin-L gene in the pea aphid, Acyrthosiphon pisum: Molting or gut phenotypes specifically induced by injection or feeding treatments. Insect Biochem. Mol. Biol. 2014, 51, 20–32. [Google Scholar] [CrossRef]
- Guo, K.; Wang, W.; Luo, L.; Chen, J.; Guo, Y.; Cui, F. Characterization of an aphid-specific, cysteine-rich protein enriched in salivary glands. Biophys. Chem. 2014, 189, 25–32. [Google Scholar] [CrossRef]
- Wang, W.; Luo, L.; Lu, H.; Chen, S.; Kang, L.; Cui, F. Angiotensin-converting enzymes modulate aphid-plant interactions. Sci. Rep. 2015, 5, 8885. [Google Scholar] [CrossRef]
- Naessens, E.; Dubreuil, G.; Giordanengo, P.; Baron, O.L.; Minet-Kebdani, N.; Keller, H.; Coustau, C. A secreted MIF cytokine enables aphid feeding and represses plant immune responses. Curr. Biol. 2015, 25, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Dai, H.; Zhang, Y.; Chandrasekar, R.; Luo, L.; Hiromasa, Y.; Sheng, C.; Peng, G.; Chen, S.; Tomich, J.M.; et al. Armet is an effector protein mediating aphid-plant interactions. FASEB J. 2015, 29, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Will, T.; Vilcinskas, A. The structural sheath protein of aphids is required for phloem feeding. Insect Biochem. Mol. Biol. 2015, 57, 34–40. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Z. Peroxiredoxin 1 protects the pea aphid Acyrthosiphon pisum from oxidative stress induced by Micrococcus luteus infection. J. Invertebr. Pathol. 2015, 127, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Fan, Y.L.; Bai, Y.; Li, X.D.; Zhang, Z.F.; Liu, T.X. Cytochrome P450 gene, CYP4G51, modulates hydrocarbon production in the pea aphid, Acyrthosiphon pisum. Insect Biochem. Mol. Biol. 2016, 76, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, B.; Grossi, G.; Falabella, P.; Liu, Y.; Yan, S.; Lu, J.; Xi, J.; Wang, G. Molecular basis of alarm pheromone detection in aphids. Curr. Biol. 2017, 27, 55–61. [Google Scholar] [CrossRef]
- Webster, C.G.; Pichon, E.; van Munster, M.; Monsion, B.; Deshoux, M.; Gargani, D.; Calevro, F.; Jimenez, J.; Moreno, A.; Krenz, B.; et al. Identification of plant virus receptor candidates in the stylets of their aphid vectors. J. Virol. 2018, 92, e00432-18. [Google Scholar] [CrossRef]
- Li, X.; Qu, M.J.; Zhang, Y.; Li, J.W.; Liu, T.X. Expression of neuropeptide F gene and its regulation of feeding behavior in the pea aphid, Acyrthosiphon pisum. Front. Physiol. 2018, 9, 87. [Google Scholar] [CrossRef]
- Ye, C.; An, X.; Jiang, Y.D.; Ding, B.Y.; Shang, F.; Christiaens, O.; Taning, C.N.T.; Smagghe, G.; Niu, J.; Wang, J.J. Induction of RNAi core machinery’s gene expression by exogenous dsRNA and the effects of pre-exposure to dsRNA on the gene silencing efficiency in the pea aphid (Acyrthosiphon pisum). Front. Physiol. 2018, 9, 1906. [Google Scholar] [CrossRef]
- Ye, C.; Jiang, Y.D.; An, X.; Yang, L.; Shang, F.; Niu, J.; Wang, J.J. Effects of RNAi-based silencing of chitin synthase gene on moulting and fecundity in pea aphids (Acyrthosiphon pisum). Sci. Rep. 2019, 9, 3694. [Google Scholar] [CrossRef]
- Wang, Q.; Yuan, E.; Ling, X.; Zhu-Salzman, K.; Guo, H.; Ge, F.; Sun, Y. An aphid facultative symbiont suppresses plant defence by manipulating aphid gene expression in salivary glands. Plant Cell Environ. 2020, 43, 2311–2322. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.Y.; Niu, J.; Shang, F.; Yang, L.; Zhang, W.; Smagghe, G.; Wang, J.J. Parental silencing of a horizontally transferred carotenoid desaturase gene causes a reduction of red pigment and fitness in the pea aphid. Pest Manag. Sci. 2020, 76, 2423–2433. [Google Scholar] [CrossRef] [PubMed]
- Shahid, S.; Shi, Y.; Yang, C.; Li, J.; Ali, M.Y.; Smagghe, G.; Liu, T.X. CCHamide2-receptor regulates feeding behavior in the pea aphid, Acyrthosiphon pisum. Peptides 2021, 143, 170596. [Google Scholar] [CrossRef]
- Zhou, X.; Ling, X.; Guo, H.; Zhu-Salzman, K.; Ge, F.; Sun, Y. Serratia symbiotica enhances fatty acid metabolism of pea aphid to promote host development. Int. J. Mol. Sci. 2021, 22, 5951. [Google Scholar] [CrossRef]
- Chang, M.; Cheng, H.; Cai, Z.; Qian, Y.; Zhang, K.; Yang, L.; Ma, N.; Li, D. miR-92a-1-p5 modulated expression of the flightin gene regulates flight muscle formation and wing extension in the pea aphid, Acyrthosiphon pisum (Hemiptera: Aphidoidea). J. Insect Sci. 2022, 22, 14. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Fan, Y.; Teng, Z.; Wang, L.; Tan, X.; Wan, F.; Zhou, H. Efficacy of RNA interference using nanocarrier-based transdermal dsRNA delivery system in the woolly apple aphid, Eriosoma lanigerum. Arch. Insect Biochem. Physiol. 2022, 110, e21888. [Google Scholar] [CrossRef]
- Mulot, M.; Boissinot, S.; Monsion, B.; Rastegar, M.; Clavijo, G.; Halter, D.; Bochet, N.; Erdinger, M.; Brault, V. Comparative analysis of RNAi-based methods to down-regulate expression of two genes expressed at different levels in Myzus persicae. Viruses 2016, 8, 316. [Google Scholar] [CrossRef]
- Gogoi, A.; Sarmah, N.; Kaldis, A.; Perdikis, D.; Voloudakis, A. Plant insects and mites uptake double-stranded RNA upon its exogenous application on tomato leaves. Planta 2017, 246, 1233–1241. [Google Scholar] [CrossRef]
- Tariq, K.; Ali, A.; Davies, T.G.E.; Naz, E.; Naz, L.; Sohail, S.; Hou, M.; Ullah, F. RNA interference-mediated knockdown of voltage-gated sodium channel (MpNav) gene causes mortality in peach-potato aphid, Myzus persicae. Sci. Rep. 2019, 9, 5291. [Google Scholar] [CrossRef]
- Mahmood, I.; Afroz, A.; Malik, M.F.; Zeeshan, N.; Khan, M.R.; Rashid, U.; Khan, M.A.U.; Ashraf, N.M.; Alam, S. RNA interference-mediated knockdown of odorant-binding protein 2 and MP58 gene causes mortality in Myzus persicae. Int. J. Trop. Insect Sci. 2021, 42, 315–326. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Ma, Z.Z.; Zhou, H.; Chao, Z.J.; Yan, S.; Shen, J. Nanocarrier-delivered dsRNA suppresses wing development of green peach aphids. Insect Sci. 2022, 29, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.Y.; Ma, Y.M.; Li, B.; Wang, Y.; Zhao, L.; Peng, J.N.; Li, M.Y.; Liu, S.; Li, S.G. Identification and functional analysis of differentially expressed genes in Myzus persicae (Hemiptera: Aphididae) in response to trans-anethole. J. Insect Sci. 2022, 22, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Feng, Z.J.; Chen, Z.S.; Zhang, Z.F.; Zhang, Y.; Liu, T.X. Use of tyrosine hydroxylase RNAi to study Megoura viciae (Hemiptera: Aphididae) sequestration of its host’s l-DOPA for body melanism. J. Insect Physiol. 2019, 114, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Lu, Y.H.; Shang, Q.L.; Song, D.L.; Gao, X.W. Gene silencing of two acetylcholinesterases reveals their cholinergic and non-cholinergic functions in Rhopalosiphum padi and Sitobion avenae. Pest Manag. Sci. 2015, 71, 523–530. [Google Scholar] [CrossRef]
- Deng, F.; Zhao, Z. Influence of catalase gene silencing on the survivability of Sitobion avenae. Arch. Insect Biochem. Physiol. 2014, 86, 46–57. [Google Scholar]
- Wang, D.; Liu, Q.; Li, X.; Sun, Y.; Wang, H.; Xia, L. Double-stranded RNA in the biological control of grain aphid (Sitobion avenae F.). Funct. Integr. Genom. 2015, 15, 211–223. [Google Scholar] [CrossRef]
- Yan, T.; Chen, H.; Sun, Y.; Yu, X.; Xia, L. RNA interference of the ecdysone receptor genes EcR and USP in grain aphid (Sitobion avenae F.) affects its survival and fecundity upon feeding on wheat plants. Int. J. Mol. Sci. 2016, 17, 2098. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, J.; Francis, F.; Chen, J. Molecular characterization and gene silencing of Laccase 1 in the grain aphid, Sitobion avenae. Arch. Insect Biochem. Physiol. 2018, 97, e21446. [Google Scholar] [CrossRef]
- Ullah, R.M.K.; Quershi, S.R.; Adeel, M.M.; Abdelnabby, H.; Waris, M.I.; Duan, S.G.; Wang, M.Q. An odorant binding protein (SaveOBP9) involved in chemoreception of the wheat aphid Sitobion avenae. Int. J. Mol. Sci. 2020, 21, 8331. [Google Scholar] [CrossRef]
- Ullah, R.M.K.; Waris, M.I.; Qureshi, S.R.; Rasool, F.; Duan, S.G.; Zaka, S.M.; Atiq, M.N.; Wang, M.Q. Silencing of an odorant binding protein (SaveOBP10) involved in the behavioural shift of the wheat aphid Sitobion avenae (Fabricius). Insect Mol. Biol. 2022, 31, 568–584. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, J.; Sun, J.-R.; Chen, J.-L. Cloning and RNA interference analysis of the salivary protein C002 gene in Schizaphis graminum. J. Integr. Agric. 2015, 14, 698–705. [Google Scholar] [CrossRef]
- Zhang, B.Z.; Ma, K.S.; Liu, J.J.; Lu, L.Y.; Chen, X.L.; Zhang, S.P.; Gao, X.W. Differential expression of genes in greenbug (Schizaphis graminum Rondani) treated by imidacloprid and RNA interference. Pest Manag. Sci. 2019, 75, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Fu, Y.; Crespo-Herrera, L.; Liu, H.; Wang, Q.; Zhang, Y.; Chen, J. Salivary effector Sm9723 of grain aphid Sitobion miscanthi suppresses plant defense and is essential for aphid survival on wheat. Int. J. Mol. Sci. 2022, 23, 6909. [Google Scholar] [CrossRef]
- Whangbo, J.S.; Hunter, C.P. Environmental RNA interference. Trends Genet. 2008, 24, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Weiberg, A.; Lin, F.-M.; Thomma, B.P.; Huang, H.-D.; Jin, H. Bidirectional cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nat. Plants 2016, 2, 16151. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Lan, C.; Capriotti, L.; Ah-Fong, A.; Nino Sanchez, J.; Hamby, R.; Heller, J.; Zhao, H.; Glass, N.L.; Judelson, H.S.; et al. Spray-induced gene silencing for disease control is dependent on the efficiency of pathogen RNA uptake. Plant Biotechnol. J. 2021, 19, 1756–1768. [Google Scholar] [CrossRef]
- Ghosh, S.K.B.; Hunter, W.B.; Park, A.L.; Gundersen-Rindal, D.E. Double-stranded RNA oral delivery methods to induce RNA interference in phloem and plant-sap-feeding hemipteran insects. J. Vis. Exp. 2018, 135, e57390. [Google Scholar] [CrossRef]
- Wang, M.; Jin, H. Spray-induced gene silencing: A powerful innovative strategy for crop protection. Trends Microbiol. 2017, 25, 4–6. [Google Scholar] [CrossRef]
- Hunter, W.B.; Glick, E.; Paldi, N.; Bextine, B.R. Advances in RNA interference: dsRNA treatment in trees and grapevines for insect pest suppression. Southwest Entomol. 2012, 37, 85–87. [Google Scholar] [CrossRef]
- Koch, A.; Biedenkopf, D.; Furch, A.; Weber, L.; Rossbach, O.; Abdellatef, E.; Linicus, L.; Johannsmeier, J.; Jelonek, L.; Goesmann, A. An RNAi-based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLoS Pathog. 2016, 12, e1005901. [Google Scholar] [CrossRef]
- Cagliari, D.; Dias, N.P.; Galdeano, D.M.; Dos Santos, E.Á.; Smagghe, G.; Zotti, M.J. Management of pest insects and plant diseases by non-transformative RNAi. Front. Plant Sci. 2019, 10, 1319. [Google Scholar] [CrossRef] [PubMed]
- Dalakouras, A.; Wassenegger, M.; Dadami, E.; Ganopoulos, I.; Pappas, M.L.; Papadopoulou, K. Genetically modified organism-free RNA interference: Exogenous application of RNA molecules in plants. Plant Physiol. 2020, 182, 38–50. [Google Scholar] [CrossRef]
- Vetukuri, R.R.; Dubey, M.; Kalyandurg, P.B.; Carlsson, A.S.; Whisson, S.C.; Ortiz, R. Spray-induced gene silencing: An innovative strategy for plant trait improvement and disease control. Crop Breed. Appl. Biotechnol. 2021, 21, e387921S387911. [Google Scholar] [CrossRef]
- Bilir, Ö.; Göl, D.; Hong, Y.; McDowell, J.M.; Tör, M. Small RNA-based plant protection against diseases. Front. Plant Sci. 2022, 13, 2973. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, J.; Zhu, K. Chitosan/double-stranded RNA nanoparticle-mediated RNA interference to silence chitin synthase genes through larval feeding in the African malaria mosquito (Anopheles gambiae). Insect Mol. Biol. 2010, 19, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Li-Byarlay, H.; Li, Y.; Stroud, H.; Feng, S.; Newman, T.C.; Kaneda, M.; Hou, K.K.; Worley, K.C.; Elsik, C.G.; Wickline, S.A. RNA interference knockdown of DNA methyl-transferase 3 affects gene alternative splicing in the honey bee. Proc. Natl. Acad. Sci. USA 2013, 110, 12750–12755. [Google Scholar] [CrossRef]
- Das, S.; Debnath, N.; Cui, Y.; Unrine, J.; Palli, S.R. Chitosan, carbon quantum dot, and silica nanoparticle mediated dsRNA delivery for gene silencing in Aedes aegypti: A comparative analysis. ACS Appl. Mater Interfaces 2015, 7, 19530–19535. [Google Scholar] [CrossRef]
- Chen, J.; Tang, B.; Chen, H.; Yao, Q.; Huang, X.; Chen, J.; Zhang, D.; Zhang, W. Different functions of the insect soluble and membrane-bound trehalase genes in chitin biosynthesis revealed by RNA interference. PLoS ONE 2010, 5, e10133. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Chandrashekar, K.; Thakur, N.; Verma, P.C.; Borgio, J.F.; Singh, P.K.; Tuli, R. RNA interference for the control of whiteflies (Bemisia tabaci) by oral route. J. Biosci. 2011, 36, 153–161. [Google Scholar] [CrossRef]
- Kennerdell, J.R.; Carthew, R.W. Use of dsRNA-mediated genetic interference to demonstrate that frizzled and frizzled 2 act in the wingless pathway. Cell 1998, 95, 1017–1026. [Google Scholar] [CrossRef]
- Timmons, L.; Fire, A. Specific interference by ingested dsRNA. Nature 1998, 395, 854. [Google Scholar] [CrossRef] [PubMed]
- Dubrovina, A.S.; Kiselev, K.V. Exogenous RNAs for gene regulation and plant resistance. Int. J. Mol. Sci. 2019, 20, 2282. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.-E.; Schwarzacher, T.; Othman, R.Y.; Harikrishna, J.A. dsRNA silencing of an R2R3-MYB transcription factor affects flower cell shape in a Dendrobium hybrid. BMC Plant Biol. 2015, 15, 194. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, J.; Dao, V.A.; Majumdar, U.; Schmitt-Engel, C.; Schwirz, J.; Schultheis, D.; Ströhlein, N.; Troelenberg, N.; Grossmann, D.; Richter, T. Large scale RNAi screen in Tribolium reveals novel target genes for pest control and the proteasome as prime target. BMC Genom. 2015, 16, 674. [Google Scholar] [CrossRef]
- Zhu, F.; Xu, J.; Palli, R.; Ferguson, J.; Palli, S.R. Ingested RNA interference for managing the populations of the Colorado potato beetle, Leptinotarsa decemlineata. Pest Manag. Sci. 2011, 67, 175–182. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, Y.; Wang, H.; Jones, H.D.; Gao, Q.; Wang, D.; Ma, Y.; Xia, L. Identifying potential RNAi targets in grain aphid (Sitobion avenae F.) based on transcriptome profiling of its alimentary canal after feeding on wheat plants. BMC Genom. 2013, 14, 560. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, X.; Wang, X.; Yu, D.; Chen, B.; Kang, L. Differential responses of migratory locusts to systemic RNA interference via double-stranded RNA injection and feeding. Insect Mol. Biol. 2013, 22, 574–583. [Google Scholar] [CrossRef]
- Camargo, R.D.A.; Herai, R.H.; Santos, L.N.; Bento, F.M.; Lima, J.E.; Marques-Souza, H.; Figueira, A. De novo transcriptome assembly and analysis to identify potential gene targets for RNAi-mediated control of the tomato leafminer (Tuta absoluta). BMC Genom. 2015, 16, 635. [Google Scholar] [CrossRef]
- Bona, A.C.D.; Chitolina, R.F.; Fermino, M.L.; de Castro Poncio, L.; Weiss, A.; Lima, J.B.P.; Paldi, N.; Bernardes, E.S.; Henen, J.; Maori, E. Larval application of sodium channel homologous dsRNA restores pyrethroid insecticide susceptibility in a resistant adult mosquito population. Parasites Vectors 2016, 9, 397. [Google Scholar] [CrossRef]
- Li, H.; Jiang, W.; Zhang, Z.; Xing, Y.; Li, F. Transcriptome analysis and screening for potential target genes for RNAi-mediated pest control of the beet armyworm, Spodoptera exigua. PLoS ONE 2013, 8, e65931. [Google Scholar] [CrossRef]
- Lomazzo, E.; Hussmann, G.P.; Wolfe, B.B.; Yasuda, R.P.; Perry, D.C.; Kellar, K.J. Effects of chronic nicotine on heteromeric neuronal nicotinic receptors in rat primary cultured neurons. J. Neurochem. 2011, 119, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Li, H.; Miao, X. Second-generation sequencing supply an effective way to screen RNAi targets in large scale for potential application in pest insect control. PLoS ONE 2011, 6, e18644. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, R.; Heschuk, D.; Kim, B.; Whyard, S. A novel paperclip double-stranded RNA structure demonstrates clathrin-independent uptake in the mosquito Aedes aegypti. Insect Biochem. Mol. Biol. 2020, 127, 103492. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.H.; Park, J.H.; Ashok, P.A.; Lee, U.; Lee, S.H. Screening of target genes for RNAi in Tetranychus urticae and RNAi toxicity enhancement by chimeric genes. Pestic. Biochem. Physiol. 2016, 130, 1–7. [Google Scholar] [CrossRef]
- Sharath Chandra, G.; Asokan, R.; Manamohan, M.; Krishna Kumar, N. Enhancing RNAi by using concatemerized double-stranded RNA. Pest Manag. Sci. 2019, 75, 506–514. [Google Scholar] [CrossRef]
- Silver, K.; Cooper, A.M.; Zhu, K.Y. Strategies for enhancing the efficiency of RNA interference in insects. Pest Manag. Sci. 2021, 77, 2645–2658. [Google Scholar] [CrossRef]
- Bolognesi, R.; Ramaseshadri, P.; Anderson, J.; Bachman, P.; Clinton, W.; Flannagan, R.; Ilagan, O.; Lawrence, C.; Levine, S.; Moar, W. Characterizing the mechanism of action of double-stranded RNA activity against western corn rootworm (Diabrotica virgifera virgifera LeConte). PLoS ONE 2012, 7, e47534. [Google Scholar] [CrossRef]
- Miller, S.C.; Miyata, K.; Brown, S.J.; Tomoyasu, Y. Dissecting systemic RNA interference in the red flour beetle Tribolium castaneum: Parameters affecting the efficiency of RNAi. PLoS ONE 2012, 7, e47431. [Google Scholar] [CrossRef]
- Clemens, J.C.; Worby, C.A.; Simonson-Leff, N.; Muda, M.; Maehama, T.; Hemmings, B.A.; Dixon, J.E. Use of double-stranded RNA interference in Drosophila cell lines to dissect signal transduction pathways. Proc. Natl. Acad. Sci. USA 2000, 97, 6499–6503. [Google Scholar] [CrossRef]
- Johnson, J.A.; Bitra, K.; Zhang, S.; Wang, L.; Lynn, D.E.; Strand, M.R. The UGA-CiE1 cell line from Chrysodeixis includens exhibits characteristics of granulocytes and is permissive to infection by two viruses. Insect Biochem. Mol. Biol. 2010, 40, 394–404. [Google Scholar] [CrossRef]
- Pridgeon, J.W.; Zhao, L.; Becnel, J.J.; Strickman, D.A.; Clark, G.G.; Linthicum, K.J. Topically applied AaeIAP1 double-stranded RNA kills female adults of Aedes aegypti. J. Med. Entomol. 2008, 45, 414–420. [Google Scholar] [CrossRef] [PubMed]
- El-Shesheny, I.; Hajeri, S.; El-Hawary, I.; Gowda, S.; Killiny, N. Silencing abnormal wing disc gene of the Asian citrus psyllid, Diaphorina citri disrupts adult wing development and increases nymph mortality. PLoS ONE 2013, 8, e65392. [Google Scholar] [CrossRef]
- Gong, L.; Chen, Y.; Hu, Z.; Hu, M. Testing insecticidal activity of novel chemically synthesized siRNA against Plutella xylostella under laboratory and field conditions. PLoS ONE 2013, 8, e62990. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Guan, R.; Miao, X. Lepidopteran insect species-specific, broad-spectrum, and systemic RNA interference by spraying ds RNA on larvae. Entomol. Exp. Appl. 2015, 155, 218–228. [Google Scholar]
- Dias, N.; Cagliari, D.; Kremer, F.S.; Rickes, L.N.; Nava, D.E.; Smagghe, G.; Zotti, M. The south American fruit fly: An important pest insect with RNAi-sensitive larval stages. Front. Physiol. 2019, 10, 794. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.-B.; Cai, W.-J.; Wang, J.-W.; Hong, G.-J.; Tao, X.-Y.; Wang, L.-J.; Huang, Y.-P.; Chen, X.-Y. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat. Biotechnol. 2007, 25, 1307–1313. [Google Scholar] [CrossRef]
- Avila, L.; Chandrasekar, R.; Wilkinson, K.; Balthazor, J.; Heerman, M.; Bechard, J.; Brown, S.; Park, Y.; Dhar, S.; Reeck, G. Delivery of lethal dsRNAs in insect diets by branched amphiphilic peptide capsules. J. Control. Release 2018, 273, 139–146. [Google Scholar] [CrossRef]
- Zhang, J.; Khan, S.A.; Heckel, D.G.; Bock, R. Next-generation insect-resistant plants: RNAi-mediated crop protection. Trends Biotechnol. 2017, 35, 871–882. [Google Scholar] [CrossRef]
- Marques, V.V.; Angelotti-Mendonça, J.; Roberto, S.R. Advances and challenges in RNA interference technology for citrus Huanglongbing vector control. Horticulturae 2021, 7, 277. [Google Scholar] [CrossRef]
- Allen, M.L.; Walker, W.B., III. Saliva of Lygus lineolaris digests double stranded ribonucleic acids. J. Insect Physiol. 2012, 58, 391–396. [Google Scholar] [CrossRef]
- Guan, R.-B.; Li, H.-C.; Fan, Y.-J.; Hu, S.-R.; Christiaens, O.; Smagghe, G.; Miao, X.-X. A nuclease specific to lepidopteran insects suppresses RNAi. J. Biol. Chem. 2018, 293, 6011–6021. [Google Scholar] [CrossRef] [PubMed]
- Katoch, R.; Thakur, N. Insect gut nucleases: A challenge for RNA interference mediated insect control strategies. Int. J. Biochem. Biotechnol. 2012, 1, 198–203. [Google Scholar]
- Kennedy, S.; Wang, D.; Ruvkun, G. A conserved siRNA-degrading RNase negatively regulates RNA interference in C. elegans. Nature 2004, 427, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Dias, N.; Cagliari, D.; Dos Santos, E.; Smagghe, G.; Jurat-Fuentes, J.; Mishra, S.; Nava, D.; Zotti, M. Insecticidal gene silencing by RNAi in the neotropical region. Neotrop. Entomol. 2020, 49, 1–11. [Google Scholar] [CrossRef]
- Cooper, A.M.; Silver, K.; Zhang, J.; Park, Y.; Zhu, K.Y. Molecular mechanisms influencing efficiency of RNA interference in insects. Pest Manag. Sci. 2019, 75, 18–28. [Google Scholar] [CrossRef]
- Laisney, J.; Rose, V.L.; Watters, K.; Donohue, K.V.; Unrine, J.M. Delivery of short hairpin RNA in the neotropical brown stink bug, Euschistus heros, using a composite nanomaterial. Pestic. Biochem. Physiol. 2021, 177, 104906. [Google Scholar] [CrossRef]
- Pugsley, C.E.; Isaac, R.E.; Warren, N.J.; Cayre, O.J. Recent advances in engineered nanoparticles for RNAi-mediated crop protection against insect pests. Front. Agron. 2021, 3, 652981. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, Y.; Kang, L.; Roossinck, M.J.; Mysore, K.S. Computational estimation and experimental verification of off-target silencing during posttranscriptional gene silencing in plants. Plant Physiol. 2006, 142, 429–440. [Google Scholar] [CrossRef]
- Reddy, K.; Rajam, M. Targeting chitinase gene of Helicoverpa armigera by host-induced RNA interference confers insect resistance in tobacco and tomato. Plant Mol. Biol. 2016, 90, 281–292. [Google Scholar]
- Senthil-Kumar, M.; Mysore, K.S. Virus-induced gene silencing can persist for more than 2 years and also be transmitted to progeny seedlings in Nicotiana benthamiana and tomato. Plant Biotechnol. J. 2011, 9, 797–806. [Google Scholar] [CrossRef]
- Ahmed, F.; Senthil-Kumar, M.; Dai, X.; Ramu, V.S.; Lee, S.; Mysore, K.S.; Zhao, P.X. pssRNAit: A web server for designing effective and specific plant siRNAs with genome-wide off-target assessment. Plant Physiol. 2020, 184, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Horn, T.; Boutros, M. E-RNAi: A web application for the multi-species design of RNAi reagents-2010 update. Nucleic Acids Res. 2010, 38, W332–W339. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Yamada, T.; Matsumiya, T.; Ui-Tei, K.; Saigo, K.; Morishita, S. dsCheck: Highly sensitive off-target search software for double-stranded RNA-mediated RNA interference. Nucleic Acids Res. 2005, 33, W589–W591. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.H.; Zhang, T.; Liu, Q.Y.; Guo, H.S. Trans-kingdom RNAs and their fates in recipient cells: Advances, utilization, and perspectives. Plant Commun. 2021, 2, 100167. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Ren, B.; Zeng, B.; Shen, J. Improving RNAi efficiency for pest control in crop species. BioTechniques 2020, 68, 283–290. [Google Scholar] [CrossRef]
- Liu, S.; Geng, S.; Li, A.; Mao, Y.; Mao, L. RNAi technology for plant protection and its application in wheat. aBIOTECH 2021, 2, 365–374. [Google Scholar] [CrossRef]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Li, P.; Jain, R.G.; Taochy, C.; Fletcher, S.J.; Carroll, B.J.; Lu, G.; Xu, Z.P. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants 2017, 3, 16207. [Google Scholar] [CrossRef]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Xu, Z.P.; Carroll, B.J. Induction of virus resistance by exogenous application of double-stranded RNA. Curr. Opin. Virol. 2017, 26, 49–55. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Wang, H.; Hu, P.; Hamby, R.; Jin, H. Small RNAs-big players in plant-microbe interactions. Cell Host Microbe 2019, 26, 173–182. [Google Scholar] [CrossRef]
- Kumar, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Hassan, A.A.; Kim, K.-H. Nano-based smart pesticide formulations: Emerging opportunities for agriculture. J. Control. Release 2019, 294, 131–153. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qian, J.; Xu, Y.; Yan, S.; Shen, J.; Yin, M. A facile-synthesized star polycation constructed as a highly efficient gene vector in pest management. ACS Sustain. Chem. Eng. 2019, 7, 6316–6322. [Google Scholar] [CrossRef]
- Fletcher, S.J.; Reeves, P.T.; Hoang, B.T.; Mitter, N. A perspective on RNAi-based biopesticides. Front. Plant Sci. 2020, 11, 51. [Google Scholar] [CrossRef]
- Cai, Q.; He, B.; Wang, S.; Fletcher, S.; Niu, D.; Mitter, N.; Birch, P.R.; Jin, H. Message in a bubble: Shuttling small RNAs and proteins between cells and interacting organisms using extracellular vesicles. Annu. Rev. Plant Biol. 2021, 72, 497. [Google Scholar] [CrossRef]
- Zuo, Y.; Wang, H.; Xu, Y.; Huang, J.; Wu, S.; Wu, Y.; Yang, Y. CRISPR/Cas9 mediated G4946E substitution in the ryanodine receptor of Spodoptera exigua confers high levels of resistance to diamide insecticides. Insect Biochem. Mol. Biol. 2017, 89, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, H.; Wang, H.; Zhao, S.; Zuo, Y.; Yang, Y.; Wu, Y. Functional validation of cadherin as a receptor of Bt toxin Cry1Ac in Helicoverpa armigera utilizing the CRISPR/Cas9 system. Insect Biochem. Mol. Biol. 2016, 76, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.-F.; Liu, X.-L.; Han, Q.; Liao, H.; Dong, X.-T.; Zhu, G.-H.; Dong, S.-L. Functional characterization of PBP1 gene in Helicoverpa armigera (Lepidoptera: Noctuidae) by using the CRISPR/Cas9 system. Sci. Rep. 2017, 7, 8470. [Google Scholar] [CrossRef]
- Dong, X.T.; Liao, H.; Zhu, G.H.; Khuhro, S.A.; Ye, Z.F.; Yan, Q.; Dong, S.L. CRISPR/Cas9-mediated PBP1 and PBP3 mutagenesis induced significant reduction in electrophysiological response to sex pheromones in male Chilo suppressalis. Insect Sci. 2019, 26, 388–399. [Google Scholar] [CrossRef]
- Zhu, G.-H.; Peng, Y.-C.; Zheng, M.-Y.; Zhang, X.-Q.; Sun, J.-B.; Huang, Y.; Dong, S.-L. CRISPR/Cas9 mediated BLOS2 knockout resulting in disappearance of yellow strips and white spots on the larval integument in Spodoptera litura. J. Insect Physiol. 2017, 103, 29–35. [Google Scholar] [CrossRef]
- Bi, H.L.; Xu, J.; Tan, A.J.; Huang, Y.P. CRISPR/Cas9-mediated targeted gene mutagenesis in Spodoptera litura. Insect Sci. 2016, 23, 469–477. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, G.; Zhang, W. Mutations in NlInR1 affect normal growth and lifespan in the brown planthopper Nilaparvata lugens. Insect Biochem. Mol. Biol. 2019, 115, 103246. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-P.; Luo, T.; Fu, H.-W.; Wang, L.; Tan, Y.-Y.; Huang, J.-Z.; Wang, Q.; Ye, G.-Y.; Gatehouse, A.M.; Lou, Y.-G. Resistance of rice to insect pests mediated by suppression of serotonin biosynthesis. Nat. Plants 2018, 4, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Seni, A. Frontier insect pest management technologies for sustainable rice production. J. Cereal Res. 2021, 13, 136–148. [Google Scholar] [CrossRef]
| Types of Gene Silencing | Aphid Species | Plant Species | Delivery Strategy | Target Genes | Molecule | Size | Main Effects | Reference |
|---|---|---|---|---|---|---|---|---|
| HIGS | Myzus persicae | Nicotiana benthamiana and A. thaliana | Transgenic N. benthamiana and A. thaliana | MpC002, Rack1 | dsRNA | 710 bp, 309 bp | Knockdown of target genes. | [17] |
| Myzus persicae | N. benthamiana and A. thaliana | Transgenic N. benthamiana and A. thaliana | MpC002, MpPIntO1 (Mp1), MpPIntO2 (Mp2) | dsRNA | 710 bp, 263 bp, 254 bp | Silencing of MpC002 and MpPIntO2 reduced nymph production. | [33] | |
| Myzus persicae | N. tabacum, A. thaliana, and N. benthamiana | Transgenic N. tabacum, A. thaliana, and N. benthamiana | Mp55 | dsRNA | >900 bp | Reduced aphid reproduction. | [34] | |
| Myzus persicae | A. thaliana | Transgenic A. thaliana | Rack1, MpC002, MpPIntO2 (Mp2) | dsRNA | 309 bp, 710 bp, 254 bp | Reduced aphid reproduction. | [35] | |
| Myzus persicae | A. thaliana | Transgenic A. thaliana | Cuticular protein MyCP | dsRNA | 327 bp | Attenuation of fecundity in aphids. | [43] | |
| Myzus persicae | Tomato | Agrobacterium-mediated transformation and transgenic tomato | Acetylcholinesterase 1 (Ace 1) | dsRNA | 571 bp | Silenced the target gene (Ace 1) and inhibited fecundity. | [36] | |
| Myzus persicae | Tobacco | Injection and feeding on transgenic tobacco | Cysteine protease Cathepsin B3 (CathB3) | dsRNA | 230 bp | Improved the performance of non-tobacco-adapted lineages on tobacco. | [37] | |
| Myzus persicae | Tobacco | Plastid-mediated RNA interference and transgenic tobacco | MpDhc64C | dsRNA | 269 bp | Reduced insect survival, impaired fecundity, and decreased weight of survivors. | [38] | |
| HIGS | Sitobion avenae | Wheat | Particle bombardment method and transgenic wheat | Carboxylesterase (CbE E4) | dsRNA | 350 bp | Suppressed CbE E4 expression impaired S. avenae larval tolerance of phoxim insecticides. | [18] |
| Sitobion avenae | Wheat | Particle bombardment method and transgenic wheat | Lipase maturation factor 2-like gene, lmf2-like | dsRNA | 543 bp | Reductions in molting number, survival, and reproduction. | [39] | |
| Sitobion avenae | Wheat | Particle bombardment method and transgenic wheat | Chitin synthase 1 (CHS1) | dsRNA | 550 bp | Decreased CHS1 expression level and reduced total and molting aphid numbers. | [40] | |
| Sitobion avenae | Wheat | Particle bombardment method and transgenic wheat | Gq protein alpha subunit (Gqα) | dsRNA | 517 bp | Reduced reproduction and molting in aphids. | [41] | |
| Sitobion avenae | Wheat | Particle bombardment method and transgenic wheat | Zinc finger protein (SaZFP) | dsRNA | 198 bp | High mortality and decreased fecundity. | [42] | |
| SIGS | Aphis glycines | Aerosolized siRNA-nanoparticle delivery method | Carotene dehydrogenase (tor), branched-chain amino acid transaminase (bcat) | siRNA | 25 nt | Knockdown of target genes. | [44] | |
| Aphis glycines | Nanocarrier-based dsRNA delivery system | TREH, ATPD, ATPE, and CHS1 | dsRNA | 431 bp, 504 bp, 536 bp, 429 bp | Silenced target gene expression and led to high mortality. | [45] | ||
| Acyrthosiphon pisum | Aerosolized siRNA-nanoparticle delivery method | Carotene dehydrogenase (tor), branched-chain amino acid transaminase (bcat) | siRNA | 25 nt | Knockdown of target genes. | [44] | ||
| Sitobion avenae | Barley | Spraying | Structural sheath protein (SHP) | dsRNA | 491 bp | Reduced shp expression level. | [46] | |
| Sitobion avenae | Barley | Spraying and feeding | Macrophage migration inhibitory factors, SaMIF1, SaMIF2, and SaMIF3 | dsRNA | 223 bp, 323 bp, 212 bp | Feeding on artificial diet led to high mortality rates; feeding from barley seedlings sprayed with naked SaMIF-dsRNAs did not alter nymph survival. | [47] | |
| Schizaphis graminum | Aerosolized siRNA-nanoparticle delivery method | Carotene dehydrogenase (tor) and branched-chain amino acid transaminase (bcat) | siRNA | 25 nt | Knockdown of target genes. | [44] | ||
| SIGS | Schizaphis graminum | Wheat | Nanocarrier-mediated transdermal dsRNA delivery system | Sg2204 | dsRNA | / | Induced a stronger wheat defense response and resulted in negative impacts on aphid feeding behavior, survival, and fecundity. | [48] |
| Other delivery method | Aphis citricidus | Feeding and citrus stem dipping | Insulin receptor genes AcInR1 and AcInR2 | dsRNA | 511 bp, 609 bp | Developmental defects and co-silencing of AcInR1 and AcInR2 resulted in high mortality. | [49] | |
| Aphis citricidus | Feeding and citrus stem dipping | Acetylcholinesterase, TcAChE1, and TcAChE2 | dsRNA | 435 bp, 421 bp | High mortality and increased the susceptibility of A. citricidus to malathion and carbaryl. | [50] | ||
| Aphis citricidus | Feeding and citrus stem dipping | Vitellogenin (AcVg), Vitellogenin receptor (AcVgR) | dsRNA | 557 bp, 577 bp | Slower embryonic development and fewer newborn nymphs. | [51] | ||
| Aphis citricidus | Feeding and citrus stem dipping | AcCP19 | dsRNA | 183 bp | Induced target gene silencing and high mortality. | [52] | ||
| Aphis citricidus | Feeding and citrus stem dipping | AcGNBP1 | dsRNA | 431 bp | Decreased the activity of immune-related phenoloxidase. | [53] | ||
| Aphis glycines | Topical application, nanocarrier, and detergent-mediated transdermal delivery system | Hemocytin, Hem | dsRNA | 555 bp | Reduced the target gene expression and aphid population density. | [54] | ||
| Aphis gossypii | Feeding | Carboxylesterase CarE | dsRNA | 686 bp | Decreased resistance to organophosphorus insecticides. | [55] | ||
| Aphis gossypii | Feeding | Cytochrome P450 monooxygenase gene CYP6A2 | dsRNA | 773 bp | Increased sensitivity to spirotetramat and alpha-cypermethrin. | [56] | ||
| Aphis gossypii | Feeding | Odorant-binding proteins AgOBP2 | dsRNA | 434 bp | Interfered with the odorant perception of aphids. | [57] | ||
| Aphis gossypii | Feeding | CYP6CY14 | dsRNA | 459 bp | Increased the resistant aphid’s susceptibility to thiamethoxam. | [58] | ||
| Other delivery method | Aphis gossypii | Feeding | CYP380C6 | dsRNA | 436 bp | Increased the sensitivity of the resistant adults and nymphs to spirotetramat. | [59] | |
| Aphis gossypii | Feeding | dsCYP6DC1, dsCYP6CY14, and dsCYP6CZ1 | dsRNA | 494 bp, 499 bp, 499 bp | Increased the Ace-R strain’s sensitivity to acetamiprid. | [60] | ||
| Aphis gossypii | Feeding | Ecdysone receptor (EcR) | dsRNA | 486 bp | Increased mortality rates and decreased longevity and fecundity. | [61] | ||
| Aphis gossypii | Injection | Crustacean cardioactive peptide (ApCCAP), crustacean cardioactive peptide receptor (ApCCAPR) | dsRNA | 339 bp, 519 bp | Developmental failure during nymph–adult ecdysis. | [62] | ||
| Acyrthosiphon Pisum | Injection | C002 | siRNA | 21-23 nt | Decreased C002 transcript level. | [63] | ||
| Acyrthosiphon Pisum | Injection | Calreticulin, cathepsin-L | dsRNA | 434 bp, 353 bp | Induced target gene silencing. | [64] | ||
| Acyrthosiphon Pisum | Feeding | ApAQP1 | dsRNA | 451 bp | Knocked down the ApAQP1 expression level, resulting in elevated hemolymph osmotic pressure. | [65] | ||
| Acyrthosiphon Pisum | injection | vATPase | dsRNA | 185 bp | Induced high levels of mortality. | [66] | ||
| Acyrthosiphon Pisum | Feeding | Hunchback | dsRNA | 524 bp, 497 bp | Reduced Aphb transcripts and increased insect lethality. | [67] | ||
| Acyrthosiphon pisum | Injection and feeding | Enzyme Cathepsin-L | dsRNA | 357 bp | Induced lethal effects. | [68] | ||
| Acyrthosiphon pisum | Injection | ACYPI39568 | dsRNA | 246 bp | Reduced ACYPI39568 expression level but did not affect the survival rate. | [69] | ||
| Acyrthosiphon Pisum | Injection | Angiotensin-converting enzymes ACE1, ACE2 | dsRNA | 313 bp, 468 bp | Knockdown of ACE1 and ACE2 caused a higher mortality rate. | [70] | ||
| Acyrthosiphon Pisum | Injection | ApMIF1 | dsRNA | 213 bp | Disturbed their ability to feed from phloem sap. | [71] | ||
| Acyrthosiphon Pisum | Injection | Armet | dsRNA | 286 bp | Disturbed feeding behavior and led to a shortened life span. | [72] | ||
| Acyrthosiphon Pisum | Injection | Structural sheath protein (SHP) | dsRNA | 491 bp | Disrupted sheath formation, prevented efficient long-term feeding from sieve tubes, and had a silencing effect on reproduction but not survival. | [73] | ||
| Other delivery method | Acyrthosiphon Pisum | Injection | Peroxiredoxins, ApPrx1 | dsRNA | 206 bp | Decreased survival rate. | [74] | |
| Acyrthosiphon Pisum | Injection and ingestion | Cytochrome P450 gene, CYP4G51 | dsRNA | 310 bp, 325 bp | Reduced CYP4G51 expression, caused reductions in internal and external long-chain hydrocarbons (HCs), and increased mortality. | [75] | ||
| Acyrthosiphon Pisum | Injection | Odorant receptors, ApisOR5, odorant-binding proteins, ApisOBP3, and ApisOBP7 | dsRNA | / | The repellent behavior of A. pisum to EBF disappeared. | [76] | ||
| Acyrthosiphon Pisum | Feeding | Cuticular protein, Stylin-01, Stylin-02 | siRNA | 19 bp | Silencing stylin-01 decreased the efficiency of cauliflower mosaic virus transmission by M. persicae. | [77] | ||
| Acyrthosiphon Pisum | Injection | Neuropeptide F (NPF), NPF receptor (NPFR) | dsRNA | 232 bp, 354 bp | Reduced aphid food intake and indicated a lower appetite for food after NPF silencing. | [78] | ||
| Acyrthosiphon Pisum | Feeding | amiD, ldcA1 | dsRNA | 311 bp, 353 bp | Reduction in Buchnera abundance and activity was accompanied by depressed aphid growth rates. | [19] | ||
| Acyrthosiphon Pisum | Injection | Gap gene Hunchback | dsRNA | 448 bp | Knockdown of target gene. | [79] | ||
| Acyrthosiphon Pisum | Injection and ingestion | Chitin synthase, CHS | dsRNA | 364 bp | Induced mortality and development deformity. | [80] | ||
| Acyrthosiphon Pisum | Injection | ApHRC | dsRNA | 263 bp | Serratia-infected aphids displayed shorter phloem-feeding durations and caused Ca2+ elevation and ROS accumulation in plants. | [81] | ||
| Acyrthosiphon pisum | Feeding and bean stem dipping | Cuticle protein gene, ApCP19 | dsRNA | 216 bp | Induced target gene silencing and high mortality. | [52] | ||
| Other delivery method | Acyrthosiphon pisum | Feeding and bean stem dipping | Carotenoid desaturase, CdeB | dsRNA | 431 bp | Reduced aphid performance and altered the age structure of the population. | [82] | |
| Acyrthosiphon pisum | Feeding and bean stem dipping | Gram-negative binding proteins, ApGNBP1, ApGNBP2 | dsRNA | 550 bp, 518 bp | Decreased the activity of immune-related phenoloxidase. | [53] | ||
| Acyrthosiphon Pisum | Injection | CCHamide-2 receptor (CCHa2-R) | dsRNA | 478 bp | Reduced CCHa2-R expression, food intake in adult aphids, and reproduction but not survival. | [83] | ||
| Acyrthosiphon Pisum | Injection | Fatty acid synthase 1 (FASN1) and diacylglycerol-o-acyltransferase 2 (DGAT2) | dsRNA | 609 bp, 388 bp | Prolonged the nymphal growth period and decreased the aphid body weight. | [84] | ||
| Acyrthosiphon pisum | Injection and nanocarrier delivery | flightin | dsRNA | 374 bp | Malformed wings, deformed dorsal longitudinal muscle (DLM) shapes, and wider and looser dorsoventral flight muscles (DVMs) were observed. | [85] | ||
| Eriosoma lanigerum Hausmann | Topical application and nanocarrier-mediated transdermal dsRNA delivery system | V-ATPase subunit D (ATPD) | dsRNA | / | Induced target gene silencing and led to high mortality. | [86] | ||
| Myzus nicotianae | Feeding | TRV-ALY, TRV-Eph | dsRNA | 182 bp, 249 bp | Inhibition of target genes. | [87] | ||
| Myzus persicae | Injection | MpMIF1 | dsRNA | 205 bp | Disturbed their ability to feed from phloem sap. | [71] | ||
| Myzus persicae | Foliar application | ZYMV HC-Pro | dsRNA | 588 bp | Insects successfully took up dsRNA; the dsRNA was processed into siRNA by the insect RNAi machinery. | [88] | ||
| Myzus persicae | Feeding | Cuticular protein, Stylin-01, Stylin-02 | siRNA | 19 bp | Silencing stylin-01 decreased the efficiency of cauliflower mosaic virus transmission by Myzus persicae. | [77] | ||
| Other delivery method | Myzus persicae | Feeding | Voltage-gated sodium channel MpNav | dsRNA | 289 bp | Induced high mortality and lower fecundity and longevity. | [89] | |
| Myzus persicae | Feeding and Brassica stem dipping | MpCP19 | dsRNA | 139 bp | Induced target gene silencing and high mortality. | [52] | ||
| Myzus persicae | Feeding and Brassica stem dipping | MpGNBP1 | dsRNA | 450 bp | Decreased the activity of immune-related phenoloxidase. | [53] | ||
| Myzus persicae | Feeding | Mp58, OBP2 | dsRNA | 423 bp, 428 bp | Induced high mortality. | [90] | ||
| Myzus persicae | Topical and root applications and nanocarrier-mediated delivery system | Vestigial (vg), Ultrabithorax (Ubx) | dsRNA | 489 bp, 359 bp | Downregulated target genes and caused wing aberration. | [91] | ||
| Myzus persicae | Injection | ATP-binding cassette transporter gene (ABCG4), DnaJ homolog subfamily C member 1 (DnaJC1) | dsRNA | ~400 bp | Increased mortality rate. | [92] | ||
| Megoura viciae | Injection | Tyrosine hydroxylase MV-TH | dsRNA | 400 bp | Reduced the L-DOPA level in aphids and a slight decrease in exuvia tanning. | [93] | ||
| Rhopalosiphum padi | Injection | Acetylcholinesterase gene RpAce1 | dsRNA | 383 bp | Increased susceptibilities to pirimicarb and malathion in R. padi and reduced fecundity. | [94] | ||
| Sitobion avenae | Feeding | Catalase CAT | dsRNA | 471 bp | Reduced survival rate and ecdysis index. | [95] | ||
| Sitobion avenae | Feeding | Unigenes DSR8, DSR32, DSR33, DSR48 | dsRNA | 162 bp, 411 bp, 439 bp, 397 bp | Downregulation of target genes and aphid mortality. | [96] | ||
| Sitobion avenae | Injection | Acetylcholinesterase gene SaAce1 | dsRNA | 400 bp | Increased susceptibility to pirimicarb in S. avenae and reduced fecundity. | [94] | ||
| Sitobion avenae | Feeding | Ecdysone receptor (SaEcR), ultraspiracle protein (SaUSP) | dsRNA | 469 bp, 411 bp | Significantly decreased the survival of aphids. | [97] | ||
| Other delivery method | Sitobion avenae | Feeding | Laccase 1, SaLac 1 | dsRNA | 613 bp | Inhibited the transcript levels of SaLac 1 and decreased the survival rate. | [98] | |
| Sitobion avenae | Feeding | Odorant-binding protein (SaveOBP9) | dsRNA | 501 bp | Reduced SaveOBP9 expression and induced a nonsignificant response in S. avenae to tetradecane, octanal, decanal, and hexadecane. | [99] | ||
| Sitobion avenae | Feeding | Odorant-binding protein (SaveOBP10) | dsRNA | 432 bp | Aphids exhibited nonattraction towards β-caryophyllene and a nonsignificant behavioral response to pentadecane, butylated hydroxytoluene, and tetradecane. | [100] | ||
| Schizaphis graminum | Feeding | SgC002 | siRNA | 476 bp | Feeding on artificial diet for 3 days followed by transfer to aphid-susceptible wheat suppressed SgC002 expression and led to lethality. | [101] | ||
| Schizaphis graminum | Feeding | MRA, GAT, TLP | dsRNA | 376 bp, 433 bp, 422 bp | Increased susceptibility to imidacloprid. | [102] | ||
| Sitobion miscanthi | Topical application and nanocarrier-mediated transdermal dsRNA delivery system | Sm9723 | dsRNA | / | Decreased fecundity and survival and negatively affected the feeding behavior. | [103] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, H.; Zhong, X.; Tian, J.; Segers, A.; Xia, L.; Francis, F. RNA-Interference-Mediated Aphid Control in Crop Plants: A Review. Agriculture 2022, 12, 2108. https://doi.org/10.3390/agriculture12122108
Zhang J, Li H, Zhong X, Tian J, Segers A, Xia L, Francis F. RNA-Interference-Mediated Aphid Control in Crop Plants: A Review. Agriculture. 2022; 12(12):2108. https://doi.org/10.3390/agriculture12122108
Chicago/Turabian StyleZhang, Jiahui, Huiyuan Li, Xue Zhong, Jinfu Tian, Arnaud Segers, Lanqin Xia, and Frédéric Francis. 2022. "RNA-Interference-Mediated Aphid Control in Crop Plants: A Review" Agriculture 12, no. 12: 2108. https://doi.org/10.3390/agriculture12122108
APA StyleZhang, J., Li, H., Zhong, X., Tian, J., Segers, A., Xia, L., & Francis, F. (2022). RNA-Interference-Mediated Aphid Control in Crop Plants: A Review. Agriculture, 12(12), 2108. https://doi.org/10.3390/agriculture12122108








