Recycling Nutrients Contained in Biomass Bottom Ash from Industrial Waste to Enhance the Fertility of an Amazonian Acidic Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Properties of Ash from Amazonian Biomasses, Phosphate Fertilizer and Lime
2.2. Sampling and Preparation of Yellow Latosol
2.3. Design and Experimental Procedures
2.4. Preparation and Analysis of Soils before and after Incubation
2.5. Statistical Analyses
3. Results and Discussion
3.1. Initial Physical and Chemical Properties of Yellow Latosol
3.2. Effects of Treatments on the Chemical Properties of Yellow Latosol
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CNA, Confederação da Agricultura e Pecuária do Brasil (Brazilian Confederation of Agriculture and Livestock). Agro maduro e Moderno (Mature and Modern Agribusiness). 2021. Available online: https://www.cnabrasil.org.br/artigos/agro-maduro-e-moderno (accessed on 9 May 2022).
- ANDA, Associação Nacional para Difusão de Adubos (Brazilian Association for the Diffusion of Fertilizers). Fertilizantes: Controle de qualidade (Fertilizers: Quality Control). 2018. Available online: http://www.anda.org.br (accessed on 8 July 2022).
- EPE, Empresa de Pesquisa Energética (Energy Research Company). Anauário Estatísitico de Energia Elétrica (Statistical Yearbook of Electric Energy). 2021. Available online: https://www.epe.gov.br/pt/imprensa/noticias/fact-sheet-anuario-estatistico-de-energia-eletrica-2022 (accessed on 5 June 2022).
- Vidal, A.C.F.; Hora, A.B. Perspectivas do Setor de Biomassa de Madeira Para a Geração de Energia (Perspectives of the Wood Biomass Sector for Energy Generation); BNDES: Brasília, Brazil, 2011; pp. 261–314. Available online: https://www.bnds.bov.br/bibliotecadigital (accessed on 15 May 2022).
- Costa, L.M.; Silva, M.F.O. A Indústria Química e o Setor de Fertilizantes (The Chemical Industry and the Fertilizer Business); BNDES: Brasília, Brazil, 2012; p. 50. [Google Scholar]
- Almeida, M.E.; Abram, M.B.; Chemale, L.T.; Paes, V.J.C.; Silveira, F.V. Diretrizes Para Avaliação dos Minerais Estratégicos: Fosfato, Potássio, Terras Raras e lítio (Guidelines for the Assessment of Strategic Minerals: Phosphate, Potassium, Rare Eath, and Lithium); CPRM—Geological Survey of Brazil: Brasília, Brazil, 2015; p. 31. [Google Scholar]
- Hansen, M.; Kepfer-Rojas, S.; Bjerager, P.E.R.; Holm, P.E.; Skov, S.; Ingerslev, M. Effects of ash application on nutrient and heavy metal fluxes in the soil and soil solution in a Norway spruce plantation in Denmark. For. Ecol. Manag. 2018, 424, 494–504. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the composition and application of biomass ash. Part 1. Phase-mineral and chemical composition and classification. Fuel 2013, 105, 40–76. [Google Scholar] [CrossRef]
- Adotey, N.; Harrell, D.L.; Weatherford, W.P. Characterization and Liming Effect of Wood Ash Generated from a Biomass-Fueled Commercial Power Plant. Commun. Soil Sci. Plant Anal. 2018, 49, 38–49. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the composition and application of biomass ash.: Part 2. Potential utilisation, technological and ecological advantages and challenges. Fuel 2013, 105, 19–39. [Google Scholar] [CrossRef]
- Demeyer, A.; Voundi Nkana, J.C.; Verloo, M.G. Characteristics of wood ash and influence on soil properties and nutrient uptake: An overview. Bioresour. Technol. 2001, 77, 287–295. [Google Scholar] [CrossRef]
- Quirantes, M.; Calvo, F.; Romero, E.; Nogales, R. Soil-nutrient availability affected by different biomass-ash applications. J. Soil Sci. Plant Nutr. 2016, 16, 159–163. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.; Cruz, N.C.; Tarelho, L.A.C.; Rodrigues, M.; Santiago, D. Use of biomass ash-based materials as soil fertilisers: Critical review of the existing regulatory framework. J. Clean. Prod. 2019, 214, 112–124. [Google Scholar] [CrossRef]
- Albuquerque, A.R.L.; Merino, A.; Angélica, R.S.; Omil, B.; Paz, S.P.A. Performance of ash from Amazonian biomasses as an alternative source of essential plant nutrients: An integrated and eco-friendly strategy for industrial waste management in the lack of raw fertilizer materials. J. Clean. Prod. 2022, 360, 132222. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Vassilev, V.S. Advantages and disadvantages of composition and properties of biomass in comparison with coal: An overview. Fuel 2015, 158, 330–350. [Google Scholar] [CrossRef]
- Albuquerque, A.R.L.; Angélica, R.S.; Merino, A.; Paz, S.P.A. Chemical and mineralogical characterization and potential use of ash from Amazonian biomasses as an agricultural fertilizer and for soil amendment. J. Clean. Prod. 2021, 295, 126472. [Google Scholar] [CrossRef]
- Romero, E.; Quirantes, M.; Nogales, R. Characterization of biomass ashes produced at different temperatures from olive-oil-industry and greenhouse vegetable wastes. Fuel 2017, 208, 1–9. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G.; Morgan, T.J. An overview of the organic and inorganic phase composition of biomass. Fuel 2012, 94, 1–33. [Google Scholar] [CrossRef]
- Embrapa. Manual de Análises Químicas de Solos, Plantas e Fertilizantes (Handbook of Chemical Analysis of Soils, Plants and Fertilizers), 2nd ed.; Embrapa—Brazilian Agricultural Research Company: Brasília, Brazil, 2009; p. 225. [Google Scholar]
- Embrapa. Recomendação de Calagem e Adubação para o Estado do Pará (Liming and Fertilization Recommendation for Pará State), 2nd ed.; Embrapa—Brazilian Agricultural Research Company: Brasília, Brazil, 2020; p. 424. [Google Scholar]
- Saarsalmi, A.; Mälkönen, E.; Piirainen, S. Effects of wood ash fertilization on forest soil chemical properties. Silva Fenn. 2001, 35, 355–368. [Google Scholar] [CrossRef] [Green Version]
- Etiegni, L.; Campbell, A.G. Physical and chemical characteristics of Wood Ash. Bioresour. Technol. 1991, 37, 173–178. [Google Scholar] [CrossRef]
- Maresca, A.; Hyks, J.; Astrup, T.F. Long-term leaching of nutrients and contaminants from wood combustion ashes. Waste Manag. 2018, 74, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Maresca, A.; Krüger, O.; Herzel, H.; Adam, C.; Kalbe, U.; Astrup, T.F. Influence of wood ash pre-treatment on leaching behaviour, liming and fertilising potential. Wast Manag. 2019, 83, 113–122. [Google Scholar] [CrossRef]
- Brasil, E.C.; Dantas, R.C.R.; Silva Júnior, M.L.; Gama, M.A.P. Phosphorus Fraction in a Yellow Latosol Cropped Under No-tilage System in the Brazilian Amazon. J. Agric. Stud. 2020, 8, 484–504. [Google Scholar] [CrossRef]
- Omil, B.; Sánchez-Rodríguez, F.; Merino, A. Effects of ash applications on soil status, nutrition, and growth of Pinus radiata D. Don plantations. Recycl. Biomass Ashes 2011, 1, 68–86. [Google Scholar] [CrossRef]
- Khan, A.A.; Jong, W.; Jansens, P.J.; Spliethoff, H. Biomass combustion in fluidized bed boilers: Potential problems and remedies. Fuel Process. Technol. 2009, 90, 21–50. [Google Scholar] [CrossRef]
- James, A.K.; Thring, R.W.; Helle, S.; Ghuman, H.S. Ash management review-Applications of Biomass Bottom Ash. Energies 2012, 5, 3856–3873. [Google Scholar] [CrossRef]
- Voshell, S.; Mäkelä, M.; Dahl, O. A review of biomass ash properties towards treatment and recycling. Renew. Sustain. Energy Rev. 2018, 96, 479–486. [Google Scholar] [CrossRef]
- Pandey, V.C.; Singh, N. Impact of fly ash incorporation in soil systems. Agric. Ecosyst. Environ. 2010, 136, 16–27. [Google Scholar] [CrossRef]

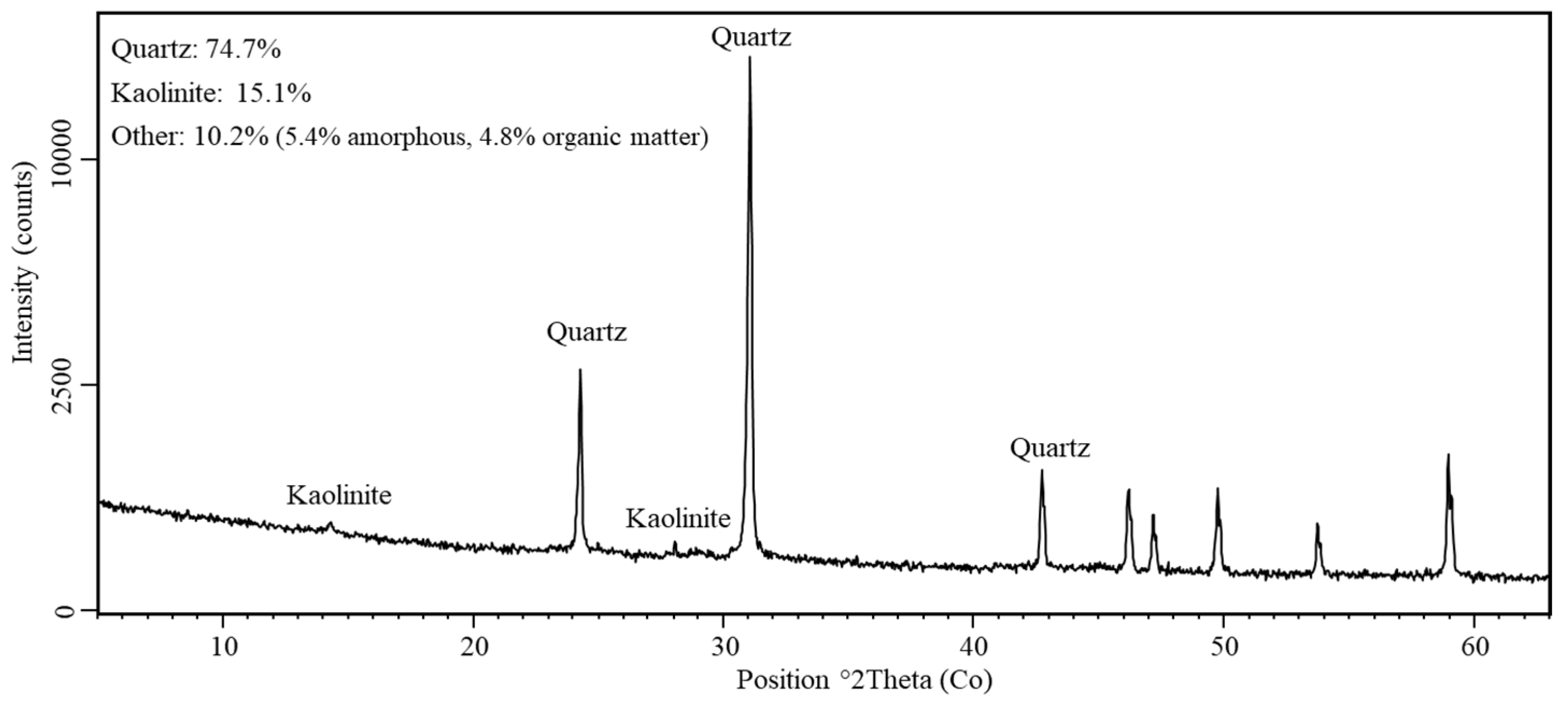

| Parameters/Elements | Total | Parameters/Elements | Total |
|---|---|---|---|
| pH (CaCl2) | 12 | mg kg−1 | |
| % | B | 39.98 | |
| Total neutralizing value | 20.5 | Co | <4.0 |
| Organic matter | 2.08 | Cu | 51.0 |
| C | 0.30 | Mn | 1082.0 |
| N | 0.17 | Fe | 37,903.0 |
| g kg−1 | Na | 4235.5 | |
| P total | 15.51 | Ni | 5.0 |
| P (2% citric acid soluble) | 11.02 | Zn | 17.0 |
| K total | 22.03 | Mo | 18.0 |
| K (2% citric acid soluble) | 7.89 | Sr | 8886.0 |
| Ca | 73.71 | Cr | 35.7 |
| Mg | 12.20 | As | <4.0 |
| S | 1.26 | Cd | <0.00 |
| Si | 170.81 | Hg | <0.01 |
| Al | 71.40 | Pb | 0.75 |
| pH 1 | pH 2 | OM | P | K | S | Ca | Mg | Al | H + Al | SB | CEC | m | V | Sd | St | Cl |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g dm−3 | mg dm−3 | cmolc dm−3 | % | |||||||||||||
| 3.9 | 3.0 | 48 | 8 | 28 | 9 | 0.2 | 0.1 | 2 | 12 | 0.4 | 13 | 87 | 2.5 | 82 | 5 | 13 |
| Treatments | pH H2O | pH CaCl2 | ||||
| 20 Days | 40 Days | 60 Days | 20 Days | 40 Days | 60 Days | |
| Ct 0 Mg ha−1 | 4.1 ± 0.14 d | 4.1 ± 0.12 d | 4.1 ± 0.17 c | 3.1 ± 0.05 d | 3.1 ± 0.05 d | 3.1 ± 0.09 d |
| A 8.75 Mg ha−1 | 4.5 ± 0.05 cd | 4.9 ± 0.12 c | 4.4 ± 0.08 c | 3.6 ± 0.05 cd | 3.8 ± 0.05 c | 3.4 ± 0.05 d |
| A 17.5 Mg ha−1 | 4.8 ± 0.12 bc | 5.0 0.12 c | 5.1 ± 0.12 b | 3.8 ± 0.05 c | 4.0 ± 0.09 bc | 3.9 ± 0.05 c |
| A 35 Mg ha−1 | 5.3 ± 0.00 b | 5.5 ± 0.08 bc | 5.4 ± 0.12 b | 4.3 ± 0.12 b | 4.4 ± 0.05 b | 4.2 ± 0.05 bc |
| A 70 Mg ha−1 | 5.4 ± 0.12 b | 5.7 0.05 b | 5.6 ± 0.29 b | 4.7 ± 0.33 b | 4.7 ± 0.05 b | 4.4 ± 0.17 b |
| CV | 11.01% | 12.18% | 13.61% | 15.69% | 15.44% | 14.79% |
| r | 0.86 | 0.86 | 0.85 | 0.91 | 0.90 | 0.88 |
| L 13 Mg ha−1 | 6.0 ± 0.05 a | 6.6 ± 0.14 a | 6.1 ± 0.50 a | 5.6 ± 0.12 a | 5.9 ± 0.12 a | 5.4 ± 0.26 a |
| Ph 2 Mg ha−1 | 4.1 ± 0.05 d | 4.2 0.12 d | 4.0 ± 0.08 c | 3.2 ± 0.05 d | 3.2 ± 0.05 d | 3.2 ± 0.05 d |
| Treatments | Ca cmolc dm−3 | Mg cmolc dm−3 | ||||
| 20 days | 40 days | 60 days | 20 days | 40 days | 60 days | |
| Ct 0 Mg ha−1 | 0.33 ± 0.05 f | 0.20 ± 0.00 f | 0.20 ± 0.00 e | 0.20 ± 0.00 e | 0.20 ± 0.00 e | 0.20 ± 0.00 e |
| A 8.75 Mg ha−1 | 1.53 ± 0.05 e | 1.27 ± 0.05 e | 0.90 ± 0.00 d | 0.53 ± 0.05 d | 0.50 ± 0.00 d | 0.50 ± 0.00 d |
| A 17.5 Mg ha−1 | 2.23 ± 0.05 d | 1.87 ± 0.05 d | 1.53 ± 0.05 c | 0.73 ± 0.05 d | 0.70 ± 0.00 cd | 0.70 ± 0.00 cd |
| A 35 Mg ha−1 | 3.37 ± 0.05 c | 2.50 ± 0.08 c | 1.97 ± 0.05 c | 1.10 ± 0.00 c | 0.97 ± 0.05 c | 0.97 ± 0.05 c |
| A 70 Mg ha−1 | 4.17 ± 0.24 b | 3.17 ± 0.19 b | 2.70 ± 0.00 b | 1.40 ± 0.08 b | 1.30 ± 0.00 b | 1.37 ± 0.05 b |
| CV | 64.75% | 63.38% | 65.88% | 59.30% | 57.68% | 59.69% |
| r | 0.94 | 0.92 | 0.95 | 0.95 | 0.96 | 0.97 |
| L 13 Mg ha−1 | 5.73 ± 0.25 a | 4.03 ± 0.12 a | 3.50 ± 0.14 a | 4.03 ± 0.21 a | 3.87 ± 0.05 a | 5.03 ± 0.25 a |
| Ph 2 Mg ha−1 | 0.67 ± 0.24 f | 0.37 ± 0.05 f | 0.37 ± 0.05 e | 0.20 ± 0.00 e | 0.20 ± 0.00 e | 0.10 ± 0.00 e |
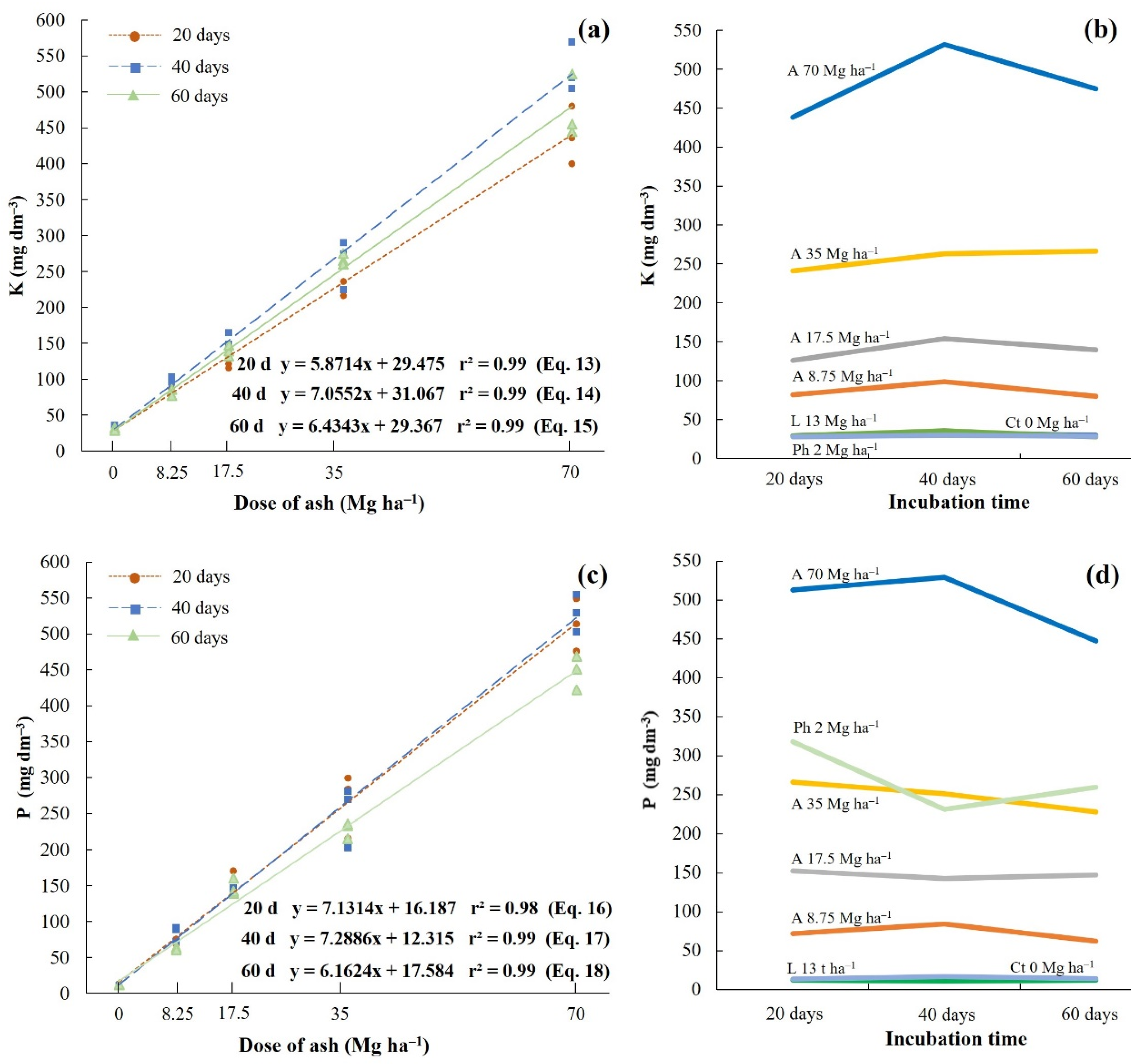
| Treatments | K mg dm−3 | P mg dm−3 | ||||
|---|---|---|---|---|---|---|
| 20 Days | 40 Days | 60 Days | 20 Days | 40 Days | 60 Days | |
| Ct 0 Mg ha−1 | 29.7 ± 1.9 d | 33.7 ± 1.7 d | 30.0 ± 1.4 d | 12.7 ± 0.5 d | 11.1 ± 0.6 d | 12.1 ± 0.4 d |
| A 8.75 Mg ha−1 | 82.3 ± 3.4 cd | 98.7 ± 3.1 c | 80.0 ± 4.2 d | 72.3 ± 2.9 cd | 84.4 ± 8.4 cd | 62.1 ± 1.3 cd |
| A 17.5 Mg ha−1 | 126.0 ± 10.8 c | 154.0 ± 7.8 c | 139.7 ± 6.5 c | 152.5 ± 12.7 c | 142.7 ± 3.8 c | 147.1 ± 9.2 bc |
| A 35 Mg ha−1 | 241.3 ± 23.2 b | 263.3 ± 27.8 b | 266.6 ± 6.2 b | 266.5 ± 36.3 b | 251.0 ± 34.5 b | 228.1 ± 9.2 b |
| A 70 Mg ha−1 | 438.7 ± 32.7 a | 531.7 ± 27.8 a | 475.0 ± 35.6 a | 512.9 ± 29.8 a | 529.0 ± 21.3 a | 447.3 ± 19.2 a |
| CV | 88.52% | 90.36% | 89.87% | 97.08% | 99.15% | 95.33% |
| r | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 1.00 |
| L 13 Mg ha−1 | 29.7 ± 1.2 d | 36.0 ± 2.9 d | 28.3 ± 1.2 d | 13.6 ± 0.3 d | 16.7 ± 0.9 d | 14.5 ± 0.4 d |
| Ph 2 Mg ha−1 | 28.3 ± 1.2 d | 30.0 ± 1.6 d | 28.7 ± 0.9 d | 318.2 ± 94.7 a | 231.2 ± 94.8 b | 259.7 ± 26.9 b |
| Treatments | V% | m% | ||||
| 20 Days | 40 Days | 60 Days | 20 Days | 40 Days | 60 Days | |
| Ct 0 Mg ha−1 | 4.62 ± 0.37 f | 3.11 ± 0.54 f | 3.20 ± 0.17 f | 80.21 ± 0.92 d | 82.34 ± 0.30 c | 82.39 ± 0.58 c |
| A 8.75 Mg ha−1 | 16.04 ± 1.01 e | 15.13 ± 0.54 e | 10.80 ± 0.41 e | 39.06 ± 2.70 c | 33.86 ± 1.60 b | 48.78 ± 1.85 b |
| A 17.5 Mg ha−1 | 22.25 ± 0.99 d | 21.30 ± 1.44 d | 17.90 ± 0.65 d | 23.90 ± 0.42 b | 3.27 ± 0.07 a | 3.72 ± 0.05 a |
| A 35 Mg ha−1 | 33.86 ± 0.57 c | 31.16 ± 0.77 c | 26.52 ± 1.17 c | 1.93 ± 0.04 a | 2.36 ± 0.11 a | 2.69 ± 0.08 a |
| A 70 Mg ha−1 | 43.19 ± 1.49 b | 41.57 ± 1.26 b | 34.97 ± 3.49 b | 1.48 ± 0.08 a | 1.69 ± 0.06 a | 1.86 ± 0.04 a |
| CV | 62.78% | 65.71% | 67.14% | 111.06% | 141.59% | 130.56% |
| r | 0.95 | 0.95 | 0.95 | −0.81 | −0.69 | −0.73 |
| L 13 Mg ha−1 | 65.11 ± 1.67 a | 69.21 ± 2.98 a | 66.57 ± 3.06 a | 1.01 ± 0.05 a | 1.24 ± 0.03 a | 1.15 ± 0.04 a |
| Ph 2 Mg ha−1 | 7.46 ± 1.50 f | 4.75 ± 0.46 f | 4.04 ± 0.42 f | 70.44 ± 5.04 d | 75.01 ± 1.62 c | 79.80 ± 1.80 c |
| Treatments | Al cmolc dm−3 | H + Al cmolc dm−3 | ||||
| 20 days | 40 days | 60 days | 20 days | 40 days | 60 days | |
| Ct 0 Mg ha−1 | 2.47 ± 0.12 d | 2.27 ± 0.05 c | 2.23 ± 0.09 c | 12.60 ± 0.75 c | 15.70 ± 3.11 c | 14.47 ± 0.78 c |
| A 8.75 Mg ha−1 | 1.47 ± 0.17 c | 1.03 ± 0.05 b | 1.53 ± 0.12 b | 11.97 ± 0.8 bc | 11.33 ± 0.21 b | 13.47 ± 0.5 bc |
| A 17.5 Mg ha−1 | 1.03 ± 0.05 b | 0.10 ± 0.00 a | 0.10 ± 0.00 a | 11.53 ± 0.9 bc | 11.00 ± 0.94 b | 11.90 ± 0.4 bc |
| A 35 Mg ha−1 | 0.10 ± 0.00 a | 0.10 ± 0.00 a | 0.10 ± 0.00 a | 9.93 ± 0.19 bc | 9.17 ± 0.69 b | 10.03 ± 0.46 b |
| A 70 Mg ha−1 | 0.10 ± 0.00 a | 0.10 ± 0.00 a | 0.10 ± 0.00 a | 8.80 ± 0.50 b | 8.20 ± 0.51 b | 9.93 ± 1.23 b |
| CV | 96.59% | 132.56% | 123.89% | 14.24% | 26.07% | 16.68% |
| r | −0.85 | −0.69 | −0.72 | −0.88 | −0.72 | −0.81 |
| L 13 Mg ha−1 | 0.10 ± 0.00 a | 0.10 ± 0.00 a | 0.10 ± 0.00 a | 5.27 ± 0.17 a | 3.57 ± 0.42 a | 4.37 ± 0.71 a |
| Ph 2 Mg ha−1 | 2.20 ± 0.08 d | 1.93 ± 0.12 c | 2.13 ± 0.05 c | 11.53 ± 0.34 c | 12.93 ± 0.52 c | 12.83 ± 0.26 c |
| Treatments | OM g dm−3 | CEC cmolc dm−3 | ||||
|---|---|---|---|---|---|---|
| 20 Days | 40 Days | 60 Days | 20 Days | 40 Days | 60 Days | |
| Ct 0 Mg ha−1 | 47.67 ± 1.25 b | 51.67 ± 2.49 b | 48.67 ± 3.86 b | 13.21 ± 0.76 a | 16.19 ± 3.11 a | 14.94 ± 0.78 a |
| A 8.75 Mg ha−1 | 53.00 ± 3.56 a | 46.00 ± 1.41 b | 53.00 ± 2.45 a | 14.24 ± 0.81 a | 13.35 ± 0.16 ab | 14.87 ± 0.54 a |
| A 17.5 Mg ha−1 | 53.67 ± 4.03 a | 58.67 ± 2.62 a | 59.33 ± 3.30 a | 14.82 ± 0.94 a | 13.96 ± 0.95 ab | 14.49 ± 0.41 a |
| A 35 Mg ha−1 | 57.00 ± 2.45 a | 49.67 ± 2.05 b | 55.67 ± 4.99 a | 15.02 ± 0.23 a | 13.31 ± 0.87 ab | 13.63 ± 0.46 a |
| A 70 Mg ha−1 | 51.33 ± 5.19 b | 48.33 ± 1.25 b | 52.67 ± 6.60 a | 15.49 ± 0.74 a | 14.03 ± 0.66 ab | 15.22 ± 1.11 a |
| CV | 6.50% | 9.48% | 7.33% | 6.01% | 8.31% | 4.16% |
| r | 0.17 | −0.21 | 0.10 | 0.63 | −0.22 | 0.05 |
| L 13 Mg ha−1 | 52.00 ± 1.41 b | 45.33 ± 3.86 b | 49.33 ± 1.89 b | 15.11 ± 0.34 a | 11.56 ± 0.26 b | 12.97 ± 1.00 a |
| Ph 2 Mg ha−1 | 49.00 ± 1.41 b | 49.67 ± 3.68 b | 46.00 ± 3.56 b | 12.47 ± 0.57 a | 13.58 ± 0.50 a | 13.37 ± 0.22 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albuquerque, A.R.L.; Gama, M.A.P.; Lima, V.M.N.; Rodrigues, A.O.; Angélica, R.S.; Paz, S.P.A. Recycling Nutrients Contained in Biomass Bottom Ash from Industrial Waste to Enhance the Fertility of an Amazonian Acidic Soil. Agriculture 2022, 12, 2093. https://doi.org/10.3390/agriculture12122093
Albuquerque ARL, Gama MAP, Lima VMN, Rodrigues AO, Angélica RS, Paz SPA. Recycling Nutrients Contained in Biomass Bottom Ash from Industrial Waste to Enhance the Fertility of an Amazonian Acidic Soil. Agriculture. 2022; 12(12):2093. https://doi.org/10.3390/agriculture12122093
Chicago/Turabian StyleAlbuquerque, Alan R. L., Marcos A. P. Gama, Vitória M. N. Lima, Andréia O. Rodrigues, Rômulo S. Angélica, and Simone P. A. Paz. 2022. "Recycling Nutrients Contained in Biomass Bottom Ash from Industrial Waste to Enhance the Fertility of an Amazonian Acidic Soil" Agriculture 12, no. 12: 2093. https://doi.org/10.3390/agriculture12122093
APA StyleAlbuquerque, A. R. L., Gama, M. A. P., Lima, V. M. N., Rodrigues, A. O., Angélica, R. S., & Paz, S. P. A. (2022). Recycling Nutrients Contained in Biomass Bottom Ash from Industrial Waste to Enhance the Fertility of an Amazonian Acidic Soil. Agriculture, 12(12), 2093. https://doi.org/10.3390/agriculture12122093





