Red Light Emitting Transition Metal Ion Doped Calcium Antimony Oxide for Plant Growth Lighting Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Procedure
2.2. Characterizations
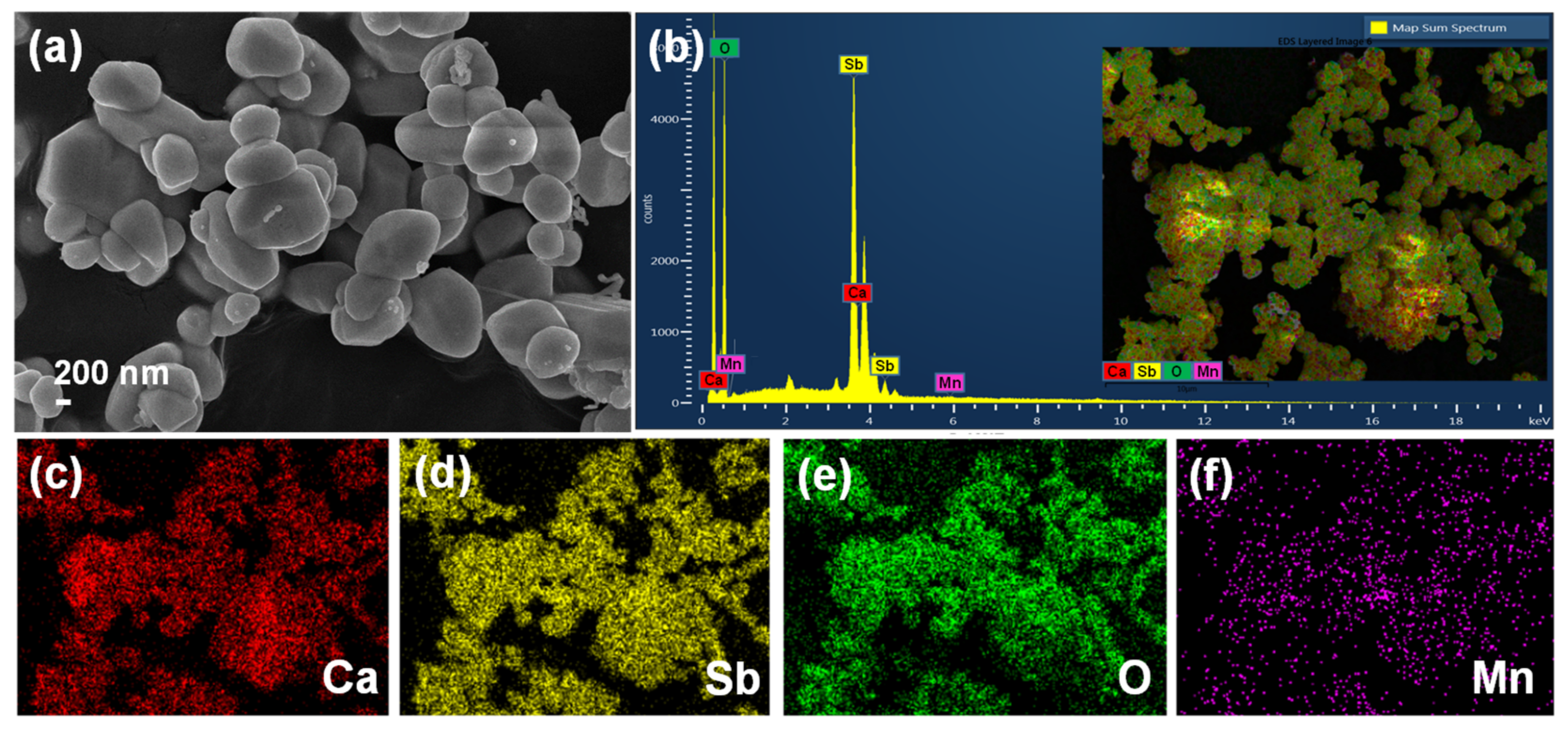
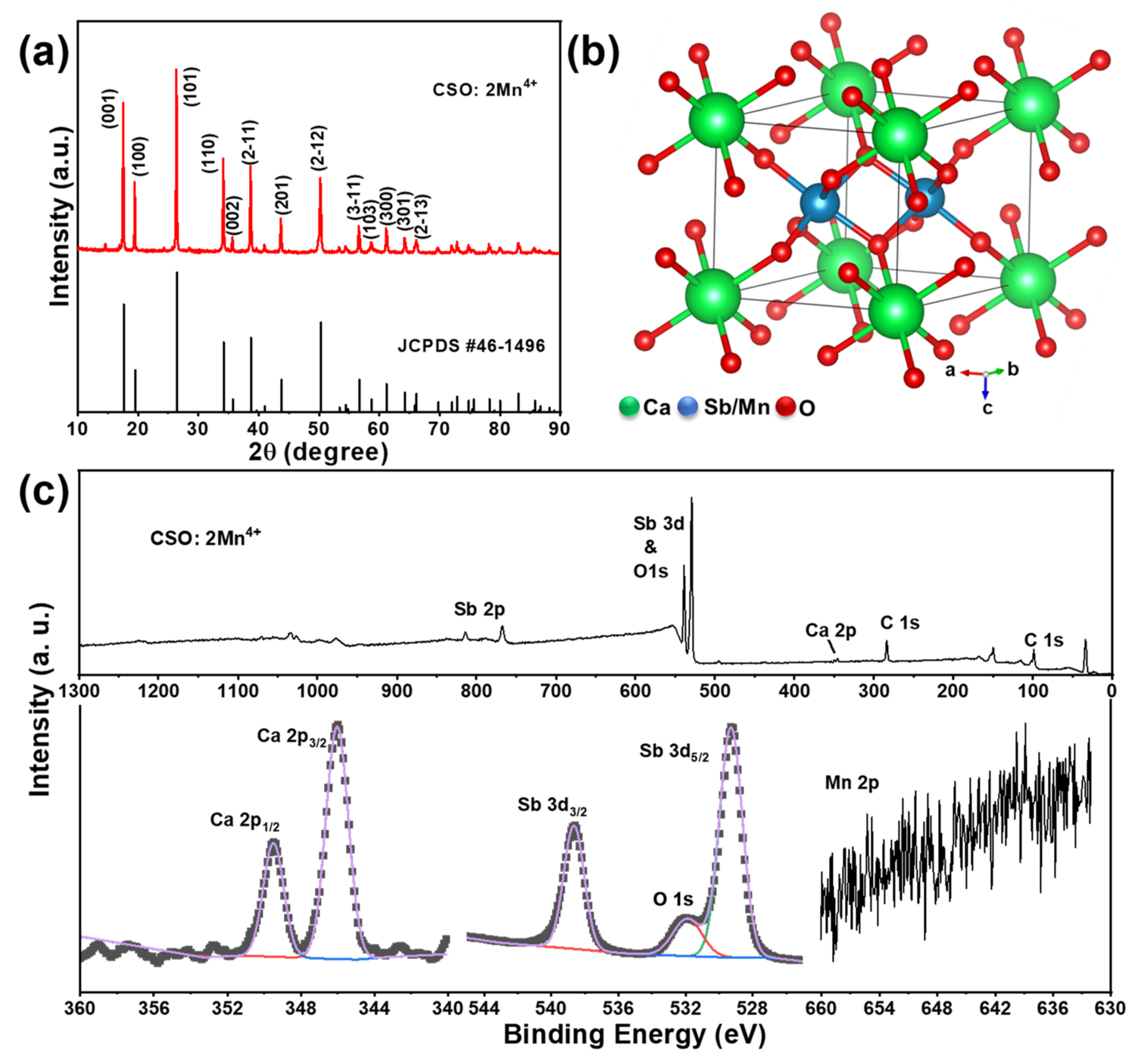
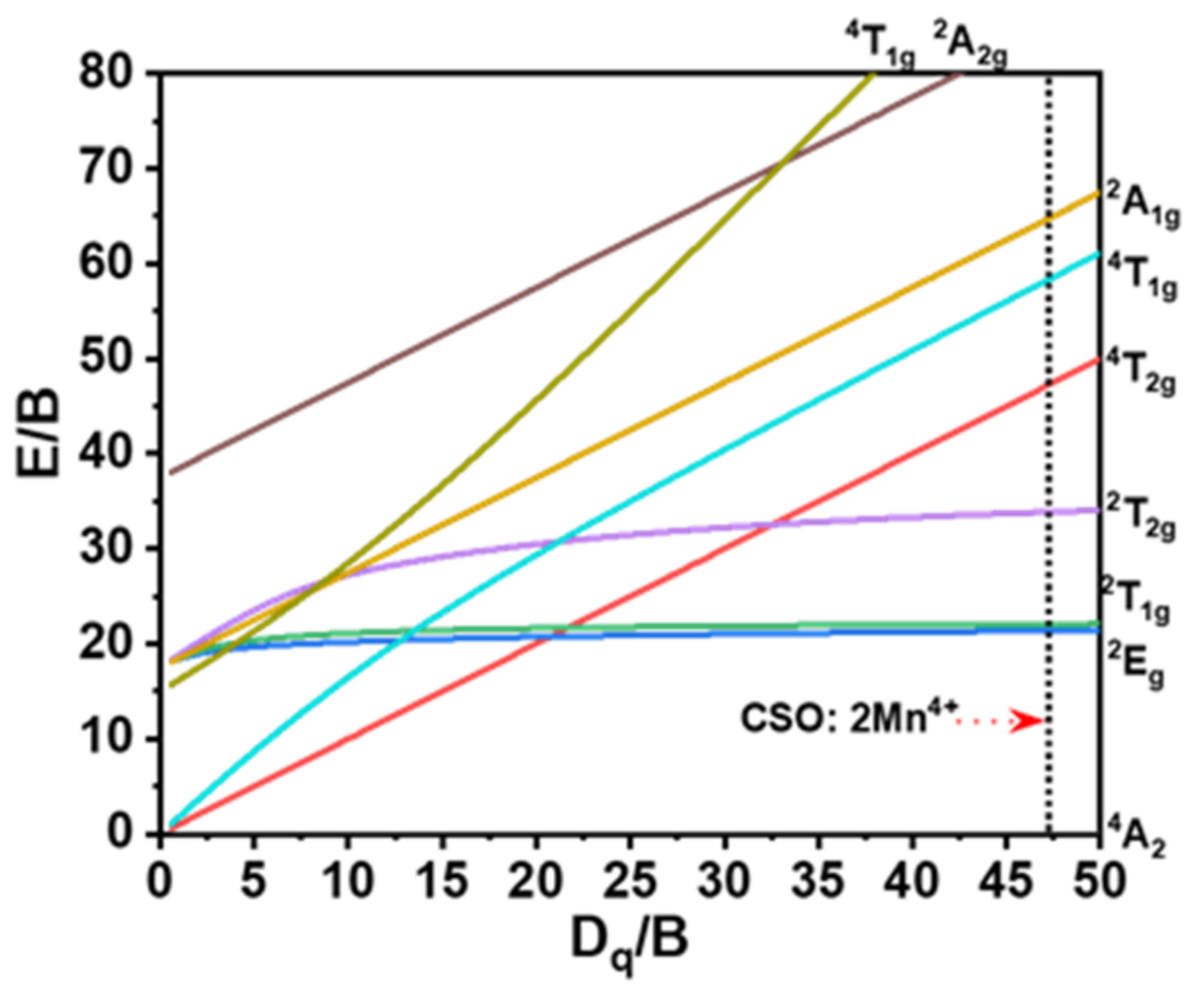
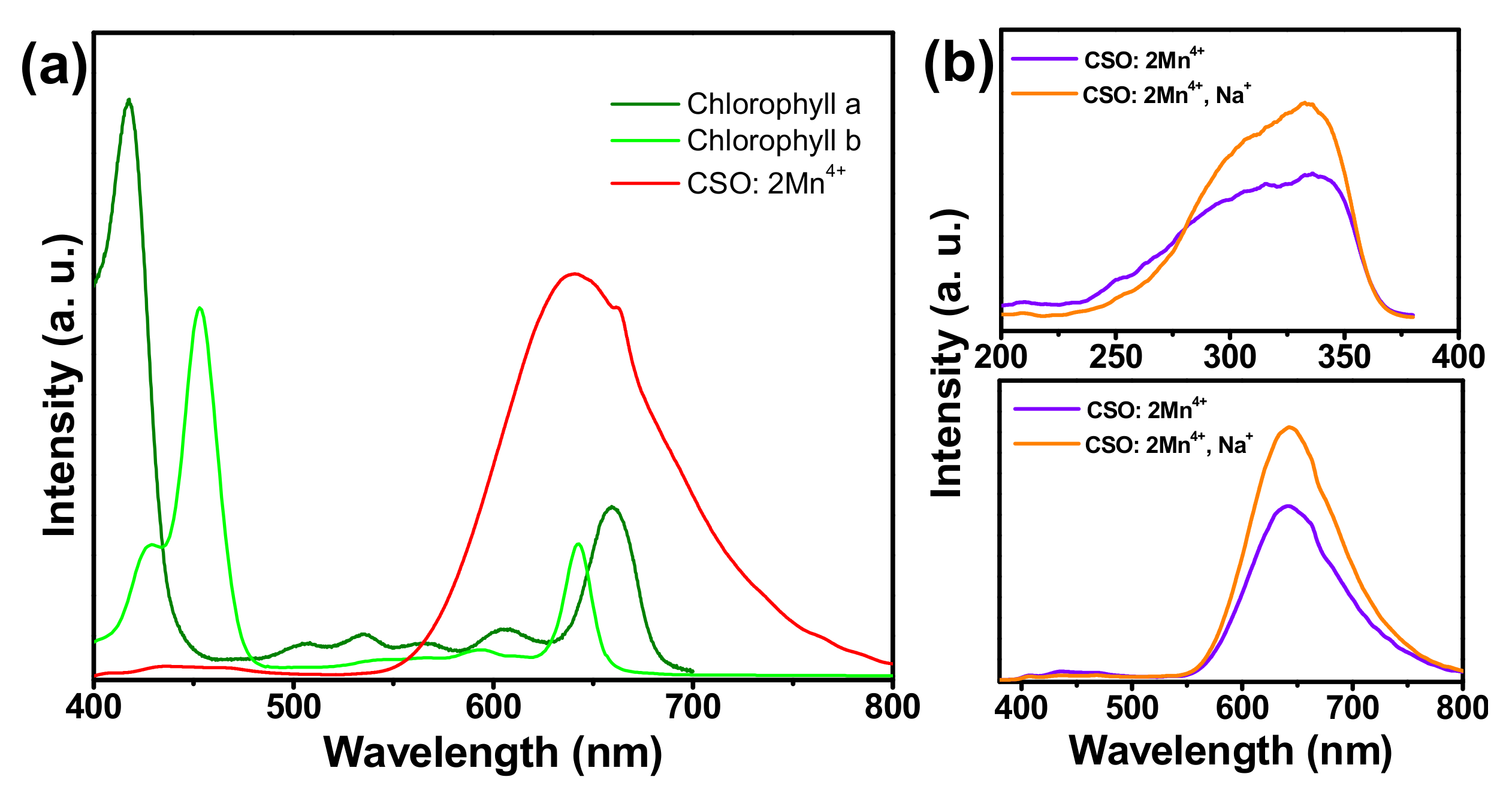
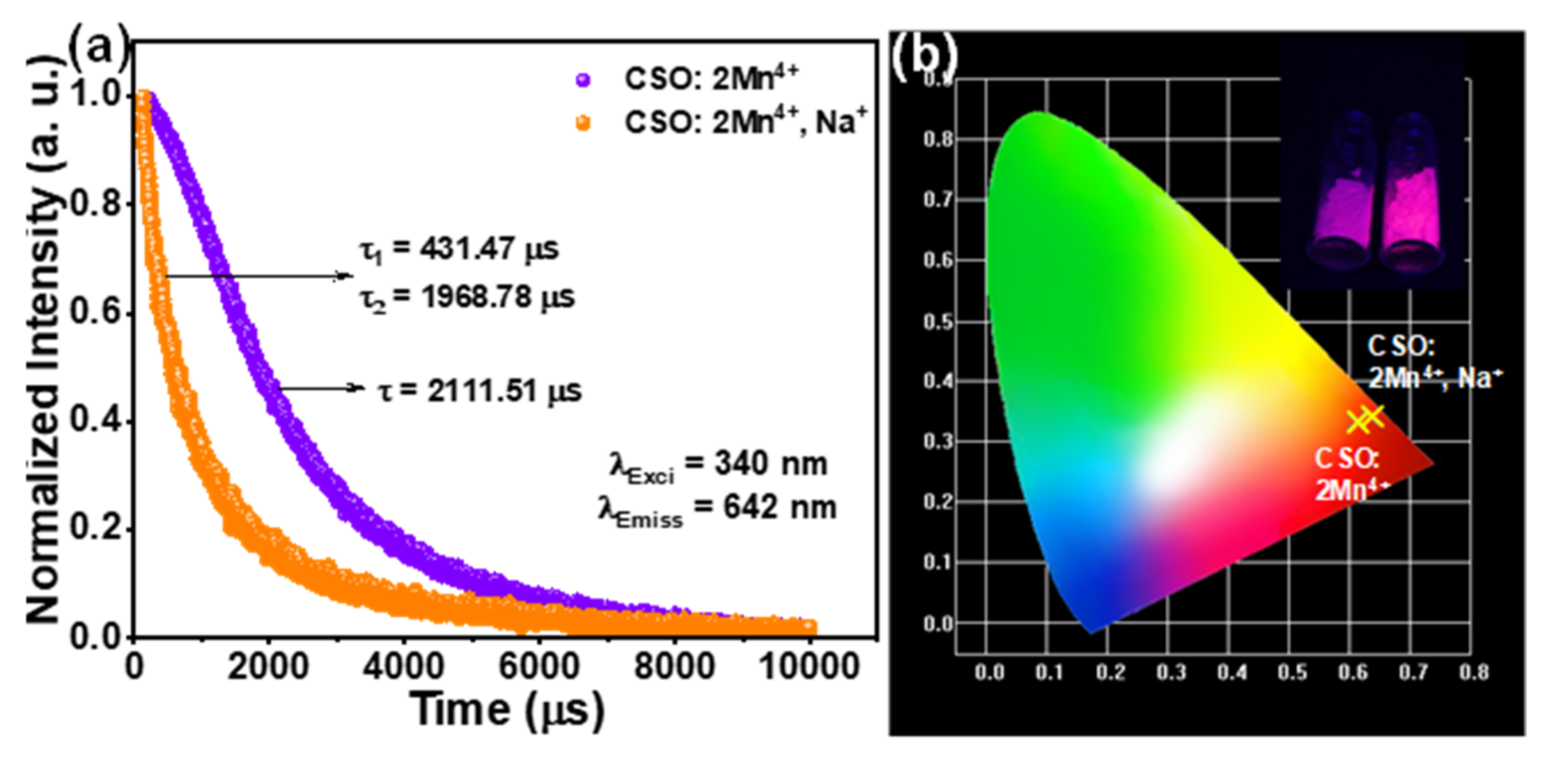
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dubey, V.; Som, S.; Kumar, V. Luminescent Materials in Display and Biomedical Applications; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Moraitis, P.; Schropp, R.E.I.; van Sark, W.G.J.H.M. Nanoparticles for Luminescent Solar Concentrators—A review. Opt. Mater. 2018, 84, 636–645. [Google Scholar] [CrossRef]
- Yu, M.; Yao, X.; Wang, X.; Li, Y.; Li, G. White-Light-Emitting Decoding Sensing for Eight Frequently-Used Antibiotics Based on a Lanthanide Metal-Organic Framework. Polymers 2019, 11, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuo, Y.; Brgoch, J. Opportunities for Next-Generation Luminescent Materials through Artificial Intelligence. J. Phys. Chem. Lett. 2021, 12, 764–772. [Google Scholar] [CrossRef]
- Timmermans, G.H.; Hemming, S.; Baeza, E.; van Thoor, E.A.J.; Schenning, A.P.H.J.; Debije, M.G. Advanced Optical Materials for Sunlight Control in Greenhouses. Adv. Opt. Mater. 2020, 8, 2000738. [Google Scholar] [CrossRef]
- Runkle, E.S.; Padhye, S.R.; Oh, W.; Getter, K. Replacing incandescent lamps with compact fluorescent lamps may delay flowering. Sci. Hortic. 2012, 143, 56–61. [Google Scholar] [CrossRef]
- Olle, M.; Viršile, A. The effects of light-emitting diode lighting on greenhouse plant growth and quality. Agric. Food Sci. 2013, 22, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Keoleian, G.A.; Saitou, K. Replacement policy of residential lighting optimized for cost, energy, and greenhouse gas emissions. Environ. Res. Lett. 2017, 12, 114034. [Google Scholar] [CrossRef]
- Singh, D.; Basu, C.; Meinhardt-Wollweber, M.; Roth, B. LEDs for energy efficient greenhouse lighting. Renew. Sustain. Energy Rev. 2015, 49, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Bayat, L.; Arab, M.; Aliniaeifard, S.; Seif, M.; Lastochkina, O.; Li, T. Effects of growth under different light spectra on the subsequent high light tolerance in rose plants. AOB Plants 2018, 10, ply052. [Google Scholar] [CrossRef]
- Monostori, I.; Heilmann, M.; Kocsy, G.; Rakszegi, M.; Ahres, M.; Altenbach, S.B.; Szalai, G.; Pál, M.; Toldi, D.; Simon-Sarkadi, L.; et al. LED Lighting–Modification of Growth, Metabolism, Yield and Flour Composition in Wheat by Spectral Quality and Intensity. Front. Plant Sci. 2018, 9, 605. [Google Scholar] [CrossRef]
- Rajapakse, N.C.; Shahak, Y. Light-Quality Manipulation by Horticulture Industry. In Annual Plant Reviews Volume 30: Light and Plant Development; Blackwell Publishing: Oxford, UK, 2007; pp. 290–312. [Google Scholar]
- Kalaitzoglou, P.; van Ieperen, W.; Harbinson, J.; van der Meer, M.; Martinakos, S.; Weerheim, K.; Nicole, C.C.S.; Marcelis, L.F.M. Effects of Continuous or End-of-Day Far-Red Light on Tomato Plant Growth, Morphology, Light Absorption, and Fruit Production. Front. Plant Sci. 2019, 10, 322. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Van Labeke, M.-C. Long-Term Effects of Red- and Blue-Light Emitting Diodes on Leaf Anatomy and Photosynthetic Efficiency of Three Ornamental Pot Plants. Front. Plant Sci. 2017, 8, 917. [Google Scholar] [CrossRef] [Green Version]
- Pavitra, E.; Raju, G.S.R.; Varaprasad, G.L.; Chodankar, N.R.; Rao, M.V.B.; Rao, N.M.; Park, J.Y.; Han, Y.-K.; Huh, Y.S. Desired warm white light emission from a highly photostable and single-component Gd2TiO5:Dy3+/Eu3+ nanophosphors for indoor illuminations. J. Alloys Compd. 2021, 875, 160019. [Google Scholar] [CrossRef]
- Rama Raju, G.S.; Pavitra, E.; Hwang, S.K.; Song, Y.H.; Park, J.Y.; Chodankar, N.R.; Ranjith, K.S.; Huh, Y.S.; Han, Y.-K. Development of dumbbell-shaped La2Si2O7:Eu3+ nanocrystalline phosphors for solid-state lighting applications. Ceram. Int. 2021, 47, 5812–5821. [Google Scholar] [CrossRef]
- Piao, X.; Horikawa, T.; Hanzawa, H.; Machida, K.-i. Photoluminescence Properties of Ca2Si5N8:Eu2+ Nitride Phosphor Prepared by Carbothermal Reduction and Nitridation Method. Chem. Lett. 2006, 35, 334–335. [Google Scholar] [CrossRef]
- Choi, K.S.; Jee, S.D.; Lee, J.P.; Kim, C.H. A Novel Synthetic Method of Sr2Si5N8:Eu2+ from SrSi2O2N2:Eu2+ by Carbo-Thermal Reduction and Nitridation. J. Nanosci. Nanotechnol. 2013, 13, 1867–1870. [Google Scholar] [CrossRef]
- Adachi, S. Review—Photoluminescence Properties of Cr3+-Activated Oxide Phosphors. ECS J. Solid State Sci. Technol. 2021, 10, 026001. [Google Scholar] [CrossRef]
- Van Quang, N.; Thi Huyen, N.; Tu, N.; Quang Trung, D.; Duc Anh, D.; Tran, M.T.; Hung, N.D.; Viet, D.X.; Huy, P.T. A high quantum efficiency plant growth LED by using a deep-red-emitting α-Al2O3:Cr3+ phosphor. Dalton Trans. 2021, 50, 12570–12582. [Google Scholar] [CrossRef] [PubMed]
- Huyen, N.T.; Tu, N.; Quang, N.V.; Quang Trung, D.; Tran, M.T.; Du, N.V.; Hung, N.D.; Viet, D.X.; Trung Kien, N.D.; Huy, P.T. Excellent Quantum Efficiency and Superior Color Purity Red-Emitting CaAl12O19–CaAl4O7–MgAl2O4:Mn4+ Phosphors for Plant Growth and High Color Rendering Index White Light-Emitting Diode Applications. ACS Appl. Electron. Mater. 2022, 4, 4322–4331. [Google Scholar] [CrossRef]
- Senden, T.; van Dijk-Moes, R.J.A.; Meijerink, A. Quenching of the red Mn4+ luminescence in Mn4+-doped fluoride LED phosphors. Light Sci. Appl. 2018, 7, 8. [Google Scholar] [CrossRef]
- Pavitra, E.; Raju, G.S.R.; Park, J.Y.; Hussain, S.K.; Chodankar, N.R.; Rao, G.M.; Han, Y.-K.; Huh, Y.S. An efficient far-red emitting Ba2LaNbO6:Mn4+ nanophosphor for forensic latent fingerprint detection and horticulture lighting applications. Ceram. Int. 2020, 46, 9802–9809. [Google Scholar] [CrossRef]
- Cao, R.; Fu, T.; Cao, Y.; Jiang, S.; Gou, Q.; Chen, Z.; Liu, P. Tunable emission, energy transfer, and charge compensation in the CaSb2O6:Eu3+, Bi3+ phosphor. J. Mater. Sci. Mater. Electron. 2016, 27, 3514–3519. [Google Scholar] [CrossRef]
- Li, P.; Peng, M.; Yin, X.; Ma, Z.; Dong, G.; Zhang, Q.; Qiu, J. Temperature dependent red luminescence from a distorted Mn4+ site in CaAl4O7:Mn4+. Opt. Express 2013, 21, 18943–18948. [Google Scholar] [CrossRef] [PubMed]
- Amarasinghe, D.K.; Rabuffetti, F.A. Bandshift Luminescence Thermometry Using Mn4+:Na4Mg(WO4)3 Phosphors. Chem. Mater. 2019, 31, 10197–10204. [Google Scholar] [CrossRef]
- Brik, M.G.; Pan, Y.X.; Liu, G.K. Spectroscopic and crystal field analysis of absorption and photoluminescence properties of red phosphor CaAl12O19:Mn4+ modified by MgO. J. Alloys Compd. 2011, 509, 1452–1456. [Google Scholar] [CrossRef]
- Medić, M.M.; Brik, M.G.; Dražić, G.; Antić, Ž.M.; Lojpur, V.M.; Dramićanin, M.D. Deep-Red Emitting Mn4+ Doped Mg2TiO4 Nanoparticles. J. Phys. Chem. C 2015, 119, 724–730. [Google Scholar] [CrossRef]
- Hu, J.; Huang, T.; Zhang, Y.; Lu, B.; Ye, H.; Chen, B.; Xia, H.; Ji, C. Enhanced deep-red emission from Mn4+/Mg2+ co-doped CaGdAlO4 phosphors for plant cultivation. Dalton Trans. 2019, 48, 2455–2466. [Google Scholar] [CrossRef]
- Sun, Z.; Lu, B.; Ren, G.; Chen, H. Synthesis of Green-Emitting Gd2O2S:Pr3+ Phosphor Nanoparticles and Fabrication of Translucent Gd2O2S:Pr3+ Scintillation Ceramics. Nanomaterials 2020, 10, 1639. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, H.; Xu, H.; Zhao, Z.; Bu, H.; Cao, X.; He, L.; Yang, Z.; Sun, J. Structure and photoluminescence of Eu3+ doped Sr2InTaO6 red phosphor with high color purity. RSC Adv. 2021, 11, 8282–8289. [Google Scholar] [CrossRef]
- Zhou, Q.; Dolgov, L.; Srivastava, A.M.; Zhou, L.; Wang, Z.; Shi, J.; Dramićanin, M.D.; Brik, M.G.; Wu, M. Mn2+ and Mn4+ red phosphors: Synthesis, luminescence and applications in WLEDs. A review. J. Mater. Chem. C 2018, 6, 2652–2671. [Google Scholar] [CrossRef]
- Haiyang, W.; Xing Wang, D. Phytochrome Signaling Mechanism. Arab. Book 2002, 2004. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Wang, Y.; Wen, Y.; Zhao, Z.; Liu, B.; Yang, Z. Tunable luminescence Y3Al5O12:0.06Ce3+, xMn2+ phosphors with different charge compensators for warm white light emitting diodes. Opt. Express 2012, 20, 21656–21664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fan, G.; Ruan, F. Charge compensating effect of alkali metal ions R+ (R = Li, Na, K) on the luminescence enhancement of CaAl11.9P0.1O19.1:Mn4+ red-emitting phosphor. Inorg. Chem. Commun. 2021, 132, 108860. [Google Scholar] [CrossRef]
- Gupta, S.K.; Sudarshan, K.; Yadav, A.K.; Gupta, R.; Bhattacharyya, D.; Jha, S.N.; Kadam, R.M. Deciphering the Role of Charge Compensator in Optical Properties of SrWO4:Eu3+:A (A = Li+, Na+, K+): Spectroscopic Insight Using Photoluminescence, Positron Annihilation, and X-ray Absorption. Inorg. Chem. 2018, 57, 821–832. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bharat, L.K.; Patnam, H.; Sokolov, A.; Gudkov, S.V.; Yu, J.S. Red Light Emitting Transition Metal Ion Doped Calcium Antimony Oxide for Plant Growth Lighting Applications. Agriculture 2022, 12, 2066. https://doi.org/10.3390/agriculture12122066
Bharat LK, Patnam H, Sokolov A, Gudkov SV, Yu JS. Red Light Emitting Transition Metal Ion Doped Calcium Antimony Oxide for Plant Growth Lighting Applications. Agriculture. 2022; 12(12):2066. https://doi.org/10.3390/agriculture12122066
Chicago/Turabian StyleBharat, Lankamsetty Krishna, Harishkumarreddy Patnam, Alexander Sokolov, Sergey V. Gudkov, and Jae Su Yu. 2022. "Red Light Emitting Transition Metal Ion Doped Calcium Antimony Oxide for Plant Growth Lighting Applications" Agriculture 12, no. 12: 2066. https://doi.org/10.3390/agriculture12122066
APA StyleBharat, L. K., Patnam, H., Sokolov, A., Gudkov, S. V., & Yu, J. S. (2022). Red Light Emitting Transition Metal Ion Doped Calcium Antimony Oxide for Plant Growth Lighting Applications. Agriculture, 12(12), 2066. https://doi.org/10.3390/agriculture12122066






